Abstract
The erythropoietin (EPO) belongs to the family of angiogenic factors, which is regulated by Hypoxia-inducible factor- 1α (HIF-1α). As known, EPO are expressed in human villi and decidua, but the function is not clear. In this study, we investigated the expression and roles of HIF-1α, EPO and its receptor (EPOR) in the biological functions of trophoblast and decidual stromal cell (DSC) in human early pregnancy. The expression of EPO, EPOR and HIF-1α was evaluated in the villi and deciduas by RT-PCR and immunohistochemistry. Thereafter, we silenced HIF-1α expression in HTR-8/SVneo cell line and decidual stromal cells (DSCs). The effects of EPO on the proliferation and apoptosis of trophoblasts and DSCs, and activation of signal molecules were investigated by BrdU proliferation assay, flow cytometry and western blot, respectively. We have observed that the HIF-1α silence results in the lower expression of EPO in trophoblasts and DSCs. The anti-EPO neutralizing antibody can inactivate the phosphorylation of STAT5 and activate p38 of these cells in a dosage-dependent manner. Furthermore, the expressions of EPO, EPOR and HIF-1α in the villi and decidua from the unexplained miscarriage were significantly lower than that of the normal early pregnancy. This study suggests that HIF-1α may regulate the expression of EPO, which plays a favorable regulatory role in the proliferation and survival of human first-trimester trophoblast cells and DSCs via inactivating p38 and activating STAT5 in an autocrine manner, while the inadequate EPO expression at maternal-fetal interface may lead to pregnancy wastage in humans.
Keywords: EPO, HIF-1, trophoblast cell, decidual stromal cell, STAT5, p38
Introduction
Successful pregnancy depends on coordinate progression of decidualization, placenta formation and embryo development. At early maternal -fetal interface, the uteroplacental vasculature transforms adequately by extravillous trophoblast following proliferation, differentiation and invasion of these cells into the maternal decidua, and this progression occurs in a relatively hypoxic environment. HIF-1α, a major tran-scriptional regulator of several hypoxia-sensitive genes, including EPO, is functionally deactivated by oxygen in a reaction catalyzed by prolyl hydroxylase. HIF-1α is essential for the production and secretion of EPO in response to hypoxia [1, 2].
EPO is a novel family of angiogenic factor, which is produced in the fetal liver, and subsequently in the adult kidney, acting on its specific receptor (EPOR) [3]. Once EPO is bound to its receptor, the EPOR activates Janus-tyrosine kinase 2 (Jak2) through phosphorylation, which results in tyrosine phosphorylation and dimerization of STAT5, ERK1/2, JNK, p38 and AKT signal pathways [4, 5]. EPO/EPOR interaction plays an important role in the growth, invasion, and metastasis of tumour in addition to erythropoiesis [6-8].
During the early pregnancy, a relative low oxygen environment is essential for normal embryonic and placental vasculature. HIF-1α is a major transcriptional regulator of EPO in hypoxic environment. Recent studies have shown that EPO and EPOR are expressed in human first-trimester trophoblast cells and decidua [9, 10], and the production of EPO is regulated by the oxygen concentration in the blood. Whether HIF-1α regulates the expression of EPO and the biological function of EPO at first maternal-fetal interface is almost unclear. In the present study, we first evaluated the expression of HIF-1α, EPO and EPOR at the maternal-fetal interface in early pregnancy by immunohistochemistry and real time PCR, and then we investigated the roles and regulation of these molecules in human trophoblasts and decidual stromal cells in early pregnancy. The different expression of HIF-1α, EPO and EPOR at the maternal-fetal interface from early pregnancy and unexplained miscarriage was also determined.
Materials and methods
Tissue collection and cell culture
All procedures in this study were carried out with the ethical committee approval of the Obstetrics and Gynecology Hospital of Fudan University, and all the participants had completed an informed consent to collect tissue samples.
Decidual tissues (n=6) and placental tissues (n=6) were from elective termination of the first-trimester pregnancies (gestational age, 6-8 weeks) for no medical reason, or the unexplained miscarriage. Decidual tissues from the first-trimester pregnancy were put immediately into ice-cold Dulbecco's modified Eagle's medium (DMEM high D-glucose; Gibco Grand Island, NY, USA), transported to the laboratory within 30 min after surgery and washed in Hank's balanced salt solution for isolation of DSCs.
