Abstract
Biologic agents targeting oncogenes have encourage researchs trying to correlate the role of tyrosine kinase in the pathogenesis of tumours. Osteosarcoma is a high grade aggressive neoplasm with poor survival. Our aim was to investigate c-kit immunoexpression, its prognostic relevance for patients with osteosarcoma, and the effect of imatinib mesylate (STI571) on proliferation and invasion of the human osteosarcoma cell line.A retrospective immu-nohistochemical study was performed on archival formalin-fixed paraffin-embedded specimens from 52 patients with high-grade primary osteosarcoma of extremities treated at the Pediatric Oncology Institute (IOP, GRAAC) and archived in the Department of Pathology, Federal University of São Paulo. Only pre-chemotherapy specimens were analyzed. Strongly stained cytoplasm and membrane cells were taken as positive. Human osteosarcoma cells from line MG-63 were incubated and the inhibitory effect of imatinib mesylate (STI571) on cell proliferation and invasion was studied. In 24 cases (46.15%), c-kit was expressed by the cells and c-kit-positive tumors exhibited lower necrosis post-chemotherapy. No correlation was found between c-kit expression and overall and disease-free survival. Imatinib mesylate decreased the rates of cell growth of osteosarcoma cells in low doses and invasion in high doses C-kit-positive tumors had worse response to chemotherapy and imatinib mesylate can play a role in blocking or decreasing the rate of growth of osteosarcoma cells, but not the invasive capacity of these neoplastic cells. These data suggested that imatinib mesylate could be a therapeutic target of strategies against osteosarcoma tumors. Further studies are necessary to confirm this indication.
Keywords: Osteosarcoma, c-kit, immunohistochemistry, in vitro assays, prognosis
Introduction
Osteosarcoma (OS) is the most common, non-hematopoietic, primary malignant tumor of bone [1]. It is a high grade neoplasm with rapid growth and early metastasis [2]. Even though effective chemotherapy for patients with OS has indeed led to a significant improvement of clinical outcome, between 30 and 50% of patients with non-metastatic disease of extremities still die from this neoplasia, in spite of having had a complete surgical removal of the tumor and intensive chemotherapy [3, 4].Currently, many immunohistochemical markers and genetic proteins have been studied, but with prognostic and therapeutic relevance doubtful [5-7].
The proto-oncogene c-kit is located in the long arm of chromosome 4 and encodes a trans-membrane tyrosine kinase receptor that binds the ligand stem cell factor. The c-kit signal seems to play a central role in regulating normal cell differentiation and proliferation. Mutation of the c-kit gene results in a constitutive activation of the c-kit protein, and it has been well documented in many tumors, such gastrointestinal stromal tumors (GIST) and myelodysplastic syndrome [8]. C-kit expression has also been found in osteosarcomas [9], with a high percentage of positive staining [8], but with divergent results about the prognostic value of this expression [10-12]. Imatinib mesylate (IM), STI571, is a selective inhibitor of the enzymatic activity of several tyrosine kinases and is successfully used in the treatment of GIST with mutations in exons 9 and 11 of the c-kit gene [13, 14]. Until now, few studies on the effect of IM on cellular growth have been reported using cell culture [15]. We tried to analyze in our homogeneous cohort of patients, whether c-kit expression can predict the evolution of patients with osteosarcoma and the effect of IM on proliferation and invasion of human osteosarcoma cell line.
Materials and methods
Fifty-two patients with high grade osteosarcomas were selected from the files of Department of Pathology at Federal University of Sao Paulo and Pediatric Oncologic Institute (GRAAC), between 2000 and 2005. These patients were treated according to the Study 2000 protocol guidelines (Tables 1 and 2). Only prechemotherapy specimens were selected (Table 3).
Table 1.
