Abstract
Multiple sclerosis is an autoimmune disease of the central nervous system characterized by neuroinflammation and demyelination. Although considered a T cell-mediated disease, multiple sclerosis involves the activation of both adaptive and innate immune cells, as well as resident cells of the central nervous system, which synergize in inducing inflammation and thereby demyelination. Differentiation, survival, and inflammatory functions of innate immune cells and of astrocytes of the central nervous system are regulated by tyrosine kinases. Here, we show that imatinib, sorafenib, and GW2580—small molecule tyrosine kinase inhibitors can each—prevent the development of disease and treat established disease in a mouse model of multiple sclerosis. In vitro, imatinib and sorafenib inhibited astrocyte proliferation mediated by the tyrosine kinase platelet-derived growth factor receptor (PDGFR), whereas GW2580 and sorafenib inhibited macrophage tumor necrosis factor (TNF) production mediated by the tyrosine kinases c-Fms and PDGFR, respectively. In vivo, amelioration of disease by GW2580 was associated with a reduction in the proportion of macrophages and T cells in the CNS infiltrate, as well as a reduction in the levels of circulating TNF. Our findings suggest that GW2580 and the FDA-approved drugs imatinib and sorafenib have potential as novel therapeutics for the treatment of autoimmune demyelinating disease.
Keywords: Imatinib, sorafenib, experimental autoimmune encephalomyelitis, tyrosine kinase inhibitors, macrophages, TNF, astrocyte proliferation
Introduction
Multiple sclerosis (MS) is an autoimmune inflammatory disease characterized by the destruction of the myelin sheath that surrounds neuronal axons in the central nervous system (CNS), a process that results in neurodegeneration and consequently in the formation of sclerotic plaques in the brain and spinal cord [1]. Adaptive [2–4] and innate [5– 7] immune cells infiltrate the CNS where they act synergistically in inducing and perpetuating local inflammation and demyelination. Deregulation of the homeostatic functions of resident CNS cells also contributes to the pathogenesis of MS [8, 9]. However, the mechanisms underlying the initiation and progression of MS remain undefined. This lack of understanding is reflected in the current treatments for MS, most of which target only symptoms or are administered together with global immuno-suppressants, which can have serious adverse side effects. Although immunoregulatory drugs that specifically target immune cells have been developed [10], they reduce the number of exacerbations only in a small proportion of patients and are beneficial only in relapsing–remitting forms of MS [11, 12]. Therefore, new therapies that target specific pathways involved in MS pathogenesis are needed.
One type of innate immune cell that plays a prominent role in MS pathogenesis is the macrophage [5]. Macrophages phagocytose myelin in brain lesions in MS [13] and in vitro of MS [14], thereby contributing to demyelination directly. They also contribute to demyelination indirectly by promoting immune cell infiltration and inflammation in the CNS. For instance, macrophages produce an array of proinflammatory cytokines, including tumor necrosis factor (TNF), a cytokine that exerts neurotoxic and chemoattractant effects in the CNS [7, 15] and is implicated in the pathogenesis of autoimmune diseases [16, 17]. Indeed, inhibiting macrophage activation [18, 19] or depleting macrophages [20, 21] attenuates disease in rodent models of MS, and this amelioration is accompanied by a reduction in TNF levels in the CNS and suppression of CNS infiltration by autoreactive T cells [22].
The differentiation, proliferation, survival, and activation of macrophages are regulated by macrophage colony-stimulating factor (MCSF; also known as CSF1) via its receptor, the tyrosine kinase colony-stimulating factor 1 receptor (c-Fms). Mice deficient in MCSF have fewer macrophages than wild-type mice [23–26], and MCSF regulates the production of cytokines by macrophages [27]. Interestingly, MCSF is upregulated in several neurological and autoimmune diseases, including MS [28, 29], and c-Fms has been proposed as a putative genetic susceptibility factor for MS [30]. Thus, by promoting the formation, survival, and activation of macrophages, c-Fms could contribute to the inflammation and demyelination characteristic of MS.
In addition to inflammatory cells infiltrating the CNS, resident cells of the CNS contribute to the pathogenesis of MS. Astrocytes, though generally considered supporting cells for neurons, could potentially promote MS pathogenesis in several ways [31–33]. In particular, excessive proliferation of astrocytes contributes to astrogliosis, a scarring process occurring in MS that prevents axonal regeneration and remyelination [34], and thus impairs tissue healing. Astrocyte proliferation depends on signaling mediated by platelet-derived growth factor receptors (PDGFRs), [35], whose ligands are upregulated in peripheral blood leukocytes in experimental autoimmune encephalomyelitis (EAE), a mouse model of MS [36]. PDGFR signaling could contribute to MS pathogenesis by promoting astrocyte proliferation and consequently astrogliosis.
The tyrosine kinases c-Fms and PDGFR are thus involved in key aspects of MS pathogenesis and may have potential as drug targets in the treatment of MS. Imatinib mesylate (imatinib)—a tyrosine kinase inhibitor prescribed for the treatment of Bcr-Abl-expressing chronic myelogenous leukemias and c-Kit-expressing gastrointestinal stromal tumors—can attenuate autoimmune arthritis [37] and autoimmune diabetes [38] in mice. Besides Abl and c-Kit, two other tyrosine kinase receptors inhibited by imatinib are the PDGFR and c-Fms receptors. Here, we test the ability of imatinib to attenuate EAE, a mouse model of MS [10, 39]. We also test the therapeutic efficacy of two other small-molecule tyrosine kinase inhibitors (TKI): sorafenib, a drug approved for the treatment of renal cell carcinoma and hepatocellular carcinoma that inhibits PDGFR, and GW2580, a relatively specific inhibitor of c-Fms that can attenuate autoimmune arthritis in mice [40]. We show that imatinib, sorafenib, and GW2580 can each effectively treat EAE. Imatinib and sorafenib abrogated platelet-derived growth factor (PDGF)-induced proliferation of astrocytes, whereas GW2580 and sorafenib suppressed TNF production by macrophages.
Materials and Methods
EAE Induction and TKI Administration
Six- to 8-week-old female C57BL/6 mice were housed in accordance with NIH guidelines. Mice were maintained on a 12-h light/dark cycle and given free access to food and water. For the induction of EAE, mice were immunized subcutaneously with myelin oligodendrocyte glycoprotein peptide (MOG33–55) emulsified in Complete Freund’s Adjuvant (CFA) and then administered pertussis toxin intravenously immediately after and 24 h after immunization. EAE progression was assessed using the following five-point system: 0, no disease; 1, limp tail; 2, partial hind-leg paralysis; 3, complete hind-leg paralysis; 4, complete hind-leg paralysis and partial front-leg paralysis; and 5, death. The mice were administered with TKI or vehicle (0.5% hydroxypropyl methylcellulose + 0.05% Tween-80) every 12 h through animal-feeding needles. In the EAE prevention studies, TKI administration was started 24 h before immunization and continued until the experiment’s termination. In the EAE treatment studies, mice were randomized into separate groups, such that each group had a mean EAE clinical score of 2.5–3, and dosed twice daily with TKI or vehicle until the experiment’s termination.
