Abstract
Background
Cancer survivors are at increased risk for secondary cancers and other diseases. Healthy dietary practices may improve cancer survivors’ health and well-being.
Objective
The durability of the effects of the FRESH START intervention, a program of sequentially-tailored mailed materials, and standardized mailed materials (for controls) on cancer survivors’ dietary outcomes was assessed over a 2-year period. Greater dietary gains were expected for FRESH START participants relative to controls.
Design
Participants were randomized to receive tailored vs. standardized 10-month mailed print interventions promoting diet and exercise behaviors. Data were collected at baseline and 1- and 2-year follow-ups.
Participants/setting
Breast and prostate cancer survivors (N = 543) were recruited from 39 states and two provinces within North America. A total of 489 participants completed the 2-year follow-up assessment (10% attrition).
Intervention
Participants were randomly assigned to either a 10-month program of tailored mailed print materials promoting fruit and vegetable (F&V) consumption, reduced total and saturated fat intake, and/or increased exercise or to a 10-month program of publicly-available mailed materials on diet and exercise.
Main outcome measures
Telephone surveys (supported with blood biomarkers) assessed dietary habits at baseline and 1- and 2-year follow-ups.
Statistical analyses performed
Paired-samples t-tests were conducted to examine the durability of the intervention’s effects on dietary outcomes within each study arm. Arm differences in follow-up outcomes were then tested with the general linear model, controlling for the baseline value of the outcome.
Results
Both arms reported decreased saturated fat intake, increased servings of F&V, and better overall diet quality at year 2 relative to baseline. However, FRESH START participants reported better overall diet quality and lower total and saturated fat intake compared to controls at the 2- year follow-up.
Conclusions
Results suggest that mailed material interventions, especially those that are tailored, can produce long-term dietary improvement among cancer survivors.
Keywords: breast carcinoma, prostate carcinoma, survivorship, diet
Advances in early detection and treatment of cancer have led to increases in the number of cancer survivors (1–2). Currently, there are more than 12 million cancer survivors in the United States, comprising roughly 4% of the population (3). Over 4.5 million of these survivors were diagnosed with breast or prostate cancer (4). The number of cancer survivors is expected to continue to grow due to the aging population and further improvement in cancer detection and care (1). Although the increase in cancer survivors is a positive trend, cancer and its treatment are associated with negative health-related sequelae. Relative to persons without a cancer history, cancer survivors are at increased risk for developing secondary cancers and other diseases or conditions, such as heart disease, osteoporosis, diabetes, and functional impairment (5–7).
A healthy diet and regular exercise may reduce cancer survivors’ comorbidities (4–5, 8–9). Some evidence suggests that a healthful diet also may reduce cancer-associated biomarkers and risk of cancer recurrence, although findings have been mixed (10–15). Furthermore, a cancer diagnosis provides a teachable moment during which many individuals are motivated to improve their diet and other health behaviors (16–21). Harnessing the teachable moment, lifestyle interventions have been effective in improving cancer survivors’ dietary behaviors (22–26).
Current dietary recommendations for cancer survivors, as well as the population at large emphasize a plant-based diet high in fruits and vegetables and low in fat (27–29). Specifically, adults’ daily total fat intake should not exceed 20%–35% of their kcal, whereas saturated fat intake should not exceed 10% of their kcal (27, 29). The current FRESH START trial tested the efficacy of an intervention that was designed to promote both additive (fruits and vegetables) and reductive (low-fat) dietary practices among cancer survivors (30).
FRESH START was the first diet and exercise intervention for cancer survivors to be delivered exclusively through mailed print materials (30). This intervention was designed to promote behavioral goals including consumption of 5 or more fruits and vegetables (F&V) per day, reduction of total and saturated fat intake to less than 30% and 10% of kcal, respectively, and 150+ minutes of moderate to vigorous exercise per week. Individuals randomly assigned to the intervention arm received sequentially-tailored mailed print materials and those assigned to the control arm received diet and exercise materials available in the public domain. At the 1-year follow-up, the intervention and control arms demonstrated significant increases in diet quality and F&V consumption and decreased fat intake (30). However, the intervention arm showed greater improvement in these areas and reduced body mass index (BMI) relative to controls. The current research examines the durability of the FRESH START and control interventions’ effects on dietary outcomes and body mass index over a 2-year period and compares the efficacy of the interventions at 2 years post-baseline. It was hypothesized that the intervention arm would show greater sustained improvement in dietary behaviors (less saturated and total fat intake, more F&V intake, and better overall diet quality) and lower BMI compared to the control arm.
