Abstract
The stunning complexity of the resident microbiota and the intricate pathways of microbial and host interactions provide a massive adaptive capacity for mammals. In this addendum we reflect on our recent publication on Toll-like receptor 2 deficiency related colonic mucosal epigenetic, immunologic and microbiomic changes. Our findings underscored the tremendous flexibility of the gut and its microbiota. This flexibility can provide means to overcome significant environmental or genetic challenges. In the meantime, the challenged intestinal system may become vulnerable to otherwise tolerable insults. In such instances, the fine-tuned mutualistic balance between the gut and its microflora may collapse leading to dysbiosis and disease. The ultimate challenge for biomedical research in these cases is to find optimal means for the restoration and maintenance of healthy host physiology.
Keywords: gut, microbiome, toll-like receptor 2, adaptation, environment, inflammatory bowel diseases, obesity
The Mammalian Gut and Its Microbiota
The discovery of penicillin by Sir Alexander Fleming was arguably the biggest breakthrough in biomedical sciences during the 20th century. The finding rooted in his ingenious recognition of antibiosis rather than a lucky accident from keeping an untidy lab as is frequently mentioned in medical history. Although Fleming's work highlighted the importance of interspecies relationships between living organisms, the explosion of molecular biology and genetics specifically has shifted the focus of the biomedical field during the second half of the last century. However, with the completion of the human genome project and the remarkably advanced technological repertoire of molecular biology, the residing microfloras of mammals and the human “superorganism” has been rediscovered.1 The era of metagenomics has arrived.2,3 We realized that our evolution was intertwined with the development and maturation of the extensively complex microbial communities (see Parker W.: http://evmedreview.com/?p = 457) present on our direct (skin) and indirect (intestinal tract) communication surfaces with the environment. The intestinal tract specifically is astonishing in respect of its intercalating relationships with the luminal microflora. Gut bacteria are recognized to influence the maturation of the intestine and the immune system,4,5 modify energy balance and metabolism,6,7 along with controlling pathogen colonization and resistance.8,9 These interactions are manifested through hundreds to thousands of bacterial species, the overwhelming majority of which belong to the Firmicutes and Bacteroidetes phyla.2,10 The less studied viral11 and fungal species12 add to the vast complexity of the mammalian commensal microflora and its communication with the host, where complex polygenic traits shape the microbiota composition.13
Adaptation to Physiologic Changes in the Gut and Its Microbes
The phenotype of an organism is the physical manifestation of its large structural/functional (“omics”) networks from genomics to metabolomics.14 In the case of multiorganic organisms such networks are vastly multifaceted. This intricacy is probably most astounding in humans where the “superorganism” can be envisioned as the result of the multiorganic physical characteristics in association with the thousands of phenotypes of its resident microbes. Considering this vast complexity, the myriads of molecular, cellular, inter-cellular and inter-organic communications (where stochastic processes are inherently present15), one can realize that each and every human is unique by phenotype.
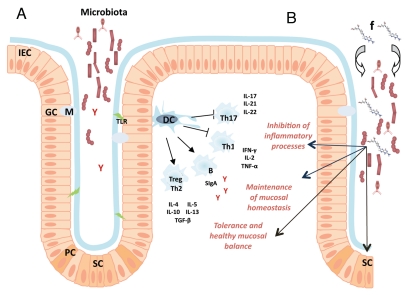
The phenotype of an organism also provides the capability for the maintenance of life (energy/metabolic support) and adaptation to environment. In mammals and humans the first layer of communication with the environment is through surfaces inhabited by commensal micro-floras composed mostly of unicellular organisms. The adaptive capacity of these microorganisms to environmental challenges and their metabolic plasticity at the cellular level is much higher than that of mammalian cells. In the meantime, mammalian differentiation into a multicellular and multiorganismic organism provides several supra-cellular layers for adaptation to-, as well as for isolation from the surroundings. A depictive example for the differences between unicellular and multiorganic adaptive capacities is calcium (Ca2+) homeostasis in yeast compared to mammals. In Saccharomyces cerevisiae, the action of a small group of intracellular Ca2+ transporters maintain the resting cytosolic Ca2+ level between 50 and 200 nM when environmental Ca2+ concentrations range from <1 µM to >100 mM (over 5 orders of magnitude).16 On the contrary, mammalian cells can only tolerate minimal shifts in extracellular Ca2+ concentration. Therefore, mammalian cells are much less adaptive to extracellular environment changes at the cellular level than unicellular organisms. However, the