Protocol for non-metastatic osteossarcoma* (Total time: 24 weeks)
| Weeks | Evaluations and Procedures |
|---|---|
| Initial evaluation: local and torax NMR or CT, cintigraphy, local and toraxX-ray, complete hemogram, TGO, TGP, alkaline phosphatase, DHL, creatinine clearance, audiometry, cardiac evaluation, pathology, genetics, tumor bank; BTF, U, Cr, biochemical tests (Na, K, Mg, Ca) | |
| 0 | CDDP DOXO |
| 3 | AD IFO |
| 5 | Cycle I - preoperative audiometry/ cardiac evaluation |
| 6 | CDDP DOXO |
| Orthopedics (consultation) | |
| 9 | AD IFO |
| 11 | PREOPERATIVE EVALUATION local and torax NMR or CT, cintigraphy, local and torax X-ray, complete hemogram, TGO, TGP, alkaline phosphatase, DHL, creatinine clearance, audiometry, cardiac evaluation. |
| 12 | SURGERY EVALUATION: Pathology and Genetics |
| 15 | CDDP DOXO |
| 18 | AD IFO |
| 20 | Cycle II - postoperative audiometry/ cardiac evaluation |
| 21 | CDDP DOXO |
| 24 | AD IFO |
| 27-28 | Final evaluation local and torax NMR orCT, cintigraphy, local and torax X-ray, complete hemogram, TGO, TGP, alkaline phosphatase, DHL, creatinine clearance, audiometry, cardiac evaluation. |
Brazilian Cooperative Group for Treatment of Bone Tumors (GBCTTO), 1999. AD: adriamycin; CDDP: cisplatin; DOXO: doxorubicin; IFO: ifosfamide.
Table 2.
Drugs and doses used in the treatment of patients with high grade osteosarcoma
| Drugs | Daily dose | Total dose/m2 |
|---|---|---|
| CDDP | 60 mg/m2 D1, D2 = 120 mg/m2 × 4 | 480 mg/m2 |
| DOXO | 40 mg/m2 D1, D2 = 80 mg/m2 × 4 | 320 mg/m2 |
| IFO | 2.7g/m2D1-D5 = 13.5g/m2 × 4 | 54g/m2 |
| MESNA | 600 mg/m2 hour 0, 3, 6 e 12 | 2.7 g/m2/day |
| Obs.: hour 12 Mesna tablets v.o. (optional) |
AD: adriamycin; CDDP: cisplatin; D1: ; D2:; DOXO: doxorubicin; IFO: ifosfamide; MESNA: sodium 2-mercaptoethane sulfonate
Table 3.
Data of 52 patients treated for high grade osteosarcoma
| Age (years) | Range Median | 5 a 29 15 | |
|---|---|---|---|
| Follow up (months) | Range Median | 9.2 a 96.8 44.7 | |
| Sex | n | % | |
| Male | 29 | 56 | |
| Female | 23 | 44 | |
| Localization of primary tumor | n | % | |
| Femur | 30 | 58 | |
| Tibia | 15 | 29 | |
| Humerus Radius | 3 | 13 | |
| Ilium | 1 | 1.92 | |
| Polyostotic | 1 | 1.92 | |
| Other | 1 | 1.92 | |
| 1 | 1.92 | ||
| Metastasis at diagnosis | n | % | |
| Yes | 16 | 31 | |
| 36 | 69 | ||
| Responders | n | % | |
| No | 33 | 63 | |
| Yes | 17 | 32 | |
| Not available | 2 | 4 | |
| Survival | n | % | |
| Alive | 25 | 48 | |
| Dead | 26 | 50 | |
| Not available | 1 | 2 | |
Immunohistochemistry
All identifiable archival material was retrieved from the Department of Pathology. In all cases, paraffin blocks from the primary site were selected. Bone specimens were decalcified with nitric acid (7.5%) and after that, were rinsed in water (30 min) and immersed in sodium bicarbonate (5%, 24 h). Sections (4 μm) were cut, deparaffinized, and rehydrated. Diluted (1:500) polyclonal anti-CD117 antibody (pAb; DAKO, A4502, Carpinteria, CA, USA) was used to stain the sections to assess expression of c-kit protein. Antigen in the sections was retrieved with high-pressure cooking treatment (5 min). Endogenous peroxidase was blocked with hydrogen peroxide (H2O2), into which the slides were immersed (5 times, 5 min, one at a time). Incubation (4°C, overnight) of the sections with the anti-CD117 pAb was followed by rinsing (30 min) with phosphate-buffered saline (PBS). Thereafter, the slides were incubated (37°C, 15 min) with the streptavidin-biotin-peroxidase complex (biotinylated peroxidase; Dako A/S) (biotinylated antibody), rinsed again in PBS and incubated (15 min) in strepatavidin-biotin-peroxidase reagent. Color was developed by incubating (37°C, 5 min) the slides with 3, 3-diaminobenzidine tetrahydrochloride (Sigma) in presence of H2O2 (0.05% in PBS). Slides were then rinsed with tap water, counterstained (5 min) with Harris hematoxylin, rinsed in ammonia water, dehydrated, and coverslipped. A case of gastrointestinal stromal tumour (GIST) with known overexpression of c-kit served as the positive control. An appropriate negative control was used throughout. All slides were examined blindly by two independent pathologists.
