Abstract
Tumors stimulate angiogenesis to meet increasing nutrient and oxygen demands. In addition to their role in vascular remodeling, pro-angiogenic cytokines and effector cells contribute to an immune-inhibitory environment associated with advanced malignancies. Despite the critical role of angiogenesis in tumor growth and dissemination, most anti-angiogenic cancer therapies have had only limited success selectively targeting one of the many factors implicated in this process. Similarly, the effectiveness of tumor immunotherapies has been limited by tumor-mediated escape mechanisms and immune suppression. By combining the two strategies, however, anti-angiogenic immunotherapy offers the possibility to more robustly inhibit tumor angiogenesis and simultaneously impact the immune-inhibitory effects of the pro-angiogenic tumor milieu. These potential synergies make the combination of immunotherapy and anti-angiogenic treatment a promising avenue for future research.
Key words: angiogenesis, vaccine, immunotherapy, GM-CSF, angiopoietin
Tumor Angiogenesis
Angiogenesis is a critical part of tumor growth and dissemination. Tumors greater than two to three millimeters have oxygen and nutrient requirements that exceed those that can be met by diffusion alone.1 Consequently, they recruit, remodel and expand the existing vasculature to meet their metabolic demands. This process is critical to development,2 but occurs only rarely in the healthy adult: during endometrial proliferation through the menstrual cycle, and during the process of wound healing. Thus, the continued cycle of angiogenesis that occurs in the tumor microenvironment has been likened to “wounds that never heal.”3 Chronic inflammation, inhibition of cellular immune responses, and a dysfunctional and abnormal vasculature are all hallmarks of this pathologic environment.
Under normal conditions, angiogenesis is a complex process involving regulated changes in endothelial cell growth, survival, proliferation, migration and tube formation.4 These multiple steps require the concerted action of numerous cytokines, cell surface receptors and intracellular signaling cascades. Vascular endothelial growth factor-A (VEGF-A), originally identified as a vascular permeability factor,5,6 is an important cytokine mediator of tumor-driven angiogenesis.7 The remainder of the VEGF family of proteins (VEGF-B, C, D and E), placental growth factor (PlGF) and the angiopoietins are additional cytokines that have also been implicated in this process.8,9
The dependence of growing tumors on new blood vessel formation has made angiogenesis an appealing target for anti-cancer therapies. Most notably, a VEGF-A blocking antibody, bevacizumab, has demonstrated clinical benefit, improving survival in metastatic colon cancer.10 Bevacizumab has also demonstrated promise and benefit in other malignancies including lung, breast, renal cancers and glioblastoma.11–14 Tyrosine kinase inhibitors that impact angiogenesis such as sorafenib and sunitinib have also proved efficacious in diseases such as hepatocellular carcinoma and renal cell cancer.15–17 Other anti-angiogenic strategies, including monoclonal antibodies, kinase inhibitors, IgG fusion proteins, RNA-aptamers and RNA-interference are being actively pursued for these and many additional malignancies.9
Despite their promise and encouraging initial results, the benefits of existing anti-angiogenic therapies have been modest, with limited improvements in survival. There are many potential explanations for this short-term overall benefit. Existing therapies generally target only one or a single family of angiogenic mediators. In response, tumors may evade this inhibition by utilizing redundant pro-angiogenic pathways and cytokines, thus eventually resuming angiogenesis unabated.18–20 Moreover, tumors can potentially bypass angiogenic inhibitors by using autocrine loops and angiogenic factors sequestered in the tumor micro-environment, and thereby inaccessible to exogenous blockade.21,22
Tumor Immunotherapy
Evidence supporting the immune system's potential role in the treatment of established malignancy is rapidly expanding. Tumor-infiltrating lymphocytes have been associated with improved outcomes in various malignancies including melanoma and cancers of the ovary, esophagus, prostate, breast, kidney and colon.23–32 Two drugs used in the treatment of high-risk and advanced melanoma, interferon-α-2b and interleukin-2, are hypothesized to work by augmenting anti-tumor immunity,33,34 and an additional drug, the immunomodulatory anti-Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) antibody ipilimumab, was recently approved by the FDA based on its ability to increase survival in metastatic melanoma patients refractory to previous therapy.35 Finally, a prostate cancer dendritic cell vaccine targeting prostatic acid phosphatase was also recently approved by the FDA after a randomized trial demonstrated its ability to increase median survival by approximately 4 months in metastatic castrate-resistant prostate cancer patients.36