Tyrosine Kinase Inhibitors
For in vivo studies, imatinib (Gleevec) and sorafenib (Nexavar) were purchased from the pharmacy, whereas GW2580 was chemically synthesized (by LC Laboratories). Pharmacological doses of imatinib [41, 42] and sorafenib [43–45] were calculated according to those attained in human blood, as reported in clinical trials and preclinical studies of the drugs showing efficacy in the treatment of cancer. For GW2580, dosing was calculated according to that used in previous mouse studies [46]. On the basis of the peak-trough and half-life of each compound, we calculated that one dose of TKI every 12 h would suffice to achieve optimal pharmacological levels of the drugs in plasma. Each dose comprised freshly prepared drug at 100 mg/kg for imatinib, 100 mg/kg for GW2580, and 30 mg/kg for sorafenib. The mice receiving a twice-daily oral dose of 100 mg/kg imatinib exhibit a pharmacokinetic profile similar to that in humans on a mid-range dose of 400 mg once daily; this dosing regimen results in mean peak plasma levels of 4.6– 6 µM in mice and 1–1.5 µM in humans, respectively [41, 42]. Sorafenib administered twice daily at 30 mg/kg exhibits a pharmacokinetic profile similar to that in humans on a mid-range dose of 400 mg once daily [43–45]. For in vitro studies, imatinib, sorafenib, and GW2580 were purchased from LC laboratories in powder form and resuspended in sterile vehicle (0.5% hydroxypropyl methylcellulose±0.05% Tween-80). Concentrations equivalent to those reached by our dosing regimen were used for all in vitro experiments.
Histology
Brains and spinal cords were collected from EAE and healthy mice immediately after sacrifice. The tissues were collected in formalin and later embedded in paraffin. We stained the tissue sections with Luxol fast blue (LFB) to highlight myelin tracts and with eosin and hematoxylin to distinguish cytoplasmic and nuclear structures. Slides were analyzed under the microscope by an expert pathologist in a blinded manner. Meningeal and parenchymal inflammatory foci were counted for all samples.
Isolation and Flow Cytometric Analysis of CNS Infiltrate
Brains and spinal cords from EAE mice treated with vehicle or GW2580 were collected in Hank’s buffered salt solution (HBSS) and passed through a 70-µM nylon mesh strainer according to a described protocol [47]. The cells were spun down at 350×g for 10 min, and then resuspended and incubated in HBSS with 300 U/ml of clostridial collagenase type IV for 60 min at 37°C. Digestion was stopped with complete Dulbecco’s modified Eagle’s medium (DMEM), and the suspension was centrifuged for 10 min at 350×g. The pellets were resuspended in 1 ml of 30% Percoll, underlayed with 1 ml of 70% Percoll, and spun at 500×g for 20 min at room temperature. The interphase containing the cells was collected and washed twice with PBS in preparation for flow cytometric analysis. Cells collected from the Percoll gradients were stained with rat anti-mouse CD3 fluorescein isothiocyanate (FITC) and rat anti-mouse F4/80 PE (eBiosciences) and analyzed using a FACScan. Data analysis was performed using Cell Quest Pro.
Cell Culture
RAW 264.7 cells were grown in RPMI media supplemented with 10% fetal calf serum (FCS), l-glutamine, penicillin/ streptomycin (P/S), and non-essential amino acids (NEAA). Primary rat astrocytes from the cerebral cortex of P2 mouse pups were grown in DMEM media supplemented with 10% FCS, 2 mM l-glutamine, P/S NEAA, sodium pyruvate, insulin (5 µg/ml), NAC (5 µg/ml), and 10 µM hydrocortisone. The C6 rat astrocyte cell line was cultured in DMEM supplemented with 10% FCS, l-glutamine, P/S, and NEAA.
Enzyme-linked Immunosorbent Assay
RAW 264.7 cells were plated on 96-well plates in RPMI with or without 10 ng/ml of MCSF or PDGFbb, in the presence or absence of TKI. Forty-eight hours after stimulation, supernatants were collected and frozen till used for the detection of TNF by enzyme-linked immunosorbent assay (ELISA) (Peprotech) according to manufacturers’ instructions. PDGFbb was purchased from Sigma and MCSF from Peprotech.
To measure the levels of serum TNF, we collected blood from vehicle- and GW258-treated mice by arterial tail bleeding immediately before sacrificing them to collect other tissues. The blood was collected in serum-separating tubes and stored at −80°C. TNF levels in serum from EAE mice treated with vehicle or GW2580 were measured using the TNF ELISA kit from Peprotech.
Proliferation Assays
C6 and primary astrocyte proliferation was assessed by 3H-thymidine incorporation. Briefly, 5×105 cells per well were plated in a 96-well plate in 200 µl of media and left incubating at 37°C overnight to allow the cells to adhere. Astrocytes were then stimulated with 10 ng/ml of PDGFbb or 10 ng/ml of TNF in the presence or absence of imatinib or sorafenib. Cells were cultured for 24 h before the addition of 1 uCi of 3H–thymidine per well. After 18 to 24 h, the cells were frozen or harvested and corrected counts per minute were counted using a betaplate reader.
Statistical Analysis
Mann–Whitney U test was used to determine statistical differences in clinical EAE scores between each TKI treatment and the vehicle control. Unpaired two-tailed Student’s t test was used to determine statistical differences between numbers of inflammatory foci and between levels of cytokines.
Results
Tyrosine kinase inhibitors imatinib, sorafenib, and GW2580 attenuate EAE
Imatinib can treat other autoimmune diseases and can inhibit signaling pathways implicated in MS, including those mediated by c-Fms and PDGFR [37, 38]. We therefore performed experiments to determine whether imatinib can attenuate autoimmune demyelinating disease in the EAE mouse model of chronic progressive MS. We also tested the therapeutic efficacy of sorafenib, a small-molecule drug that inhibits PDGFR, and GW2580, a small-molecule that inhibits c-Fms and can attenuate autoimmune arthritis in mice [40]. We induced EAE in C57BL/6 mice by immunizing them with purified MOG33–55 emulsified in CFA, and then injecting them intravenously with pertussis toxin immediately after immunization and 24 h after immunization [39]. Mice were dosed orally twice daily with 100 mg/kg of imatinib, 30 mg/kg of sorafenib, 100 mg/kg of GW2580, or vehicle on the basis of published pharmacokinetic profiles of imatinib and sorafenib metabolism in mice and humans [41, 42, 48–51] and GW2580 metabolism in mice [46, 49, 52] (see “Methods” section).
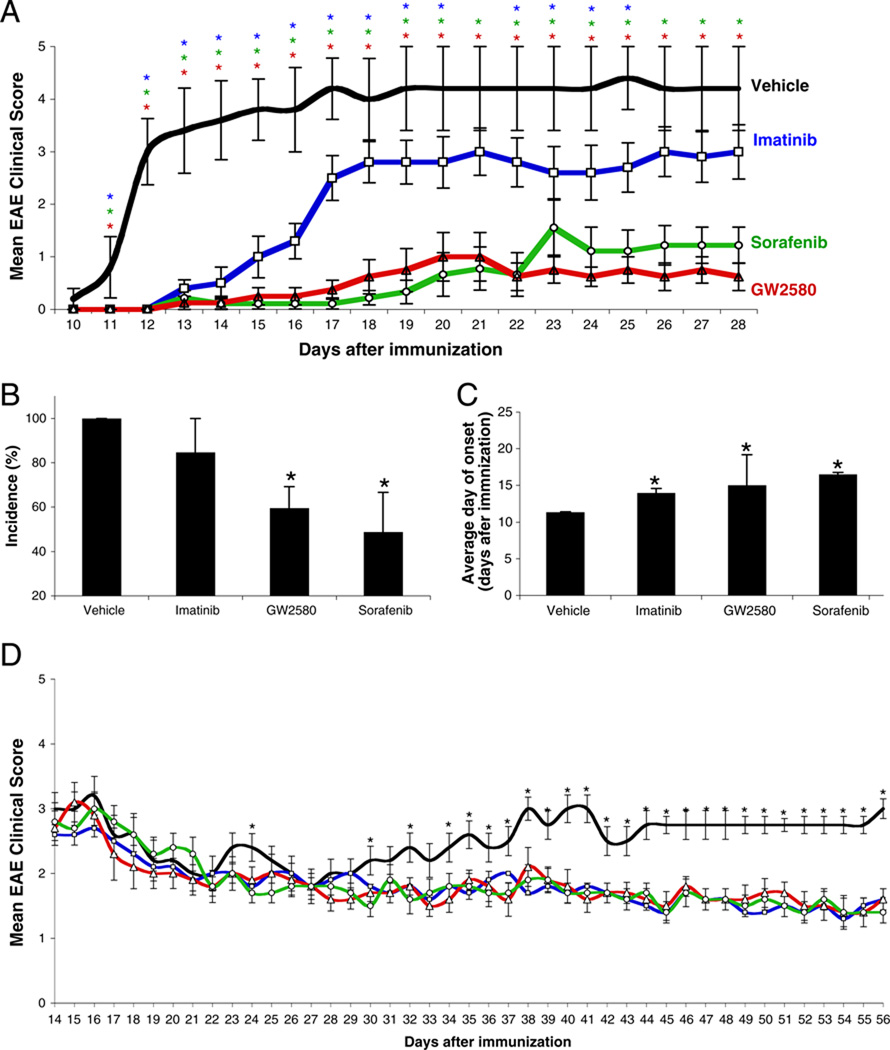
To determine whether the TKI can prevent the development of EAE, we started administering the TKI 1 day before immunizing the mice with MOG33–55. After immunization, EAE was less severe (Fig. 1a), EAE incidence was lower (Fig. 1b), and EAE onset was delayed (Fig. 1c) in TKI-treated compared to vehicle-treated mice. There were no apparent toxicities or adverse effects in any of the mice receiving any of the TKI.