The specimens were scored according to both the percentage of positively stained tumor cells and their immunostaining intensity. The immunostaining intensity was codified as negative (0), weak (+), moderate (++), and strong (+++) expression. Only slides with moderate (++) and strong (+++) expression were considered as positive. Specimens with moderate expression showed cytoplasm stained in a diffuse pattern. Specimens with strong protein expression exhibited both cytoplasm and membrane stained in a diffuse pattern. A cut-off point of 10% was used.
In vitro assays
Cell culture. MG-63 cell line was incubated (37°C) in a humidified CO2-enriched (5%) atmosphere. Cells were cultured in RPMI-1640 medium (Invitrogen, Burlington, Ontario, Canada), supplemented with heat-inactivated fetal bovine serum (FBS; 5%), fungizone (1%), and penicillin-streptomycin (1%) purchased from Invitrogen (Burlington, Ontario, Canada). Cells were cultured as a monolayer in 25 cm2 flasks (Fisher, Whitby, Ontario, Canada) and evaluated twice a week, at every media change, for normal growth by phase-contrast microscopy. The culture was grown to confluence and underwent a treatment (37°C) with trypsin (0.05%) in ethylene diamine tretraacetic acid (EDTA; Fisher) and washed in RPMI-1640 medium (7 ml) before being centri-fuged (120 x g, 10 min) to form a pellet. Cells were then suspended in RPMI-1640 medium (1 ml) and counted using the Trypan Blue-dye exclusion test.
Invasion assay. A modified Boyden chamber consisting of a polyethylene terephthalate membrane (PET) with 8-μm diameter pores, pre-coated with Matrigel, an artificial basement membrane (Beckton Dickenson Labware, Bedford, MA, USA), was used as previously described [13] to assay for cell invasive ability. A PET membrane without Matrigel was used as a control.
Cells (1.25×105) suspended in RPMI-1640 medium with FBS (0.1%) were added to the upper chamber. RPMI-1640 medium with FBS (10%) was added to the lower chamber, which acted as a chemoattractant, to obtain the baseline invasive ability of the cell lines. Inhibition of cell invasion was assayed by adding IM (50 μM) to the RPMI-1640 medium supplemented with FBS (10%) in the upper chamber. MI concentration (50 μM) for 50% inhibition of cell invasion (IC50) was semi-quantitativelly estimated. All cells were killed with a concentration of 1000 μM, which would make the invasion measurement not feasible. Half of the cells were killed (letal dose of 50%) with a concentration of 100 μM, which was still too strong to measure invasion. A significant effect of cell invasion was not observed with a concentration of 10 μM. Therefore, regarding the distinct effects caused by IM doses of 100 e 10 μM on cell invasion, 50 μM was considered a convenient concentration, which neither killed nor destroyed the cells yet displaying a cytostatic effect.
Non-invading cells were removed from the upper chamber by gently wiping the membrane surface with a moist cotton swab. The membranes were removed and stained with a Diff-Quick staining kit, which stains cell nuclei purple and cytoplasm pink. Stained cells were randomly counted under microscope in 20 high-powered fields. Only cells whose nuclei had completely invaded through the membrane were counted. Each experimental condition, including the control, was performed in triplicate and the average number of invading cells was then calculated for all experimental conditions.
Percent invasion was determined for each cell line under each experimental condition using the following formula: % invasion = 100 x (mean number of cells invading through the Matrigel membrane) / (mean number of cells migrating through the control PET membrane). The cell lines were then ranked according to their invasive ability.