The DSCs were isolated according to the method of Nagle with slight improvement [11]. The decidua tissues were fully washed in Ca2+Mg2+-free PBS, dissected and minced, and were stayed in a solution of 0.1% Collagenase IV (sigma, USA) for 90 min at 37°C. Next, the suspension was filtered through sterile gauzes (100 and 300 μm), and centrifuged at 1000 rpm for 10 min. The supernatant was discarded, and the cell pellet was suspended in PBS solution, and centrifuged on a discontinuous gradient of 20%, 40% and 60% Ficoll for 20 min at 2000 rpm. The cells were collected from the 20%-40% interface containing mainly DSCs, and then suspended in RPMI 1640, washed, and cultured in the complete RPMI medium with 10% FBS. After culture for 30 min, the non-adherent lymphocytes were removed, leaving a highly purified population of DSCs. Immunocytochemistry has showed that the vimentin-positive cells made up for above 98%.
The HTR-8/SVneo extravillous trophoblasts line was kindly provided by Professor Charles H. Graham (Queen's University) [12], it was grown in RPMI 1640 supplemented with 5% fetal bovine serum (FBS), under standard culture conditions of 5% CO2 in air at 37°C with medium renewal every 2-3 days.
Immuocytochemistry
The cells (HTR8/SVneo cells and DSCs) (n=6) growing on coverslips were cultured for 48h. The coverslips were fixed in 4% (vol/vol) paraformaldehyde for 20 min at room temperature, washed in PBS and permeabilized for 25 min with 0.25% (vol/vol) Triton-100 in PBS. The cells were then incubated with 1% BSA in PBS/Tween (PBST) for 30 min to block non-specific binding of antibodies. The mouse anti-human vimentin monoclonal antibody (1:100; Dingguo, Beijing) as marker for DSCs, and HLA-G (Applied Biosystems, USA) and cytokeratin-7 antibody (1:100; Dingguo, Beijing) as markers for trophoblast cells were then added. The mouse anti-human HIF-1α mouse monoclonal antibody (1:500; Abcam, US), rabbit anti-EPO antibody (1:50; Santa Cruz, US) or rabbit anti-EPOR antibody (1:50; Santa Cruz, US) was used to detect the expression of HIF-1α, EPO and EPOR protein, respectively. The cells were incubated with primary antibody or isotypic control overnight at 4° C, and then incubated with a peroxidase-conjugated secondary antibody for 60 min at 37°C. The slides were stained with DAB, and counterstained with haematoxylin. The experiments were repeated five times.
Immunohistochemistry
Immunohistochemical analysis was performed for decidua and villi from the early pregnancy (n=6) and miscarriage (n=6) (gestational age, 6-8 weeks), respectively. For each of the samples, briefly, 5mm sections of formalin-fixed, paraffin-embedded tissues were dewaxed in xylene twice for 5 min; rehydrated in 100% ethanol twice for 3 min, followed by 95% ethanol for 3 min and 80% ethanol solution for 3 min; then rehydrated in Tris-buffered saline (TBS); for each specimen an antigen retrieval step was required (Dingguo, Beijing), which was achieved by microwave heating for 20 min in target retrieval solution; washed twice. Endogenous hydrogen peroxidase activity was quenched by using 3% H2O2 for 30 min at room temperature, and the specimens were then rinsed in PBST. Non-specific binding was prevented by preincubation with a non-immune block (1%BSA/PBST). The sections were incubated with mouse immunoglobulin (IgG, as isotype), and mouse anti-human HIF-1α monoclonal antibody (1:500; Abcam, US), rabbit anti-EPO antibody (1:50; Santa Cruz, US), rabbit anti-EPOR antibody (1:50; Santa Cruz, US). Thereafter, the secondary peroxidase-conjugated goat anti-mouse or anti-rabbit antibodies (1:1000; Dingguo, Beijing) in the non-immune block were applied overnight at 4°C. After stringent washing with PBST, the slides were subsequently incubated for 10 min in DAB (EnVisiontw kit, US). The slides were counter-stained using Harris’ hematoxylin (Sigma Chemical Company, US), and then washed in water for 2 min. Slides were mounted for microscopic examination. The experiments were repeated five times.