METHODS
Participants
Individuals diagnosed with early-stage (in situ, localized, or regional) breast or prostate cancer within the prior 9 months participated in the FRESH START trial between July 2002 and October 2005. Individuals were excluded from participation if they had conditions precluding unsupervised exercise (recent myocardial infarction, uncontrolled angina or congestive heart failure, plans for hip or knee replacement surgery, pulmonary difficulties requiring oxygen use or hospitalization, or walker or wheelchair use) or if they had conditions that prohibited a high F&V diet (chronic warfarin use or renal failure). Potential participants also were excluded if they had advanced cancer or an additional primary cancer or lacked English fluency.
Procedure
The FRESH START trial design and methods were published previously (30–33). Following approval of study methods by the Duke University Health System Institutional Review Board, participants were recruited via self-referral, cancer registries of participating medical centers, or oncology practices in 39 states and 2 provinces in North America. Written informed consent was obtained prior to study participation. Survivors were identified and underwent eligibility screening within 9 months of diagnosis. Eligible individuals began the intervention following receipt of primary treatment. Survivors were excluded from the intervention trial if they met two or more goal behaviors (exercising 150+ minutes per week, eating 5 or more servings of F&V each day, limiting total and saturated fat intakes to less than 30% and 10% of kcal, respectively). Participants were randomized to receive one of two 10-month interventions designed to improve diet and exercise practices. The interventions involved an initial workbook followed by a series of seven newsletters at 6-week intervals. Between mailings, participants in both study arms received brief surveys and a $5 incentive for each returned survey. The surveys for control participants assessed the perceived helpfulness of the brochures, whereas the surveys for experimental participants assessed current diet and exercise practices and readiness to change these practices. Information from the surveys was used to individually tailor each newsletter and provide feedback to experimental participants. The FRESH START intervention was based upon Social Cognitive Theory (34) that emphasizes confidence building and goal setting. Mailings were tailored to the experimental participants’ demographic characteristics (age, race, and sex) (30), cancer coping style (e.g., ‘fighting spirit,’ ‘anxious preoccupation’) (35), stage of readiness (36), barriers to lifestyle changes, and progress toward goal behaviors (150+ minutes of exercise, adherence to a low fat or high F&V diet) (31). Each experimental participant received two 5-month mailings on F&V consumption, low fat dietary practices, or exercise and only received materials in areas where they did not meet the goal behavior. Participants who did not adhere to any goal behaviors at baseline were randomly assigned to two of the three mailings. Additional newsletter content included information on the health benefits of lifestyle change.
Control participants received an initial workbook that included the “Facing Forward” booklet from the National Cancer Institute and subsequent materials on exercise, F&V consumption, and low fat dietary practices that are available in the public domain (31). These materials were mailed on a similar schedule to the FRESH START intervention arm. Current smokers in both study arms were sent the American Lung Association ‘Quitting for Life’ brochure (2003PS96328).
Measures
Computer-assisted telephone interviews of 45 to 55 minutes each were conducted at baseline and 1- and 2-year follow-ups and included measures of weight status and the Diet History Questionnaire (37–38), which was modified to include regional cuisine (e.g., okra, hominy). Reliability and validity of the DHQ has been demonstrated (37–38). The DHQ assesses dietary fat intake and consumption of a variety of foods and nutrients over the past year. Dietary outcome variables for the current research included the 100-point Diet Quality Index-Revised score (39), with higher numbers indicating better adherence to dietary recommendations, number of daily servings of F&V, and percentages of kcal from fat and saturated fat. On a 23% subset, anthropometric measures (height and weight), and blood samples were drawn and tested for plasma carotenoids. Measured values confirmed those of self report, i.e., BMI and fruit and vegetable consumption.
Throughout the study, participants reported adverse events by calling a toll-free number. Adverse events also were reported during the 1- and 2-year follow-up interviews and were classified as serious (life-threatening, permanently debilitating, or requiring hospitalization overnight) or non-serious (all other events) by research team members blinded to random assignment status.
Data Analysis
Paired-samples t-tests were used to determine the durability of the intervention’s effects on dietary outcomes and body mass index within each study arm. The extent to which these outcomes were stable between the 1- and 2-year follow-up assessments and improved at year 2 relative to baseline values was examined. Our hypothesis that the intervention arm would show better outcomes than the control arm at 2 years post-baseline was tested with the general linear model, controlling for the baseline value of the outcome. All reported p values were two-tailed, and a ps value < .05 was considered statistically significant. Data were analyzed with SPSS statistical software (version 18.0, 2009, SPSS, Chicago, IL).
Two potential moderators of the intervention’s effects were examined and excluded from the final analyses. Gender, a variable confounded with cancer type, and body mass index (normal weight vs. overweight) did not significantly interact with study arm to predict outcomes (data not shown).
RESULTS
Sample Characteristics
Descriptions of the sample and statistical analyses of the accrual procedures have been previously reported (30). To summarize, significant differences (all p values < 0.01) were found between participants and non-participants with respect to gender (54% vs. 46% female, respectively), race (19% vs. 38% minority, respectively), and age (58 vs. 62 years, respectively).
