Abstract
Background
Youth with bipolar disorder (BD) show behavioral and neural deficits in cognitive flexibility; however, whether such deficits exist among youths at risk for BD has not been explored.
Methods
The current fMRI study examined the neural basis of cognitive flexibility in BD youth (n=28), unaffected youth at risk for BD (AR; n=13), and healthy volunteer youth (HV; n=21) by comparing brain activation patterns while participants performed the change task. On change trials, subjects must inhibit a prepotent response and execute an alternate one.
Results
During successful change trials, both BD and AR youth had increased right ventrolateral prefrontal and inferior parietal activity, compared to HV youth. During failed change trials, both BD and AR youth exhibited increased caudate activation relative to HV youth, but BD youth showed increased activation in the subgenual anterior cingulate cortex (ACC) relative to the other two groups.
Conclusions
Abnormal activity in ventrolateral prefrontal cortex, inferior parietal cortex, and striatum during a cognitive flexibility task may represent a potential BD endophenotype, but subgenual ACC dysfunction may represent a marker of BD illness itself.
Keywords: bipolar disorder, relatives, fMRI, cognitive flexibility, cognitive control
Introduction
In this study, to examine a potential neurobiological endophenotype of bipolar disorder (BD), we compared neural activity in youth with BD, unaffected youth at familial risk for BD, and healthy volunteers while they completed a cognitive flexibility task. Cognitive flexibility, the ability to adapt one’s behavior to changes in the environment, is essential to higher cognitive functions such as decision-making, problem-solving, reward processing and emotion regulation (Davidson et al., 2006; Dempster, 1992; Stemme et al., 2005). Cognitive flexibility deficits have been found in both children and adults with BD across mood states (Arts et al., 2008; Dickstein et al., 2007), as well as in other psychiatric disorders including schizophrenia (Daban et al., 2006) and attention-deficit hyperactivity disorder (ADHD) (Walshaw et al., 2010). Such deficits may limit patients’ ability to consider and execute alternative response options (Goldberg et al., 2009), thus leading to severe impairment in decision making and social functioning (Leibenluft et al., 2008; Pavuluri et al., 2005).
Cognitive flexibility is measured using various paradigms that involve switching stimuli, responses, rules and/or tasks. Across such paradigms, regions activated during cognitive flexibility include ventrolateral prefrontal cortex (VLPFC), dorsolateral prefrontal cortex (DLPFC), parietal association cortex, and striatum. The striatum primarily mediates motor control during such tasks (Vink et al., 2005), while VLPFC (BA44/45/47) and DLPFC (9/46) play a role in response inhibition and switching (Aron et al., 2004; Bunge, 2004; Rubia, 2010). Together, the prefrontal and parietal cortex mediate top-down attention control (Barber et al., 2005; Sohn et al., 2000).
In this study, we used the change task to study the circuitry mediating cognitive flexibility. The change task is adapted from the stop-signal paradigm developed by Logan et al. (1997). It is a response switching task that requires individuals to inhibit a prepotent response and switch rapidly to an alternative response when the change cue is presented (Kenner et al., 2010). Therefore, the change task engages three major components of cognitive flexibility: attention control, response inhibition, and response switching. A previous study using the change task in healthy adults demonstrated that cognitive flexibility during the task is associated with recruitment of VLPFC, DLPFC, and parietal cortex, as well as striatum (Kenner et al., 2010).
Data indicate that both youth and adults with BD show behavioral deficits on cognitive flexibility tasks, including the change (Dickstein et al., 2007; McClure et al., 2005), Wisconsin Card Sort (Fleck et al., 2008; Martinez-Aran et al., 2004), reversal learning (Gorrindo et al., 2005), set-shifting (McKirdy et al., 2009), and response inhibition tasks (McClure et al., 2005; Pavuluri et al., 2006). Functional MRI studies suggest that such deficits are mediated by dysfunction in a variety of regions that participate in flexible responding including DLPFC, VLPFC, parietal association cortex, and striatum (Blumberg et al., 2003; Chang et al., 2004; Dickstein et al., 2010; Passarotti et al., 2010; Singh et al., 2010; Strakowski et al., 2005). For example, a study using the change task found hyperactivity, which may reflect inefficiency, in DLPFC among BD youth compared to healthy controls during successful response substitution (Nelson et al., 2007). In addition, abnormalities in VLPFC, parietal, striatal activity have been found among BD youth during successful response inhibition (Leibenluft et al., 2007; Passarotti et al., 2010).
In addition to these findings in probands, unaffected adult relatives of adults with BD exhibit deficits in cognitive flexibility (Balanza-Martinez et al., 2008; Bora et al., 2009), although findings are somewhat inconsistent (Schulze et al., 2011). The only existing study of cognitive function in unaffected youth at familial risk for BD found behavioral impairment on attention control (Brotman et al., 2009) and a working memory/interference control task (Doyle et al., 2009). Recent fMRI studies reveal altered prefrontal and parietal activation among relatives of BD adult patients during working memory (Drapier et al., 2008; Thermenos et al., 2009). Effective deployment of working memory is an important component of cognitive flexibility (Bunge et al., 2007). Specifically, working memory deficits may lead to failed inhibition of goal-irrelevant responses and inability to select appropriate alternative responses. However, to our knowledge, no published fMRI study has yet examined neural activity during a cognitive flexibility task in unaffected first-degree relatives (either children or adults) of patients with BD.
Thus, the current study aims to identify neurobiological deficits during a cognitive flexibility task in unaffected, medication-naïve, psychopathology-free youth at risk for BD. Such deficits would be a candidate endophenotype for BD (Gottesman et al., 2003). Compared to studying adults at risk for BD, examining youths at risk for the illness has two advantages. First, the adult relatives of subjects with BD have passed the age of risk for the illness, so findings in that population may reflect resilience as much as risk. Second, data in unaffected youth at familial risk for BD may contribute to efforts to prevent such youth from developing BD or other mood disorders.