RT-PCR
The total RNA was isolated from villi and decidual tissues of the early pregnancy (n=6) and miscarriage (n=6) with Trizol reagent (Invitrogen, USA). The complementary DNA (cDNA) was generated with oligo (dT) primers using Revertra Ace-α-TM First Strand cDNA Synthesis Kit (TOYOBO, Japan) following the protocol. The cDNA was reverse transcribed from total RNA using the correspondent primer. The 50 μl PCR amplification of the single-strand cDNA was performed by pre-denaturation (94°C) for 5min, and 28 cycles of denaturation (94°C) for 45 s, annealing for 45 s and elongation (72°C) for 45 s using 2.5 U Taq polymerase (Takara, Japan). The primer sequences were indicated in Table 1 (Shenggong, Shanghai). The amplified DNA was fractionated by 2% agarose gel (Oxiod, UK) electrophoresis, and ethidium bromide-stained bands were photographed. Experiments were performed on threefold dilutions of cDNA and the amount of cDNA was normalized by actin.
Table 1.
Primer sequences of EPO, EPOR, HIF-1α and GAPDH
| Gene name | Primer sequences | Anneal temperature | Length (bp) |
|---|---|---|---|
| EPO | Sense: GAGGCCGAGAATATCACGAC | ||
| Anti-sense: TGCGGAAAGTGTCAGCAGTG | 57 °C | 358 | |
| EPOR | Sense: TCTCCTACCAGCTCGAGGAT | ||
| Anti-sense: GCGTCTAGGAGCACTACTTC | 55°C | 205 | |
| HIF-1α | Sense: AGAAACCACCTATGACCTGCT | ||
| Anti-sense: AAG CATCCTGTACTGTCCTGTG | 53°C | 287 | |
| GAPDH | Sense: CGGAGTCAACGGATTTGGTCGTAT | ||
| Anti-sense: AGCCTTCTCCATGGTGGTGAAGA | 57 °C | 307 |
Knockdown of HIF-1α with siRNA transfection
Hairpin loop constructs that produce specific small-interfering RNA (siRNA) and a nonspecific control siRNA were designed in Table 2. We silenced HIF-1α with plasmid DNA by using Lipofectamine2000 (Invitrogen, US) according to the manufacturer's recommendations. One day before transfection, the cells were cultured in the medium without antibiotics so that they would be 90-95% confluent at the time of transfection. The recovered cells were mixed gently with plasmid DNA and Lipofectamine™2000 (The proportion of plasmid DNA and Lipofec-tamine™2000 was 1:2 or 1:2.5), and then incubated at 37°C in a CO2 incubator, and then the culture medium was replaced after 6 hours.
Table 2.
Primer sequences of HIF-1α, HIF-1α siRNA and Negative Control
| Gene name | Primer sequences |
|---|---|
| Sense:5'- CCAGTTATGATTGTGAAGTTA -3' | |
| HIF-1α | |
| Anti-sense:3'- TAACTTCACAATCATAACTGG -5' | |
| Sense:5'-CACCGCCAGTTATGATTGTGAAGTTATTCAAGAGATAACTTCACAATCATAACTGGTTTTTTG-3' | |
| HIF-1α siRNA | |
| Anti-sense:5-GATCCAAAAAACCAGTTATGATTGTGAAGTTATCTCTTGAATAACTTCACAATCATAACTGGC-3 | |
| Sense:5'-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3' | |
| Negative Control | |
| Anti-sense:5'-GATCCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGACGTGACACGTTCGGAGAAC-3'. | |
Western blot assay
Western blot analysis was performed by using anti-EPO (1:100; Santa cluz, US), anti-HIF-1α (1:1000; Abcam, US), anti-STAT5 (1:10000; CST, US), anti-p-STAT5 (1:10000; CST, US), anti-AKT (1:10000; CST, US), anti-p-AKT (1:10000; CST, US), anti-p38 (1:10000; CST, US), anti-p-p38 (1:10000; CST, US), anti-ERK1/2 (1:10000; CST, US), anti-p-ERK1/2 (1:10000; CST, US), anti-JNK (1:10000; CST, US), anti-p-JNK (1:10000; CST, US), or anti-GAPDH antibody (1:10000; Kangcheng, Shanghai), respectively. After having been ground smoothly, the cells were lysed in 1% NP-40, 50 mM Tris HCl (pH 8.0), 150 mM NaCl, 100 μg/ml PMSF, 1 μg/ml Aprotinin, and 0.1% SDS for 10 min at 4°C. Nuclei were removed by centrifugation at 12000 g at 4°C for 10 min, and cell lysates were assayed for protein contents using the Bradford protein assay. Proteins (50 μg) were resuspended in sample buffer (2% SDS, 62.5 mM Tris, pH 6.8, 0.1% bromophenol blue and 2.5% 2-mercaptoethanol, 10% glycerol), separated on 10% SDS-polyacrylamide gels. The proteins were transferred to a nitrocellulose membrane by electrotransfer for 1 h. After being soaked in blocking buffer (1x TBS with blocking reagent), the membrane was incubated with the primary antibody overnight at 4°C. Blots were developed by using the HRP-linked secondary antibody and a chemiluminescent detection system (LI-COR Biosciences, US). The experiments were repeated three times.