A total of 306 breast cancer patients and 237 prostate cancer patients participated in the randomized trial. Participants were primarily Caucasian (83%) or African American (13%) and well educated (88% with at least some college) (see Table 1). The average age of participants was 57 years (SD = 10.8) and the average time since diagnosis was 3.8 months (SD = 2.7) at the point of study entry. Most participants (85%) had undergone surgery and received adjuvant treatment, including radiation (44%), chemotherapy (27%), and hormonal therapy (38%).
Table 1.
Baseline characteristics of study participants
| Total Sample (N = 489) |
FRESH START Intervention (n = 236) |
Attention Control (n = 253) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. of participants |
% | No. of participants |
% | No. of participants |
% | |||
| Age, years | |||||||||
| Mean | 57.2 | 57.4 | 57.1 | ||||||
| SD | 10.7 | 10.1 | 11.2 | ||||||
| Range | 22–85 | 30–78 | 22–85 | ||||||
| Race | |||||||||
| White | 413 | 85 | 200 | 85 | 213 | 84 | |||
| Black | 59 | 12 | 25 | 11 | 34 | 13 | |||
| Other | 17 | 4 | 11 | 5 | 6 | 2 | |||
| Source of ascertainment | |||||||||
| Self-referral | 185 | 38 | 90 | 38 | 95 | 38 | |||
| Cancer registry | 304 | 62 | 146 | 62 | 158 | 63 | |||
| Type and clinical stage of cancer | |||||||||
| Breast | |||||||||
| Stage 0 | 39 | 14 | 18 | 13 | 21 | 15 | |||
| Stage I | 148 | 53 | 78 | 57 | 70 | 50 | |||
| Stage II | 78 | 28 | 33 | 24 | 45 | 32 | |||
| Stage III | 13 | 5 | 8 | 6 | 5 | 4 | |||
| Prostate | |||||||||
| Stage I | 83 | 39 | 41 | 41 | 42 | 38 | |||
| Stage II | 111 | 53 | 51 | 52 | 60 | 54 | |||
| Unknown | 17 | 8 | 7 | 7 | 10 | 9 | |||
| Treatment | |||||||||
| Surgery | 416 | 85 | 206 | 87 | 210 | 83 | |||
| Radiation therapy | 221 | 45 | 108 | 46 | 113 | 45 | |||
| Chemotherapy | 127 | 26 | 64 | 27 | 63 | 25 | |||
| Hormonal therapy | 195 | 40 | 99 | 42 | 96 | 38 | |||
| Other | 17 | 4 | 7 | 3 | 10 | 4 | |||
| No. of comorbid conditions | |||||||||
| Mean | 2.1 | 2.0 | 2.2 | ||||||
| SD | 1.7 | 1.8 | 1.7 | ||||||
| Range | 0–11 | 0–10 | 0–11 | ||||||
| Education | |||||||||
| ≤ High school graduate | 56 | 11 | 28 | 12 | 28 | 11 | |||
| Some college or associate | 145 | 30 | 64 | 27 | 81 | 32 | |||
| College graduate/postgraduate | 288 | 59 | 144 | 61 | 144 | 57 | |||
Attrition and Adverse Events
An excellent retention rate (90%) was observed, with 519 patients completing the 1-year follow-up and 489 patients completing the 2-year follow-up. Reasons for attrition included participant withdrawal (n = 17), death (n = 8), familial illness (n = 7), and inability to reach the participant (n = 22). Attrition did not differ by sex or level of education; however, ethnic minority participants had a significantly higher attrition rate than Caucasians (16% vs. 9%, respectively, p = .02). Reasons for attrition among ethnic minority participants included inability to reach the participant (n = 9), death (n = 4), and familial illness (n = 1). In addition, the intervention arm had a significantly higher attrition rate than the control arm (13% vs. 7%, respectively, p = .02).
No differences in adverse events were found between study arms; intervention participants reported 211 total events (41 serious and 170 non-serious), and control participants reported 238 total events (56 serious and 182 non-serious).
Change in Dietary Outcomes and Body Mass Index
Baseline and follow-up data for study variables are listed in Table 2. Demographic and medical variables and dependent measures did not differ between study arms at baseline. As previously reported (30), both arms showed increased F&V consumption, decreased total and saturated fat intake, and better overall diet quality at the 1-year follow-up (all p-values < .01). However, significantly greater improvement in dietary outcomes occurred in the intervention arm, which also showed a significant reduction in BMI at the 1-year follow-up relative to controls (−0.2 vs. +0.1 kg/m2, respectively, p < .01).
Table 2.