In the current study, we compared brain activation during the change task among unaffected at-risk youth with a first-degree relative with BD, BD youth, and healthy volunteer youth. We focused on group differences in brain activations on three contrasts – (1) successful response substitution (successful change) vs. successful execution of the prepotent response (successful go); (2) unsuccessful response substitution (unsuccessful change) vs. successful go; (3) successful change vs. unsuccessful change contrast. Based on previous studies in BD probands and adult relatives of BD patients (Drapier et al., 2008; Leibenluft et al., 2007; Nelson et al., 2007; Passarotti et al., 2010; Singh et al., 2010; Thermenos et al., 2009), we hypothesized that BD youth and unaffected at-risk youth would show altered activation relative to controls in VLPFC, DLPFC, parietal, and striatal regions, regions known to mediate cognitive flexibility during the change task.
Methods
Participants
Participants were patients with pediatric bipolar disorder (BD), at-risk (AR) youth, and healthy volunteer (HV) youth. Participants aged 8-17 were enrolled in an Institutional Review Board-approved protocol at the National Institute of Mental Health. Parents and youth provided written informed consent and assent, respectively. BD patients were recruited through advertisements placed on support groups’ websites and distributed to psychiatrists nationwide. AR youth were recruited by advertisement or through a relative participating in another NIMH study. HV youth were recruited by advertisement in the community. None of the participants were biologically related.
All participants were assessed with a standardized semi-structured interview, the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL) (Kaufman et al., 1997). K-SADS-PL was administered separately to children and parents by clinicians with established inter-rater reliability (κ ≥ 0.9). To evaluate mood state in BD patients, the same clinicians administered the Children’s Depression Rating Scale (CDRS) (Poznanski et al., 1984) and the Young Mania Rating Scale (YMRS) (Young et al., 1978) within 48 hours of scanning. Pediatric BD patients all met the criteria for “narrow phenotype” BD (Leibenluft et al., 2003), with at least one full-duration hypomanic (≥ 4 days) or manic (≥ 7 days) episode characterized by abnormally elevated or expansive mood and at least three other DSM-IV-TR criterion B mania symptoms.
AR youth had either a full biological sibling with “narrow phenotype” BD or a parent with DSM-IV-TR bipolar I or II disorder. K-SADS-PL was used to confirm a bipolar I or II disorder for siblings. Parental BD diagnosis was determined using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (First et al., 2002) or the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994). All the AR participants were medication-naïve and free of psychopathology. Healthy volunteer (HV) youth had no lifetime psychiatric diagnoses, as determined by K-SADS-PL interview with parent and youth, and no first-degree relatives with mood disorders, as determined by interview with the parent. HV youth were also medication free.
All the participants had IQ >70, and no history of neurological disorder, pervasive developmental disorder, chronic medical illness, or current substance abuse and dependence. The Wechsler Abbreviated Scale of Intelligence (Weschler, 1999) was administered to determine IQ. After scanning, some of the total 110 youth who participated in the current study were excluded from all the analysis due to excessive movement during scanning (>3mm, >3 degree rotation in any direction; BD=14, AR=4, HV=10), scanner malfunction (BD=3, AR=2, HV=5), or behavioral accuracy rate on go trials below 50% (Nelson et al., 2007) (BD=6, AR=2, HV=2). Thus, the final numbers of the participants included in the analysis were 28 BD, 13 AR, and 21 HV youth. There was no difference in the number of excluded participants between three groups (p=.47). Data from 20 of 28 BD youth (Nelson et al., 2007) and all of the HVs (Thomas et al., 2011) have been published previously. Data from all AR subjects (n=13) and 8 BD youth have never been published previously.
Behavioral Paradigm
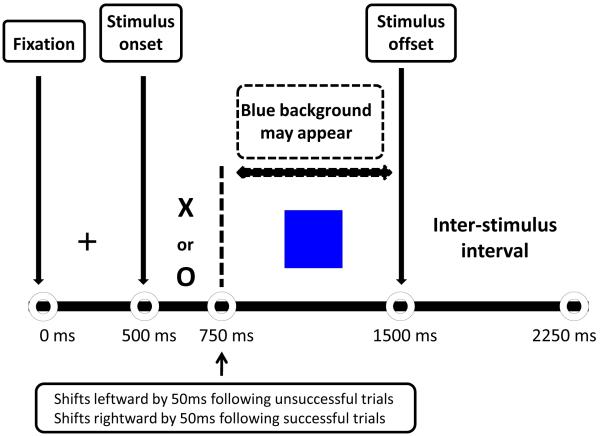
The change task (Dickstein et al., 2007; Logan et al., 1997; McClure et al., 2005; Nelson et al., 2007) consisted of two trial types: go and change. On all trials, a fixation cross was presented for 500 ms followed by a target stimulus (“X” or “O”) for 1000 ms. Go trials were the predominant trial type and established the prepotent response. On go trials, participants were instructed to press 1 if an “X” appeared and 2 if an “O” appeared. Like go trials, change trials began with the appearance of the target stimulus, but this was followed by a change signal (i.e., the stimulus background changed to blue; for change signal timing, see below). This change signal cued participants to press 3 instead of 1 or 2. Consequently, change trials required participants to replace a prepotent stimulus-response pattern with an alternative response to a less frequently occurring stimulus, and thus to display cognitive flexibility (Fig. 1).
Figure 1.
Behavioral paradigm of the Change task. First, a fixation cross is presented for 500ms. Next, participants are instructed to press ‘1’ if an X appears and ‘2’ if and O appears. These constitute prepotent go trials. If a blue background appears, participants are to press a ‘3’ instead of a ‘1’ or ‘2’. These constitute change trials. The onset of the change signal varies from trial to trial. If subjects respond correctly on a change trial, the inhibit delay (the interval between the onsets of the go and change signals) on the next change trial increases by 50 ms, making it more difficult for the subject to change successfully. If subjects respond incorrectly on a change trial, the inhibit delay on the next change trial decreases by 50 ms, making the task easier. A blank screen is displayed during the inter-stimulus interval.
To control for task difficulty across participants, the interval between presentation of the target stimulus and the change signal varied from trial to trial. Correct responses on change trials resulted in a 50 ms delay in the onset of the change signal. This increased task difficulty by increasing the time elapsed between the “go” and “change” signals and thus requiring the participant to alter the prepotent “go” response further into its execution. Conversely, incorrect responses on change trials resulted in a decrease of 50 ms in the interval between the “go” and “change” signals, decreasing the time elapsed between the initiation of the “go” response and the onset of the “change” response and thus making the task easier. Importantly, to avoid strategic delays in response execution, participants were required to maintain a reaction time of >1000 ms across all runs. Across all subjects, accuracy on change trials was maintained at approximately 50%, suggesting that the algorithm worked correctly.
