Abstract
The first full length IgG produced in Pichia pastoris was reported in late 1980. However, use of a wild-type Pichia expression system to produce IgGs with human-like N-linked glycans was not possible until recently. Advances in glycoengineering have enabled organisms such as Pichia to mimic human N-glycan biosynthesis and produce IgGs with human glycans on an industrial scale. Since there are only a few reports of the analytical characterization of Pichia-produced IgG, we summarize the results known in this field, and provide additional characterization data generated in our laboratories. The data suggest that Pichia-produced IgG has the same stability as that produced in Chinese hamster ovary (CHO) cells. It has similar aggregation profiles, charge variant distribution and oxidation levels as those for a CHO IgG. It contains human N-linked glycans and O-linked single mannose. Because of the comparable biophysical and biochemical characteristics, glycoengineered Pichia pastoris is an attractive expression system for therapeutic IgG productions.
Key words: Pichia pastoris, IgG, N-linked glycan, O-linked glycan, analytical characterization
Introduction
The first IgG was produced in yeast Saccharomyces cerevisiae by two independent groups in the 1980s.1,2 However, it was not until 1999 that Ogunjimi et al. reported the first fully functional IgG produced in methylotrophic yeast Pichia pastoris,3 although there were many reports in the 1990s and later of single chain IgG fragment (scFv), fusion scFc and Fab fragments produced in Pichia pastoris.4–23
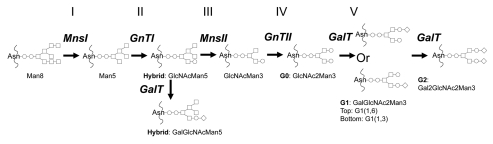
Wild-type yeast glycoproteins contain N-linked high mannose glycans which are immunogenic and have poor pharmacokinetic/pharmacodynamic properties in primates and humans. In the 2000s, both GlycoSwitch and GlycoFi technologies have made tremendous progress such that it is possible for Pichia to produce therapeutic IgG with human-like N-linked glycans.24–33 In both technologies, the secretory pathway of Pichia pastoris is genetically engineered to replicate human-like N-linked glycan biosynthesis. Genes responsible for yeast high mannose glycans, e.g., och1, are disrupted, and a series of glycosidases and glycosyltransferases are introduced (Fig. 1). The first step of the humanized N-linked glycan synthesis involves an early Golgi mannosidase that trims Man8 to Man5 core structure (Step I). Man5 is then glycosylated to a hybrid structure GlcNAcMan5 by a Golgi-residing fusion protein N-acetylglucosaminyltransferase I (Step II). Next, a mannosidase II (MnsII) is introduced in Golgi and quantitatively converts the hybrid structure to GlcNAcMan3 (Step III). GlcNAcMan3 is then further glycosylated to G0 structure by N-acetylglucosaminyltransferase II (GnTII) (Step IV). The last step involves the addition of Gal sugars to the non-reducing end of terminal GlcNAc and is achieved by the introduction of galactosyltransferase (GalT), as well as ways that increase the cellular pool of UDP-galactose substrate (Step V).
Figure 1.
The schematic outline of N-glycan biosynthesis pathway in glycoengineered Pichia pastoris that mimics human N-glycan synthesis. MnsI: α-1,2-mannosidase; GnTI: β-1,2-N-acetylglucosaminyltransferase I; MnsII: mannosidase II; GnTII: β-1,2-N-acetylglucosaminyltransferase II; GalT: β-1,4-galactosyltransferase. ○: GlcNAc; □: Man; ◊: Gal. To simplify nomenclature, the two GlcNAc sugars at the reducing end of all glycans are omitted.
Because of the extensive genetic engineering in Pichia, one of the perceived challenges for industrial scale Pichia IgG production is genetic stability. In the last two years a robust and scalable fermentation process for glycoengineered Pichia with titers of more than 1 g/L of fully assembled IgG1 with uniform N-linked glycosylation was reported.34,35 Potgieter et al. showed that the N-linked glycan fidelity can be maintained for up to 64 generations, which is double the passage numbers required for a 2,000 L fermentation scale.34 In addition, the authors have demonstrated that both the productivity and N-linked glycan quality can be maintained across a range of fermentation conditions. The genetic stability of this Pichia strain has laid a solid foundation for industrial scale IgG production.
Since Pichia technology for IgG production is relatively new, there are only a few publications on biochemical and biophysical characterization of Pichia-produced IgG. In early reports of IgG fragments expressed in Pichia pastoris, most groups used simple biochemical methods, such as sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), western blot and ELISA, for product characterization, although some used size-exclusion chromatography (SEC),9 Biacore,9,21 nuclear magnetic resonance,13 peptide mapping, mass spectrometry (MS) and N-terminal sequencing methods.15 More in-depth characterization of Pichia-derived IgG has been reported recently in reference 36. In this review, we summarize the current knowledge of Pichia-derived IgG and present additional characterization data of a Pichia-derived IgG1 in direct comparison with the Chinese hamster ovary (CHO) cell-derived counterpart.
Biochemical and Biophysical Characterization of IgG1 Produced in Pichia pastoris
Characterization is typically done to assess product attributes, including aggregation, charge heterogeneities, methionine oxidation, purity, N-linked glycan occupancy and composition, O-linked glycosylation, secondary and tertiary structures, thermal stability and stability during storage.