Changes in outcome measures over time between the intervention and attention control arms
| FRESH START Intervention (n = 236) |
Attention Control (n = 253) |
P (Intervention vs. Attention Control) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | 1 Year | 2 Years | |
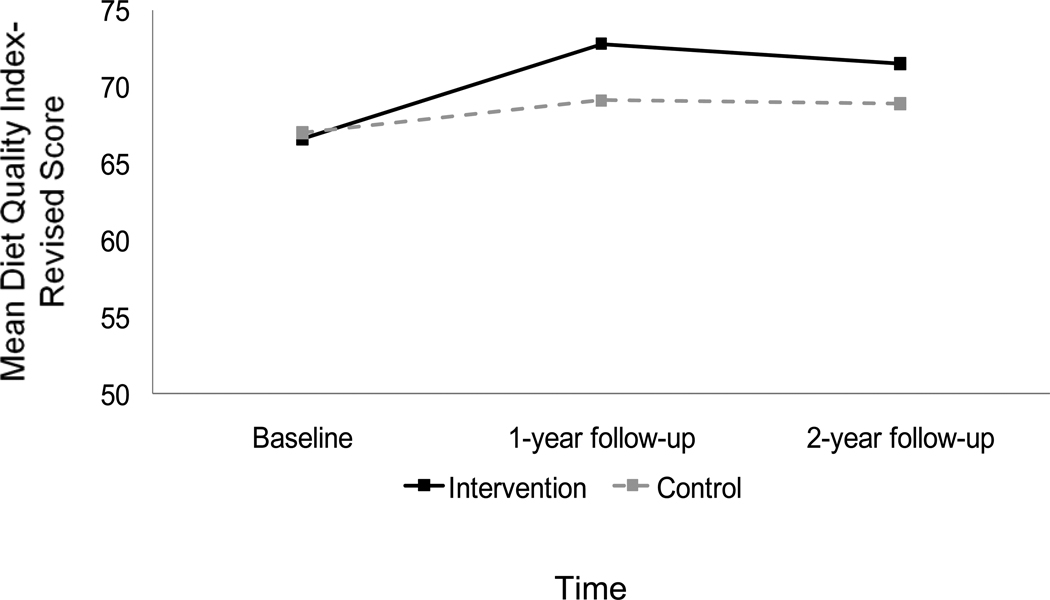
| Diet Quality Index-Revised scorea | <.001 | <.001 | ||||
| Baseline | 66.6 | 11.1 | 67.0 | 9.4 | ||
| 1-year follow-up | 72.8 | 10.6 | 69.1 | 10.8 | ||
| 2-year follow-up | 71.5 | 10.5 | 68.9 | 10.6 | ||
| Total % calories from fat | <.001 | .001 | ||||
| Baseline | 38.0 | 5.7 | 37.8 | 5.6 | ||
| 1-year follow-up | 33.6 | 5.8 | 35.7 | 4.6 | ||
| 2-year follow-up | 36.5 | 6.6 | 38.0 | 5.4 | ||
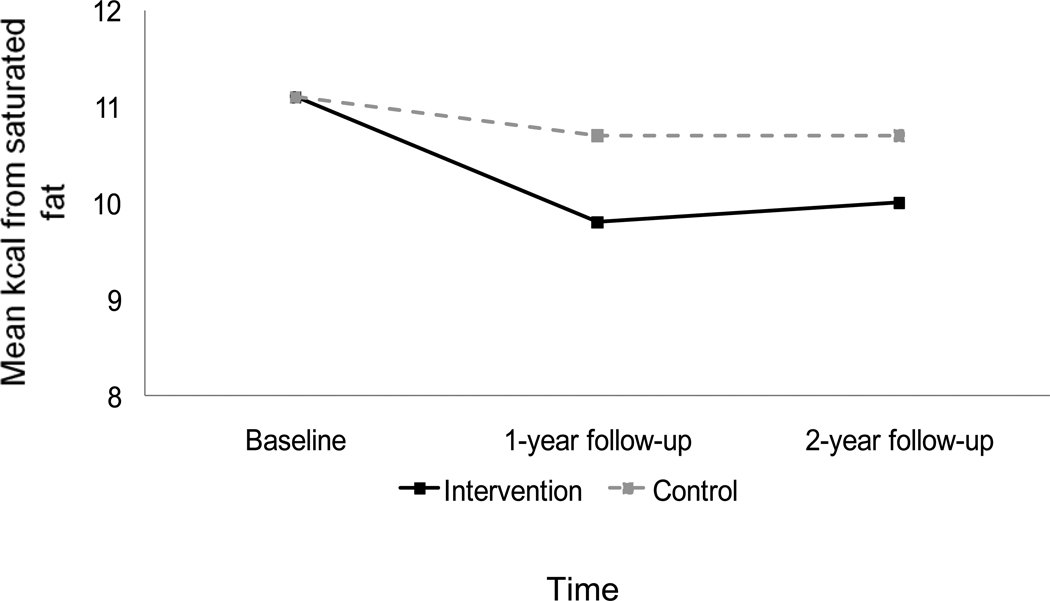
| Total % calories from saturated fat | <.001 | <.001 | ||||
| Baseline | 11.1 | 2.1 | 11.1 | 2.0 | ||
| 1-year follow-up | 9.8 | 2.3 | 10.7 | 2.2 | ||
| 2-year follow-up | 10.0 | 2.1 | 10.7 | 2.1 | ||
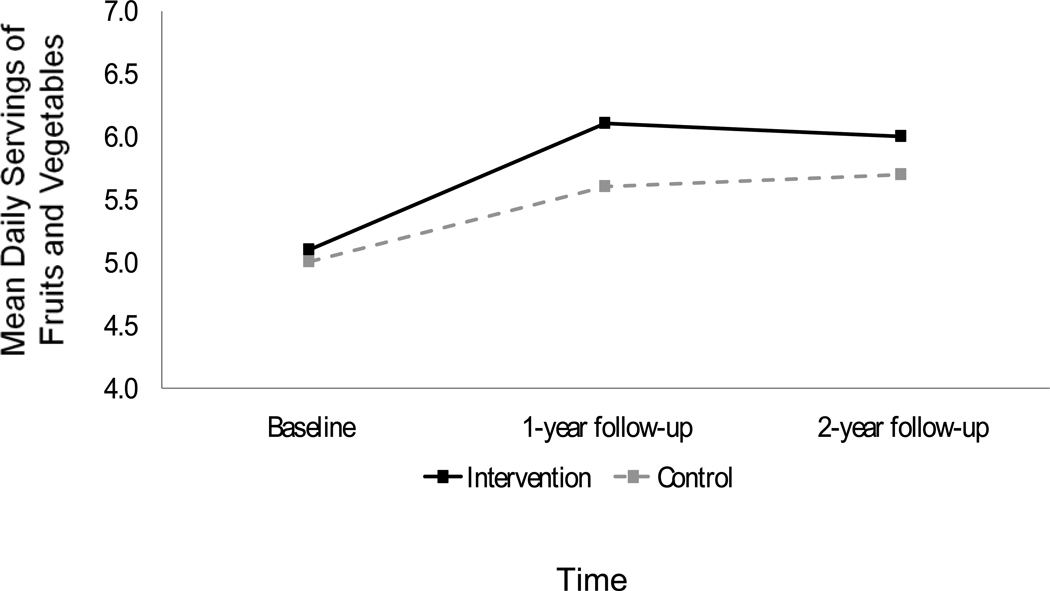
| No. of daily servings of F&V | .014 | .152 | ||||
| Baseline | 5.1 | 2.7 | 5.0 | 2.3 | ||
| 1-year follow-up | 6.1 | 2.8 | 5.6 | 2.5 | ||
| 2-year follow-up | 6.0 | 2.7 | 5.7 | 2.8 | ||
| BMI, kg/m2 | ||||||
| Baseline | 27.2 | 5.1 | 27.6 | 5.4 | .008 | .710 |
| 1-year follow-up | 27.0 | 5.0 | 27.7 | 5.4 | ||
| 2-year follow-up | 27.5 | 5.2 | 27.8 | 5.3 | ||
Note. Arm differences in 1- and 2-year follow-up outcomes were tested with the general linear model, controlling for baseline values of the outcome.
A 100-point scale with higher scores indicating better diet quality.
Abbreviations: SD, standard deviation; F&V, fruits and vegetables; BMI, body mass index.
Although both arms significantly increased their total fat intake between the 1- and 2-year follow-ups (p-values < .001), only the intervention arm continued to show a reduction in total fat intake compared to baseline (p < .001). Other dietary outcomes showed sustained improvement relative to baseline in both arms. Specifically, both groups maintained their increased F&V intake, reduced saturated fat intake, and enhanced overall diet quality at the 2-year follow-up (see Figures 1, 2, and 3; all p-values < .01). Overall diet quality and total and saturated fat intake continued to be better for the intervention arm relative to controls (see Table 2). Weight status was fairly stable; both the intervention and control arms reported small increases in BMI over the study period (+0.2 vs. +0.1).
Figure 1.
Change in number of daily servings of fruits and vegetables by study arm
Figure 2.
Change in total percentage of calories from saturated fat by study arm
Figure 3.