highly differentiated mammalian body (with its absorptive/hormonal/skeletal systems) can maintain the strict regulation of extracellular Ca2+ required by its cells. Hence, the supra-cellular layers of mammalian regulation not only match, but even surpass the adaptive capacity of unicellular organisms. Naturally, the multiorganismic regulation not only provides added capacity for adaptation, but also facilitates differentiated levels of functioning such as nerve conduction and muscle contraction, which actually rely on the tight control of the extracellular environment (as in the case of Ca2+, for instance). On one hand, the adaptive capacity for single mammalian cells is limited secondary to their differentiated state. On the other hand, this differentiation provides a higher level of physiologic functioning for the multi-organic organism and supports its separation from the environment. Within this separation, the residing microbiota at environmental communication surfaces of humans provides the first line of buffering by its vast unicellular adaptive capacity in concert with host molecules and cells that have a prioritized function in regards to microbial pattern recognition (Fig. 1A). The sophisticated network of host-microbial interactions contribute to immunologic maturation and homeostasis.17 This interaction manifests itself both through the innate and the adaptive immune system12,18,19 and is highly responsive to environmental/nutritional changes supporting “super-organismic” adaptation.20–22 The nutritional supplementation of folate may be a good example to illustrate this complexity of physiologic responses to environmental stimuli. While many bacterial species can produce folic acid, others require it from an exogenous source. Observations in insect gut microbiota revealed symbiotic relationships between bacteria based on their capacity for folate production.23 Folic acid may also support the growth of mutant microbe strains that are otherwise nonviable.24 Such biological interactions are likely to occur in the mammalian intestinal microbiome. Exogenous folate supplementation may affect these relationships and influence microbiota composition (Fig. 1B). Folate derivatives are essential cofactors in one-carbon (C1) transfer reactions. C1 transfer is required for the synthesis of a variety of different compounds (methionine and purines)25 and is necessary for epigenetic modifications, such as DNA methylation both in prokaryotes and eukaryotes,26 and histone methylation in eukaryotes.27 C1 dependent processes in the host may be modified by folate supplementation in critical periods of development.28 These processes may impact inter-microbial communications (such as defense mechanism against phages in bacteria29), intestinal development, immunity, and modify microbiota composition (Fig. 1B).30–32 Not surprisingly, plasma folate levels have been related to fecal bacterial composition changes in pregnant women recently.33 These studies on one single vitamin/micronutrient model and illustrate the close to incomprehensible complexity of host-environment interactions in the human “superorganism”.
Figure 1.
Schematic presentation of environmental, microbial and host interactions at the colonic mucosa. (a) The mucosa associated microbiota, the mucosal anatomy and the communicating immune system. Intestinal epithelial cells (IEC) interact with the residing microbiota through the cell surface mucous (CSM) layer, both playing essential roles in the maintenance of mucosal homeostasis.43,44 Commensal bacteria interact with intraepithelial lymphocytes,45 promote tolerogenic dendritic cells (DC) and induce the stimulation of regulatory T cells (Treg) and T helper 2 (Th2) cells.12,46 Anti-inflammatory cytokines and mediators, such as interleukin (IL) -4, -5, -10, -13 and transforming-growth-factor-β (TGFβ), are released and these molecules help maintain the healthy balance of the intestinal mucosa.47 Tolerogenic DCs also inhibit pro-inflammatory responses mediated by Th1 and Th17 cells.48 Toll-like receptor (TLR and green lightning) signaling pathways induce upregulation of antimicrobial peptides and chemoattractants, promote IEC survival (by activation of anti-apoptotic genes) and help maintain the mucosal barrier.49 Harmful bacteria are also restricted by soluble immunoglobulin-A (SIgA, red Y) produced by mucosal B cells (B).50 These mechanisms contribute to sustaining mucosal homeostasis, immunologic tolerance and prevent inflammation in a healthy organism. (B) Exogenous folate (f and also depicted by its graphic chemical structure) can act on multiple layers of the intricate network described in (A) and in the text of the manuscript. Folate can influence microbial interactions (thick arrows) and IEC regeneration through the modification of one carbon transfer mediated reactions.51 It may also impact dendritic cell responses and epithelial cell regeneration through stem cells (SC) by influencing epigenetic processes52,53 (black linear arrows).