Aggregation by Size Exclusion Chromatography
IgG is typically formulated for the high-concentration doses required to achieve therapeutic efficacy. However, IgG has a tendency to form aggregates such as dimers, trimers and multimers when concentrated, and the high-order aggregates could have deleterious consequence, e.g., reduced activity, increased immunogenicity,37–40 poor solubility. The tendency toward formation of aggregates is governed by several factors, including intrinsic properties and external conditions during product development. For example, during process development, IgG can experience temperature, pressure, pH and ionic strength changes that can cause aggregation. A number of methods can be used to measure aggregations,41–43 but SEC is considered the workhorse for detection and quantification of IgG aggregations. The SEC profile of one IgG1 produced in P. pastoris is shown in Figure 2; SEC method used is described in Cohen et al.44 The early elution peak represents aggregations, while the monomer form elutes at about 16 min. The fragment is below the quantitation limit for this IgG1 molecule. The amount of aggregation in Pichia expressed IgG is less than 5%, which is similar to typical IgGs produced in either CHO or NS0 cell lines.
Figure 2.
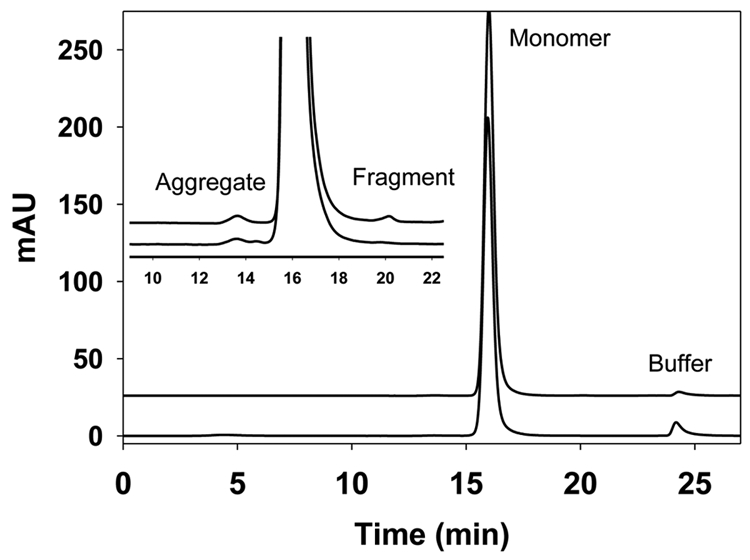
Size exclusion chromatography (SEC) profiles of IgG1 produced from CHO cell lines (upper trace) and from Pichia (lower trace). Insert is a zoomed view of the SEC profiles. Peak eluting at 16 min represents the IgG monomer, while peak at 13.5 min represents the aggregate and peak at 20 min represents the fragment. In both cases, the percentage of the monomer is above 99%, and the aggregate and fragment are below 0.5% individually. SEC was performed using a TSK-gelG3000SW column with UV detection at 280 nm and flow rate of 0.5 mL/min. The mobile phase contained 25 mM sodium phosphate, 300 mM sodium chloride and 0.05% sodium azide at pH 6.8.
Charge Heterogeneities
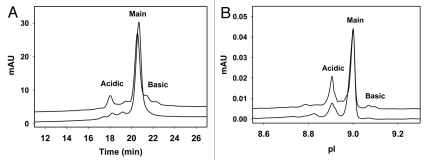
Charge variant heterogeneities are generated through several pathways such as chemical modification, incomplete enzymatic reaction, and other post-translational modifications. In IgG, these modifications result in charge-based heterogeneities such as deamidation, acetylation, N-terminal cyclization to pyroglutamate, incomplete C-terminal lysine cleavage, glycation, phosphorylation and sialylation. Deamidation, in particular, is of great interest as it is one of the major degradation pathways for IgG, and IgG with deamidation in the complementarity-determining region is shown to have reduced biological activity. Deamidation contributes to the majority of the acidic variants of an IgG and is typically monitored through cation-exchange chromatography (CEX) and capillary isoelectric focusing (cIEF). The two methods are complementary, and each has its own advantages and disadvantages.45–47 Figure 3 illustrates the charge heterogeneity of a pair of IgG1 with the same sequence produced from CHO and P. pastoris, respectively, as determined by CEX (Fig. 3A) and cIEF (Fig. 3B) methods. The overall features between the IgGs produced in the two expression systems are quite similar. For this specific IgG1, Pichia-produced material has less acidic and less basic variants than that of the CHO-produced IgG1 by both analytical techniques. The difference in charge heterogeneity may be due to differences in C-terminal lysine variants, sialylation and process conditions. The CEX method used was similar to that described in Vlasak et al. (gradient was slightly modified),48 while the cIEF method used was similar to that described in Li et al.49
Figure 3.