The in-scanner task consisted of 4 runs of 44 go trials, 20 change trials and 22 fixation trials (trial order was randomized within each run). All participants completed practice trials before entering the scanner.
Scanning acquisition and fMRI data preprocessing
Scanning took place in a General Electric 3 Tesla magnet scanner (Milwaukee, WI). The experimental images were displayed via Avotec Silent Vision goggles (Stuart, FL) mounted on the head coil above participants’ eyes. Functional data was acquired (echoplanar single shot gradient echo T2* weighted, TR = 2000 ms, TE = 40ms, flip angle = 90, field of view = 240mm, matrix size 64 × 64, 23 axial slices, 5mm thick, voxels = 3.75 × 3.75 × 5mm). Anatomical T1-weighted echo-planar images with a standardized magnetization-prepared rapid gradient echo sequence (MPRAGE; 180 axial slices, 1mm thick, flip angle = 6, field of view= 256mm, number of acquisitions (NEX) = 1, TR=11.4, TE=4.4ms, matrix size 256×256, T1=300 ms, bandwidth = 130 Hz/pixel, 33kHz/256 pixels) were acquired to be coplanar with the functional scans for spatial registration.
Functional imaging data were preprocessed and analyzed using SPM8 (Statistical Parametric Mapping 8; Wellcome Trust Center for Neuroimaging, University College, London, UK; http://www.fil.ion.ucl.ac.uk/spm) and Matlab 7 (The MathWorks, Natick, MA). Four images at the beginning of each fMRI run were discarded to account for magnetic equilibrium. After slice time correction, images within each run were realigned to the fifth image of the run to correct for movement. After motion correction, the MPRAGE high resolution T1 anatomical images were co-registered to realigned functional images. The high resolution T1 anatomical images were spatially normalized to the SPM8 MNI template using the default setting. The normalized functional images were resampled 2 × 2 × 2 mm. Images were then spatially smoothed using a Gaussian filter with a full-width half-maximum value of 8mm.
Behavioral data analysis
Separate means were calculated for the following variables: accuracy on go and change trials, the average interval between the target and change signal (mean inhibit delay), and reaction time on correct go trials. Trials with a reaction time faster than 100ms were excluded from the analysis because these responses were executed prior to stimulus delivery. In addition, we calculated the change signal reaction time (CSRT) which represents the speed at which one can execute the flexible response, incorporating both speed and accuracy (Williams et al., 1999). When the change accuracy is 50%, CSRT equals the participant’s mean reaction time on change trials minus the mean inhibit delay. The mean inhibit delay is the duration between the initial target “go” signal and the change signal, which was adjusted for each subject on a trial by trial basis (see Methods). Often, individual accuracy rates deviated slightly from 50%, in which case an interpolation algorithm was used to calculate CSRT. Specifically, CSRT is the “go” reaction time at the Xth percentile of go trials (where X is the participant’s accuracy on change trials), minus the participant’s mean inhibit delay. Thus, the CSRT represents an individually adjusted measure of the speed of response flexibility. Separate univariate analysis of variance (ANOVA) analyses were used to assess between-group differences for each behavioral variable.
fMRI data analysis
At the individual subject level, event-related response amplitudes were estimated using the general linear model for each event type. A high pass filter (0.0078 Hz) was used. Event types included successful change, unsuccessful change, and successful go trials. Unsuccessful go trials were rare and were excluded from the analysis. Thus, hereafter, “go trials” refers only to successful go trials. For individual subjects, pair-wise comparison of event-related response amplitudes created contrast images of the blood oxygen level-dependent (BOLD) signal change associated with the three main contrasts, successful change minus go, unsuccessful change minus go, and successful change minus unsuccessful change.
For the group-level analysis, contrast images for individual subjects were entered into a random-effects analysis to produce group activation maps. For each of the three main contrasts, a univariate ANOVA with group (BD, AR, or HV) as a between-subject factor was performed. The whole-brain analysis was conducted first using a conservative statistical threshold, p<.05, false discovery rate (FDR) corrected for multiple comparisons. However, researchers have suggested that, when complex cognitive processes are examined, the corrected statistical threshold may be too conservative. Specifically, Lieberman et al. (2009) argued that the less conservative statistical threshold of p<.005, uncorrected with an extent threshold of 10 consecutive voxels achieves a desirable balance between Types I and II error rates. Thus, we repeated the whole-brain analysis with a less conservative threshold. We employed a threshold of p<.001 uncorrected, with a cluster threshold of 10 voxels, which was more strict than that suggested by Lieberman et al (2009) and was consistent with Nelson et al. (2007), in which we reported data from the same paradigm in a partly overlapping sample of pediatric BD patients.
Areas of activation were identified using the Talairach Daemon atlas after translating coordinates from MNI to Talairach space (Talairach et al., 1988) using mni2tal (http://imaging.mrc-cbu.cam.ac.uk/download/MNI2tal). Findings of group differences in the ANOVA were decomposed within SPSS. Estimates of signal change for each contrast averaged across the entire suprathreshold region were extracted from areas of activations for each participant using MarsBaR (Marseille boîte à région d’intérêt) (Brett et al., 2002). Post-hoc Bonferroni-corrected analyses were performed using SPSS (SPSS, Inc., Chicago, Ill) to identify which pair of groups showed significant differences at a statistical threshold of p<.05.
Furthermore, in addition to the primary contrasts of change and go trials, we performed an additional set of post-hoc analyses to decompose the primary contrasts into those of each event type vs. baseline (fixation). Specifically, in regions identified in the primary contrasts, univariate ANOVAs were used to test for between-group differences in successful change vs. fixation, go vs. fixation, and unsuccessful change vs. fixation. Post-hoc Bonferroni-corrected analyses identified which pair of groups differed at a statistical threshold of p<.05.