Change in overall diet quality by study arm
DISCUSSION
This study examined the durability of dietary outcomes of the FRESH START intervention at two years post-baseline. FRESH START was the first diet and exercise intervention for cancer survivors delivered exclusively through mailed print materials. At the 2-year follow-up, both the intervention and control arms showed greater F&V intake, lower saturated fat intake, and better overall diet quality relative to baseline. However, intervention participants showed lower total and saturated fat intake and better overall diet quality compared to controls at the 2-year follow-up. These results did not vary by cancer type or body mass index. In addition, although both arms increased their total fat intake between the 1- and 2-year follow-ups, only the intervention arm showed a sustained reduction in total fat intake relative to baseline. These findings suggest that tailored intervention materials resulted in better long-term dietary outcomes than standard materials, although both fostered improved adherence to healthy dietary behaviors in cancer survivors. Results are consistent with evidence that tailored interventions are more effective than standardized interventions in modifying various lifestyle practices, including mammography, smoking, exercise, and dietary behaviors (24, 40–43). Tailored messages are thought to produce behavior change by providing personally relevant information.
Although this trial did not focus on weight loss, the intervention group showed a significant decrease in BMI when compared to the control group at the 1-year follow-up. Both study arms, however, reported small increases in BMI over the 2-year study period. These increases in BMI were roughly half that generally observed for the adult population over a 2-year time period (44), which suggests that the interventions may have promoted weight stability.
Although a number of diet and exercise interventions have focused on cancer survivors (12, 45–48), the FRESH START trial is noteworthy for a variety of reasons. First, FRESH START accrued a large North American sample of cancer survivors, whereas most trials accrued a smaller sample from one institution. Second, the FRESH START intervention targeted both prostate and breast cancer survivors, whereas most lifestyle intervention trials have targeted survivors of a single cancer type. In addition, the intervention was delivered exclusively via mailed print materials, whereas most studies have examined hospital-based or telephone-based interventions. Other strengths of this trial include the use of a rigorous attention control group, the long-term follow-up assessments, and the low attrition rate.
Several extensions of the present findings warrant future research attention. First, the extent to which booster materials may help cancer survivors sustain reductions in fat intake and improve other dietary outcomes should be evaluated. Second, technology-based delivery of lifestyle interventions (e.g. text, podcast, or Web-based approaches) should be explored, as the majority of U.S. adults have Internet access (49). The feasibility of these approaches with older adults warrants further study. Finally, the health events and health care costs associated with mailed material interventions such as FRESH START and technology-based interventions should be examined to determine their potential cost savings.
Limitations of this study also should be noted. Although a large North American sample was recruited, ethnic minorities, older adults, and individuals of lower educational attainment were underrepresented. Future research is needed to evaluate the effects of similar interventions in samples that are entirely representative of the population of cancer survivors. In addition, differential dropout occurred as a function of study arm (13% in the intervention arm and 7% in the control arm) and race (16% for ethnic minority participants and 9% for Caucasian participants). However, given the low overall attrition rate, this limitation may be a minor issue. The primary reason for dropout of ethnic minority participants was an inability to reach them, which may be due to passive refusal or changes in contact information. Cultural tailoring of intervention materials may increase retention of ethnic minority individuals. Finally, study outcomes were self-reported. Although self-reported dietary outcomes were strongly supported by biomarker data (e.g., plasma alpha-carotene) from a 23% subsample at the 1-year follow-up (30), further collection of biomarker data from a larger subsample would have been desirable.
Conclusions
Our findings inform clinical care by showing that a minimal, mailed intervention can produce long-term dietary improvement among cancer survivors. This print intervention may be readily disseminated to geographically dispersed survivors. Further research is needed to examine the cost-effectiveness of mailed material approaches and to compare their efficacy to that of less expensive technology-based approaches (e.g., Web-based interventions).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gapstur SM, Thun MJ. Progress in the war on cancer. JAMA. 2010;303:1084–1085. doi: 10.1001/jama.2010.284. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Cancer advances in focus. [Accessed on March 15, 2011]; Available at: http://www.cancer.gov/cancertopics/factsheet/cancer-advances-in-focus/cancer. Updated November 29, 2010.