(A) Charge heterogeneity of IgG1 revealed by cation exchange chromatography. In this assay, the Pichia-produced IgG1 (lower trace) has 15% acidic variants, 84% main fraction and 1% basic variants. The CHO produced IgG1 (upper trace) has 23%, 61% and 16% acidic, main and basic fractions, respectively. The CEX separation was performed using Dionex ProPac WCX-10 column (4 × 250 mm) with a salt gradient elution and UV detection at 280 nm wavelength. The mobile phase A contained 25 mM sodium phosphate at pH 6.5 and the mobile phase B contained 25 mM sodium phosphate, 300 mM sodium chloride and 0.05% sodium azide at pH 6.5. The salt gradient elution was 4%–22% mobile phase B in 28 min at 1 mL/min flow rate. (B) Charge heterogeneity of IgG1 revealed by imaged capillary isoelectric focusing. The acidic, main and basic fractions assessed by this assay are 24, 75, 1% for Pichia-produced IgG1 (lower trace) and 33, 62, 5% for CHO-produced IgG1 (upper trace), respectively. Imaged capillary isoelectric focusing was carried out using Convergent Bioscience iCE280 analyzer. Samples were diluted to 0.25 mg/mL in a solution containing 0.35% methylcellulose, a mixture of pH 3–10 and 8–10 carrier ampholytes, and two pI markers of 7.6 and 9.5. The prepared samples were focused in a 5 cm long, 100 µm ID × 200 µm OD silica capillary and detected at 280 nm. Focusing time was 1 min at 1.5 kV then 8 min at 3 kV.
Methionine Oxidation
Methionine oxidation is another post-translational modification that can occur either during antibody production or storage. Two conserved methionine residues in human IgG1, Met 252 and Met 428, are particularly prone to oxidation.50–52 Oxidation of these two methionine residuals has been shown to decrease the binding affinity of IgG to FcRn,53 which consequently reduces its serum half-life.54 Methionine oxidation can be quantified by either Protein A high-performance liquid chromatography or peptide mapping.53,55 Using the peptide mapping method as described in Bertolotti-Ciarlet et al.53 we demonstrated that Pichia-produced IgG1 has a level of oxidation comparable to the oxidation level typically observed in CHO-produced IgG1.
Purity and N-Linked Glycan Occupancy by Capillary Electrophoresis-SDS Gel
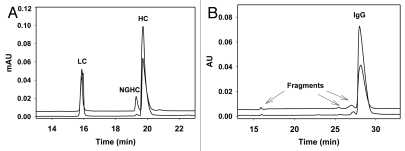
Capillary electrophoresis (CE)-SDS gel is becoming a standard technique to evaluate IgG purity under both reducing and non-reducing conditions, replacing the older, labor-intensive and manual slab SDS-PAGE gel. Similar as SDS-PAGE, the separation principal of the CE-SDS gel is based on molecular weight, but CE-SDS gel has the advantages of automatability, reproducibility, easy quantitation, robustness, high resolution and throughput. Unique to the CE-SDS method under reducing condition, the glycosylated heavy chain can be well separated from the non-glycosylated heavy chain (NGHC). Therefore, this method can provide information regarding N-linked glycan occupancy, in addition to purity and percentage of fragmentation. Furthermore, non-reducing CE-SDS gel provides orthogonal information to SEC regarding covalent aggregations and intact IgG.56,57 We have compared CHO- and P. pastoris-produced IgGs using CE-SDS gel under both reducing and non-reducing conditions. The electropherograms under reducing conditions show the profile of light chain (LC), heavy chain (HC) and NGHC (Fig. 4A). High purity with (LC + HC + NGHC) >99% and no fragmentation are achieved for both IgGs. However, with this particular Pichia strain and the fermentation conditions used, the NGHC from the Pichia-produced IgG is ∼10%, higher than the level normally observed for IgG produced in CHO or NS0 (<2%). The majority of the observed NGHC could pair with a glycosylated HC and form hemi-glycosylated IgG. Since hemi-glycosylated IgG has impaired binding affinity to FcγRs, the quantity of NGHC could be important for some applications where effector functionality is required.58 As shown in Figure 4B, under non-reducing condition the intact IgG percentage for Pichia-produced IgG is >96%, comparable with IgG produced in CHO or NS0. There are no covalently linked aggregations observed for either of the IgGs. The CE-SDS method used was similar to the one described in Rustandi et al.57
Figure 4.
(A) CE-SDS gel electropherograms of IgG1 produced in CHO (lower trace) and Pichia (upper trace) under reducing condition. Both have typical IgG pattern with LC, HC and NGHC. This particular Pichia-produced IgG1 contains about 10% NGHC compared to 1% of NGHC in CHO IgG1. Samples (1 mg/mL) were reduced in beta mercaptoethanol (2-ME) containing 0.5% SDS and heated at 70°C for 10 min. CE-SDS gel was performed using Beckman PA800 CE system (220 nm detection) in bare-fused silica capillary. Sample was injected at anode with reverse polarity using -5 kV for 25 s. The separation was performed at -15 kV reverse polarity with 20 psi at both ends of capillary for 30 min. (B) CE-SDS gel electropherograms of IgG1 produced in CHO (lower trace) and Pichia (upper trace) under non-reducing condition. Both have >96% intact IgG with no covalently linked aggregates. Non-reducing samples (1 mg/mL) were treated with 25 mM iodoacetamide in the presence of 0.5% SDS and heated at 70°C for 10 min. CE separation was the same as described in reducing samples above.
N-Linked Glycan Composition
Human IgG is glycosylated at heavy chain Asn297. The N-linked glycan of human serum IgGs contains predominantly biantennary complex-type structures, with the majority being core-fucosylated—G0F/G1F/G2F. A small percentage of IgG can have sialylated termini—A2F/A1F or contain a bisecting GlcNAc,59,60 and N-linked glycosylation can also be found in the Fab region.61 Current commercial IgGs produced in CHO or NS0 cell lines contain mostly core-fucosylated glycan structures on Asn297, similar to glycoforms present in human IgGs. However, CHO or NS0 can add abnormal sugars to IgGs, which may lead to immunogenic reactions. A recent report by Chung et al. on the hypersensitivity reaction towards cetuximab (Erbitux®) has highlighted the importance of glycan structures.62 Thus, industry guidelines for the thorough characterization of the glycan profile of IgG products have been provided by regulatory agencies.