Cell proliferation assay
The 1×104 cells (HTR8/SVneo cells or DSCs) were plated in each well of 96 well plates. In culture for 12h, the anti-EPO neutralizing antibody (R&D, US) in the concentration of 0, 0.2, 1 and 5 ng/ml was added respectively into the wells, and the plates were incubated for 24, 48, 72h, respectively, and finally the cells proliferation was analyzed with Brdu Proliferation Assay kit according to the manufacturer's protocol (Millipore, US).
Annexin V and PI staining for cell apoptosis
To determine the cell apoptosis, an Apoptosis Detection Kit was used for Annexin V binding and PI staining (Invitrogen, USA). The 2×105 HTR8/SVneo cells or DSCs were plated in 12 well plates, incubated for 12 h, and then treated with 1ng/ml anti-EPO neutralizing antibody. In culture for 48h, the cells were washed and re-suspended in 100 μl annexin-binding buffers, and then added by 5 μl Alexa Fluor 488 annexin V (Component A) and 1 μl PI working solution. The cells were mixed and then incubated in the dark for 15 min at room temperature. At the end of incubation, a further 400 μl binding buffer was added, and the cells were analyzed immediately by flow cytometry (BD Bioscience).
Statistics
Data were expressed as mean+SEM. Statistical significance was determined by one-way ANOVA followed by Dunnett's multiple comparisons test. A P<0.05 is recognized statistically significant.
Results
Expression of HIF-1α, EPO, EPOR at human maternal-fetal interface in the early pregnancy
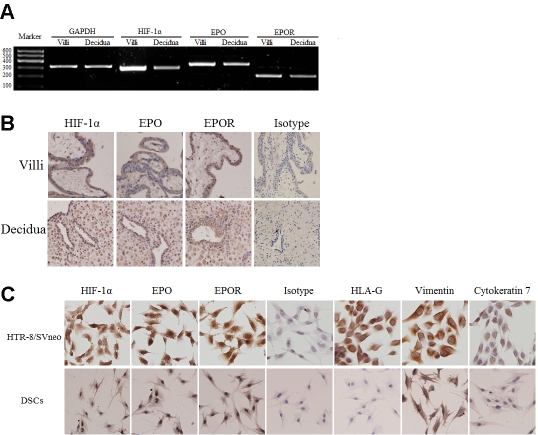
We first determined whether expression of HIF-1α, EPO and EPOR was presented in both human villi and decidua by RT-PCR and immunohistochemistry. The result has showed that HIF-1α, EPO and its receptor were transcribed in villi and decidua of early pregnancy. The proteins could be detected in the nuclear, cytoplasma and membrane of trophoblasts, decidual stromal cells as well as glandular epithelial cells (Figure 1A, B). We further testified the expression of HIF-1α, EPO and EPOR in HTR-8/SVneo cells and DSCs by immuocytochemistry with HLA-G and cytokeratin7 as markers of the extra-villous trophoblast and vimentin as marker of DSCs, respectively. HTR-8/SVneo cell line also expresses vimentin, as previous studies demonstrated [13]. It has been shown in Figure 1C that all these cells expressed HIF-1α, EPO and EPOR.
Figure 1.
Expression of HIF-1α, EPO, EPOR at human maternal-fetal interface in the early pregnancy. The expression of HIF-1α, EPO and EPOR on villi and deciduas was detected by RT-PCR (A) and immunohistochemisty (B). The expression of HIF-1α, EPO and EPOR on primary DSCs and HTR-8/SVneo cells was detected by immuocytochemistry (C). HIF-1α, EPO and EPOR were high expressed, and localized to the plasma and nucleus (x200). Trophoblast cells were stained strongly by anti- cytokerat-in7 (CK7) and anti-HLA-G monoclonal antibody (mAb), not by anti-vimentin mAb. Decidual stromal cells (DSCs) were positive for vimentin and negative for CK7 and HLA-G. Magnification: x200. Results were highly reproducible in three independent experiments performed in triplicate.