We conducted exploratory analyses to rule out the potentially confounding effects of having a parent vs. a sibling with BD among AR youth; comorbid ADHD among BD patients; mood state and of medication. To conduct these post-hoc analyses, tests were performed in SPSS comparing brain activity between (1) AR youth with a BD sibling vs. HV youth; (2) AR youth with a BD parent vs. HV youth; (3) BD patients without ADHD youth vs. HV youth; (4) euthymic BD patients vs. HV youth; and (5) unmedicated BD patients vs. HV youth. In addition, the whole-brain analysis revealed that BD youths exhibited subgenual anterior cingulate cortex (sgACC) hyperactivity compared to AR and HV youths. Given the evidence suggesting that stimulants (Peterson et al., 2009) and antipsychotic drugs (Pavuluri et al., 2011) impact sgACC activity, sgACC activity was compared between BD patients and HV youths after excluding those receiving stimulants or antipsychotic medications. Finally, we examined whether the differences in neural activity between BD and HV groups may be due to mood symptoms in the BD group. Using SPSS, we performed bivariate correlations in BD patients between YMRS or CDRS scores and activation in clusters where we observed differences between BD and HV.
Results
Demographic, clinical and behavioral data
Participant groups did not differ on age, race, IQ, or gender (Table 1). Clinical characteristics of BD youth, including co-morbid illnesses, mood state, and medications are reported in Table 1. Means and standard deviations of behavioral performance are also reported in Table 1. No between-group differences were found for any behavioral measure, including percent accuracy on go trials, F(2,59)= 0.79, or on change trials, F(2,59)= 0.55, mean inhibit delay, F(2,59)= 2.24, mean reaction time on go, F(2,59)= 0.48, or change trials, F(2,59)= 1.33, or mean CSRT, F(2,59)= 1.00 (p >.10 for all).
Table 1.
Demographic, clinical characteristics and behavioral performance on the change task of the bipolar disorder, at risk, and healthy volunteer youth
| BD (N = 28) | AR (N = 13) | HV (N = 21) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 14.37 (2.63) | 13.90 (2.02) | 13.73 (1.96) |
| WASI IQ | 109.36 (15.60) | 107.92 (12.82) | 113.67 (14.21) |
| YMRS | 10.46 (8.43) | --- | --- |
| CDRS | 25.79 (8.52) | --- | --- |
| Number of medications | 1.74 (0.34) | --- | --- |
| Behavioral Performance | |||
| Go accuracy (%) | 74.99(13.65) | 80.72(12.17) | 77.49(14.35) |
| Change accuracy (%) | 32.95(13.24) | 29.15(16.40) | 34.21(9.43) |
| Inhibit delay (ms) | 398.07(82.07) | 402.86(131.24) | 460.14(119.61) |
| Go reaction time (ms) | 674.96(70.67) | 682.69(108.90) | 700.81(106.54) |
| Change reaction time (ms) | 833.61(66.36) | 875.23(93.92) | 867.76(116.52) |
| Change signal reaction time (ms) |
206.68(52.63) | 197.45(80.75) | 183.43(44.20) |
| N (%) | N (%) | N (%) | |
| Male | 12 (43) | 6 (46) | 13 (62) |
| Bipolar Typea | |||
| Bipolar I | 22(79) | --- | --- |
| Bipolar II | 3 (11) | --- | --- |
| Mood State | |||
| Euthymic | 19(68) | --- | --- |
| Comorbid Conditions | |||
| ADHD | 18(64) | --- | --- |
| ODD or CD | 9(32) | --- | --- |
| Anxiety | 10(36) | --- | --- |
| Medicationb | |||
| Unmedicated | 10(37) | 13(100) | 21(100) |
| Antipsychotic | 12(44) | --- | --- |
| Lithium | 7(26) | --- | --- |
| Antiepileptic | 14(52) | --- | --- |
| Antidepressant | 6(22) | --- | --- |
| Stimulants | 6(22) | --- | --- |
BD = bipolar disorder, AR = at risk, HV = healthy volunteer, WASI= Weschler Abbreviated Scale of Intelligence, YMRS = Young Mania Rating Score, CDRS = Children’s Depression Rating Score, ADHD = Attention Deficit Hyperactivity Disorder; ODD = Oppositional Defiant Disorder; CD = Conduct Disorder
missing data from 3 bipolar patients
missing data form 1 bipolar patient
fMRI data
In the whole-brain analysis on three primary contrasts, no region showed significant between-group differences at a threshold of p<.05, FDR corrected. Thus, we used a statistical threshold of p<.001 uncorrected with a cluster threshold of 10 voxels (see Methods) to identify between-group differences in brain activation on the contrasts of successful change vs. go, unsuccessful change vs. go, and successful change vs. unsuccessful change (Table 2).
Table 2.
Significant between-group differences in brain activations on the successful change vs. go, unsuccessful change vs. go and successful change vs. unsuccessful change contrasts, and in the post-hoc analyses of successful change vs. fixation, unsuccessful change vs. fixation, go vs. fixation, and unsuccessful change vs. fixation contrasts.