Proliferation assay
The sulforhodamine-B (SRB)-based assay kit (TOX-6, Sigma-Aldrich) was performed as per the National Cancer Institute protocol [15]. Briefly, 2.5×103 cells of the MG-63 line were seeded into each well, in a minimum of six wells. A row of 8 wells exposed only to the RPMI-1640 medium was used as a control. Twenty-four hours following cell seeding, IM was added to the experimental wells where cells were allowed to incubate (24 h). IM concentrations were 1000, 100, 10, 1, 0.1, 0.01, and 0.001 μM [14]. After incubation, cells were fixed to the bottom of the wells using a trichloroacetic acid (TCA) solution (50%, 1 h, 4°C). Plates were then rinsed with distilled water, to remove TCA and medium, and air dried. The SRB dye was added to each well and allowed to stain (25 min). The dye was then removed by washing with acetic acid solution (10%) and allowed to air dry. The dye, which was incorporated into the fixed cells at the bottom of the wells, was solubilized in a tris-hydroxymethyl-amino-methane solution (10 mM) for quantification. The absorbance values for the solute were read using a microplate reader (ƛ= 510 nm). This procedure allowed a comparison between the proliferation rates of cells exposed to IM at given doses and the control over 48 h.
Statistical analysis
Kaplan Meier and log rank tests were utilized to compare the overall survival rates between patients with positive versus negative staining for c-kit. All analyses were performed using the Prism 4.0 program (GraphPad Software Inc., San Diego, CA, USA). Differences between invasion rates for the MG-63 cell line under experimental conditions were determined using the ANOVA test. The growth rates with and without IM were determined using the Student's t-test. A p value of less than 0.05 was considered to be statistically significant in these tests. Calculations were computer-based (SPSS 11.5, SPSS Inc., Chicago, IL, USA).
Results
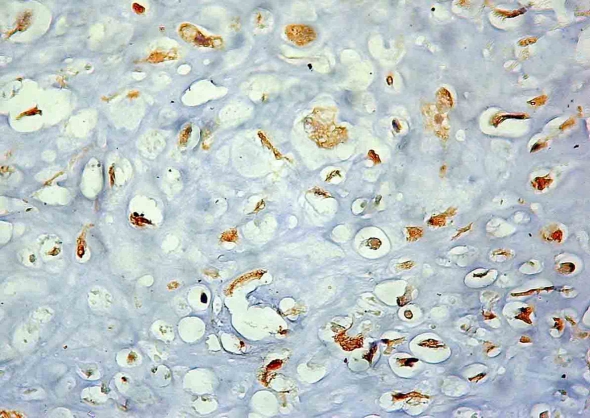
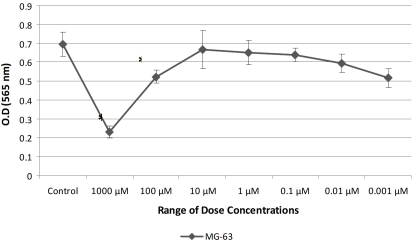
Twenty-four of the 52 pediatric patients (46.15%) showed immunoexpression of c-kit (Figure 1). These expressions were statistically correlated with bad response to chemotherapy (p=0.0355). No correlation with c-kit positivity and recurrence, death, and metastases at diagnosis was observed. No correlation between c-kit immunoexpression and poor survival (p=0.643) was observed when only non-metastatic pediatric patients at diagnosis (15 cases) were analyzed isolatedly (Figure 2). Addition of IM inhibited growth of osteosarcoma cell line in doses of 1000, 100, 0.1, 0.01, and 0.001 μM (Figure 3) and cell invasion in doses of 50 μM.
Figure 1.
Immnoexpression of c-kit. - Membranous and cytoplasmic staining.
Figure 2.