Fig. 1.
The TKI imatinib, sorafenib, and GW2580 can prevent and treat EAE. a–c EAE prevention. C57BL/6J mice (n=10–15 mice per group) were dosed orally with 100 mg/kg imatinib (blue), 30 mg/kg sorafenib (green), 100 mg/kg GW2580 (red), or vehicle (black) twice a day, starting 1 day before the induction of EAE. a Disease severity was assessed by a visual scoring system. b The incidence of disease at experiment’s termination and c the average day of EAE onset. d EAE treatment. Once mice developed overt EAE (mean clinical score of 2.5–3), they were randomized and administered TKI every 12 h until the experiment’s termination (n=10–15 mice per group). Results are representative of two independent experiments and are shown as the mean±SEM. *P<0.05 at each data point by Mann–Whitney U test comparing each treatment with vehicle
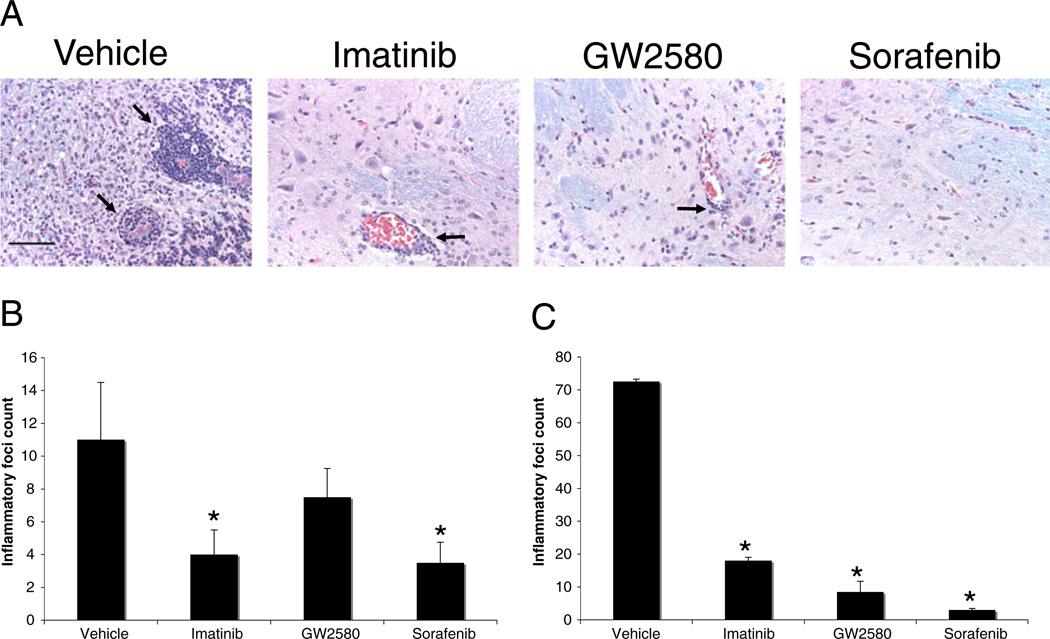
To determine whether the TKI can treat established EAE, we randomized mice with established clinical EAE (mean clinical score of 2.5–3) and treated them with 100 mg/kg imatinib, 30 mg/kg of sorafenib, 100 mg/kg of GW2580, or vehicle. All the TKI tested suppressed the progression and reduced the severity of established EAE (Fig. 1d). Histopathologic analysis of brains and spinal cords harvested from mice used in these experiments demonstrated that EAE mice treated with imatinib, sorafenib, or GW2580 had significantly fewer inflammatory foci in both the EAE prevention (Fig. 2a, b) and the treatment (Fig. 2c) studies than did vehicle-treated mice.
Fig. 2.
TKI treatment suppresses formation of inflammatory foci in the CNS during EAE. (a) Representative H&E/LFB-stained brainstem and cerebellum sections from C57BL/6 mice from a prevention EAE study at day 17 after immunization. Scale bar=50 µM. Mean number of inflammatory foci in meninges and parenchyma of TKI- or vehicle-treated mice from (b) an EAE prevention study (n=10) and (c) an EAE treatment study (n=4). Results are shown as the mean± SEM. *P<0.05 by Student’s t test, compared to vehicle-treated mice
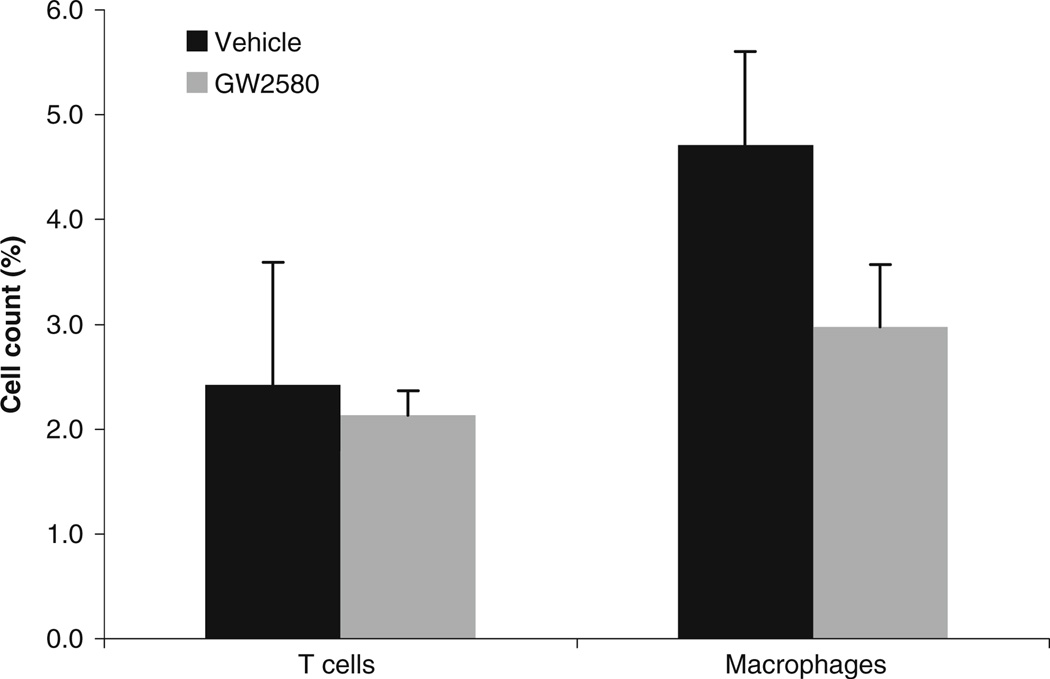
GW2580 reduces the proportion of macrophages in the CNS of EAE mice
To assess the effect of GW2580 on the infiltration of inflammatory cells into the CNS in EAE, we performed flow cytometric analysis of the mononuclear cell infiltrate isolated from brains and spinal cords of EAE mice treated prophylactically with GW2580 or vehicle. Because inflammatory cells are not abundant in the CNS even under inflammatory conditions, infiltrates from two to three brains and spinal cords were pooled for the analysis. Cells were stained with anti-CD3 FITC antibodies and anti-F4/80 PE antibodies for the detection of T cells and macrophages, respectively. As shown in Fig. 3, the proportion of macrophages was lower in the CNS infiltrate from GW250-treated mice than that from vehicle-treated mice (2.97%± 0.59 vs 4.71%±0.89). The proportion of T cells was not significantly different in the CNS infiltrate of GW2580-treated mice compared to vehicle-treated mice (2.13%± 0.23 vs 2.42%±1.71).