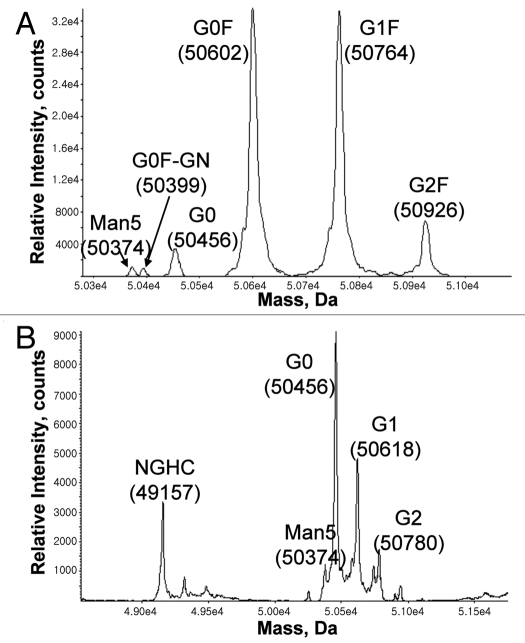
The N-linked glycan composition can be analyzed by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) and electrospray ionization (ESI)-MS using either intact or PNGase digested IgG.63,64 N-glycan profiles for both Pichia- and CHO-derived anti-Her2 mAb have been compared.36,65 While CHO-derived IgG contains predominantly fucosylated G0F and G1F glycans, Pichia-derived IgG is 100% afucosylated and contains mainly G0 and G1 glycans. N-glycan composition can also be analyzed by capillary electrophoresis with laser-induced fluorescence detection.66,67 The ESI-TOF spectra of reduced HCs for both CHO and Pichia-produced IgGs are shown in Figure 5, which illustrates that the Pichia-produced IgG contains mainly G0 and small amount of G1, G2 and Man5. Man5 is also present in the commercially-available, CHO-produced IgG.
Figure 5.
ESI-TOF spectra of the heavy chain in (A) CHO- and (B) Pichia-produced IgG1. As expected, Pichia-produced IgG1 has no core fucose and contains predominantly G0 glycan. Samples were reduced in the presence of 1 mM DTT at 75°C for 15 minutes, then separated on a PS-DVB column (0.5 mm × 50 mm) at 80°C with a gradient elution of mobile A and B and a flow rate of 20 µL/min. The mobile phase A contained water with 0.1% formic acid, and the mobile phase B contained acetonitrile with 0.1% formic acid. The liquid chromatography separation was coupled to an Agilent MSD-TOF MS and the mass was determined using TOF Protein Confirmation software.
O-Linked Glycosylation
O-glycosylation is an important post-translational modification on the Ser/Thr residuals. Mammalian proteins have mainly O-linked GalNAc (mucin-type), Fuc and Glu sugars, while those from yeast have distinctive O-linked oligomannose. The majority of yeast cell wall proteins are O-mannosylated, which is essential for maintaining a stable cell wall. In 1998, Duman et al. showed direct evidence that not only the yeast cellular proteins, but also recombinant proteins from the yeast host, contain O-mannosylation.68 The O-linked glycans from Pichia-expressed recombinant protein comprise from dimeric to pentameric α-1,2-linked mannose, while dimeric and trimeric mannose are the two major components of the O-linked glycans.68
Although O-mannosylation is highly abundant in yeast, it is less frequent in mammals. Protein O-mannosylation in mammals has been identified in brain, nerve and muscular tissues.69–72 The most well-characterized human O-mannosylated protein is dystroglycan.73 It has a highly O-mannosylated extracellular domain, with a high abundance of Neu5Ac-Gal-GlcNAc-Man glycan structure. Its impaired O-glycosylation has been linked to several muscular dystrophies.
Although most marketed therapeutic IgGs do not have O-glycosylation, endogenous IgG is known to contain O-glycosylations. Approximately 40% of mouse IgG2b heavy chain hinge region is O-glycosylated, containing mainly the mucin-type tetrassacharide.74 Arnold et al. have shown that human serum IgA and IgD have multiple O-linked glycosylation within their hinge region.75 O-mannosylated IgG is uncommon; however, an IgG2 produced in both CHO cell line and COS transient cells has been reported to be O-mannosylated on Ser66 of the LC.76
The challenge for the Pichia expression system is how to eliminate yeast-like O-mannosylation on the expressed IgGs. Because O-glycosylation is essential to cell survival, complete elimination of O-glycosylation is lethal to yeast.77 However, progress has been made in inhibiting O-glycosylation using small molecule protein-O-mannosyltransferase inhibitors (PMTi).78
As there is no specific enzyme to cleave O-glycans from IgG, the most common release is via chemical β-elimination reaction catalyzed by base. The reaction is then followed immediately with reduction to prevent peeling reaction. Subsequently, the released O-glycans are analyzed with high-performance anion-exchange chromatography with pulsed amperometric detection.79 In general, there is only one type of O-glycan, mannose, observed in Pichia-produced IgG, and its total occupancy can be controlled to a low level of less than 2 mole of mannose per mole of IgG.