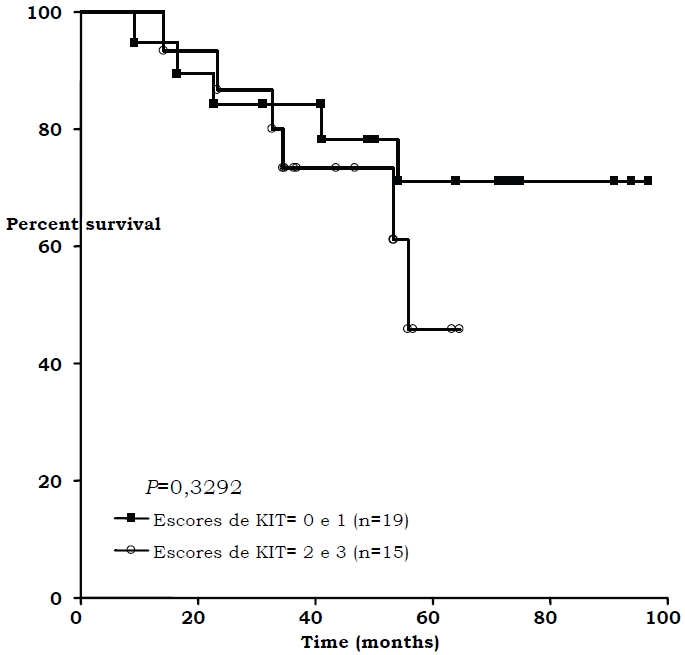
Overall survival and c-kit expression (only non-metastatic patients)
Figure 3.
Proliferation assay (24 h) with the MG-63 cell line IC50 (50 μM) was determined experimentally.
Discussion
A strong immunoexpression of c-kit was found in osteosarcomas (46.15%), comparable to those reported by Entz-Werle et al [10] (57%), Wei et al [12] (62,5%), and Smithey et al [9] (39%) Our positive cases showed a statistically high correlation with bad response to chemotherapy. However, such high expression was not correlated with overall survival and disease-free survival. In the literature, correlation between expression of c-kit and necrosis post-chemotherapy was reported only by Sulzbacher et al [11], who found no correlation of these variables, and overall and disease-free survival. Although these authors studied a considerable number of patients (100) with osteosarcoma, 15% of the tumors were axial. Also, the patients were treated with four different protocols, and only 20% of the cases expressed the protein c-kit. The use of different methodologies to evaluate c-kit expression and the nonhomogeneous cohort may explain the different results. Moreover, in the present study, patients who overexpressed c-kit protein did not show a higher probability to develop metastases than c-kit-negative patients.
Sulzabacher et al [11], Entz-Werle et al [10], and Wei et al [12] searched for mutations in the c-kit gene, but none could be found. Since the essential of studies on the mutation status of c-kit gene is evaluation of tumor response to IM, we decided to study the ability of IM to inhibit cell growth in the osteosarcoma cell line in vitro. ST1571 inhibited cell growth in the osteosarcoma cell line in doses of 1000, 100, 0.1, 0.01, and 0.001 μM and cell invasion in doses of 50 μM. Yoshitani et al [15] demonstrated cell growth inhibition in the rat osteosarcoma cell line by IM in a concentration of 10 μM, the maximal tolerated concentration. Knowledge on the capacity of invasion by tumor cells is important both to evaluate the ability of these cells to cross the basal membrane and to calculate the ability of tumor cells to develop metastases. Our study showed that IM inhibited invasion of osteosarcoma line cells only in high concentration (50 μM).
Conclusions
C-kit protein is strongly immunoexpressed in human osteosarcoma and the positive cases indicate a group of patients who will have a poor response to chemotherapy. The c-kit gene might be involved in osteosarcoma carcinogenesis since this tumor immunoexpress the protein and ST1571 could inhibit the growth of human osteosarcoma cell line in vitro, even at low doses. Seeing that IM could inhibit the invasion of cell lines in vitro, only in high doses, and that c-kit expression do not influence the tumor capacity to metastasize, we can suggest that c-kit gene is not involved in the progression of this disease.
Acknowledgments
The authors acknowledge Dr. Paulo Boschcov, former professor at UNIFESP, whose suggestions contributed to improve the quality of the final version of the manuscript.