HIF-1α regulates EPO expression in trophoblast cells and DSCs in the early pregnancy
To investigate the regulation of HIF-1α on EPO expression at maternal-fetal interface, the HTR-8/SVneo cells and DSCs were silenced for HIF-1α with non-silenced vector as control. Western Blot analysis has showed that the HIF-1α gene was successfully silenced in HTR-8/SVneo cells and DSCs in 1:2.5 ratio of vector: Lipofectamine™ 2000 (Figure 2A). It results in Figure 2B, which has showed that EPO expression was significantly decreased in HTR-8/SVneo cells and primary DSCs when HIF-1α was silenced (P<0.01).
Figure 2.
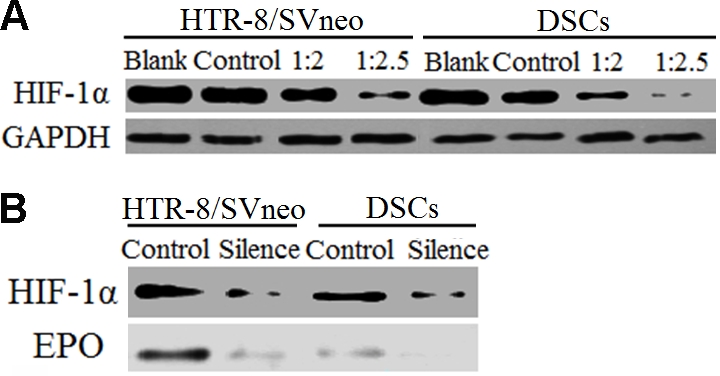
HIF-1α regulates EPO expression in tro-phoblast cells and DSCs. Lipofectamine™2000 was used to transfect the HIF-1α silenced plasmid into cells, Mixed plasmid DNA and Lipofectamine™2000 gently at 1:2, 1:2.5, then detected the protein expression by Western Blot, the result was shown that basal HIF-1α protein was markedly reduced after transfec-tion, especially the ratios of DNA (μg) to Lipofec-tamine™ 2000 (μ1) was 1:2.5 (A). After the expression of HIF-1α was silenced, the expression of EPO in HTR-8/SVneo cells and DSCs was significantly decreased (B). Results were highly reproducible in three independent experiments performed in triplicate. Blank: no transfection; control: DNA plasmid includes a nonspecific control siRNA; silence: HIF-1α was knocked down.
EPO promotes the proliferation, and inhibits the apoptosis of trophoblast cells and DSCs
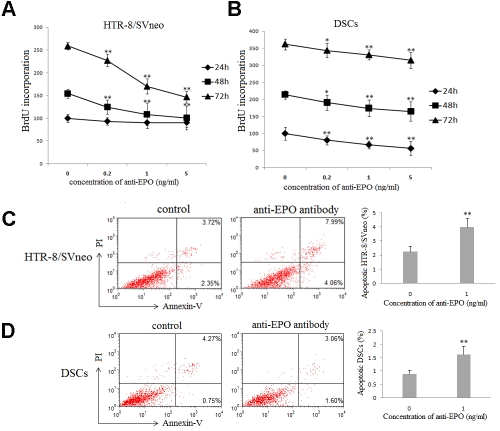
To testify the roles of EPO at maternal-fetal interface, BrdU incorporation was used to analyze proliferation of the HTR-8/SVneo cells and primary DSCs after treated with different concentration of anti-human EPO neutralizing antibody for 24, 48 or 72h, respectively. The anti-human EPO neutralizing antibody could significantly inhibit the proliferation of HTR8/SVneo cells (Figure 3A) and DSCs in concentration-dependent manner (Figure 3B) (P<0.01 compared to the control treatment). The apoptosis of the HTR-8/SVneo cells and primary DSCs after treated by 1.0 μg/ml anti-human EPO neutralizing antibody were also determined by Annexin V and PI staining and flow cytometry analysis. As shown in Figure 3C and 3D, treatment with neutralizing antibody of EPO significantly increased apoptosis of HTR8/SVneo cells and DSCs (p<0.01). These data suggests that EPO could promote the proliferation and inhibit trophoblasts and DSCs.
Figure 3.