Fig. 3.
GW2580 reduces the proportion of macrophages in the CNS of mice with EAE. Brains and spinal cords from EAE mice treated prophylactically with vehicle or GW2580 were collected 17 days after MOG35–55 immunization. Mononuclear cells were isolated and stained for T cells with anti-CD3 antibodies and for macrophages with anti-F4/80 antibodies. Results are representative of five mice per treatment group; for each treatment group, brain and spinal cords from 2–3 mice were pooled and analyzed by flow cytometry. Results are shown as the mean±SEM
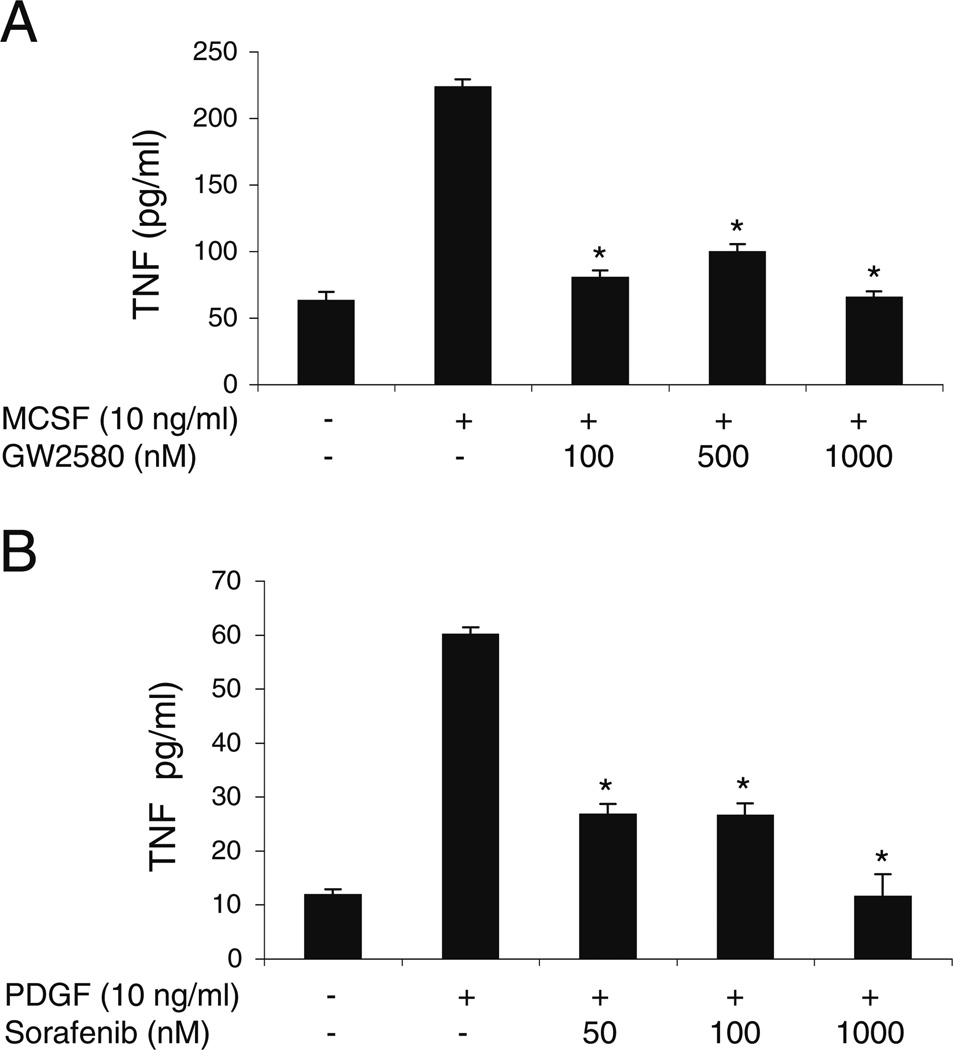
Sorafenib and GW2580 inhibit macrophage production of proinflammatory cytokines
Macrophages contribute to the pathogenesis of MS by producing proinflammatory cytokines [5], and c-Fms regulates macrophage activation [52]. We therefore examined the effect of GW2580 on the activation of the macrophage cell line RAW264.7 cells. We stimulated the RAW264.7 cells with 10 ng/ml MCSF for 48 h in the presence of 10–1000 nM GW2580 and then measured TNF levels in culture supernatants by ELISA. GW2580 reduced MCSF-stimulated production of TNF to basal levels (Fig. 4a). Because macrophages also express PDGFR, we tested sorafenib’s ability to reduce PDGFbb-mediated macrophage TNF secretion. Sorafenib was able to decrease PDGFbb-induced TNF release by macrophages (Fig. 4b).
Fig. 4.