Adaptation to Pathologic or Uncommon Changes in the Gut and its Microbiota
In physiology, where events usually follow a Gaussian (bell shaped) distribution, normal and abnormal are relative terms. These terms are commonly separated by their frequency in a population and their relative negative effect. For example, geneticists define a change in DNA that occurs with less than 1% frequency in a single species as a mutation. Changes occurring more frequently are designated as polymorphisms. Mutations or abnormalities are usually considered to have negative effects, although it is well know that those can induce positive deviation from “normal” as well. For example, we generally do not think of geniuses the same way as of mentally impaired individuals. In the meantime, both are equally rare and are “abnormal” deviations from the average, but in opposite directions. In both cases, the phenotype related to a mutation or an abnormality is the direct result of the absent or abnormal structure/function as well as the comprehensive physical manifestation of the compensatory mechanisms of the mutated/abnormally inflicted organism. The Ca2+ homeostasis example of the previous chapter can demonstrate the differences in adaptive capacity between unicellular and multiorganismic living beings to such mutations/abnormalities. Saccharomyces cerevisiae strains can easily tolerate the deletion of pmr1, the primary Ca2+ ATPase of the Golgi apparatus. Pmr1 knockouts only show a phenotype in extremely low or high extracellular Ca2+ environments. Their adaptive capacity is restricted, but this is not apparent under usual circumstances. Some strains of S. cerevisiae can even tolerate the deletion of their second major Ca2+ ATPase, pmc1 (i.e., viable pmr1/pmc1 double knockouts). In such strains the genetic absence or pharmacological inhibition of the third major intracellular Ca2+ transporter (Ca2+/H+ antiporter, vcx1) is lethal. These observations underscore that the phenotype of pmr1Δ S. cerevisiae is not only the result of Pmr1 absence, but also the concerted interaction of Pmc1 and Vcx1 to maintain viability in respect to Ca2+ homeostasis.16 The mammalian ortholog of pmr1 is the secretory pathway Ca2+ ATPase, Spca1.34 The complete absence of Spca1 induces midgestational lethality in mice35 demonstrating a significantly decreased adaptive capacity to the loss of this molecule in mammals compared to yeast. Haploinsufficiency of Spca1 induces squamous cell tumors in heterozygous adult mice,35 and it leads to Hailey-Hailey disease, an epidermal acantholytic disorder in humans, but all other tissues are phenotypically normal.36 Therefore, while mammals have a decreased adaptive capacity to tolerate the loss of Spca1 compared to unicellular organisms, their differentiation at the cellular and organ level has provided the adaptive capacity (no obvious deviation from the “normal”, i.e., normal phenotype) to the partial loss of this Ca2+ transporter (except for the skin).36 Even in the skin, it is only with age or noxious insults that symptomatology develops. This example also demonstrates that the fine-tuned physiology of the mammalian skin significantly differs between murine and human (tumors versus epidermal acantholysis in response to Spca1/hSPCA1 haploinsufficiency) “superorganisms”. Consequently, while murine models are extremely valuable in respect to human physiology and pathophysiology, it is obvious that results from mouse experiments should be interpreted with caution. Nevertheless, the Pmr1/Spca1 illustration nicely demonstrates the complex adaptive capacity to pathologic changes both at the microorganism/microbiota and the host level in mammals. This example also shows that once a negative mutation/abnormality occurs, the phenotype of the organism has a restricted capacity to tolerate further biological or environmental challenges.