EPO promotes the proliferation, and inhibits the apoptosis of trophoblast cells and DSCs. Brdu proliferation assay was used to detect the proliferation of HTR-8/SVneo cells and primary human DSCs, which were treated with different concentrations of anti-human EPO neutralizing antibody (0, 0.2, 1 and 5 ng/ml) after 24, 48 or 72h. It was shown that the proliferation of HTR8/SVneo cells (A) and DSCs (B) is significantly lower as compared with that of the control (p<0.01). After incubated the HTR-8/SVneo cells and primary human DSCs with or without 1ug/ml anti-human EPO neutralizing antibody for 48h, the Annexin V and PI staining was used to detect the apoptosis of these cells. As shown in Figure, the apoptosis of HTR8/SVneo cells (C) and DSCs (D) is significantly higher as compared with that of the control (P<0.01). Results were highly reproducible in three independent experiments performed in triplicate. Error bars depict the standard error of the mean. *P<0.05, **P<0.01 compared to the control.
The effects of EPO in early maternal-fetal interface through activating STAT5 and inactivating p38 signaling pathway
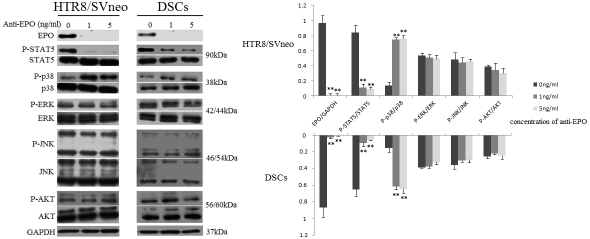
We further investigated the signal pathway of EPO in the primary DSCs and HTR8/SVneo cells. After the HTR-8/SVneo cells and DSCs were treated with anti-EPO antibody for 48h, the phosphorylation of STAT5 was significantly down -regulated, and p38 was up-regulated (P<0.01), but the neutralizing antibody did not change the phosphorylation level of AKT, JNK and ERK1/2 (Figure 4), which suggests that EPO might regulate the proliferation and survival of human first-trimester trophoblast cells and DSCs via inactivating p38 and activating STAT5.
Figure 4.
EPO plays roles through activating STAT5 and inactivating p38 signaling pathway at maternal-fetal interface. Anti-EPO antibody was used to treat HTR-8/SVneo cells and DSCs for 48h. The bands were scanned and analyzed with LI-COR software. The ratio of phosphorylation/total protein indicates the relative activating protein level. Only the phosphorylation level of STAT5 (P<0.01) and p38 (P<0.01) is decreased. These pictures are representatives of three individual experiments performed in triplicate. Error bars depict the standard error of the mean. **P<0.01 compared to the control.
The expressions of EPO and EPOR were decreased in decidua and villi of miscarriage
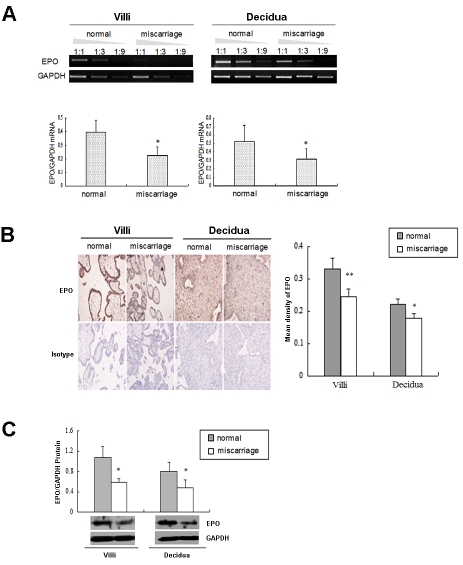
To further demonstrate the association of EPO and EPOR expression at human maternal-fetal interface with pregnancy outcome, we further evaluated the expression of EPO and EPOR in the decidua and villi from normal early pregnancy and the unexplained miscarriage. As shown in Figure 5A, the mRNA levels of EPO and EPOR in the decidua and villi from unexplained miscarriage were much lower than that of the normal early pregnancy (P<0.05). Consistently, the protein expression was significantly decreased in decidua and villi from unexplained miscarriage (P<0.05, Figure 5B, Figure 5C).
Figure 5.
The expressions of EPO and EPOR are decreased in decidua and villi of miscarriage. The EPO mRNA and protein levels in human villi and decidua from normal early pregnancy and the unexplained miscarriage, were analyzed by (A) RT-PCR, (B) Immunochemistry (C) western blot, respectively. The pictures were scanned and analyzed with Image J software. The villus and decidua from normal early pregnancy had a statistically higher EPO expression than that of the miscarriage (P<0.05 or P<0.01). These pictures are representatives of three individual experiments performed in triplicate. Normal: villi and decidua from normal early pregnancy; Miscarriage: villi and decidua from the unexplained miscarriage. Error bars depict the standard error of the mean. *P<0.05, **P<0.01 compared to the normal control.