| Area of Activation | Brodmann area |
Side | cluster size |
MNI coordinates |
F (2, 59) |
p value |
Between-group differences | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Successful change vs Go | |||||||||
|
| |||||||||
| Ventrolateral PFC (IFG) | 47 | R | 47 | 42 | 22 | 0 | 10.86 | AR>BD***, AR>HV*** | |
| Successful change vs Fixation | 5.19 | ** | AR>BD**, AR>HV* | ||||||
| Ventrolateral PFC (IFG) | 44 | R | 51 | 52 | 12 | 18 | 10.97 | AR>BD***, AR>HV*** | |
| Successful change vs Fixation | 6.29 | ** | AR>BD*, AR>HV** | ||||||
| Inferior Parietal Gyrus | 40 | R | 30 | 36 | −42 | 46 | 10.19 | AR>BD*, AR>HV***, BD>HV* | |
| Successful change vs Fixation | 4.76 | * | AR>HV*, BD>HV† | ||||||
| Cerebellum | -- | L | 176 | −8 | −54 | −28 | 14.53 | AR>BD***, AR>HV*** | |
| Successful change vs Fixation | 4.47 | * | AR>BD†, AR>HV* | ||||||
| Go vs Fixation | 6.24 | ** | AR>BD**, AR>HV** | ||||||
| Cerebellum | -- | L | 20 | -2 | -56 | -10 | 10.04 | AR>BD*, AR>HV***, BD>HV* | |
| Successful change vs Fixation | 3.69 | * | AR>HV* | ||||||
| Go vs Fixation | 3.53 | * | AR>HV†, BD>HV† | ||||||
|
| |||||||||
| Unsuccessful change vs Go | |||||||||
|
| |||||||||
| Subgenual Anterior Cingulate Gyrus | 25 | R | 15 | 2 | 0 | −8 | 9.89 | BD>AR***, BD>HV* | |
| Unsuccessful change vs Fixation | 12.74 | *** | BD>AR***,BD>HV† | ||||||
| Caudate | -- | R | 29 | 14 | 24 | 10 | 8.73 | AR>HV*, BD>HV*** | |
| Unsuccessful change vs Fixation | 3.98 | * | BD>HV* | ||||||
| Go vs Fixation | 4.72 | * | HV>AR*, HV>BD* | ||||||
| Cerebellum | -- | L | 44 | −14 | −40 | −26 | 10.67 | AR>HV***, BD>HV** | |
| Unsuccessful change vs Fixation | 6.81 | ** | AR>HV**, BD>HV* | ||||||
|
| |||||||||
| Successful change vs Unsuccessful change | |||||||||
|
| |||||||||
| Ventrolateral PFC (IFG) | 45 | R | 14 | 48 | 14 | 18 | 9.53 | AR>BD†, AR>HV***, BD>HV* | |
| Successful change vs Fixation | 6.06 | ** | AR>BD*, AR>HV** | ||||||
p< .10
p<.05
p<.01
p<.001
All clusters were determined at the significance threshold p<.001, voxel ≥ 10
BD = bipolar disorder, AR = at risk, HV = healthy volunteer, PFC = prefrontal cortex, IFG = Inferior frontal gyrus, MFG = middle frontal gyrus x,y, and z coordinates refer to the voxel with maximum signal intensity
Note: The post-hoc analyses of successful change, go or unsuccessful change vs. fixation were conducted in regions identified by the primary contrasts (successful change vs. go, unsuccessful change vs. go and successful change vs. unsuccessful change
Successful Change vs. Go
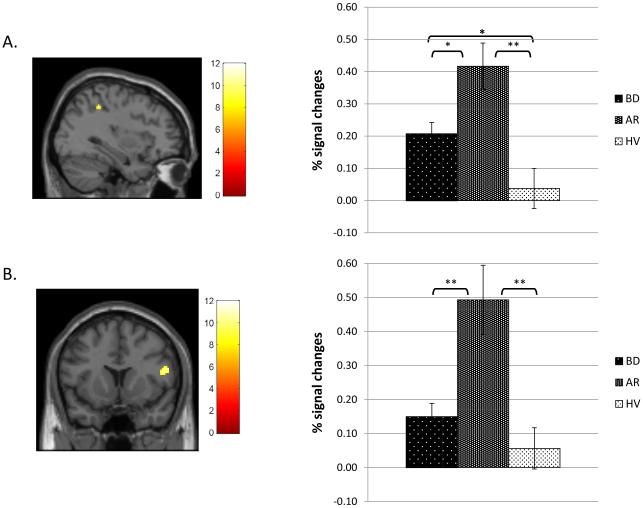
Between-group differences were found in five regions: two in right VLPFC (BA47 and BA44), one in right inferior parietal lobe (IPL; BA40), and two left cerebellar regions. Post-hoc analyses revealed that the AR group showed greater brain activation than BD and HV groups in all five regions including right IPL (Fig. 2A) and right VLPFC (BA44; Fig. 2B). Compared to HV, BD showed greater activation in the IPL (Fig. 2A) and one cerebellar region.
Figure 2.
Brain areas showing between-group differences on the successful change vs successful go contrast among bipolar disorder (BD), at risk (AR), and healthy volunteer (HV) groups. Color bars represent F values of the contrast. All clusters were determined at the significance threshold of p < .001 (uncorrected), voxel ≥ 10. Percent signal change estimates for each contrast were averaged across the entire cluster for each participant. Post-hoc Bonferroni-corrected analyses were performed to decompose between-group differences at a statistical threshold of p < .05.
A. right inferior parietal lobe (BA40; 36, −42, 46); B. right ventrolateral prefrontal cortex (BA44; 52, 12, 18). **p<.001, *p<.05.
On the successful change vs. fixation contrast, the groups differed in activation in all five regions, consistent with the pattern on the successful change vs. go contrast. On the go vs. fixation contrast, groups differed only in two cerebellar regions. Thus, group differences in the successful change vs. go contrast were driven primarily by group differences during successful change trials.
Unsuccessful Change vs. Go
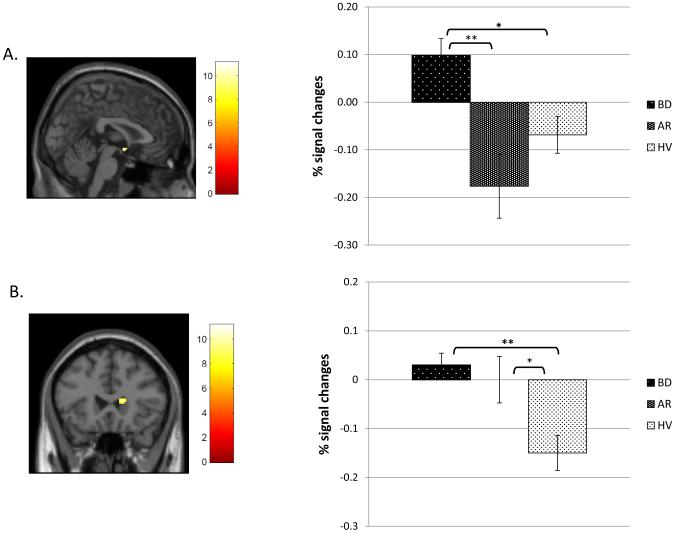
Between-group differences were found in three regions: right sgACC (BA25), right caudate, and left cerebellum. The BD group showed elevated activation compared to the AR and HV groups in right sgACC (Fig. 3A). Both BD and AR groups had increased activity relative to HV in right caudate (Fig. 3B) and in left cerebellum.