Secondary and Tertiary Structures
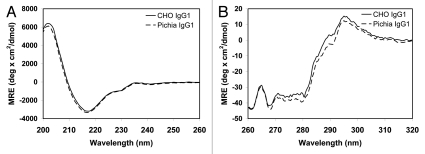
Circular dichroism (CD) has been routinely used to evaluate protein secondary and tertiary structures. We have compared the far-ultraviolet (UV: 200–260 nm) and the near-UV (260–320 nm) CD spectra (Fig. 6A and B, respectively) of CHO- and Pichia-produced IgG1. The far-UV CD spectra of the two IgG1 overlap with each other, indicating that the two IgG1 have comparable secondary structures regardless of the expression system. The similarity between the near-UV CD spectra indicates the comparable tertiary structures of the two IgG1. The CD method is similar to that described in Wang et al. with minor modifications.80
Figure 6.
Far-UV (A) and near-UV (B) circular dichroism (CD) spectra of CHO- and Pichia-produced IgG1. The two antibodies have similar spectra, indicating that Pichia-produced IgG1 has similar secondary and tertiary structures compared to the CHO-produced counterpart. The CD measurements were performed on a Jasco J-810 Spectropolarimeter. Samples were dialyzed into PBS buffer and their concentrations were measured by UV. CD measurements were performed using a 1 cm quartz cuvette. A total of five spectral scans were collected at ambient temperature and signals were averaged. The buffer spectrum was collected under the same condition and was subtracted from the sample spectrum prior to the mean residue ellipticity calculation.
Thermal Stability by Differential Scanning Calorimetry
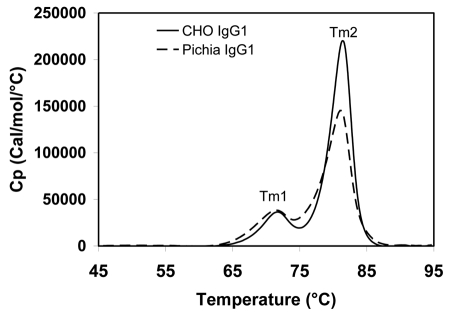
Differential scanning calorimetry (DSC) is an important tool to characterize the thermal stability of IgGs. Since antigen-binding fragments (Fab), CH2 and CH3 domains unfold with increasing temperature, the DSC profile has been used to evaluate the structural integrity and stability of these domains. The DSC thermograms of one IgG1 expressed from both CHO cells and Pichia pastoris are shown in Figure 7. The first transition peak (Tm1) represents the unfolding of the CH2 domain, and the second transition peak (Tm2) represents the unfolding of both the Fab and CH3 domains. CHO- and Pichia-derived IgG1 have the same Tm1 (72°C) and Tm2 (81°C), indicating that Pichia-produced antibody has similar thermal stability compared to the CHO-produced counterpart. The DSC method used is described in Ionescu et al.81
Figure 7.
Temperature-induced unfolding of IgG1 produced from CHO and Pichia pastoris. CHO- and Pichia-produced IgG1 have identical Tm1 and Tm2 melting temperatures. DSC was conducted on a VP-DSC Capillary Cell Microcalorimeter with a scan rate of 1°C/min from 25°C to 95°C. The thermograms were processed using the Origin 7.0 software and normalized to the molar concentration of the IgG sample.
Stability Over Storage
Pichia-produced IgGs have demonstrated good physical stability. No significant aggregation or degradation has been detected for the Pichia-derived anti-HER2 mAb solution during six-week storage at 4, 25 and 37°C.36 We have compared head-to-head the chemical degradation of CHO- and Pichia-produced IgG1 in liquid formulation under different temperatures (4, 25, 37 and 45°C) for two months. Both IgG1 demonstrate similar chemical degradation profiles, and similar activation energies can be derived from the temperature dependent chemical degradation profiles using the Arrhenius model.
Conclusion
As the market for therapeutic IgGs continues to grow rapidly, there is a high demand for industrial scale IgG production. Currently, all marketed therapeutic IgGs are produced from mammalian cell cultures. However, mammalian cell culture is expensive, lengthy (e.g., typical two-week cultivation), and sensitive to fermentation process parameters. The glycoengineered Pichia pastoris expression system can substantially reduce the cultivation time34 and the cost associated with fermentation facility, raw material and viral clearance. We have shown that Pichia produces stable IgGs with comparable aggregation, charge variant and oxidation profile compared to the CHO-produced counterpart. Because of the comparable biophysical and biochemical product characteristics, glycoengineered Pichia pastoris is becoming an attractive alternative to the traditional mammalian expression system for therapeutic IgGs.
While the mammalian expression system offers little control of glycosylation, the Pichia expression system has great flexibility in modulating the N-linked glycan composition. It has been shown that IgG with afucosylated N-linked glycosylation binds to FcγRIIIa about 20- to 40-fold tighter than fucosylated variant32,65,82 and elicits higher ADCC response compared with fucosylated IgG.65,83 A fucosyltransferase knock-out CHO cell line (Potelligent™ technology) has been explored by several pharmaceutical companies to produce IgGs that require enhanced ADCC function. Glycoengineered Pichia produces 100% afucosylated IgG. A direct comparison of ADCC function has shown that Pichia-produced anti-HER2 antibody elicits 4-fold higher ADCC function with PBMC effector cells than CHO-produced counterpart.65 Thus, glycoengineered Pichia offers a promising platform for the production of IgGs with a higher ADCC function.