TKI suppress MSCF- and PDGFbb-induced TNF production by macrophages. TNF levels were measured by ELISA in culture supernatants from RAW 264.7 cells stimulated for 48 h with (a) 10 ng/ml of MCSF in the presence of 0–1,000 nM of GW2580 or (b) 10 ng/ml of PDGFbb in the presence of 0–1,000 nM of sorafenib. Results are representative of three independent experiments. The mean±SEM of triplicates is shown. *P<0.05 by Student’s t test, compared with cells stimulated in the absence of inhibitor
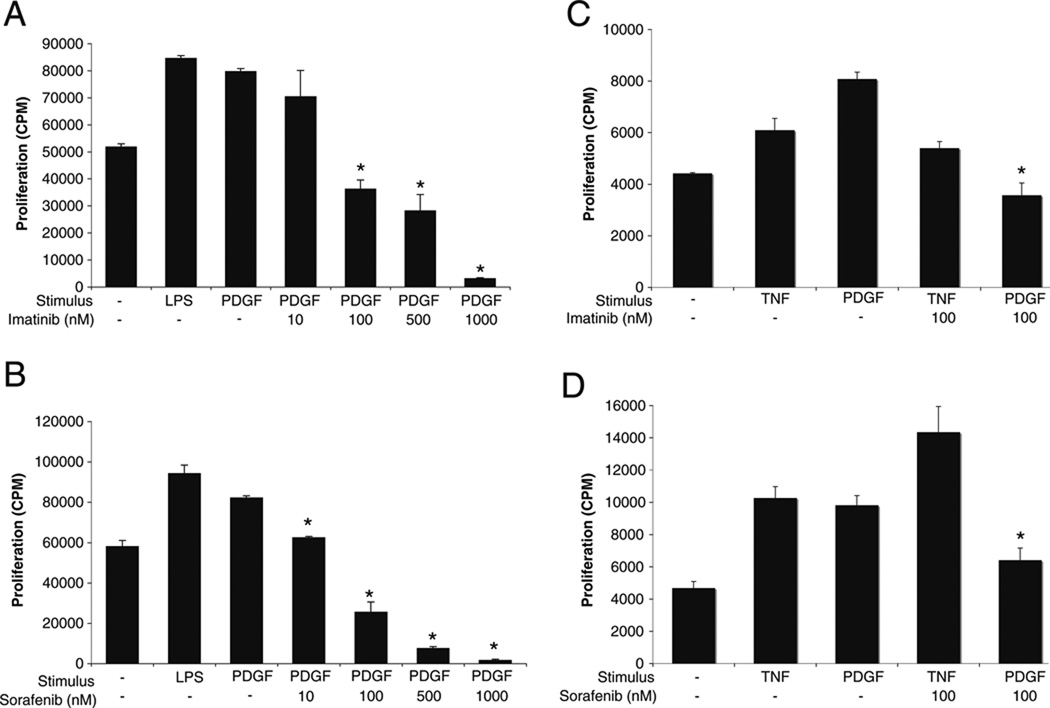
Imatinib and Sorafenib Inhibit Proliferation of Rat Astrocytes
We next determined whether imatinib or sorafenib could decrease astrocyte proliferation, a process involved in the astrogliosis typical of MS. Rat astrocyte cells from the C6 clone were stimulated with 10 ng/ml of PDGFbb or with 100 ng/ml of LPS as a positive control, whereas primary cortical rat astrocytes were stimulated with 10 ng/ml of PDGFbb or with 10 ng/ml of TNF as a positive control. PDGFbb was added to the astrocytes in the presence or absence of different concentrations of imatinib or sorafenib within the therapeutic range for each drug, and proliferation was measured by 3H–thymidine incorporation. Both imatinib and sorafenib were able to inhibit PDGF-induced astrocyte proliferation (Fig. 5). In contrast, GW2580 had no effect on astrocyte proliferation (data not shown), confirming that GW2580 does not inhibit PDGFR.
Fig. 5.
Imatinib and sorafenib suppress PDGF-mediated astrocyte proliferation. Astrocytes of the C6 cell line were stimulated with 10 ng/ml of PDGFbb in the presence of increasing concentrations of (a) imatinib or (b) sorafenib. LPS (100 ng/ml) was used as a positive control. Primary cortical rat astrocytes were stimulated with 10 ng/ml PDGFbb in the presence of (c) imatinib or (d) sorafenib. TNF was used as a physiological positive control. *P<0.05 by Student’s t test, compared with cells stimulated in the absence of inhibitor. Results are representative of five independent experiments and are shown as the mean±SEM of triplicates
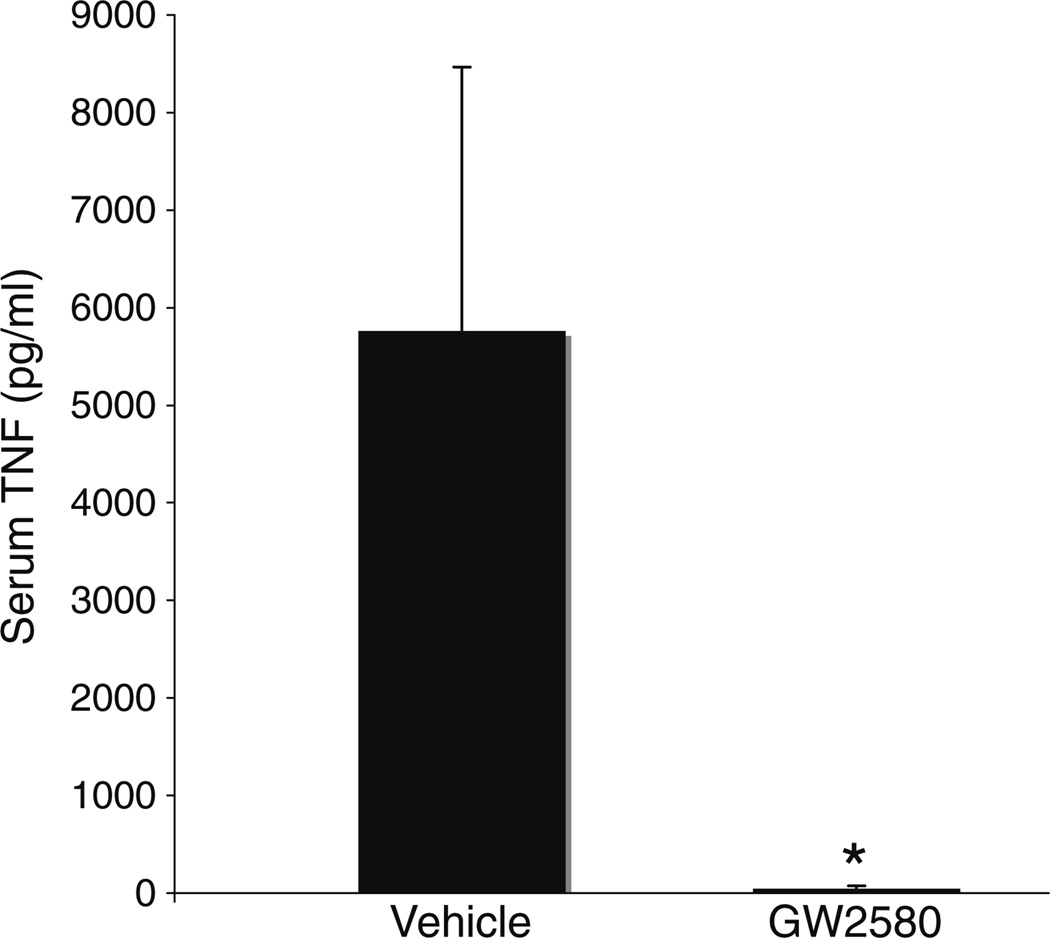
GW2580 Decreases Serum TNF Levels in EAE Mice
Because GW2580 decreased macrophage TNF production in vitro, we asked whether GW2580 decreases TNF levels in vivo in mice with EAE. We measured TNF levels in serum collected 17 days after MOG immunization from mice treated prophylactically with GW2580 or vehicle. The levels of TNF were significantly higher in serum from vehicle-treated EAE mice compared to GW2580-treated mice (Fig. 6).
Fig. 6.
GW2580 reduces levels of circulating TNF in mice with EAE. Serum levels of TNF in EAE mice treated prohylactically with GW2580 (n=5) or vehicle (n=3) were measured by ELISA. Serum was collected 17 days after MOG immunization. Results shown are the mean of five GW2580-treated mice and three vehicle-treated mice. The mean±SEM is shown. *P≤0.05 by Student’s t test
Discussion
In this study, we demonstrate that the tyrosine kinase inhibitors imatinib, sorafenib, and GW2580 cannot only attenuate the development of EAE but also treat established EAE. We show that GW2580 and sorafenib can suppress TNF production by macrophages, while imatinib and sorafenib can abrogate PDGF-induced proliferation of astrocytes. Moreover, the administration of GW2580 to mice with EAE reduced the circulating levels of TNF and reduced the infiltration of the CNS by macrophages. Together, our data suggest that imatinib, sorafenib, and GW2580 potently inhibit cellular responses that contribute to inflammation and astrogliosis in the CNS in autoimmune demyelinating disease.