Figure 3.
Brain areas showing between-group differences on the unsuccessful change vs successful go contrast among bipolar disorder (BD), at risk (AR), and healthy volunteer (HV) groups. Color bars represent F values of the contrast. All clusters were determined at the significance threshold of p < .001 (uncorrected), voxel ≥ 10. Percent signal change estimates for each contrast were averaged across the entire cluster for each participant. Post-hoc Bonferroni-corrected analyses were performed to decompose between-group differences at a statistical threshold of p < .05.
A. right subgenual cingulate cortex (2, 0, −8); B. right caudate (14, 24, 10). **p<.001, *p<.05.
Between-group differences were found in all three regions on the unsuccessful change vs. fixation contrast and were largely consistent with differences found in the main contrast. On the go vs. fixation contrast, HV group had greater activity than BD or AR in the right caudate. Thus, group differences in the unsuccessful change vs. go contrast were driven primarily by group differences during unsuccessful change trials.
Successful Change vs. Unsuccessful Change
On this contrast, the groups differed in activation in right VLPFC (BA45). Post-hoc analyses revealed that both BD and AR groups showed increased activation relative to the HV group. The AR group also showed greater activation than the BD group. AR youth showed hyperactivation relative to the other two groups on the successful change vs. fixation contrast, but not on the unsuccessful change vs. fixation contrast.
Effects of familial at risk status, comorbid ADHD, mood states, and medication
We tested whether the differences we observed between AR and HV youth were driven by offspring of BD parents, or siblings of youth with BD. Seven of thirteen AR youth (54%) had a BD sibling and the rest had a BD parent. When AR youth with a BD sibling (N=7) were compared to HV, differences were found in all of the regions identified in the primary analyses (ts(26) > 2.54, ps < .05) except for right caudate, where the difference was a trend, p<.10. Similarly, when AR youth with a BD parent (N=6), but no affected sibling, were compared to HV, all of the regions identified in the primary analyses remained significant (ts(25) > 2.18, ps < .05).
In four of the six regions identified in the primary analyses, BD patients without comorbid ADHD (N=18) differed from the HV group (ts(29) > 3.00, ps < .05). In the remaining two regions (left cerebellum on the successful change vs. go contrast and right VLPFC (BA45) on the successful change vs. unsuccessful change contrast), the differences between BD patients without comorbid ADHD and HV youth was a trend, ps < .10.
When only euthymic BD patients (N=19) were compared to HV youth, differences remained significant in all of the regions identified in the primary analyses (ts(38)>2.97, ps<.05), with the exception that, in three instances, previously significant findings became trends (ps < .10). These regions were in right IPL and left cerebellum on the successful change vs. go contrast, and right VLPFC (BA45) on the successful change vs. unsuccessful change contrast.
Activation was greater among unmedicated BD (N=10) vs. HV youth in the regions where BD youth differed from HV youths in the primary analysis, ts(29) > 2.56, ps < .05, except in three regions where were trends, ps<.10. Those regions were right IPL and left cerebellum on successful change vs. go, and right VLPFC (BA45) on successful change vs. unsuccessful change.
Potential effects of stimulants and antipsychotic medications on the sgACC activity were tested. Stimulant-free BD patients (N=21) showed greater activity than HV youths, t(40) = 3.40, p< .005. Activation between BD patients who were free of antipsychotic medications (N=15) and HV youths also differed, t(34) = 2.62, p< .05.
Lastly, in BD patients, we examined correlations between mood state and activation in clusters where we observed activation differences between BD and HV. These regions were right IPL and left cerebellum on the correct change vs. go contrast, right sgACC and caudate on the incorrect change vs. go contrast, and right VLPFC on the correct vs. incorrect change contrast. There was no correlation between YMRS or CDRS scores and activation in these regions.
Discussion
The current study examined group differences in the neural correlates of cognitive flexibility among BD youth, youth at risk for BD (AR), and healthy volunteers (HV) using the change task. Despite having similar behavioral performance on the task, during successful response switching, BD youth showed greater activation in right VLPFC (BA45) than HV youth, while AR youth showed greater activation in right VLPFC (BA44/45/47) than both HV and BD youth. In addition, compared to HV, both BD and AR youth exhibited increased activity in right IPL during successful response switching and in right caudate during failed response switching. These findings were present in at-risk youth irrespective of whether risk was conferred by having a parent or sibling with BD. Since hyperactivity in VLPFC, IPL, and caudate are present in both probands and youth at risk for BD, dysfunction in these regions may be a risk marker for the illness. In contrast, during failed response switching, BD youth showed increased activity in right sgACC relative to both AR and HV youth, indicating that sgACC hyperactivity may appear only after the onset of the illness. These findings did not vary with mood state, medication status, or ADHD comorbidity.
Both probands and youth at-risk for BD exhibited hyperactivation in brain regions mediating response inhibition and flexibility. Our finding of hyperactivity in the VLPFC, IPL, and caudate among youth with BD is consistent with prior findings (Blumberg et al., 2003; Dickstein et al., 2010; Passarotti et al., 2010). Because BD, AR and HV youth did not differ in task performance in this study, increased recruitment of the VLPFC and IPL in BD and AR youth may reflect decreased efficiency in attentional control during successful performance of the cognitive flexibility task. Similarly, increased caudate activity among BD and AR youth may reflect motor control inefficiency during failed response switching.
Our findings suggest that hyperactivity in the right VLPFC, IPL and caudate during cognitive flexibility may be a neurobiological endophenotype of BD. An illness endophenotype is an abnormality that is (1) associated with the illness, (2) state-independent, (3) found in unaffected relatives at a higher rate than in the general population, (4) heritable, and (5) co-segregates with illness within families (Gottesman et al., 2003). Previous studies provide evidence that behavioral deficits in cognitive flexibility meet the first three criteria (Arts et al., 2008; Bora et al., 2009). In the current study, we add to that literature by reporting that aberrant activity within brain circuit (VLPFC, IPL and caudate) activation during cognitive flexibility also meets these three criteria i.e., they are associated with BD, state-independent, and present in unaffected relatives. To our knowledge, no studies have tested whether behavioral performance or neural activity during a cognitive flexibility task is heritable, so that would be a topic for future research in twin samples or extended pedigrees. In addition, to test whether VLPFC, IPL and caudate hyperactivity during a cognitive flexibility task meets the fifth criterion for an endophenotype, it is necessary to have longitudinal studies that test whether at-risk youth with such hyperactivity are at higher risk to develop BD than at-risk youth without hyperactivity.
