Abstract
In an ongoing study of streptococcal skin infection and acute glomerulonephritis (AGN) begun in 1964, C′3 determinations were done in 784 patients. There were 126 patients with acute poststreptococcal nephritis, 172 of their siblings, and 486 patients with uncomplicated impetigo from families without an index case of nephritis.
90% of the patients with nephritis were infected with one of the four prevalent streptococcal serotypes associated with nephritis in this population; only 12% of patients with uncomplicated impetigo were infected with similar serotypes.
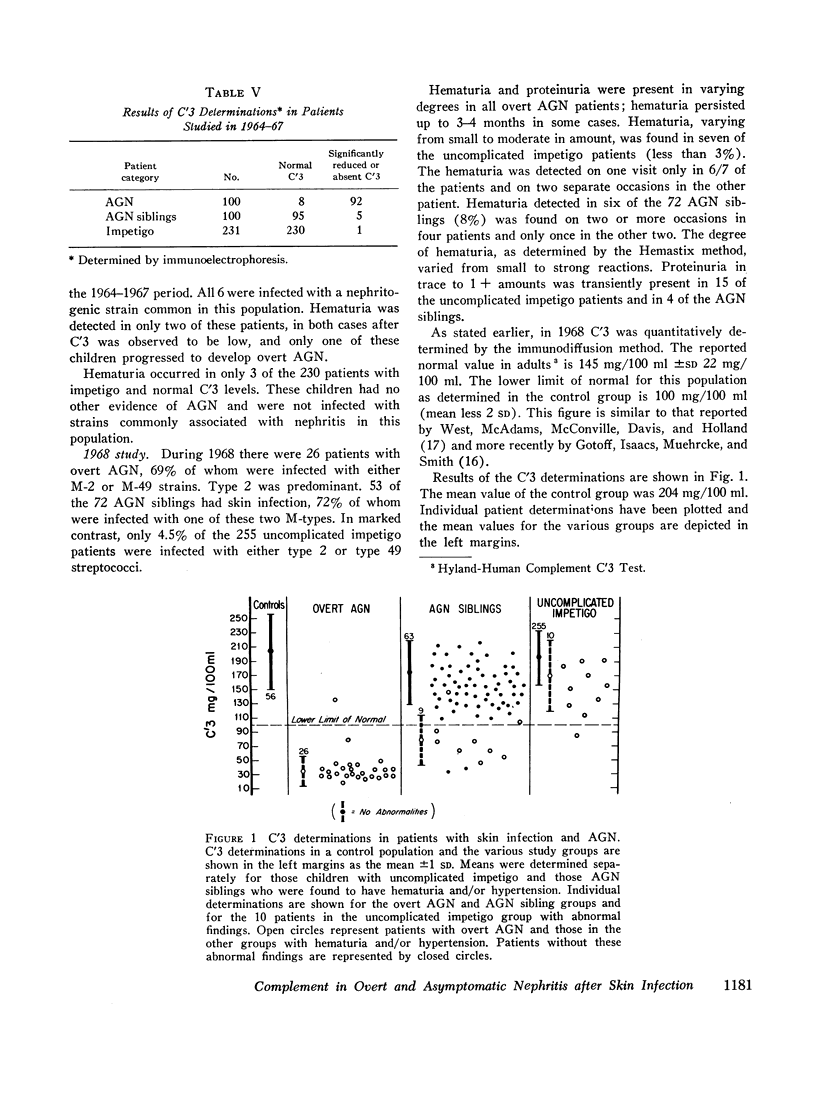
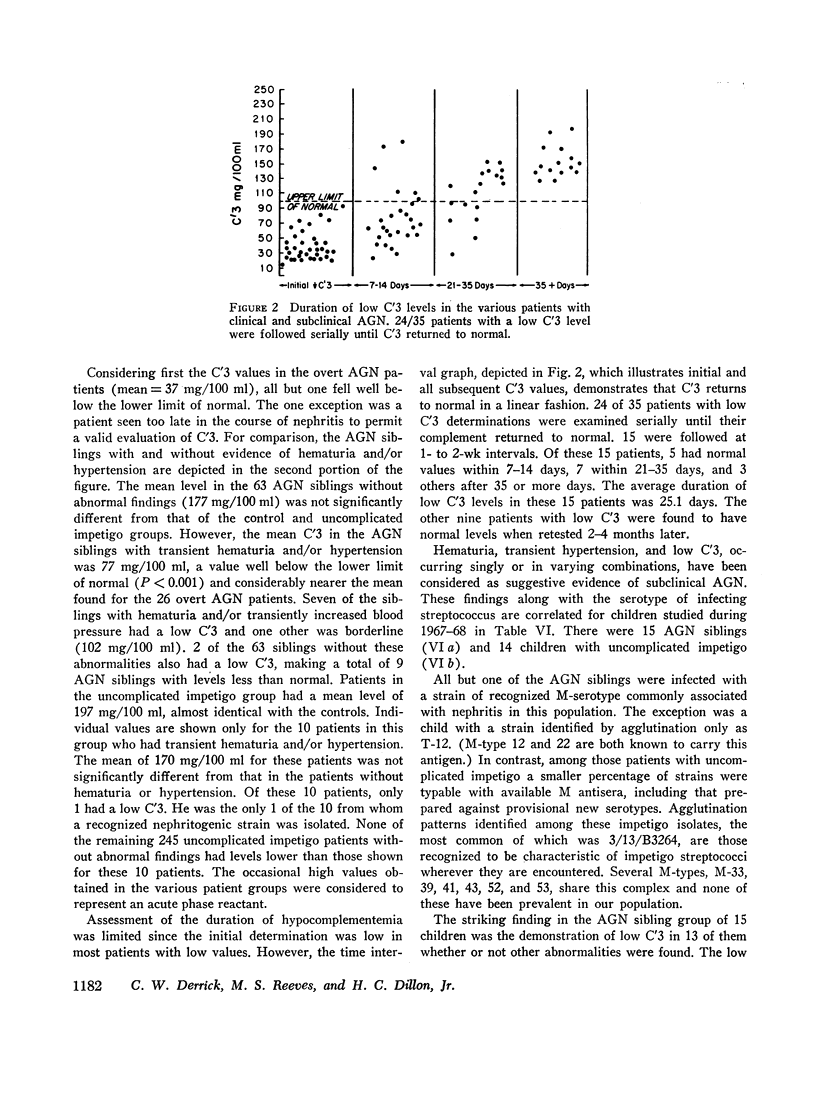
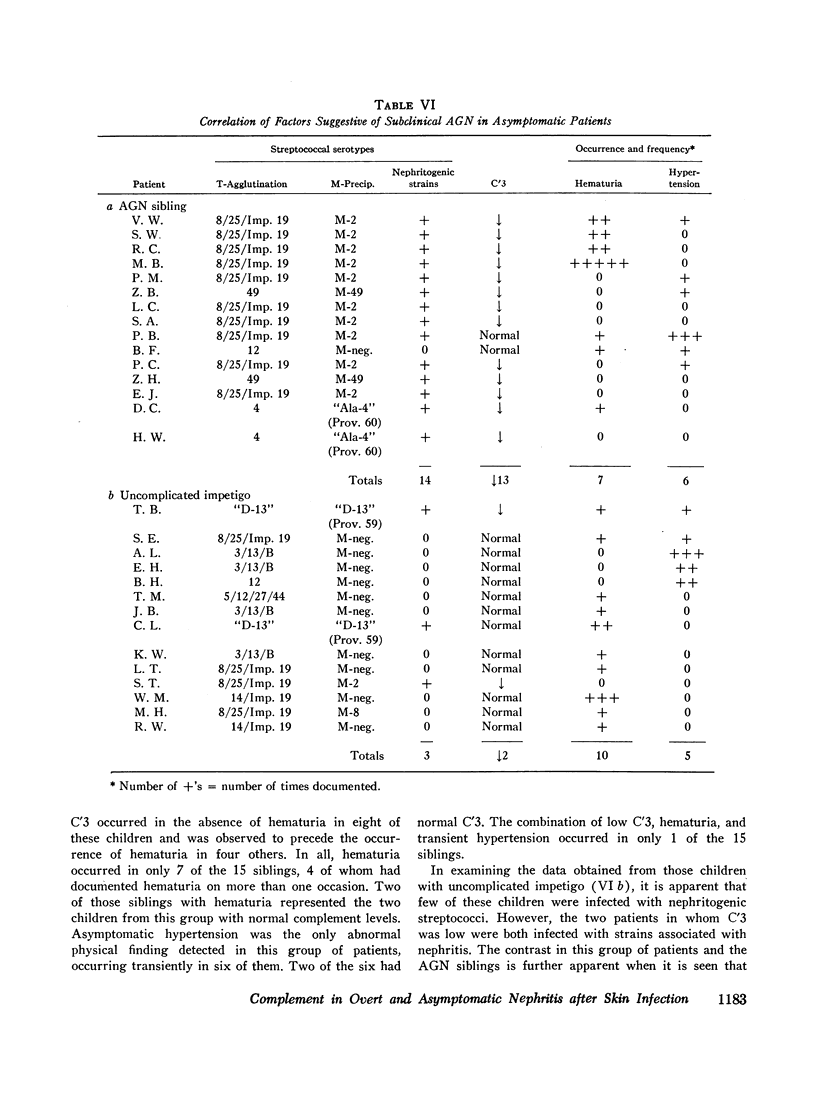
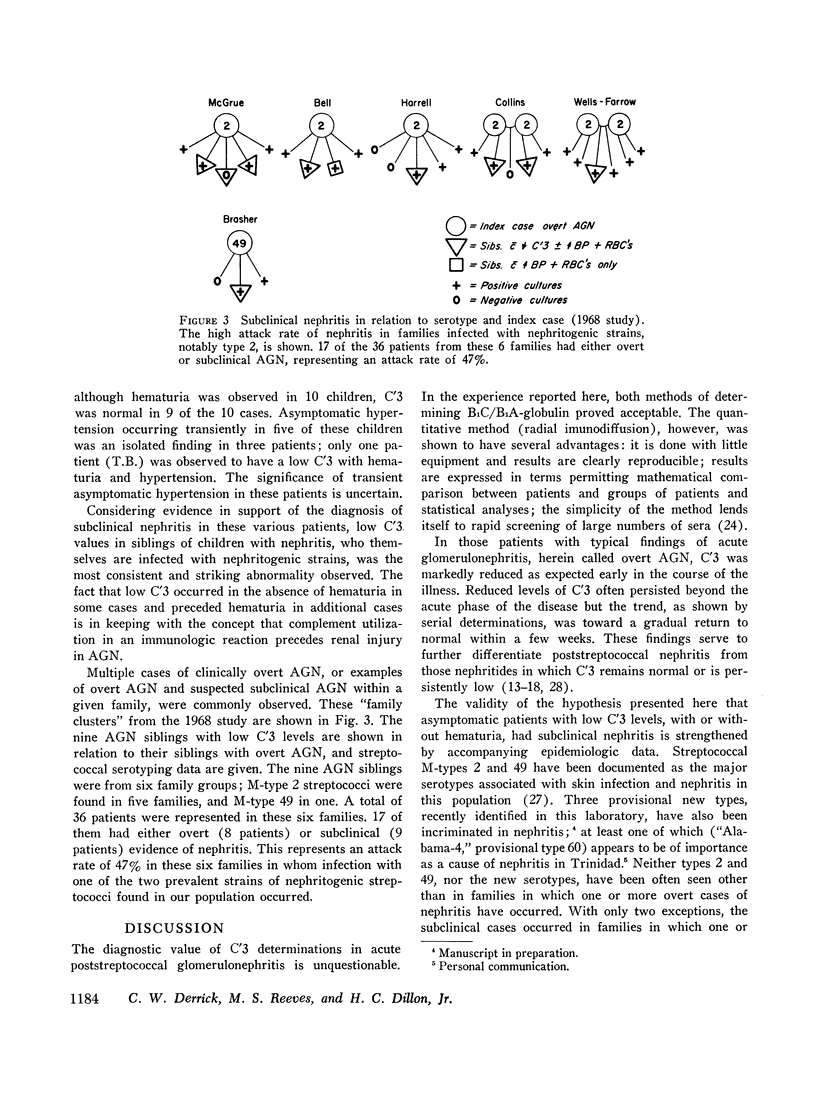
93% of the patients with overt nephritis had diminished complement levels. Low complement was more often observed (8%) in AGN siblings than was transient hypertension and/or hematuria (5%). Considering the relationship of low C′3 alone and low C′3 preceded hematuria in four others. Two (0.4%) of the patients with uncomplicated impetigo had low complement values, both of whom were infected with nephritogenic strains. Transient hematuria and/or hypertension was less frequently observed (2.7%) among patients with uncomplicated impetigo. Serial determinations in patients with low complement revealed a return to normal in a linear fashion within 2-12 wk.