Although our ability to manipulate the immune system and identify potential tumor antigens has increased, tumor immunotherapies are still limited by the ability of advanced malignancies to evade immune recognition. Many potential tumor antigens are also present on normal tissue and can therefore induce immune tolerance; moreover, tumors can mutate and shift their antigenic profile, thereby evading immune attack.37 Additionally, in part because of a significant tumor burden, patients with advanced malignancies are often relatively immunocompromised. Cancers can themselves be immunosuppressive in a variety of ways: by releasing immunosuppressive cytokines; by inhibiting antigen processing and presentation; by increasing numbers of inhibitory T-regulatory cells; by recruiting tumor-infiltrating macrophages and myeloid-derived suppressor cells; and by attenuating immune-mediated cytotoxicity.37–50
Angiogenesis as a Target for Tumor-Immunotherapy
Pro-angiogenic cytokines, receptors and effector molecules may be particularly good targets of immune-based cancer therapy. As mentioned above, although angiogenesis is a normal physiologic process, it is tightly regulated4 and infrequently occurs in the healthy, non-menstruating adult. Consequently, anti-angiogenic immunotherapy can target a wide variety of tumor types yet be tumor-specific. In contrast to other tumor-specific therapies, targeting the tumor-associated vasculature in addition to the tumor itself may make anti-angiogenic immunotherapy more resistant to immune-escape mechanisms.51 The mediators of angiogenesis, including a number of normal cell types such as the vascular endothelium, do not likely possess the same degree of mutability as cancer cells.
Immune-based cancer therapies that target angiogenesis may potentiate a broader anti-tumor immune response52 by interfering with tumor-mediated immune inhibition. VEGF may functionally inhibit the immune system in part by preventing the maturation of dendritic cells53,54 and inhibiting early T-cell development.55 In a study of 19 patients with colon cancer, inhibiting VEGF with bevacizumab increased the antigen-presenting capacity of peripheral blood dendritic cells.56 The angiopoietins impact inflammation57 and affect immune trafficking due to their ability to increase expression of platelet endothelial cell adhesion molecule-1 (PECAM-1) and vascular endothelial (VE)-cadherin and decrease expression of vascular-cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1) and endothelial leukocyte adhesion molecule 1 (ELAM-1).58 Consequently, inhibiting angiopoietins or other angiogenic mediators may restore normal immune cell trafficking and increase numbers of tumor-infiltrating lymphocytes.59,60 For instance, mice with tumors engineered to express the inhibitory soluble angiopoietin receptor, tie-2, demonstrated increased survival dependent on increased numbers of tumor-infiltrating leukocytes.58
Angiogenic cytokines also help recruit and stimulate immune-inhibiting monocytes and myeloid suppressor cells. Angiopoietin-2 stimulates tie-2 expressing monocytes to suppress T-cell activation and promote regulatory T-cell activity.61,62 Populations of myeloid-suppressor cells that release immunosuppressive cytokines can be recruited by VEGF expression.63,64 Inhibition of VEGF with bevacizumab can reduce levels of these cells in the peripheral blood.56
In addition to the potential for antiangiogenic therapy to increase anti-tumor immunity, there is also evidence to suggest that immunologic approaches may be a more successful way of inhibiting tumor angiogenesis. As previously mentioned, existing anti-angiogenic therapies that only target a single agent are limited when tumors eventually utilize other pro-angiogenic mediators.65 Anti-angiogenic immunotherapy, in contrast, could potentially target multiple angiogenic mediators and attack tumor-associated stroma in addition to tumor cells.66,67 Indeed, poor penetration and resistance of tumor-associated stroma may contribute to the limited effectiveness of systemic anti-angiogenic therapy.22 Targeting immune-inhibiting monocytes68 and myeloid-suppressor cells could also more successfully inhibit tumor angiogenesis, as these cell types have been associated with resistance to anti-VEGF therapies.69 Finally, anti-angiogenic treatment may be most successful when administered constantly, preventing the re-growth of tumor vasculature during breaks in treatment.70 Immunotherapy could deliver this constant therapy without the need for repeated drug administration.