Compared to healthy youth, both BD and AR youth showed hyperactivity in right VLPFC and IPL during successful change trials. However, it is important to note that we also observed differences between AR and BD youth in these regions i.e., AR youth showed greater VLPFC and IPL activation than BD youth. This may be due to medication exposure in BD youth, since data suggest that medication may normalize neural activation (Leibenluft et al., 2007; Nelson et al., 2007; Pavuluri et al., 2010; Phillips et al., 2008). For example, in previous analyses, some in a sample overlapping with this study, we reported that differences between unmedicated BD patients and healthy controls were more marked than those between medicated patients and controls in striatum and ACC during response inhibition, and in DLPFC and motor cortex during cognitive flexibility (Leibenluft et al., 2007; Nelson et al., 2007). Unfortunately, given the severity of BD in youth (Axelson et al., 2006) and ethical proscriptions against withdrawing severely ill children from medication for research purposes, it is difficult to recruit large samples of unmedicated youth with BD. Thus, it is difficult to disambiguate whether differences between AR and BD children are due to medication or are sequelae of illness in the BD sample. To do so, future research should follow unaffected youth at risk for BD longitudinally to characterize neural activation before and after the onset of BD and before and after the institution of medication treatment.
Although hyperactivity in prefrontal, parietal and striatal regions was shared between BD and AR youth, hyperactivity in right sgACC was observed only in BD youth. Specifically, on the unsuccessful change vs. go contrast, BD youth exhibited greater sgACC activation than both AR and HV youth. The sgACC projects to the amygdala and plays an important role in emotion regulation (Drevets et al., 2008). Speculatively, increased sgACC activity in BD youth during failed change trials may be due to the interference of emotions on the performance of the cognitive flexibility task. Our finding of sgACC dysfunction in patients with BD is consistent with previous studies (Bauer et al., 2005; Drevets et al., 1997; Sharma et al., 2003). Our findings add to the prior literature by indicating that this abnormality in sgACC activity is present among patients with BD, but not among unaffected youth at risk for BD. There is evidence suggesting that sgACC hyperactivity among BD patients may be related to medication. In one study, ventral ACC activity increased from pre- to post-treatment of risperidone (Pavuluri et al., 2011). Evidence in ADHD children also suggest that activity in sgACC, which is part of the default mode network, may be altered by stimulants (Peterson et al., 2009). However, in the current study, after excluding BD youths taking stimulant or antipsychotic medication, the greater sgACC activity among BD youths compared to HV youths remained significant. A longitudinal study is needed to determine whether sgACC hyperactivity is related to medication exposure, or manifests only after the onset of BD or, alternatively, whether it is present in the subset of AR youth who ultimately develop the illness and thus is an additional risk marker for BD.
The current study differed from our prior report in a partially overlapping sample (Nelson et al., 2007), in that here we did not find differences between BD and HV youth in DLPFC activation during the successful change vs. go contrast. Differences between Nelson et al. (2007) and the current study may be due to the inclusion here of a larger sample of BD youth and a group of AR youth. Although there are other studies suggesting abnormal DLPFC activation during response inhibition among BD youth (Passarotti et al., 2010; Singh et al., 2010), increased DLPFC activation during cognitive flexibility tasks is not found consistently across studies (Barber et al., 2005).
The results should be considered in light of the study’s limitations. The study has a relatively small sample size, particularly in the AR group, because we included only the unaffected AR youth who were medication-naïve, and psychopathology-free. Such subjects are difficult to recruit, particularly for fMRI studies. Some of the AR youth in our sample had a BD parent whereas others had a BD sibling, although post-hoc analyses indicated that the deficits that we identified in the entire at-risk sample were also present in each of these sub-samples. There was also heterogeneity among BD patients in terms of comorbid conditions; however, when the sample of BD youth was limited to ones without comorbid ADHD (the most common comorbidity), differences in neural activity between BD and HV youth remained consistent. A limitation of these exploratory post-hoc analyses is that they were performed only in those regions where between-group differences were identified in the primary analysis. Future studies are needed to focus directly on the effect of the at-risk familial status, comorbidity or medication, using larger and/or better targeted samples. Finally, in the current study, we found no differences in behavioral indicators of cognitive flexibility between BD and HV youth, whereas previous out-of-scanner behavioral studies found that BD youth had a slower CSRT than controls (McClure et al., 2005). Other studies have failed to replicate in the scanner between-group differences in behavior identified in the clinic, perhaps because of the impact of the different testing environments (Dickstein et al., 2010; Nelson et al., 2007). Lastly, it should be noted that potential cognitive endophenotypes of BD are certainly not limited to cognitive flexibility. Behavioral and neural evidence suggest that other cognitive domains, including working and verbal memory and sustained attention, may also be endophenotypes of BD (Arts et al., 2008; Brotman et al., 2009; Drevets et al., 2008). Studies examining dysfunction in the neural systems mediating a variety of cognitive domains are important in evaluating a number of potential endophenotypes in BD.