- 3.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2007, National Cancer Institute. Bethesda, MD: National Cancer Institute; 2010. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 4.American Cancer Society. Atlanta, GA: American Cancer Society; 2010. [Accessed March 15, 2011]. Cancer Facts and Figures, 2010. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf. [Google Scholar]
- 5.Aziz NM. Cancer survivorship research: Challenge and opportunity. J Nutr. 2002;132:3494S–3503S. doi: 10.1093/jn/132.11.3494S. [DOI] [PubMed] [Google Scholar]
- 6.Rowland J, Mariotto A, Aziz N, Tesauro G, Feuer EJ, Blackman B, Thompson P, Pollack LA. Cancer survivorship-United States, 1971–2001. MMWR Morbid Mortal Wkly Rep. 2004;53:526–529. [PubMed] [Google Scholar]
- 7.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: Findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 8.Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: Challenge and opportunity. Semin Radiat Oncol. 2003;13:248–266. doi: 10.1016/S1053-4296(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 9.Pinto B, Trunzo J. Health behaviors during and after a cancer diagnosis. Cancer. 2005;104:2614–2623. doi: 10.1002/cncr.21248. [DOI] [PubMed] [Google Scholar]
- 10.Aronson WJ, Barnard RJ, Freedland SJ, Henning S, Elashoff D, Jardack PM, Cohen P, Heber D, Kobayashi N. Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J Urol. 2010;183:345–350. doi: 10.1016/j.juro.2009.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, Goodman MT, Giuliano AE, Karanja N, McAndrew P, Hudis C, Butler J, Merkel D, Kristal A, Caan B, Michaelson R, Vinciguerra V, Del Prete S, Winkler M, Hall R, Simon M, Winters BL, Elashoff RM. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 12.George SM, Neuhouser ML, Mayne ST, Irwin ML, Albanes D, Gail MH, Alfano CM, Bernstein L, McTiernan A, Reedy J, Smith AW, Ulrich CM, Ballard-Barbash R. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19:2220–2228. doi: 10.1158/1055-9965.EPI-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold EB, Pierce JP, Natarajan L, Stefanick ML, Laughlin GA, Caan BJ, Flatt SW, Emond JA, Saquib N, Madlensky L, Kealey S, Wasserman L, Thomson CA, Rock CL, Parker BA, Karanja N, Jones V, Hajek RA, Pu M, Mortimer JE. Dietary pattern influences breast cancer prognosis in women without hot flashes: The women's healthy eating and living trial. J Clin Oncol. 2009;27:352–359. doi: 10.1200/JCO.2008.16.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rock CL, Flatt SW, Natarajan L, Thomson CA, Bardwell WA, Newman VA, Hollenbach KA, Jones L, Caan BJ, Pierce JP. Plasma carotenoids and recurrence-free survival in women with a history of breast cancer. J Clin Oncol. 2005;23:6631–6638. doi: 10.1200/JCO.2005.19.505. [DOI] [PubMed] [Google Scholar]
- 15.Rock CL, Natarajan L, Pu M, Thomson CA, Flatt SW, Caan BJ, Gold EB, Al-Delaimy WK, Newman VA, Hajek RA, Stefanick ML, Pierce JP. Longitudinal biological exposure to carotenoids is associated with breast cancer-free survival in the Women's Healthy Eating and Living Study. Cancer Epidemiol Biomarkers Prev. 2009;18:486–494. doi: 10.1158/1055-9965.EPI-08-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfano CM, Day JM, Katz ML, Herndon JE, II, Bittoni MA, Oliveri JM, Donohue K, Paskett ED. Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psychooncology. 2009;18:128–133. doi: 10.1002/pon.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell MK, Carr C, Devellis B, Switzer B, Biddle A, Amamoo MA, Walsh J, Zhou B, Sandler R. A randomized trial of tailoring and motivational interviewing to promote fruit and vegetable consumption for cancer prevention and control. Ann Behav Med. 2009;38:71–85. doi: 10.1007/s12160-009-9140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 19.Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, Reece GP. Successes and failures of the teachable moment: Smoking cessation in cancer patients. Cancer. 2006;106:17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt ME, Greenfield S, Stovall E. Institute of Medicine and National Research Council: From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 21.McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: The context of cancer care and survivorship. Cancer Control. 2003;10:325–333. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]
- 22.Demark-Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz N, Snyder DC, Giguere JK, Shaw E. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin Breast Cancer. 2008;8:70–79. doi: 10.3816/CBC.2008.n.005. [DOI] [PubMed] [Google Scholar]
- 23.Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: Potential for prevention and questions that remain. J Clin Oncol. 2006;24:5125–5131. doi: 10.1200/JCO.2006.06.6175. [DOI] [PubMed] [Google Scholar]
- 24.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, Miller P, Mitchell DC, Demark-Wahnefried W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons JK, Newman V, Mohler JL, Pierce JP, Paskett E, Marshall J. The Men's Eating and Living (MEAL) study: A Cancer and Leukemia Group B pilot trial of dietary intervention for the treatment of prostate cancer. Urology. 2008;72:633–637. doi: 10.1016/j.urology.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 26.Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50:167–178. doi: 10.3109/0284186X.2010.529822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietary Guidelines for Americans, 2010. 7th Edition. Washington, DC: U.S. Government Printing Office; 2010. Dec, [Accessed on March 16, 2011]. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Available at: http://www.eatright.org/Public/content.aspx?id=206. [Google Scholar]
- 28.American Dietetic Association. Eat right: Food, nutrition, and health tips from the American Dietetic Association. [Accessed on March 16, 2011]; Available at: http://www.eatright.org/Public/content.aspx?id=206.