The Remarkable Adaptation of the Colonic Mucosa to Toll-Like Receptor 2 Deficiency in Mice
It was with the afore described concepts in genetics, microbiology and mammalian biology that we recently approached the colonic mucosa of Toll-like receptor 2 (Tlr2) deficient C57BL/6 murine “superorganisms”.37 Just as pmr1 knockout yeast or Hailey-Hailey disease patients in remission, the phenotype of Tlr2-/- mice is normal under regular housing conditions (normal environment). However, in environmental or nutritional stress Tlr2-/- mice have variable phenotypes compared to wild type (WT). Additionally, murine strains respond to the loss of Tlr2 differently arguing for genetic background (or “complex polygenic traits”13) to influence the phenotype of the animals. Therefore, the adaptive capacity to Tlr2 loss is diverse even between strains of the same species of mouse “superorganisms”. C57BL/6 Tlr2-/- mice specifically are resistant to high fat and carbohydrate induced obesity.38 This is an abnormality even if the phenotype is appealing in the current obesity epidemic. In the meantime, C57BL/6 Tlr2-/- mice do not show histologically differing colitis upon dextran sulfate sodium exposure as opposed to other strains (C57BL/10: protected; second generation of 129/SvJ X C57BL/6: sensitized).37 It was in C57BL/6 Tlr2-/- animals that we examined genome wide colonic mucosal DNA methylation (methylation of cytosines/C/followed by a guanine/G/in CpG dinucleotides, the most stable epigenetic mark in mammals usually correlating with transcriptional downregulation of associated genes) and gene expression changes compared to WT. We found that transcriptional variation (both correlating to- and independent from DNA methylation) in genes relevant for immune regulation occurred in response to Tlr2 deficiency.37 Thereafter, we turned to metagenomic analyses of the mucosal microbiome by massively parallel pyrosequencing. Interestingly, Tlr2 deficiency associated with metagenomic changes resembling gut microbiota of lean rodents and humans compared to WT at the phylum level. The Firmicutes/Bacteroidetes ratio in Tlr2-/- mice was lower than in WT (Fig. 2) on regular chow without any obvious difference in body composition. This may be one reason for the previously observed resistance to diet induced obesity in these animals.38 In the meantime, we also found genera changes that resembled microbiome variation detected earlier in human ulcerative colitis. This result was particularly interesting since TLR2 has been associated with severe forms of UC implicating its role as a modifier in inflammatory bowel diseases.39 Overall, we could conclude that the genetic deficiency of a molecule with distinctive roles in bacterial pattern recognition (Tlr2) induces a comprehensive response at the level of the colonic mucosa that involves the reconstitution of both the epithelial transcriptional network (including epigenetic modifications) and the associated microbiota. This reconstitution is likely sensitive to the genetic background of the host and may be responsive to nutritional exposures (such as folate supplementation) during specific stages of development.32
Figure 2.

The ratio of Firmicutes and Bacteroidetes in Tlr2-/- mice compared to Wt. Colonic mucosal Firmicutes to Bacteroidetes ratio was ∼8.1 in WT and ∼4.3 in Tlr2-/- animals. Obesity has been associated with decreasing proportion of Bacteroidetes and increasing of Firmicutes (i.e., increasing Firmicutes/Bacteroidetes (F/B) ratio) in human and rodent gut microbiota.54–56 The Tlr2-/- colonic microbiome showed a leaner type of composition based on F/B ratio.
In summary, our manuscript emphasized the sophisticated network of molecular and cellular interactions that bridge genotype to phenotype in mammalian “superorganisms”. This intercalating network has the capacity to overcome significant genetic/environmental insults (such as the homozygous absence of Tlr2 in mice) with no obvious phenotype change under usual environmental conditions. In the meantime, the adjusted/pressured “superorganism” is restricted in its capacity for adaptation. Upon further offenses the fine tuned “superorganismic” balance can collapse and disease along with dysbiosis may ensue. The disruption of the dynamic and mutualistic relationship between the host and its microbes may contribute to the development of common immunologically mediated human disorders, such as inflammatory bowel diseases,40 celiac disease,41 and asthma,42 for example. A major challenge for medicine in such instances in the face of multifaceted/multietiologic common human disorders is that the key culprits for the disease have to be identified from the collapsed architecture of the “superorganism”. Additionally, while the disease may be similar in appearance, the perpetrators may be different in each unique, individual human “superorganism”. Finding means to identify and correct the key pathologic components in an individualized manner is our ultimate task. Such means can support the delineation of preventative measures for specific disorders and promote health for all.
Acknowledgments
R.K. would like to acknowledge and express his gratitude to all of his former and current mentors including, but not restricted to Miklos Kellermayer, Ferenc Gallyas, Bela Kocsis, David Bedwell, Theodore Putnam, Pearay Ogra, Howard Faden, Gyorgy Kosztolanyi, Robert Waterland, George Ferry, Mark Gilger, Scot Dowd, James Versalovic and C. Wayne Smith.
Grant Support
This work was supported in part by the Crohn's and Colitis Foundation of America-Children's Digestive Health and Nutrition Foundation/North American Society of Pediatric Gastroenterology Hepatology and Nutrition (CCFA Ref #2426), the Broad Medical Research Program, the Broad Foundation (IBD-0252); the Child Health Research Career Development Agency of the Baylor College of Medicine (NIH # 5K12 HD041648); and a Public Health Service grant DK56338, funding the Texas Medical Center Digestive Diseases Center.