Although imatinib and sorafenib both inhibit PDGFR signaling, sorafenib was more efficacious than imatinib in preventing the development of EAE. In contrast, imatinib and sorafenib were equally efficacious in treating established EAE. This can be explained by differences in the drugs’ ability to penetrate the CNS: Unlike sorafenib [53], imatinib does not readily cross an intact blood–brain barrier (BBB) [52–54]. In a treatment setting, however, the BBB is compromised, as a result of the EAE pathology [55]. Thus, in prevention studies, imatinib’s effects are restricted to the periphery, whereas in treatment studies, imatinib might additionally target cells within the CNS.
Infiltrating inflammatory cells, including T cells and macrophages, contribute to the neuroinflammation in MS, and we found that imatinib, sorafenib, or GW2850 decreased the number of inflammatory foci in the CNS of mice with EAE. Moreover, we show that GW2580 decreased the proportion of infiltrating macrophages in the CNS, a decrease that may result from GW2580’s ability to suppress c-Fms-mediated macrophage differentiation [40]. Although we saw only a trend towards a decrease in number of infiltrating T-cells in the CNS of GW2580-treated mice, previous studies have shown that depleting EAE mice of macrophages suppresses T-cell infiltration of the CNS [20], and amelioration of EAE with a different c-Fms inhibitor, Ki20227, was associated with the suppression of myeloid cell expansion and reduction in MOG-specific T-cell proliferation [56]—results suggesting that molecules that inhibit macrophage formation or activation could indirectly inhibit T-cell activity.
Besides its ability to inhibit macrophage differentiation [40], GW2580 abrogated the production of TNF by cultured macrophages and markedly suppressed serum levels of TNF in mice with EAE. It is possible that levels of TNF in the serum do not accurately reflect levels of TNF in the CNS. Nevertheless, we found that the proportion of macrophages—the main TNF-producing cells in EAE [20]—is diminished in the CNS of GW2580-treated EAE mice. Together, these findings suggest that GW2580 ameliorates EAE at least in part by reducing levels of macrophage-derived TNF.
TNF may contribute to the pathogenesis of autoimmune demyelination by recruiting inflammatory cells to the CNS [7] and exerting toxic effects on oligodendrocyte progenitor cells [15]. Indeed, the administration of TNF exacerbates, whereas anti-TNF treatment attenuates, EAE [17]. The role of TNF in MS, however, is less clear. Although TNF levels in cerebrospinal fluid may positively correlate with disease activity in MS [57, 58], case studies suggest that anti-TNF treatment in rheumatoid arthritis patients is associated with the development of MS-like lesions in the CNS [59]. We speculate that anti-TNF antibodies induce MS pathology because they are unable to penetrate the CNS, where TNF inhibition might be beneficial, and are restricted to the periphery, where TNF inhibition may be detrimental [60]. c-Fms inhibitors suppress TNF production by macrophages specifically, and macrophages produce TNF only within tissues (such as CNS tissues in EAE). We show, however, that amelioration of EAE by GW2580 is associated with a reduction in peripheral levels of TNF. The role of TNF in the CNS and periphery remains to be further defined.
Although we show that GW2580 can decrease macrophage numbers in the CNS of EAE mice, reduce circulating TNF levels in vivo, and suppress MCSF-induced macrophage TNF production in vitro, it remains possible that GW2580 attenuates EAE by affecting macrophage functions other than TNF production. In MS, macrophages produce not only TNF but also matrix metalloproteinases (MMPs) [61], which break down the extracellular matrix required for the integrity of the BBB [13]. Macrophages also contribute to MS pathology by phagocytosing myelin, as well as by producing nitric oxide [62], IL-1β, and Th1-polarizing osteopontin [1, 60]. Because macrophages depend on c-Fms signaling for their differentiation and activation [63], inhibiting c-Fms inhibition might also suppress some of these functions, further contributing to the beneficial effects of TKI seen in EAE.
Astrocytes are generally considered support cells for neurons—yet they too could promote demyelinating disease in several ways, e.g., by promoting astrogliosis and producing proinflammmatory cytokines and chemokines. We show that imatinib and sorafenib can suppress PDGF-induced proliferation of astrocytes. Their ability to suppress astrocyte proliferation may account, at least partially, for these TKI’s therapeutic efficacy in EAE because astrocyte proliferation promotes astrogliosis and scar formation in autoimmune demyelinating disease [34]. It is also possible that these inhibitors provide benefit in EAE by suppressing PDGFR activity in other cell types or by modulating other astrocyte functions. Astrocytes are mediators of glutamate homeostasis [33, 64], and imbalances in glutamate secretion and reabsorption play an important role in the demyelinating stages of MS [8, 9, 61]. Astrocytes can also contribute to the breakdown of the BBB by producing MMPs [61, 62]. By inhibiting PDGFR-mediated proliferation of astrocytes, imatinib and sorafenib could indirectly suppress these potentially pathogenic processes, in addition to directly suppressing astrogliosis.
We show that GW2580 inhibits c-Fms-mediated TNF production and that imatinib and sorafenib inhibit PDGFR-mediated cell proliferation. However, despite being a relatively specific inhibitor of c-Fms, GW2580 can also inhibit TrkA [65]. TrkA belongs to the family of neurotrophin receptors, which is also implicated in MS [66]; inhibition of TrkA could therefore account for some of GW2580’s beneficial effects in EAE. Furthermore, besides PDGFR, another prominent target of imatinib and sorafenib is c-kit, a tyrosine kinase receptor expressed on mast cells. Mast cells also play an important role in CNS demyelination, by promoting BBB breakage in the early stages of MS and recruiting other inflammatory cells [7]. The beneficial effects of imatinib and sorafenib in EAE may reflect the simultaneous inhibition of several tyrosine kinases in several cell types.
Conclusion
We show that the administration of sorafenib, imatinib, or GW2580 ameliorates EAE, raising the possibility that these TKI—two of which are already FDA-approved drugs—could serve as novel therapeutics for the treatment of MS. Further elucidation of the specific pathways targeted by these TKI could shed light on the pathogenesis of MS and allow the development of more specific drugs that may have fewer side effects than the current therapies for MS.
Acknowledgments
We thank Ben Barres’ group, in particular Maria Fabian, for kindly providing the rat primary astrocytes. We also thank Jane Eaton for providing guidance through the histopathology tissue-staining process. This work was supported by National Institutes of Health (NIH) National Heart Lung and Blood Institute contract N01-HV-00242, NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR-054822, and Veterans Affairs Health Care System funding awarded to WHR. OC received funding from the NIH training grant 5 T32 AI07290 for Molecular and Cellular Immunobiology.
Abbreviations
- MS
Multiple sclerosis
- EAE
Experimental autoimmune encephalomyelitis
- MOG
Myelin oligodendrocyte glycoprotein
- TKI
Tyrosine kinase inhibitor
- PDGFR
Platelet-derived growth factor receptor
- PDGF
Platelet-derived growth factor
- c-Fms
Colony-stimulating factor 1 receptor
- MCSF
Macrophage colony-stimulating factor
- CFA
Complete Freund’s adjuvant
- TNF
Tumor necrosis factor
- IL
Interleukin
- CNS
Central nervous system
- FCS
Fetal calf serum
- NEAA
Non-essential amino acids
- LFB
Luxol fast blue
- HBSS
Hank’s buffered salt solution
Contributor Information
Oliver Crespo, Division of Immunology and Rheumatology, Department of Medicine, Stanford University School of Medicine, CCSR, 269 Campus Drive, Stanford, CA 94305, USA; VA Palo Alto Health Care System, Palo Alto, CA, USA.
Stacey C. Kang, Division of Immunology and Rheumatology, Department of Medicine, Stanford University School of Medicine, CCSR, 269 Campus Drive, Stanford, CA 94305, USA
Richard Daneman, Department of Developmental Biology, Stanford University, School of Medicine, Stanford, CA, USA.