- 29.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, McTiernan A, Rock CL, Thompson C, Gansler T, Andrews KS. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 30.Demark-Wahnefried W, Clipp EC, Lipkus IM, Lobach D, Snyder DC, Sloane R, Peterson B, Macri JM, Rock CL, McBride CM, Kraus WE. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 31.Demark-Wahnefried W, Clipp EC, McBride C, Lobach DF, Lipkus I, Peterson B, Snyder DC, Sloane R, Arbanas J, Kraus WE. Design of FRESH START: a randomized trial of exercise and diet among cancer survivors. Med Sci Sports Exerc. 2003;35:415–424. doi: 10.1249/01.MSS.0000053704.28156.0F. [DOI] [PubMed] [Google Scholar]
- 32.Macri JM, Downs SM, Demark-Wahnefried W, Snyder DC, Lobach DF. A simple, flexible and scalable approach for generating tailored questionnaires and health education messages. Comput Inform Nurs. 2005;23:316–321. doi: 10.1097/00024665-200511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demark-Wahnefried W. Print-to-practice: Designing tailored print materials to improve cancer survivors' dietary and exercise practices in the FRESH START trial. Nutr Today. 2007;42:131–138. doi: 10.1097/01.NT.0000277790.03666.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice Hall; 1977. [Google Scholar]
- 35.Watson MM, Law M, dos Santos M, Greer S, Baruch J, Bliss J. The Mini-MAC: Further development of the Mental Adjustment to Cancer scale. J Psychosoc Oncol. 1994;12:33–45. [Google Scholar]
- 36.Prochaska JO, Velicer WF, Rossi JS, Goldstein MG, Marcus BH, Rakowski W, Fiore C, Harlow LL, Redding CA, Rosenbloom D, Ross SR. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- 37.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 38.Thompson FE, Subar AF, Brown CC, Smith AF, Sharbaugh CO, Jobe JB, Mittl B, Gibson JT, Ziegler RG. Cognitive research enhances accuracy of food frequency questionnaire reports: Results of an experimental validation study. J Am Diet Assoc. 2002;102:212–225. doi: 10.1016/s0002-8223(02)90050-7. [DOI] [PubMed] [Google Scholar]
- 39.Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index revised: A measurement instrument for populations. J Am Diet Assoc. 1999;99:697–704. doi: 10.1016/S0002-8223(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 40.Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Prev Med. 2010;51:214–221. doi: 10.1016/j.ypmed.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sternfeld B, Block C, Quesenberry CP, Jr, Block TJ, Husson G, Norris JC, Nelson M, Block G. Improving diet and physical activity with ALIVE: A worksite randomized trial. Am J Prev Med. 2009;36:475–483. doi: 10.1016/j.amepre.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 42.Walker SN, Pullen CH, Boeckner L, Hageman PA, Hertzog M, Oberdorfer MK, Rutledge MJ. Clinical trial of tailored activity and eating newsletters with older rural women. Nurs Res. 2009;58:74–85. doi: 10.1097/NNR.0b013e31818fcee1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Vries H, Kremers SPJ, Smeets T, Brug J, Eijmael K. The effectiveness of tailored feedback and action plans in an intervention addressing multiple health behaviors. Am J Health Promot. 2008;22:417–425. doi: 10.4278/ajhp.22.6.417. [DOI] [PubMed] [Google Scholar]
- 44.Rosell M, Appleby P, Spencer E, Key T. Weight gain over 5 years in 21,966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int J Obes (Lond) 2006;30:1389–1396. doi: 10.1038/sj.ijo.0803305. [DOI] [PubMed] [Google Scholar]
- 45.Adamsen L, Quist M, Andersen C, Moller T, Herrstedt J, Kronborg D, Baadsgaard MT, Vistisen K, Midtgaard J, Christiansen B, Stage M, Kronborg MT, Rorth M. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fillion L, Gagnon P, Leblond F, Gelinas C, Savard J, Dupuis R, Duval K, Larochelle M. A brief intervention for fatigue management in breast cancer survivors. Cancer Nurs. 2008;31:145–159. doi: 10.1097/01.NCC.0000305698.97625.95. [DOI] [PubMed] [Google Scholar]
- 47.Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, Dipietro L, Mayne ST, Yu H. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: The Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch BM, Cerin E, Owen N, Aitken JF. Associations of leisure-time physical activity with quality of life in a large, population-based sample of colorectal cancer survivors. Cancer Causes Control. 2007;18:735–742. doi: 10.1007/s10552-007-9016-6. [DOI] [PubMed] [Google Scholar]
- 49.National Cancer Institute. Health information national trends survey. [Accessed on March 16, 2011];2007 Available at: http://hints.cancer.gov/questions/question-details.jsp?qid=752&dataset=2007&method=combined.