In summary, using a cognitive flexibility task, we compared neural activation among unaffected pediatric relatives of BD patients, youth with BD, and healthy subjects. Our results suggest that children at risk for BD as well as children with BD showed abnormal activity in right VLPFC, IPL and caudate during a cognitive flexibility task. We also found that sgACC dysfunction is present only in youth with BD, but not in unaffected at risk youth. Future work should include larger samples and a longitudinal design to determine whether VLPFC, IPL and caudate hyperactivity during a cognitive flexibility task predicts onset of BD in youth at risk for the illness. In addition, future work is needed to examine the association between cognitive flexibility deficits and clinical course of BD. Specifically, such deficits may impact patients’ ability to adapt to new environments and exercise good judgment (e.g., regarding treatment compliance), and thus may be associated with adverse clinical outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–73. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological Medicine. 2008;38:771–85. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry. 2006;63:1139–48. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- Balanza-Martinez V, Rubio C, Selva-Vera G, Martinez-Aran A, Sanchez-Moreno J, Salazar-Fraile J, et al. Neurocognitive endophenotypes (endophenocognitypes) from studies of relatives of bipolar disorder subjects: a systematic review. Neuroscience and Biobehavioral Reviews. 2008;32:1426–38. doi: 10.1016/j.neubiorev.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cerebral Cortex. 2005;15:899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Bauer M, London ED, Rasgon N, Berman SM, Frye MA, Altshuler LL, et al. Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Molecular Psychiatry. 2005;10:456–69. doi: 10.1038/sj.mp.4001647. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. American Journal of Psychiatry. 2003;160:1345–7. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: A meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox [abstract]; Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Brotman M, Rooney M, Skup M, Pine D, Leibenluft E. Increased intra-subject variability in response time in youths with bipolar disorder and at-risk family members. Journal of the American Academy of Child and Adolescent Psychiatry. 2009 doi: 10.1097/CHI.0b013e3181a27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cognitive, Affective and Behavioral Neuroscience. 2004;4:564–79. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Current Opinion in Neurobiology. 2007;17:243–50. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Archives of General Psychiatry. 2004;61:781–92. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Balanza-Martinez V, Salazar-Fraile J, et al. Specificity of cognitive deficits in bipolar disorder versus schizophrenia. A systematic review. Psychotherapy and Psychosomatics. 2006;75:72–84. doi: 10.1159/000090891. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–78. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disorders. 2010;12:707–19. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, Brotman MA, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:341–55. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Wozniak J, Wilens TE, Henin A, Seidman LJ, Petty C, et al. Neurocognitive impairment in unaffected siblings of youth with bipolar disorder. Psychological Medicine. 2009;39:1–11. doi: 10.1017/S0033291708004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier D, Surguladze S, Marshall N, Schulze K, Fern A, Hall MH, et al. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biological Psychiatry. 2008;15:513–20. doi: 10.1016/j.biopsych.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr., Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Insistute, Biometrics Research; New York: 2002. [Google Scholar]
- Fleck DE, Shear PK, Madore M, Strakowski SM. Wisconsin Card Sorting Test performance in bipolar disorder: effects of mood state and early course. Bipolar Disorders. 2008;10:539–45. doi: 10.1111/j.1399-5618.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Chengappa KN. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disorders. 2009;11(Suppl 2):123–37. doi: 10.1111/j.1399-5618.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. American Journal of Psychiatry. 2005;162:1975–7. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kenner NM, Mumford JA, Hommer RE, Skup M, Leibenluft E, Poldrack RA. Inhibitory motor control in response stopping and response switching. Journal of Neuroscience. 2010;30:8512–8. doi: 10.1523/JNEUROSCI.1096-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. American Journal of Psychiatry. 2003;160:430–7. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA. Pediatric bipolar disorder. Annu Rev Clin Psychol. 2008;4:163–87. doi: 10.1146/annurev.clinpsy.4.022007.141216. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. American Journal of Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsitivy and inhibitory control. Psychological Science. 1997;8:60–4. [Google Scholar]
- Martinez-Aran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, et al. Cognitive function across manic or hypomanic, depressed and euthymic states in bipolar disorder. American Journal of Psychiatry. 2004;161:262–70. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. American Journal of Psychiatry. 2005;162:1644–51. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, McIntosh AM. Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychological Medicine. 2009;39:1289–93. doi: 10.1017/S0033291708004935. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, Dickstein DP, et al. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disorders. 2007;9:810–9. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Research. 2010;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:846–71. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. Journal of Clinical Psychiatry. 2010;71:1526–34. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Lu LH, Carbray JA, Sweeney JA. Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder: fMRI outcomes. Psychiatry Research. 2011;193:28–37. doi: 10.1016/j.pscychresns.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. American Journal of Psychiatry. 2006;163:286–93. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, et al. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. American Journal of Psychiatry. 2009;166:1286–94. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008;165:313–20. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. Journal of the American Academy of Child Psychiatry. 1984;23:191–7. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Rubia K. “Cool” Inferior Frontostriatal Dysfunction in Attention-Deficit/Hyperactivity Disorder Versus “Hot” Ventromedial Orbitofrontal-Limbic Dysfunction in Conduct Disorder: A Review. Biological Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Schulze KK, Walshe M, Stahl D, Hall MH, Kravariti E, Morris R, et al. Executive functioning in familial bipolar I disorder patients and their unaffected relatives. Bipolar Disorders. 2011;13:208–16. doi: 10.1111/j.1399-5618.2011.00901.x. [DOI] [PubMed] [Google Scholar]
- Sharma V, Menon R, Carr TJ, Densmore M, Mazmanian D, Williamson PC. An MRI study of subgenual prefrontal cortex in patients with familial and non-familial bipolar I disorder. Journal of Affective Disorders. 2003;77:167–71. doi: 10.1016/s0165-0327(02)00109-x. [DOI] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Mazaika P, Garrett A, Adleman N, Kelley R, et al. Neural correlates of response inhibition in pediatric bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20:15–24. doi: 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: the role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci. 2000;97:13448–53. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemme A, Deco G, Busch A, Schneider WX. Neurons and the synaptic basis of the fMRI signal associated with cognitive flexibility. Neuroimage. 2005;26:454–70. doi: 10.1016/j.neuroimage.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. American Journal of Psychiatry. 2005;162:1697–705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Thermenos HW, Goldstein JM, Milanovic SM, Whitfield-Gabrieli S, Makris N, Laviolette P, et al. An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2009;153B:120–31. doi: 10.1002/ajmg.b.30964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, Hall JM, Skup M, Jenkins SE, Pine DS, Leibenluft E. A developmental neuroimaging investigation of the change paradigm. Dev Sci. 2011;1:148–61. doi: 10.1111/j.1467-7687.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Human Brain Mapping. 2005;25:336–44. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walshaw PD, Alloy LB, Sabb FW. Executive function in pediatric bipolar disorder and attention-deficit hyperactivity disorder: in search of distinct phenotypic profiles. Neuropsychology Review. 2010;20:103–20. doi: 10.1007/s11065-009-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. Weschler Abbreviated Scale of Intelligence. The Psychological Corporation; Austin, TX: 1999. [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35:205–13. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]