The validity of the hypothesis that the asymptomatic patients with low complement levels, with or without hematuria, likely had subclinical nephritis is strengthened by the accompanying epidemiologic data. The finding of low complement before the onset of, or in the absence of, hematuria or other evidence of nephritis supports the concept that an immunologic mechanism may precipitate the renal injury of acute streptococcal nephritis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres G. A., Accinni L., Hsu K. C., Zabriskie J. B., Seegal B. C. Electron microscopic studies of human glomerulonephritis with ferritin-conjugated antibody. Localization of antigen-antibody complexes in glomerular structures of patients with acute glomerulonephritis. J Exp Med. 1966 Feb 1;123(2):399–412. doi: 10.1084/jem.123.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony B. F., Perlman L. V., Wannamaker L. W. Skin infections and acute nephritis in American Indian children. Pediatrics. 1967 Feb;39(2):263–279. [PubMed] [Google Scholar]
- BERMAN L. B., VOGELSANG P. Poststreptococcal glomerulonephritis without proteinuria. N Engl J Med. 1963 Jun 6;268:1275–1277. doi: 10.1056/NEJM196306062682306. [DOI] [PubMed] [Google Scholar]
- Becker C. G., Murphy G. E. The experimental induction of glomerulonephritis like that in man by infection with group A streptococci. J Exp Med. 1968 Jan 1;127(1):1–24. doi: 10.1084/jem.127.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN J. A., LEVIT M. F. Acute glomerulonephritis with few urinary abnormalities. Report of two cases proved by renal biopsy. N Engl J Med. 1963 Apr 4;268:749–753. doi: 10.1056/NEJM196304042681402. [DOI] [PubMed] [Google Scholar]
- Dillon H. C., Jr Impetigo contagiosa: suppurative and non-suppurative complications. I. Clinical, bacteriologic, and epidemiologic characteristics of impetigo. Am J Dis Child. 1968 May;115(5):530–541. doi: 10.1001/archpedi.1968.02100010532002. [DOI] [PubMed] [Google Scholar]
- Dillon H. C., Jr, Moody M. D., Maxted W. R., Parker M. T. The epidemiology of impetigo and acute glomerulonephritis. Results of serological typing of group A streptococci. Am J Epidemiol. 1967 Nov;86(3):710–723. doi: 10.1093/oxfordjournals.aje.a120779. [DOI] [PubMed] [Google Scholar]
- Dillon H. C., Jr Pyoderma and nephritis. Annu Rev Med. 1967;18:207–218. doi: 10.1146/annurev.me.18.020167.001231. [DOI] [PubMed] [Google Scholar]
- Dillon H. C., Reeves M. S., Maxted W. R. Acute glomerulonephritis following skin infection due to streptococci of M-type 2. Lancet. 1968 Mar 16;1(7542):543–545. doi: 10.1016/s0140-6736(68)92826-2. [DOI] [PubMed] [Google Scholar]
- Dixon F. J. The pathogenesis of glomerulonephritis. Am J Med. 1968 Apr;44(4):493–498. doi: 10.1016/0002-9343(68)90050-8. [DOI] [PubMed] [Google Scholar]
- Dudding B. A., Dillon H. C., Wannamaker L. W., Kilton R. M., Chapman S. S., Anthony B. F. Postepidemic surveillance studies of a food-borne epidemic of streptococcal pharyngitis at the United States Air Force Academy. J Infect Dis. 1969 Aug;120(2):225–236. doi: 10.1093/infdis/120.2.225. [DOI] [PubMed] [Google Scholar]
- Dunn M. J. Acute glomerulonephritis with normal results from urinalyses. A report of two cases and comments on four additional cases with atypical findings from urinalyses. JAMA. 1967 Sep 18;201(12):933–937. doi: 10.1001/jama.201.12.933. [DOI] [PubMed] [Google Scholar]
- Feldman J. D., Mardiney M. R., Shuler S. E. Immunology and morphology of acute post-streptococcal glomerulonephritis. Lab Invest. 1966 Jan;15(1 Pt 2):283–301. [PubMed] [Google Scholar]
- Freedman P., Meister H. P., Co B. S., Markowitz A. S., Dubin A. Subclinical renal response to streptococcal infection. N Engl J Med. 1966 Oct 13;275(15):795–802. doi: 10.1056/NEJM196610132751501. [DOI] [PubMed] [Google Scholar]
- Gewurz H., Pickering R. J., Mergenhagen S. E., Good R. A. The complement profile in acute glomerulonephritis systemic lupus erythematosus and hypocomplementemic chronic glomerulonephritis. Contrasts and experimental correlations. Int Arch Allergy Appl Immunol. 1968;34(6):556–570. doi: 10.1159/000230149. [DOI] [PubMed] [Google Scholar]