Tamsin M. Lindstrom, Division of Immunology and Rheumatology, Department of Medicine, Stanford University School of Medicine, CCSR, 269 Campus Drive, Stanford, CA 94305, USA VA Palo Alto Health Care System, Palo Alto, CA, USA.
Peggy P. Ho, Department of Neurological Sciences and Pediatrics, Stanford University, Stanford, CA, USA
Raymond A. Sobel, Department of Pathology, Stanford University, School of Medicine, Stanford, CA, USA
Lawrence Steinman, Department of Neurological Sciences and Pediatrics, Stanford University, Stanford, CA, USA.
William H. Robinson, Email: wrobins@stanford.edu, Division of Immunology and Rheumatology, Department of Medicine, Stanford University School of Medicine, CCSR, 269 Campus Drive, Stanford, CA 94305, USA; VA Palo Alto Health Care System, Palo Alto, CA, USA.
References
- 1.Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 2.Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125:161–169. doi: 10.1111/j.1365-2567.2008.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh S, Cudrici C, Ito T, Rus H. B-cells humoral immunity in multiple sclerosis. Implications for therapy. Immunol Res. 2007;40(3):224–234. doi: 10.1007/s12026-007-8009-6. [DOI] [PubMed] [Google Scholar]
- 4.Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43:465–471. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- 5.Benveniste E. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75(3):165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 6.Huitinga I, Ruuls SR, Jung S, Van Rooijen N, Hartung HP, Dijkstra CD. Macrophages in T cell line-mediated, demyelinating, and chronic relapsing experimental autoimmune encephalomyeli-tis in Lewis rats. Clin Exp Immunol. 1995;100:344–351. doi: 10.1111/j.1365-2249.1995.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184(12):6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 8.Vercellino M, Merola A, Piacentino C, Votta B, Capello E, Mancardi GL, et al. Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J Neuropathol Exp Neurol. 2007;66:732–739. doi: 10.1097/nen.0b013e31812571b0. [DOI] [PubMed] [Google Scholar]
- 9.Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- 10.Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005;26:565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Healy BC, Engler D, Gholipour T, Weiner H, Bakshi R, Chitnis T. Accounting for disease modifying therapy in models of clinical progression in multiple sclerosis. J Neurol Sci. 2011;303(1– 2):109–113. doi: 10.1016/j.jns.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Liblau R. Glatiramer acetate for the treatment of multiple sclerosis: evidence for a dual anti-inflammatory and neuro-protective role. J Neurol Sci. 2009;287:S17–S23. doi: 10.1016/S0022-510X(09)71296-1. [DOI] [PubMed] [Google Scholar]
- 13.Vos CM, van Haastert ES, de Groot CJ, van der Valk P, de Vries HE. Matrix metalloproteinase-12 is expressed in phagocytotic macrophages in active multiple sclerosis lesions. J Neuroimmunol. 2003;138:106–114. doi: 10.1016/s0165-5728(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 14.Bruck W, Friede RL. L-fucosidase treatment blocks myelin phagocytosis by macrophages in vitro. J Neuroimmunol. 1990;27:217–227. doi: 10.1016/0165-5728(90)90072-u. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Haaf M, Todorich B, Grosstephan E, Schieremberg H, Surguladze N, et al. Cytokine toxicity to oligodendrocyte precursors is mediated by iron. Glia. 2005;52:199–208. doi: 10.1002/glia.20235. [DOI] [PubMed] [Google Scholar]
- 16.Kassiotis G, Kollias G. TNF and receptors in organ-specific autoimmune disease: multi-layered functioning mirrored in animal models. J Clin Invest. 2001;107:1507–1508. doi: 10.1172/JCI13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollias G, Douni E, Kassiotis G, Kontoyiannis D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol Rev. 1999;169:175–194. doi: 10.1111/j.1600-065x.1999.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin M, Wu M, Tsirka SE. Modulation of microglial/macrophage activation by macrophage inhibitory factor (TKP) or tuftsin (TKPR) attenuates the disease course of experimental autoimmune encephalomyelitis. BMC Immunol. 2007;8:10. doi: 10.1186/1471-2172-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martiney JA, Rajan AJ, Charles PC, Cerami A, Ulrich PC, Macphail S, et al. Prevention and treatment of experimental autoimmune encephalomyelitis by CNI-1493, a macrophage-deactivating agent. J Immunol. 1998;160:5588–5595. [PubMed] [Google Scholar]
- 20.Brosnan CF, Bornstein MB, Bloom BR. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J Immunol. 1981;126:614–620. [PubMed] [Google Scholar]
- 21.Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol. 1998;161:3767–3775. [PubMed] [Google Scholar]
- 23.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120(6):1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki A, Yokoo H, Naito M, Kaizu C, Shultz LD, Nakazato Y. Effects of macrophage-colony-stimulating factor deficiency on the maturation of microglia and brain macrophages and on their expression of scavenger receptor. Neuropathology. 2000;20:134–142. doi: 10.1046/j.1440-1789.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 26.Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/ op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982;156:1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pradervand S, Maurya M, Subramaniam S. Identification of signaling components required for the prediction of cytokine release in RAW 264.7 macrophages. Genome Biol. 2006;7(2):R11. doi: 10.1186/gb-2006-7-2-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell IK, Rich MJ, Bischof RJ, Hamilton JA. The colony-stimulating factors and collagen-induced arthritis: exacerbation of disease by M-CSF and G-CSF and requirement for endogenous M-CSF. J Leukoc Biol. 2000;68:144–150. [PubMed] [Google Scholar]
- 29.Gallo P, Pagni S, Giometto B, Piccinno MG, Bozza F, Argentiero V, et al. Macrophage-colony stimulating factor (M-CSF) in the cerebrospinal fluid. J Neuroimmunol. 1990;29:105–112. doi: 10.1016/0165-5728(90)90152-d. [DOI] [PubMed] [Google Scholar]
- 30.D’Alfonso S, Nistico L, Zavattari P, Marrosu MG, Murru R, Lai M, et al. Linkage analysis of multiple sclerosis with candidate region markers in Sardinian and Continental Italian families. Eur J Hum Genet. 1999;7:377–385. doi: 10.1038/sj.ejhg.5200301. [DOI] [PubMed] [Google Scholar]
- 31.Campbell IL, Eddleston M, Kemper P, Oldstone MB, Hobbs MV. Activation of cerebral cytokine gene expression and its correlation with onset of reactive astrocyte and acute-phase response gene expression in scrapie. J Virol. 1994;68:2383–2387. doi: 10.1128/jvi.68.4.2383-2387.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ransom B, Behar T, Nedergaard M. New roles for astrocytes (stars at last) Trends Neurosci. 2003;26:520–522. doi: 10.1016/j.tins.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Williams A, Piaton G, Lubetzki C. Astrocytes—friends or foes in multiple sclerosis? Glia. 2007;55:1300–1312. doi: 10.1002/glia.20546. [DOI] [PubMed] [Google Scholar]
- 34.Bannerman P, Hahn A, Soulika A, Gallo V, Pleasure D. Astrogliosis in EAE spinal cord: derivation from radial glia, and relationships to oligodendroglia. Glia. 2006;55(1):57–64. doi: 10.1002/glia.20437. [DOI] [PubMed] [Google Scholar]