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- 3.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Are A, Aronsson L, Wang S, Greicius G, Lee YK, Gustafsson JA, et al. Enterococcus faecalis from newborn babies regulate endogenous PPARgamma activity and IL-10 levels in colonic epithelial cells. Proc Natl Acad Sci USA. 2008;105:1943–1948. doi: 10.1073/pnas.0711734105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martens EC, Chiang HC, Gordon JI. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 2008;4:447–457. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X, Tian Y, Guo L, Lux R, Zusman DR, Shi W. Oral-derived bacterial flora defends its domain by recognizing and killing intruders—a molecular analysis using Escherichia coli as a model intestinal bacterium. Microb Ecol. 2010;60:655–664. doi: 10.1007/s00248-010-9708-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preidis GA, Hill C, Guerrant RL, Ramakrishna BS, Tannock GW, Versalovic J. Probiotics, enteric and diarrheal diseases and global health. Gastroenterology. 2011;140:8–14. doi: 10.1053/j.gastro.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker A. Gut metagenomics goes viral. Nat Rev Microbiol. 2010;8:841. doi: 10.1038/nrmicro2476. [DOI] [PubMed] [Google Scholar]
- 12.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci USA. 2010;107:18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellermayer R. Genetic drift. “Omics” as the filtering gateway between environment and phenotype: The inflammatory bowel diseases example. Am J Med Genet A. 2010;152:3022–3025. doi: 10.1002/ajmg.a.33726. [DOI] [PubMed] [Google Scholar]
- 15.Kellermayer R. Genetic drift. Physiologic noise obscures genotype-phenotype correlations. Am J Med Genet A. 2007;143:1306–1307. doi: 10.1002/ajmg.a.31825. [DOI] [PubMed] [Google Scholar]
- 16.Kellermayer R, Aiello DP, Miseta A, Bedwell DM. Extracellular Ca(2+) sensing contributes to excess Ca(2+) accumulation and vacuolar fragmentation in a pmr1Delta mutant of S. cerevisiae. J Cell Sci. 2003;116:1637–1646. doi: 10.1242/jcs.00372. [DOI] [PubMed] [Google Scholar]
- 17.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulder IE, Schmidt B, Stokes CR, Lewis M, Bailey M, Aminov RI, et al. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009;7:79. doi: 10.1186/1741-7007-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjogren YM, Tomicic S, Lundberg A, Bottcher MF, Bjorksten B, Sverremark-Ekstrom E, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy. 2009;39:1842–1851. doi: 10.1111/j.1365-2222.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 20.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6–14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic micro-biota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, et al. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141:1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graber JR, Breznak JA. Folate cross-feeding supports symbiotic homoacetogenic spirochetes. Appl Environ Microbiol. 2005;71:1883–1889. doi: 10.1128/AEM.71.4.1883-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guldener U, Koehler GJ, Haussmann C, Bacher A, Kricke J, Becher D, et al. Characterization of the Saccharomyces cerevisiae Fol1 protein: starvation for C1 carrier induces pseudohyphal growth. Mol Biol Cell. 2004;15:3811–3828. doi: 10.1091/mbc.E03-09-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucock M. Is folic acid the ultimate functional food component for disease prevention? BMJ. 2004;328:211–214. doi: 10.1136/bmj.328.7433.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratel D, Ravanat JL, Berger F, Wion D. N6-methyladenine: the other methylated base of DNA. Bioessays. 2006;28:309–315. doi: 10.1002/bies.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nijhout HF, Reed MC, Ulrich CM. Mathematical models of folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:45–82. doi: 10.1016/S0083-6729(08)00402-0. [DOI] [PubMed] [Google Scholar]