Evidence Supporting Anti-Angiogenic Immunotherapy
Studies have demonstrated the potential effectiveness of using immune therapy to target mediators of angiogenesis. Endothelial cell vaccines inhibited tumor growth and led to tumor destruction in mice.71–74 Other studies have used a variety of means to generate a specific anti-VEGF immune response that was successful in inhibiting implanted tumor cell-types including: colorectal, rhabdomyosarcoma, fibrosarcoma, hepatoma, melanoma, lung, ovarian, pancreatic and mammary cancer cells in mice75–78 and spontaneously arising sarcomas in dogs.79 A number of other pro-angiogenic molecules and receptors including VEGF receptor-2 (VEGFR2), tie-2, fibroblast growth factor (FGF) receptor-1, integrin Beta-3, vascular endothelial (VE)-cadherin and matrix-metalloproteinase-2 (MMP-2) were similarly targeted with success.77,80–85
Combined treatment with angiogenic inhibitors and anti-tumor immunotherapy has demonstrated initial efficacy in animal models. Vaccination with dendritic cells transfected with angiogenic cytokine and receptor mRNA in addition to total tumor mRNA demonstrated a synergistic anti-tumor effect.86 In a murine prostate cancer model, concomitant treatment with the anti-angiogenic tyrosine kinase inhibitor SU6668 and the recombinant immunomodulatory B7.2-IgG fusion protein significantly inhibited tumor growth to a greater degree than either treatment alone.87 Lymphocytes obtained from mice that received the combined treatment demonstrated a higher proliferative response to CD3 stimulation. Administration of a GM-CSF-secreting tumor cell vaccine in combination with VEGF-blockade significantly increased the survival of mice implanted with B16 melanoma and CT26 colon carcinoma cells, increasing overall numbers of tumor-infiltrating T-cells while decreasing tumor-infiltrating regulatory T cells.88 Treatment of mice with implanted NT2.5 breast cancer cells with DC101, an anti-angiogenic monoclonal antibody targeting VEGFR-2, increased the numbers of tumor-infiltrating lymphocytes. When combined with Her2/neu vaccination, DC101 induced tumor regression by augmenting anti-tumor immune responses,89 an effect that was not present in mice tolerant to Her2/neu. In another study, VEGF inhibition increased the infiltration of adoptively transferred T cells into tumor implants, increasing the efficacy of this treatment.90 Adding the CTLA-4 inhibiting antibody 9H10 to treatment of mice with DC101 and a dendritic cell vaccine led to rejection of established tumors, an effect that required the combination of all three treatments.91 Finally, the use of the immune-stimulating cytokines GM-CSF and IL-12 with anti-angiogenic factors endostatin and pigment epithelium-derived factor had synergistic anti-tumor effects in an established woodchuck hepatoma model.92 The combined treatment was able to induce activation of natural-killer cells and reduce expression of immune inhibitory CTLA-4 and programmed death (PD)-1 receptors compared with animals treated with immunotherapy alone.
In humans, the use of an autologous tumor cell vaccine engineered to express high levels of GM-CSF has demonstrated success in generating a coordinated anti-tumor immune response including a lymphocytic infiltrate in tumor metastases.93,94 Interestingly, some long-term surviving patients were also noted to develop tumor-associated vasculopathy with associated lymphocytic and granulocytic invasion. Further investigation identified VEGF family members and the angiopoietins as targets of immune recognition in these and other patients who demonstrated a prolonged response to the autologous vaccine.95 These generated antibodies demonstrated functional abilities to inhibit binding to the tie-2 receptor, downstream signaling, endothelial cell tube formation and macrophage chemotaxis. Of note was the ability of this autologous tumor cell vaccine to generate antibodies directed against a panel of angiogenic cytokines, potentially preventing the tumor-associated vasculature from eventually escaping immune recognition and angiogenic blockade in these patients. Autologous vaccination also led to antibodies directed against macrophage inhibitory factor (MIF) that functionally inhibited MIF-induced expression of tie-2 on monocytes and also inhibited the production of MMP-9. Thus, functional inhibition of monocytes as a result of this anti-MIF antibody response may act in concert with antibodies directed pro-angiogenic cytokines to inhibit tumor angiogenesis.