References
- 1.Fletcher CDM, Unni KK, Mertens F. Lyon: IARC Press; 2002. World Health Organization, International Academy of Pathology: Pathology and genetics of tumours of soft tissue and bone. [Google Scholar]
- 2.Unni KK, Dahlin DC. 5. Philadelphia: Lippincott-Raven; 1996. Dahlin's bone tumors: general aspects and data on 11,087 cases. [Google Scholar]
- 3.Bacci G, Ferrari S, Longhi A, Forni C, Zavatta M, Versari M, Smith K. High-grade osteosarcoma of the extremity: differences between localized and metastatic tumors at presentation. J Pediatr Hematol Oncol. 2002;24:27–30. doi: 10.1097/00043426-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Petrilli AS, de Camargo B, Filho VO, Bruniera P, Brunetto AL, Jesus Garcia R, Camargo OP, Pena W, Péricles P, Davi A, Prospero JD, Alves MT, Oliveira CR, Macedo CR, Mendes WL, Almeida MT, Borsato ML, dos Santos TM, Ortega J, Consentino E. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24:1161–1168. doi: 10.1200/JCO.2005.03.5352. [DOI] [PubMed] [Google Scholar]
- 5.Meyers PA, Heller G, Healey JH, Huvos A, Applewhite A, Sun M, LaQuaglia M. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol. 1993;11:449–453. doi: 10.1200/JCO.1993.11.3.449. [DOI] [PubMed] [Google Scholar]
- 6.Baldini N, Scotlandi K, Serra M, Picci P, Bacci G, Sottili S, Campanacci M. P-glycoprotein expression in osteosarcoma: a basis for risk-adapted adjuvant chemotherapy. J Orthop Res. 1999;17:629–632. doi: 10.1002/jor.1100170502. [DOI] [PubMed] [Google Scholar]
- 7.Benassi MS, Molendini L, Gamberi G, Sollazzo MR, Ragazzini P, Merli M, Magagnoli G, Sangiorgi L, Bacchini P, Bertoni F, Picci P. Altered G1 phase regulation in osteosarcoma. Int J Cancer. 1997;74:518–522. doi: 10.1002/(sici)1097-0215(19971021)74:5<518::aid-ijc7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–495. doi: 10.1053/hupa.2002.124124. [DOI] [PubMed] [Google Scholar]
- 9.Smithey BE, Pappo AS, Hill DA. C-kit expression in pediatric solid tumors: a comparative immunohistochemical study. Am J Surg Pathol. 2002;26:486–492. doi: 10.1097/00000478-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Entz-Werlé N, Marcellin L, Gaub MP, Guerin E, Schneider A, Berard-Marec P, Kalifa C, Brugiere L, Pacquement H, Schmitt C, Tabone MD, Jeanne-Pasquier C, Terrier P, Dijoud F, Oudet P, Lutz P, Babin-Boilletot A. Prognostic significance of allelic imbalance at the c-kit gene locus and c-kit overexpression by immunohistochemistry in pediatric osteosarcomas. J Clin Oncol. 2005;23:2248–2255. doi: 10.1200/JCO.2005.03.119. [DOI] [PubMed] [Google Scholar]
- 11.Sulzbacher I, Birner P, Toma C, Wick N, Mazal PR. Expression of c-kit in human osteosarcoma and its relevance as a prognostic marker. J Clin Pathol. 2007;60:804–807. doi: 10.1136/jcp.2005.032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei H, Zhao MQ, Dong W, Yang Y, Li JS. Expression of c-kit protein and mutational status of the c-kit gene in osteosarcoma and their clinicopathological significance. J Int Med Res. 2008;36:1008–1014. doi: 10.1177/147323000803600518. [DOI] [PubMed] [Google Scholar]
- 13.Guilhot F. Indications for imatinib mesylate therapy and clinical management. Oncologist. 2004;9:271–281. doi: 10.1634/theoncologist.9-3-271. [DOI] [PubMed] [Google Scholar]
- 14.Pindolia VK, Zarowitz BJ. Imatinib mesylate, the first molecularly targeted gene suppressor. Pharmacotherapy. 2002;22:1249–1265. doi: 10.1592/phco.22.15.1249.33482. [DOI] [PubMed] [Google Scholar]
- 15.Yoshitani K, Honoki K, Morishita T, Kido A, Miyauchi Y, Mii Y, Takakura Y. Growth inhibition of rat osteosarcoma and malignant fibrous histiocytoma cells by tyrosine kinase inhibitor. STI571 In Vivo. 2003;17:255–258. [PubMed] [Google Scholar]