Discussion
Successful pregnancy depends on the proper development of the fetoplacental vasculature in the villous core, which provides structure for the delivery of oxygen and nutrients from the inter-villous space to the fetus [14]. During early pregnancy, trophoblast differentiation occurs in a relatively hypoxic environment that is a key regulator of a variety of biological events during early trophoblast differentiation [15-17]. HIF-1 has been known to activate the gene transcription in response to hypoxia, and likely plays a key role in the placental, cardiovascular, and hematopoietic development [18, 19].
In low oxygen, the stable HIF-1α subunit dimerizes with HIF-1β to form an active HIF-1 complex [20], which can regulate the expression of more than 20 genes [21], including erythropoietin (EPO) [22], vascular endothelial growth factor (VEGF) and glycolytic enzymes [23, 24].
In order to learn the expression of HIF-1α and EPO in early pregnancy, we analyzed their expression in mRNA and protein levels, and found that HIF-1α and EPO were highly expressed in both human first trimester villi and decidua, especially in trophoblasts and DSCs. The HIF-1α silence results in the lower expression of EPO in HTR-8/SVneo cells and DSCs. Our study proves that HIF-1α regulates the expression of EPO in human early pregnancy. Relatively hypoxic environment is essential for early maternal-fetal interface, the production and autocrine role of EPO are regulated by HIF-1α.
In human placenta, the proliferation and differentiation of both trophoblasts and DSC cells are important to the early stages of placental development. EPO is proved to be associated with several pathologic pregnancy, including preeclampsia [25], intrauterine growth restriction [26], intrauterine hypoxia [27] and diabetic pregnancy. Angiogenic growth factors are considered to be the main mediators of these processes. EPO is a novel family of angiogenic factors, which participates in the development of placenta vessels, as well as the angiogenesis and metastasis of tumors. We have found that EPO is expressed in trophoblasts and DSCs, the main functional cells at maternal-fetal interface. To testify the function of EPO, we investigated the proliferation and apoptosis of human HTR-8/SVneo cells and DSCs. Only in lower concentration of anti-human EPO antibody, the growth of HTR8/SVneo cells and DSCs is significantly suppressed, while the apoptosis is elevated. Here we have demonstrated the high expression of EPO participates in regulation of proliferation and apoptosis of trophoblasts and DSCs, which is beneficial to the placental and fetal development in human early pregnancy.
EPO can promote angiogenesis, anti-apoptosis and anti-hypoxia when binding its receptor via intracellular Janus family tyrosine protein kinase 2 (Jak 2) molecules transphosphorylated, and then phosphorylation of tyrosine residues in the cytoplasmic domain of the EPOR [28]. EPOR phosphorylation provides docking sites for a variety of signalling molecules, such as signal transducer and activator of transcription 5 (STAT5), phosphatidylinositol-3 kinase (PI3K) and RAS/mitogen-activated protein kinase (MAPK, including ERK, JNK, p38) [29]. STAT5 enters the nucleus and induces transcription of target genes involved mainly in inhibition of apoptosis and cell proliferation. PI3K inhibits apoptosis by activating its downstream effector AKT [30], while p38 is mostly mediated by both negative regulation of cell cycle progression and induction of apoptosis [31]. Our study indicates that the induction of EPO also seems to be an important regulator, and may serve to render the balance between proliferation and apoptosis at maternal-fetal interface by regulating the STAT5 and p38 signal pathway.
We have also found the different expression of EPO in human normal early pregnancy and unexplained miscarriage, which suggests that EPO acts as a positive regulator in early placental development. The decreased expression of EPO may lead to pregnancy wastage owing to insufficient proliferation of trophoblasts and DSCs. Our research is helpful to elucidate the physiological mechanism of the early pregnancy and the pathological mechanisms of miscarriage.
Acknowledgments
We thank our colleagues in the Obstetrics and Gynecology Hospital of Fudan University. This study is supported by National Basic Research Program of China 2006CB944007, Key Project (30730087) and Major International Joint Research Project (30910103909) of NSFC, NSFC 30670787, National and Shanghai Leading Academic Discipline Project (211XK22), and Program for Outstanding Medical Academic Leader (to D-J Li), and by grant from the Science Foundation of Nantong University Medical College (YKC09029).