- 35.Luo J, Miller MW. Platelet-derived growth factor-mediated signal transduction underlying astrocyte proliferation: site of ethanol action. J Neurosci. 1999;19:10014–10025. doi: 10.1523/JNEUROSCI.19-22-10014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koehler NK, Roebbert M, Dehghani K, Ballmaier M, Claus P, von Hoersten S, et al. Up-regulation of platelet-derived growth factor by peripheral-blood leukocytes during experimental allergic encephalomyelitis. J Neurosci Res. 2008;86:392–402. doi: 10.1002/jnr.21497. [DOI] [PubMed] [Google Scholar]
- 37.Paniagua RT. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116(10):2633–2642. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louvet C, Szot G, Lang J, Lee M, Martinier N, Bollag G, et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008;105(48):18895–18900. doi: 10.1073/pnas.0810246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 40.Paniagua RT, Chang A, Mariano MM, Stein EA, Wang Q, Lindstrom TM, et al. c-Fms-mediated differentiation and priming of monocyte lineage cells play a central role in autoimmune arthritis. Arthritis Res Ther. 2010;12(1):R32. doi: 10.1186/ar2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Druker B, Talpaz M, Resta D, Peng B, Buchdunger E, Ford J, Sawyers C, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. (2001) [DOI] [PubMed] [Google Scholar]
- 42.Wolff NC, Randle DE, Egorin MJ, Minna JD, Ilaria RL., Jr Imatinib mesylate efficiently achieves therapeutic intratumor concentrations in vivo but has limited activity in a xenograft model of small cell lung cancer. Clin Cancer Res. 2004;10:3528–3534. doi: 10.1158/1078-0432.CCR-0957-03. [DOI] [PubMed] [Google Scholar]
- 43.Huynh H, Lee JW, Chow PK, Ngo VC, Lew GB, Lam IW, et al. Sorafenib induces growth suppression in mouse models of gastrointestinal stromal tumor. Mol Cancer Ther. 2009;8:152–159. doi: 10.1158/1535-7163.MCT-08-0553. [DOI] [PubMed] [Google Scholar]
- 44.Chung EJ, Yoo S, Lim HJ, Byeon SH, Lee JH, Koh HJ. Inhibition of choroidal neovascularisation in mice by systemic administration of the multikinase inhibitor, sorafenib. Br J Ophthalmol. 2009;93:958–963. doi: 10.1136/bjo.2008.149187. [DOI] [PubMed] [Google Scholar]
- 45.Strumberg D, Voliotis D, Moeller JG, Hilger RA, Richly H, Kredtke S, et al. Results of phase I pharmacokinetic and pharmacodynamic studies of the Raf kinase inhibitor BAY 43- 9006 in patients with solid tumors. Int J Clin Pharmacol Ther. 2002;40:580–581. doi: 10.5414/cpp40580. [DOI] [PubMed] [Google Scholar]
- 46.Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci USA. 2005;102(44):16078–16083. doi: 10.1073/pnas.0502000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katz-Levy Y, Neville KL, Girvin AM, Vanderlugt CL, Pope JG, Tan LJ, et al. Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler’s virus-infected mice. J Clin Invest. 1999;104:599–610. doi: 10.1172/JCI7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, De Valeriola D, et al. Phase I safety and pharmacokinetics of BAY 43–9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92(10):1855–1861. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilhelm S, Chien DS. BAY 43–9006: preclinical data. Curr Pharm Des. 2002;8:2255–2257. doi: 10.2174/1381612023393026. [DOI] [PubMed] [Google Scholar]
- 50.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7(10):3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tong FK, Chow SHD. Pharmacodynamic monitoring of BAY 43–9006 (Sorafenib) in phase I clinical trials involving solid tumor and AML/MDS patients, using flow cytometry to monitor activation of the ERK pathway in peripheral blood cells. Cytometry B Clin Cytom. 2006;70(3):107–114. doi: 10.1002/cyto.b.20092. [DOI] [PubMed] [Google Scholar]
- 52.Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci USA. 2005;102:16078–16083. doi: 10.1073/pnas.0502000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valcamonico F, Ferrari V, Amoroso V, Rangoni G, Simoncini E, Marpicati P, et al. Long-lasting successful cerebral response with sorafenib in advanced renal cell carcinoma. J Neurooncol. 2009;9(1):47–50. doi: 10.1007/s11060-008-9676-4. [DOI] [PubMed] [Google Scholar]
- 54.Czyzewski K, Styczynski J. Imatinib is a substrate for various multidrug resistance proteins. Neoplasma. 2009;56:202–207. doi: 10.4149/neo_2009_03_202. [DOI] [PubMed] [Google Scholar]
- 55.Tonra JR, Reiseter BS, Kolbeck R, Nagashima K, Robertson R, Keyt B, et al. Comparison of the timing of acute blood-brain barrier breakdown to rabbit immunoglobulin G in the cerebellum and spinal cord of mice with experimental autoimmune enceph-alomyelitis. J Comp Neurol. 2001;430:131–144. [PubMed] [Google Scholar]
- 56.Uemura Y, Ohno H, Ohzeki Y, Takanashi H, Murooka H, Kubo K, et al. The selective M-CSF receptor tyrosine kinase inhibitor Ki20227 suppresses experimental autoimmune encephalomyelitis. J Neuroimmunol. 2008;195:73–80. doi: 10.1016/j.jneuroim.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 57.Lock C, Oksenberg J, Steinman L. The role of TNFalpha and lymphotoxin in demyelinating disease. Ann Rheum Dis. 1999;58 Suppl 1:I121–I128. doi: 10.1136/ard.58.2008.i121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- 59.Sicotte NL, Voshkul RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57(10):1885–1888. doi: 10.1212/wnl.57.10.1885. [DOI] [PubMed] [Google Scholar]
- 60.Robinson WH, Genovese MC, Moreland LW. Demyelinating and neurologic events reported in association with tumor necrosis factor alpha antagonism: by what mechanisms could tumor necrosis factor alpha antagonists improve rheumatoid arthritis but exacerbate multiple sclerosis? Arthritis Rheum. 2001;44:1977–1983. doi: 10.1002/1529-0131(200109)44:9<1977::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 61.El Chartouni C, Benner C, Eigner M, Lichtinger M, Rehli M. Transcriptional effects of colony-stimulating factor-1 in mouse macrophages. Immunobiology. 2010;215(6):466–474. doi: 10.1016/j.imbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Dalton D, Wittmer S. Nitric-oxide-dependent and independent mechanisms of protection from CNS inflammation during Th1-mediated autoimmunity: evidence from EAE in iNOS KO mice. J Neuroimmunol. 2005;160(1–2):110–121. doi: 10.1016/j.jneuroim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Rovida E, Baccarini M, Olivotto M, Dello Sbarba P. Opposite effects of different doses of MCSF on ERK phosphorylation and cell proliferation in macrophages. Oncogene. 2002;21:3670–3676. doi: 10.1038/sj.onc.1205409. [DOI] [PubMed] [Google Scholar]
- 64.Karpiak VC, Bridges RJ, Eyer CL. Organotins disrupt components of glutamate homeostasis in rat astrocyte cultures. J Toxicol Environ Health A. 2001;63:273–87. doi: 10.1080/15287390151143668. [DOI] [PubMed] [Google Scholar]
- 65.Conway J, Pink H, Bergquist M, Han B, Depee S, Tadepalli S, et al. Effects of the cFMS kinase inhibitor 5-(3-methoxy-4-((4-methoxybenzyl)oxy)benzyl)pyrimidine-2,4-diamine (GW2580) in normal and arthritic rats. J Pharmacol Exp Ther. 2008;326(1):41–50. doi: 10.1124/jpet.107.129429. [DOI] [PubMed] [Google Scholar]
- 66.Valdo P, Stegagno C, Mazzucco S, Zuliani E, Zanusso G, Moretto G, et al. Enhanced expression of NGF receptors in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2002;61:91–98. doi: 10.1093/jnen/61.1.91. [DOI] [PubMed] [Google Scholar]