Acknowledgments
We gratefully thank Assunta Ng for providing SEC aggregation data, Anna Mach for CE-SDS data, Feng Wang for CEX data, Brian Peklansky for cIEF data, Yi Du for ESI-TOF data, Bei Wang for DSC data, Yunsong (Frank) Li for CD data and Sarita Mittal for chemical degradation data. We also would like to thank our colleagues from GlycoFi for providing technical assistance and colleagues from BPRD for providing all IgG materials.
Abbreviations
- IgG
immunoglobulin
- NGHC
non-glycosylated heavy chain
- HC
heavy chain
- LC
light chain
- CEX
cation exchange chromatography
- SEC
size exclusion chromatography
- cIEF
capillary isoelectric focusing
- DSC
differential scanning calorimetry
- CE-SDS
capillary electrophoresis sodium dodecyl sulfate
- ADCC
antibody-dependent cellular cytotoxicity
- FcγR
human Fcγ receptor
- FcRn
neonatal Fc receptor
References
- 1.Wood CR, Boss MA, Kenten JH, Calvert JE, Roberts NA. The synthesis and in vivo assembly of functional antibodies in yeast. Nature. 1985;314:446–449. doi: 10.1038/314446a0. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz AH, Chang CP, Better M, Hellstrom KE, Robinson RR. Secretion of functional antibody and Fab fragment from yeast cells. Proc Natl Acad Sci USA. 1988;85:8678–8682. doi: 10.1073/pnas.85.22.8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogunjimi AA, Chandler JM, Gooding CM, Recinos A, Choudary PV. High-level secretory expression of immunologically active intact antibody from the yeast Pichia pastoris. Biotechnol Lett. 1999;21:561–567. [Google Scholar]
- 4.Ridder R, Schmitz R, Legay F, Gram H. Generation of rabbit monoclonal antibody fragments from a combinatorial phage display library and their production in the yeast Pichia pastoris. Nature Biotechnol. 1995;13:255–260. doi: 10.1038/nbt0395-255. [DOI] [PubMed] [Google Scholar]
- 5.Luo D, Mah N, Krantz M, Wilde K, Wishart D, Zhang Y, et al. VI-linker-Vh orientation-dependent expression of single chain Fv containing an engineered disulfide-stabilized bond in the framework regions. J Biochem. 1995;118:825–831. doi: 10.1093/oxfordjournals.jbchem.a124986. [DOI] [PubMed] [Google Scholar]
- 6.Ando K, Arunwanich P, Kai K, Shinkai M, Honda H, Kobayashi T. Production of Fv fragment of monoclonal antibody from recombinant methylotrophic yeast, Pichia pastoris. J Chem Eng Jpn. 1996;29:390–392. [Google Scholar]
- 7.Luo D, Geng M, Noujaim AA, Madiyalakan R. An engineered bivalent single-chain antibody fragment that increases antigen binding activity. J Bicohem. 1997;121:831–834. doi: 10.1093/oxfordjournals.jbchem.a021661. [DOI] [PubMed] [Google Scholar]
- 8.Luo D, Mah N, Krantz M, Wishart D, Jacobs F, Martin L. High level secretion of single-chain antibody in Pichia exression system. Biotechnol Tech. 1997;11:759–761. [Google Scholar]
- 9.Goel A, Beresford GW, Colcher D, Pavlinkova G, Booth BJM, Baranowska-Kortylewicz J, et al. Divalent forms of CC49 single-chain antibody constructs in Pichia pastoris: expression, purification and characterization. J Biochem. 2000;127:829–836. doi: 10.1093/oxfordjournals.jbchem.a022676. [DOI] [PubMed] [Google Scholar]
- 10.Andrade EV, Albuquerque FC, Moraes LMP, Brigido MM, Santos-Silva MA. Single-chain Fv with Fc fragment of the human IgG1 tag: construction, Pichia pastoris expression and antigen binding characterization. J Biochem. 2000;128:891–895. doi: 10.1093/oxfordjournals.jbchem.a022838. [DOI] [PubMed] [Google Scholar]
- 11.Freyre FM, Vazquez JE, Ayala M, Canaan-Haden L, Bell H, Rodriguez I, et al. Very high expression of an anti-carcinoembryonic antigen single chain Fv antibody fragment in the yeast Pichia pastoris. J Biotechnol. 2000;76:157–163. doi: 10.1016/s0168-1656(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Yuuki T, Takai T, Ra C, Okumura K, Yokota T, et al. Production of humanized Fab fragment against human high affinity IgE receptor in Pichia pastoris. Biosci Biotechnol Biochem. 2000;64:2138–2144. doi: 10.1271/bbb.64.2138. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Wang K, Jette DC, Wishart DS. Production of an anti-prostate-specific antigen single-chain antibody fragment from Pichia pastoris. Protein Expres Purif. 2001;23:419–425. doi: 10.1006/prep.2001.1521. [DOI] [PubMed] [Google Scholar]