Future Prospects and Conclusion
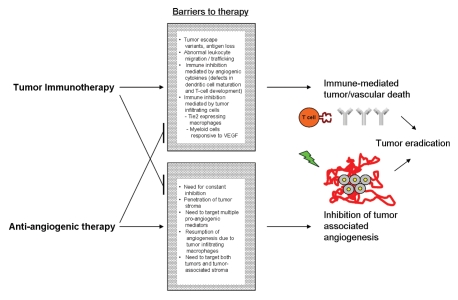
The number of anti-angiogenic therapies available or undergoing active investigation has rapidly expanded in recent years, as has the potency and potential impact of anti-tumor immunotherapies. Whether these existing and investigational therapies can be used in tandem for a synergistic benefit is of much interest. Combining anti-angiogenic treatment with tumor immunotherapy may help circumvent the weaknesses of either treatment administered individually (Fig. 1).
Figure 1.
combined use of tumor immunotherapy and anti-angiogenic therapy may help overcome barriers to successful tumor eradication.
Future work should delineate the specific types of anti-angiogenic treatments and anti-tumor immunotherapies that work best together and the optimal timing of each therapy when used in combination. Alternatively, angiogenic mediators can be targeted directly with immunotherapeutic approaches. Anti-angiogenic immunotherapy may also be successful when combined with conventional tumor-directed treatments such as chemotherapy and radiation, thus increasing the ability to target the tumor and tumor-stroma simultaneously.96 Although the antitumor effects of these different treatments may be additive or even synergistic, their toxicities are likely to be very different, thereby increasing the potential therapeutic benefit.
Research continues to identify the complex interactions that exist between cancer, angiogenesis and the immune system. As wounds that never heal, cancer's continued angiogenic drive incites inflammation and recruits systemic mediators to aid in tumor growth and metastasis. These factors contribute to the abnormal tumor microenvironment and at least partially mediate the resistance of advanced malignancy to conventional and experimental treatments. Anti-angiogenic immunotherapy could potentially circumvent multiple aspects of this resistance to provide more effective cancer treatment.
Acknowledgments
Supported by the HHMI (J.S.) and CA78378, CA111506, AI29530, the Leukemia and Lymphoma Society, the Melanoma Research Alliance, and the Research Foundation for the Treatment of Ovarian Cancer (G.D.).
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 5.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 6.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69:4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 8.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 9.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 12.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 13.Vredenburgh JJ, Desjardins A, Herndon JE, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 14.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow LQM, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 16.Hasskarl J. Sorafenib. Results Cancer Res. 2010;184:61–70. doi: 10.1007/978-3-642-01222-8_5. [DOI] [PubMed] [Google Scholar]
- 17.Longo R, D'Andrea MR, Sarmiento R, Salerno F, Gasparini G. Integrated therapy of kidney cancer. Ann Oncol. 2007;18:141–148. doi: 10.1093/annonc/mdm244. [DOI] [PubMed] [Google Scholar]
- 18.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Gaur P, Bose D, Samuel S, Ellis LM. Targeting tumor angiogenesis. Semin Oncol. 2009;36:12–19. doi: 10.1053/j.seminoncol.2009. [DOI] [PubMed] [Google Scholar]
- 20.Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PAE, Robinson BA, et al. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200:183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- 21.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 22.Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281:951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 23.Clark WH, Elder DE, Guerry D, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 24.Clemente CG, Mihm MC, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AIDCNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Marrogi AJ, Munshi A, Merogi AJ, Ohadike Y, El-Habashi A, Marrogi OL, et al. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer. 1997;74:492–501. doi: 10.1002/(SICI)1097-0215 (19971021)74:5<492::AID-IJC3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Mihm MC, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 27.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 28.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 29.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 31.Vesalainen S, Lipponen P, Talja M, Syrjänen K. Histological grade, perineural infiltration, tumourinfiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30:1797–1803. doi: 10.1016/0959-8049(94) E0159-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 33.Moschos SJ, Edington HD, Land SR, Rao UN, Jukic D, Shipe-Spotloe J, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 34.Riker AI, Radfar S, Liu S, Wang Y, Khong HT. Immunotherapy of melanoma: a critical review of current concepts and future strategies. Expert Opin Biol Ther. 2007;7:345–358. doi: 10.1517/14712598.7.3.345. [DOI] [PubMed] [Google Scholar]