- Gotoff S. P., Isaacs E. W., Muehrcke R. C., Smith R. D. Serum beta-1C globulin in glomerulonephritis and systemic lupus erythematosus. Ann Intern Med. 1969 Aug;71(2):327–333. doi: 10.7326/0003-4819-71-2-327. [DOI] [PubMed] [Google Scholar]
- KLEINMAN H. Epidemic acute glomerulonephritis at Red Lake. Minn Med. 1954 Jul;37(7):479–passim. [PubMed] [Google Scholar]
- Koffler D., Paronetto F. Immunofluorescent localization of immunoglobulins, complement, and fibrinogen in human diseases. II. Acute, subacute, and chronic glomerulonephritis. J Clin Invest. 1965 Oct;44(10):1665–1671. doi: 10.1172/JCI105273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler P. F., Ten Bensel R. Serial complement component alterations in acute glomerulonephritis and systemic lupus erythematosus. Clin Exp Immunol. 1969 Feb;4(2):191–202. [PMC free article] [PubMed] [Google Scholar]
- LANGE K., WASSERMAN E., SLOBODY L. B. The significance of serum complement levels for the diagnosis and prognosis of acute and subacute glomerulonephritis and lupus erythematosus disseminatus. Ann Intern Med. 1960 Oct;53:636–646. doi: 10.7326/0003-4819-53-4-636. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McCluskey R. T., Vassalli P., Gallo G., Baldwin D. S. An immunofluorescent study of pathogenic mechanisms in glomerular diseases. N Engl J Med. 1966 Mar 31;274(13):695–701. doi: 10.1056/NEJM196603312741301. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Jr, Drummond K. N., Good R. A., Vernier R. L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Invest. 1966 Feb;45(2):237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northway J. D., McAdams A. J., Forristal J., West C. D. A "silent" phase of hypocomplementemic persistent nephritis detectable by reduced serum beta-1c-globulin levels. J Pediatr. 1969 Jan;74(1):28–38. doi: 10.1016/s0022-3476(69)80005-3. [DOI] [PubMed] [Google Scholar]
- RAMMELKAMP C. H., Jr, WEAVER R. S. Acute glomerulonephritis, the significance of the variations in the incidence of the disease. J Clin Invest. 1953 Apr;32(4):345–358. doi: 10.1172/JCI102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMMELKAMP C. H., Jr, WEAVER R. S., DINGLE J. H. Significance of the epidemiological differences between acute nephritis and acute rheumatic fever. Trans Assoc Am Physicians. 1952;65:168–175. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SEEGAL B. C., ANDRES G. A., HSU K. C., ZABRISKIE J. B. STUDIES ON THE PATHOGENESIS OF ACUTE AND PROGRESSIVE GLOMERULONEPHRITIS IN MAN BY IMMUNOFLUORESCEIN AND IMMUNOFERRITIN TECHNIQUES. Fed Proc. 1965 Jan-Feb;24:100–108. [PubMed] [Google Scholar]
- STETSON C. A., RAMMELKAMP C. H., Jr, KRAUSE R. M., KOHEN R. J., PERRY W. D. Epidemic acute nephritis: studies on etiology, natural history and prevention. Medicine (Baltimore) 1955 Dec;34(4):431–450. doi: 10.1097/00005792-195512000-00002. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Plentil A. A., Adamsons K. Exchange of carbon dioxide in the pregnant rhesus monkey: multicompartmental analysis of carbon dioxide kinetics. J Clin Invest. 1969 Nov;48(11):1967–1982. doi: 10.1172/JCI106163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UPDYKE E. L., MOORE M. S., CONROY E. Provisional new type of group A streptococci associated with nephritis. Science. 1955 Feb 4;121(3136):171–172. doi: 10.1126/science.121.3136.171. [DOI] [PubMed] [Google Scholar]
- WANNAMAKER L. W., PIERCE H. C. Family outbreak of acute nephritis associated with type 49 streptococcal infection. J Lancet. 1961 Dec;81:561–571. [PubMed] [Google Scholar]
- WEST C. D., NORTHWAY J. D., DAVIS N. C. SERUM LEVELS OF BETA-1C GLOBULIN, A COMPLEMENT COMPONENT, IN THE NEPHRITIDES, LIPOID NEPHROSIS, AND OTHER CONDITIONS. J Clin Invest. 1964 Aug;43:1507–1517. doi: 10.1172/JCI105027. [DOI] [PMC free article] [PubMed] [Google Scholar]