- 14.Lange S, Schmitt J, Schmid RD. High-yield expression of the recombinant, atrazine-specific Fab fragment K411B by the methylotrophic yeast Pichia pastoris. J Immunol Methods. 2001;255:103–114. doi: 10.1016/s0022-1759(01)00351-9. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro RI, Wen D, Levesque M, Hronowski X, Gill A, Garber EA, et al. Expression of sonic hedgehog-Fc fusion protein in Pichia pastorsi. Identification and control of post-translational, chemical and proteolytic modifications. Protein Expres Purif. 2003;29:272–283. doi: 10.1016/s1046-5928(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 16.Hu S, Li L, Qiao J, Guo Y, Cheng L, Liu J. Codon optimization, expression and characterization of an internalizing anti-ErbB2 single-chain antibody in Pichia pastoris. Protein Expres Purif. 2006;47:249–257. doi: 10.1016/j.pep.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Tanfous NGB, Kallel H, Jarboui MA, Fathallah DM. Expression in Pichia pastoris of a recombinant scFv form of mAb 107, an anti human CD11b integrin antibody. Enzyme Microb Technol. 2006;38:636–642. [Google Scholar]
- 18.Gasser B, Maurer M, Gach J, Kunert R, Mattanovich D. Engineering of Pichia pastoris for improved production of antibody fragments. Biotechnol Bioeng. 2006;94:353–361. doi: 10.1002/bit.20851. [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Kim T, Xiong F, Yang X. Enhancing the production of Fc fusion protein in fed-batch fermentation of Pichia pastoris by design of experiments. Biotechnol Prog. 2007;23:621–625. doi: 10.1021/bp0603199. [DOI] [PubMed] [Google Scholar]
- 20.Cai J, Li F, Wang SZ. Expression of secreted human single-chain fragment variable antibody against human amyloid beta peptide in Pichia patoris. Neural Regen Res. 2008;3:910–913. [Google Scholar]
- 21.Chang HJ, Choi SW, Chun HS. Expression of functional single-chain variable domain fragment antibody (scFv) against mycotoxin zearalenone in Pichia pastoris. Biotechnol Lett. 2008;30:1801–1806. doi: 10.1007/s10529-008-9770-x. [DOI] [PubMed] [Google Scholar]
- 22.Kunert R, Gach J, Katinger H. Expression of a Fab fragment in CHO and Pichia pastoris. BioProcess Int. 2008;6:34–36. [Google Scholar]
- 23.Schoonooghe S, Kaigorodov V, Zawisza M, Dumolyn C, Haustraete J, Grooten J, et al. Efficient production of human bivalent and trivalent anti-MUCI Fab-scFv antibodies in Pichia pastoris. BMC Biotechnol. 2009;9:70. doi: 10.1186/1472-6750-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callewaert N, Laroy W, Cadirgi H, Geysens S, Saelens X, Jou WM, Contreras R. Use of HDEL-tagged Trichoderma reesei mannosyl oligosaccharide 1,2-α-D-mannosidase for N-glycan engineering in Pichia pastoris. FEBS Lett. 2001;503:173–178. doi: 10.1016/s0014-5793(01)02676-x. [DOI] [PubMed] [Google Scholar]
- 25.Vervecken W, Kaigorodov V, Callewaert N, Geysens S, Vusser KD, Contreras R. In vivo synthesis of mammalian-like, hybrid-type N-glycans in Pichia pastoris. Appl Environ Microbiol. 2004;70:2639–2646. doi: 10.1128/AEM.70.5.2639-2646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi BK, Bobrowicz P, Davidson RC, Hamilton SR, Kung DH, Li H, et al. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc Natl Acad Sci USA. 2003;100:5022–5027. doi: 10.1073/pnas.0931263100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton SR, Bobrowicz P, Bobrowicz B, Davidson RC, Li H, Mitchell T, et al. Production of complex human glycoproteins in yeast. Science. 2003;301:1244–1246. doi: 10.1126/science.1088166. [DOI] [PubMed] [Google Scholar]
- 28.Bobrowicz P, Davidson RC, Li H, Potgieter TI, Nett JH, Hamilton SR, et al. Engineering of an artificial glycosylation pathway blocked in core oligosaccharide assembly in the yeast Pichia pastoris: production of complex humanized glycoproteins with terminal galactose. Glycobiology. 2004;14:757–766. doi: 10.1093/glycob/cwh104. [DOI] [PubMed] [Google Scholar]
- 29.Gerngross TU. Advances in the production of human therapeutic proteins in yeasts and filamentous fungi. Nature Biotechnol. 2004;22:1409–1414. doi: 10.1038/nbt1028. [DOI] [PubMed] [Google Scholar]
- 30.Wildt S, Gerngross TU. The humanization of N-glycosylation pathways in yeast. Nat Rev Microbiol. 2005;3:119–128. doi: 10.1038/nrmicro1087. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton SR, Davidson RC, Sethuraman N, Nett JH, Jiang Y, Rios S, Bobrowicz P, et al. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science. 2006;313:1441–1443. doi: 10.1126/science.1130256. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Sethuraman N, Stadheim TA, Zha D, Prinz B, Ballew N, et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nature Biotechnol. 2006;24:210–215. doi: 10.1038/nbt1178. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs PP, Geysens S, Vervecken W, Contreras R, Callewaert N. Engineering complex-type N-glycosylation in pichia pastoris using GlycoSwitch technology. Nat Protocols. 2009;4:58–70. doi: 10.1038/nprot.2008.213. [DOI] [PubMed] [Google Scholar]