- 28.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luria SE, Human ML. A nonhereditary, host-induced variation of bacterial viruses. J Bacteriol. 1952;64:557–569. doi: 10.1128/jb.64.4.557-569.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellermayer R, Balasa A, Zhang W, Lee S, Mirza S, Chakravarty A, et al. Epigenetic maturation in colonic mucosa continues beyond infancy in mice. Hum Mol Genet. 2010;19:2168–2176. doi: 10.1093/hmg/ddq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaible TD, Harris RA, Dowd SE, Smith CW, Kellermayer R. Maternal methyl-donor supplementation induces prolonged murine offsping colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr044. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santacruz A, Collado MC, Garcia-Valdes L, Segura MT, Martin-Lagos JA, Anjos T, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 34.Kellermayer R. Hailey-Hailey disease as an orthodisease of PMR1 deficiency in Saccharomyces cerevisiae. FEBS Lett. 2005;579:2021–2025. doi: 10.1016/j.febslet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, et al. Loss of the Atp2c1 secretory pathway Ca(2+)-ATPase (SPCA1) in mice causes Golgi stress, apoptosis and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J Biol Chem. 2007;282:26517–26527. doi: 10.1074/jbc.M703029200. [DOI] [PubMed] [Google Scholar]
- 36.Szigeti R, Kellermayer R. Autosomal-dominant calcium ATPase disorders. J Invest Dermatol. 2006;126:2370–2376. doi: 10.1038/sj.jid.5700447. [DOI] [PubMed] [Google Scholar]
- 37.Kellermayer R, Dowd SE, Harris RA, Balasa A, Schaible TD, Wolcott RD, et al. Colonic mucosal DNA methylation, immune response and microbiome patterns in Toll-like receptor 2-knockout mice. FASEB J. 2011;5:1449–1460. doi: 10.1096/fj.10-172205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010;24:731–739. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology. 2009;137:209–220. doi: 10.1053/j.gastro.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Palma G, Nadal I, Medina M, Donat E, Ribes-Koninckx C, Calabuig M, et al. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010;10:63. doi: 10.1186/1471-2180-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Couzin-Frankel J. Bacteria and asthma: untangling the links. Science. 2010;330:1168–1169. doi: 10.1126/science.330.6008.1168. [DOI] [PubMed] [Google Scholar]
- 43.Roda G, Sartini A, Zambon E, Calafiore A, Marocchi M, Caponi A, et al. Intestinal epithelial cells in inflammatory bowel diseases. World J Gastroenterol. 2010;16:4264–4271. doi: 10.3748/wjg.v16.i34.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stremmel W, Braun A, Hanemann A, Ehehalt R, Autschbach F, Karner M. Delayed release phosphatidylcholine in chronic-active ulcerative colitis: a randomized, double-blinded, dose finding study. J Clin Gastroenterol. 2010;44:101–107. doi: 10.1097/MCG.0b013e3181c29860. [DOI] [PubMed] [Google Scholar]
- 45.Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182:3047–3054. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 47.Broere F, du Pre MF, van Berkel LA, Garssen J, Schmidt-Weber CB, Lambrecht BN, et al. Cyclooxygenase-2 in mucosal DC mediates induction of regulatory T cells in the intestine through suppression of IL-4. Mucosal Immunol. 2009;2:254–264. doi: 10.1038/mi.2009.2. [DOI] [PubMed] [Google Scholar]
- 48.Gabriele L, Ozato K. The role of the interferon regulatory factor (IRF) family in dendritic cell development and function. Cytokine Growth Factor Rev. 2007;18:503–510. doi: 10.1016/j.cytogfr.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 50.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Biasco G, Zannoni U, Paganelli GM, Santucci R, Gionchetti P, Rivolta G, et al. Folic acid supplementation and cell kinetics of rectal mucosa in patients with ulcerative colitis. Cancer Epidemiol Biomarkers Prev. 1997;6:469–471. [PubMed] [Google Scholar]
- 52.Fedulov AV, Kobzik L. Allergy Risk is Mediated by Dendritic Cells with Congenital Epigenetic Changes. Am J Respir Cell Mol Biol. 2011;44:285–292. doi: 10.1165/rcmb.2009-0400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graham TA, Humphries A, Sanders T, Rodriguez-Justo M, Tadrous PJ, Preston SL, et al. Use of Methylation Patterns to Determine Expansion of Stem Cell Clones in Human Colon Tissue. Gastroenterology. 2011;140:1241–1250. doi: 10.1053/j.gastro.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 54.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 55.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009;4:7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sefcikova Z, Kmet V, Bujnakova D, Racek L, Mozes S. Development of gut microflora in obese and lean rats. Folia Microbiol (Praha) 2010;55:373–375. doi: 10.1007/s12223-010-0061-2. [DOI] [PubMed] [Google Scholar]