- 35.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2011;201:2517–2526. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 37.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodmer WF, Browning MJ, Krausa P, Rowan A, Bicknell DC, Bodmer JG. Tumor escape from immune response by variation in HLA expression and other mechanisms. Ann NY Acad Sci. 1993;690:42–49. doi: 10.1111/j.1749-6632.1993.tb43994.x. [DOI] [PubMed] [Google Scholar]
- 39.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 40.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 41.Hatfield P, Merrick A, Harrington K, Vile R, Bateman A, Selby P, et al. Radiation-induced cell death and dendritic cells: potential for cancer immunotherapy? Clin Oncol. 2005;17:1–11. doi: 10.1016/j.clon.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–331. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 47.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/S0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 48.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mulé JJ, Rosenberg SA, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viguier M, Lemaître F, Verola O, Cho MS, Gorochov G, Dubertret L, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 50.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 51.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 52.Seavey MM, Paterson Y. Anti-Angiogenesis immunotherapy induces epitope spreading to Her-2/neu resulting in breast tumor immunoediting. Breast Cancer (London) 2009;1:19–30. doi: 10.2147/bctt.s6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 54.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappaB activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 55.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 56.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Melani C, Stoppacciaro A, Foroni C, Felicetti F, Caré A, Colombo MP. Angiopoietin decoy secreted at tumor site impairs tumor growth and metastases by inducing local inflammation and altering neoangiogenesis. Cancer Immunol Immunother. 2004;53:600–608. doi: 10.1007/s00262-004-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dirkx AEM, oude Egbrink MGA, Castermans K, van der Schaft DWJ, Thijssen VLJL, Dings RPM, et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621–630. doi: 10.1096/fj.05-4493com. [DOI] [PubMed] [Google Scholar]
- 60.Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 61.Coffelt SB, Chen YY, Muthana M, Welford AF, Tal AO, Scholz A, et al. Angiopoietin 2 Stimulates TIE2-Expressing Monocytes To Suppress T Cell Activation and To Promote Regulatory T Cell Expansion. J Immunol. 2011;186:4183–4190. doi: 10.4049/jimmunol.1002802. [DOI] [PubMed] [Google Scholar]
- 62.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, et al. Angiopoietin-2 Regulates Gene Expression in TIE2-Expressing Monocytes and Augments Their Inherent Proangiogenic Functions. Cancer Res. 2010;70:5270–5280. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- 63.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 65.Kerbel RS. Therapeutic implications of intrinsic or induced angiogenic growth factor redundancy in tumors revealed. Cancer Cell. 2005;8:269–271. doi: 10.1016/j.ccr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Franses JW, Baker AB, Chitalia VC, Edelman ER. Stromal endothelial cells directly influence cancer progression. Sci Transl Med. 2011;3:66. doi: 10.1126/scitranslmed.3001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 68.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 70.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–740. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 71.Chen XY, Zhang W, Zhang W, Wu S, Bi F, Su YJ, et al. Vaccination with viable human umbilical vein endothelial cells prevents metastatic tumors by attack on tumor vasculature with both cellular and humoral immunity. Clin Cancer Res. 2006;12:5834–5840. doi: 10.1158/1078-0432.CCR-06-1105. [DOI] [PubMed] [Google Scholar]
- 72.Okaji Y, Tsuno NH, Kitayama J, Saito S, Takahashi T, Kawai K, et al. Vaccination with autologous endothelium inhibits angiogenesis and metastasis of colon cancer through autoimmunity. Cancer Sci. 2004;95:85–90. doi: 10.1111/j.1349-7006.2004.tb03175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scappaticci FA, Nolan GP. Induction of anti-tumor immunity in mice using a syngeneic endothelial cell vaccine. Anticancer Res. 2003;23:1165–1172. [PubMed] [Google Scholar]