References
- 1.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 2.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5:125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacombe C, Mateux P. Biology of erythropoietin. Haematologica. 1998;83:724–732. [PubMed] [Google Scholar]
- 4.Jie KE, Verhaar MC, Cramer MJ, van der Putten K, Gaillard CA, Doevendans PA, Koomans HA, Joles JA, Braam B. Erythropoietin and the cardiorenal syndrome: cellular mechanisms on the cardiorenal connectors. Am J Physiol Renal Physiol. 2006;291:932–944. doi: 10.1152/ajprenal.00200.2006. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westenfelder C, Baranowski RL. Erythropoietin stimulates proliferation of human renal carcinoma cells. Kidney Int. 2000;58:647–657. doi: 10.1046/j.1523-1755.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 7.Acs G, Zhang PJ, Rebbeck TR, Acs P, Verma A. Immunohistochemical expression of erythropoietin and erythropoietin receptor in breast carcinoma. Cancer. 2002;95:969–981. doi: 10.1002/cncr.10787. [DOI] [PubMed] [Google Scholar]
- 8.Batra S, Perelman N, Luck LR, Shimada H, Malik P. Pediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survival. Lab Invest. 2003;83:1477–1487. doi: 10.1097/01.lab.0000090156.94795.48. [DOI] [PubMed] [Google Scholar]
- 9.Conrad KP, Benyo DF, Westerhausen Larsen A, Miles TM. Expressionof erythropoietin by the human placenta. FASEB J. 1996;10:760–768. doi: 10.1096/fasebj.10.7.8635693. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda Y, Sasaki T, Takagawa M, Maeda M, Yasuda M, Atsumi T, Fujita Y, Fujita H. Erythropoietin contributes to implantation: ectopic hemoglobin synthesis in decidual cells of mice. Congenit Anom. 2007;47:22–33. doi: 10.1111/j.1741-4520.2006.00137.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagle RB. Intermediate filaments: a review of the basic biology. Am J Surg Path. 1988;12:4–16. [PubMed] [Google Scholar]
- 12.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended life-span. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 13.Straszewski Chavez SL, Abrahams VM, Alvero AB, Aldo PB, Ma Y, Guller S, Romero R, Mor G. The Isolation and Characterization of a Novel Telomerase Immortalized First Trimester Trophoblast Cell Line, Swan 71. Placenta. 2009;30:939–948. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajakumar A, Conrad KP. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol Reprod. 2000;63:559–569. doi: 10.1095/biolreprod63.2.559. [DOI] [PubMed] [Google Scholar]
- 15.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 16.Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21:25–30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Jiang YZ, Chen DB, Zheng J. Hypoxia enhances FGF2- and VEGF-stimulated human placental artery endothelial cell proliferation: roles of MEK1/2/ERK1/2 and PI3K/AKT1 pathways. Placenta. 2009;30:1045–1051. doi: 10.1016/j.placenta.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang GL, Semenza GL. General involvement of hypoxia inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia inducible factor 1 is a basic helix-loophelix PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol. 2000;59:47–53. doi: 10.1016/s0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY. Cellular and developmental control of oxygen homeostasis by hypoxiain-ducible factor 1α. Genes and Development. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troeger C, Holzgreve W, Ladewig A, Zhong XY, Hahn S. Examination of maternal plasma erythropoietin and activin A concentrations with regard to circulatory erythroblast levels in normal and preeclamptic pregnancies. Fetal Diagn Ther. 2006;21:156–160. doi: 10.1159/000089068. [DOI] [PubMed] [Google Scholar]
- 26.Girsen A, Mäkikallio K, Hiilesmaa V, Hämäläinen E, Teramo K, Räsänen J. Umbilical artery erythropoietin and human fetal cardiovascular hemodynamics in intrauterine growth restriction. Am J Obstet Gynecol. 2007;196:e1–6. doi: 10.1016/j.ajog.2006.12.032. 467. [DOI] [PubMed] [Google Scholar]
- 27.Teramo KA, Widness JA. Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology. 2009;95:105–116. doi: 10.1159/000153094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossert J, Eckardt KU. Erythropoietin receptors: their role beyond erythropoiesis. Nephrol Dial Transplant. 2005;20:1025–1028. doi: 10.1093/ndt/gfh800. [DOI] [PubMed] [Google Scholar]
- 29.Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med. 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 30.Van der Meer P, Voors AA, Lipsic E, van Gilst WH, van Veldhuisen DJ. Erythropoietin in cardiovascular diseases. Eur Heart J. 2004;25:285–291. doi: 10.1016/j.ehj.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]