- 74.Wei YQ, Wang QR, Zhao X, Yang L, Tian L, Lu Y, et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med. 2000;6:1160–1166. doi: 10.1038/80506. [DOI] [PubMed] [Google Scholar]
- 75.Bequet-Romero M, Ayala M, Acevedo BE, Rodríguez EG, Ocejo OL, Torrens I, et al. Prophylactic naked DNA vaccination with the human vascular endothelial growth factor induces an anti-tumor response in C57Bl/6 mice. Angiogenesis. 2007;10:23–34. doi: 10.1007/s10456-006-9062-9. [DOI] [PubMed] [Google Scholar]
- 76.Rad FH, Le Buanec H, Paturance S, Larcier P, Genne P, Ryffel B, et al. VEGF kinoid vaccine, a therapeutic approach against tumor angiogenesis and metastases. Proc Natl Acad Sci USA. 2007;104:2837–2842. doi: 10.1073/pnas.0611022104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang B, Kaumaya PTP, Cohn DE. Immunization with synthetic VEGF peptides in ovarian cancer. Gynecol Oncol. 2010;119:564–570. doi: 10.1016/j.ygyno.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei YQ, Huang MJ, Yang L, Zhao X, Tian L, Lu Y, et al. Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci USA. 2001;98:11545–11550. doi: 10.1073/pnas.191112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamstock D, Elmslie R, Thamm D, Dow S. Evaluation of a xenogeneic VEGF vaccine in dogs with soft tissue sarcoma. Cancer Immunol Immunother. 2007;56:1299–1309. doi: 10.1007/s00262-007-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hou J, Tian L, Wei Y. Cancer immunotherapy of targeting angiogenesis. Cell Mol Immunol. 2004;1:161–166. [PubMed] [Google Scholar]
- 81.Liu JY, Wei YQ, Yang L, Zhao X, Tian L, Hou JM, et al. Immunotherapy of tumors with vaccine based on quail homologous vascular endothelial growth factor receptor-2. Blood. 2003;102:1815–1823. doi: 10.1182/blood-2002-12-3772. [DOI] [PubMed] [Google Scholar]
- 82.Luo Y, Wen YJ, Ding ZY, Fu CH, Wu Y, Liu JY, et al. Immunotherapy of tumors with protein vaccine based on chicken homologous Tie-2. Clin Cancer Res. 2006;12:1813–1819. doi: 10.1158/1078-0432.CCR-05-1990. [DOI] [PubMed] [Google Scholar]
- 83.Okaji Y, Tsuno NH, Saito S, Yoneyama S, Tanaka M, Nagawa H, et al. Vaccines targeting tumour angiogenesis—a novel strategy for cancer immunotherapy. Eur J Surg Oncol. 2006;32:363–370. doi: 10.1016/j.ejso.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 84.Wang YS, Wang Gq, Wen YJ, Wang L, Chen Xc, Chen P, et al. Immunity against tumor angiogenesis induced by a fusion vaccine with murine beta-defensin 2 and mFlk-1. Clin Cancer Res. 2007;13:6779–6787. doi: 10.1158/1078-0432.CCR-07-1587. [DOI] [PubMed] [Google Scholar]
- 85.Zhao Z, Yao Y, Ding Z, Chen X, Xie K, Luo Y, et al. Antitumour immunity mediated by mannan-modified adenovirus vectors expressing VE-cadherin. Vaccine. 2011;29:4218–4224. doi: 10.1016/j.vaccine.2011.03.109. [DOI] [PubMed] [Google Scholar]
- 86.Nair S, Boczkowski D, Moeller B, Dewhirst M, Vieweg J, Gilboa E. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood. 2003;102:964–971. doi: 10.1182/blood-2002-12-3738. [DOI] [PubMed] [Google Scholar]
- 87.Huang X, Raskovalova T, Lokshin A, Krasinskas A, Devlin J, Watkins S, et al. Combined antiangiogenic and immune therapy of prostate cancer. Angiogenesis. 2005;8:13–23. doi: 10.1007/s10456-005-2893-y. [DOI] [PubMed] [Google Scholar]
- 88.Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12:6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 89.Manning EA, Ullman JGM, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–3959. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 90.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pedersen AE, Buus S, Claesson MH. Treatment of transplanted CT26 tumour with dendritic cell vaccine in combination with blockade of vascular endothelial growth factor receptor 2 and CTLA-4. Cancer Lett. 2006;235:229–238. doi: 10.1016/j.canlet.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 92.Huang KW, Wu HL, Lin HL, Liang PC, Chen PJ, Chen SH, et al. Combining antiangiogenic therapy with immunotherapy exerts better therapeutical effects on large tumors in a woodchuck hepatoma model. Proc Natl Acad Sci USA. 2010;107:14769–14774. doi: 10.1073/pnas.1009534107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schoenfeld J, Jinushi M, Nakazaki Y, Wiener D, Park J, Soiffer R, et al. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 2010;70:10150–10160. doi: 10.1158/0008-5472.CAN-10-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]