- 34.Potgieter TI, Cukan M, Drummond JE, Houston-Cummings NR, Jiang Y, Li F, et al. Production of monoclonal antibodies by glycoengineered Pichia pastoris. J Biotechnol. 2009;139:318–325. doi: 10.1016/j.jbiotec.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Barnard GC, Kull AR, Sharkey NS, Shaikh SS, Rittenhour AM, Burnina I, et al. High-throughput screening and selection of yeast cell lines expressing monoclonal antibodies. J Ind Microbiol Biotechnol. 2010;37:961–971. doi: 10.1007/s10295-010-0746-1. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Li F, Zha D, Potgieter T, Mitchell T, Moore R, et al. Purification process development of a recombinant monoclonal antibody expressed in glycoengineered Pichia pastoris. Protein Expr Purif. 2011;76:7–14. doi: 10.1016/j.pep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Pinckard RN, Weir DM, McBride WH. Factors influencing the immune response. I. Effects of the physical state of the antigen and of lymphoreticular cell proliferation on the response to intravenous injection of bovine serum albumin in rabbits. Clin Exp Immunol. 1967;2:331–341. [PMC free article] [PubMed] [Google Scholar]
- 38.Moore WV, Leppert P. Role of aggregated human growth hormone (hGH) in development of antibodies to hGH. J Clin Endocrinol Metabolism. 1980;51:691–697. doi: 10.1210/jcem-51-4-691. [DOI] [PubMed] [Google Scholar]
- 39.Robbins DC, Cooper SM, Fineberg SE, Mead PM. Antibodies to covalent aggregates of insulin in blood of insulin-using diabetic patients. Diabetes. 1987;36:838–841. doi: 10.2337/diab.36.7.838. [DOI] [PubMed] [Google Scholar]
- 40.Frost H. Antibody-mediated side effects of recombinant proteins. Toxicology. 2005;209:155–160. doi: 10.1016/j.tox.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 41.Arakawa T, Philo JS, Ejima D, Tsumoto K, Arisaka F. Aggregation analysis of therapeutic proteins, Part 1: General aspects and techniques for assessment. Bioprocess Int. 2006;4:32–43. [Google Scholar]
- 42.Arakawa T, Philo JS, Ejima D, Tsumoto K, Arisaka F. Aggregation analysis of therapeutic proteins, Part 2: Analytical Ultracentrifugation and dynamic light scattering. Bioprocess Int. 2007;5:36–50. [Google Scholar]
- 43.Krishnamurthy R, Sukumar M, Das TK, Lacher NA. Emerging analytical technologies for biotherapeutics development. BioProcess Int. 2008;6:32–43. [Google Scholar]
- 44.Cohen SL, Price C, Vlasak J. β-elimination and peptide bond hydrolysis: two distinct mechanisms of human IgG1 hinge fragmentation upon storage. J Am Chem Soc. 2007;129:6976–6977. doi: 10.1021/ja0705994. [DOI] [PubMed] [Google Scholar]
- 45.Santora LC, Krull IS, Grant K. Characterization of recombinant human monoclonal tissue necrosis factor-α antibody using cation-exchange HPLC and capillary isoelectric focusing. Anal Biochem. 1999;275:98–108. doi: 10.1006/abio.1999.4275. [DOI] [PubMed] [Google Scholar]
- 46.Harris RJ, Kabakoff B, Macchi FD, Shen FJ, Kwong M, Andya JD, et al. Identification of multiple sources of charge heterogeneity in a recombinant antibody. J Chrom B. 2001;752:233–245. doi: 10.1016/s0378-4347(00)00548-x. [DOI] [PubMed] [Google Scholar]
- 47.Mario N, Baudin B, Aussel C, Giboudeau J. Capillary isoelectric focusing and high-performance cation-exchange chromatography compared for qualitative and quantitative analysis of hemoglobin variants. Clin Chem. 1997;43:2137–2142. [PubMed] [Google Scholar]
- 48.Vlasak J, Bussat MC, Wang S, Wagner-Rousset E, Schaefer M, Klinguer-Hamour C, et al. Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal Biochem. 2009;392:145–154. doi: 10.1016/j.ab.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 49.Li N, Kessler K, Bass L, Zeng D. Evaluation of the iCE280 Analyzer as a potential high-throughput tool for formulation development. J Pharm Biomed Anal. 2007;43:963–972. doi: 10.1016/j.jpba.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Gaza-Bulseco G, Xiang T, Chumsae C. Structural effect of deglycosylation and methionine oxidation on a recombinant monoclonal antibody. Mol Immunol. 2008;45:701–708. doi: 10.1016/j.molimm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Lam XM, Yang JY, Cleland JL. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER2. J Pharm Sci. 1997;86:1250–1255. doi: 10.1021/js970143s. [DOI] [PubMed] [Google Scholar]
- 52.Chumsae C, Gaza-Bulseco G, Sun J, Liu H. Comparison of methionine oxidation in thermal stability and chemically stressed samples of a fully human monoclonal antibody. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850:285–294. doi: 10.1016/j.jchromb.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 53.Bertolotti-Ciarlet A, Wang W, Lownes R, Pristatsky P, Fang Y, McKelvey T, et al. Impact of methionine oxidation on the binding of human IgG1 to FcRn and Fc γ receptors. Mol Immunol. 2009;46:1878–1882. doi: 10.1016/j.molimm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Vlasak J, Li Y, Pristatsky P, Fang Y, Pittman T, et al. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol Immunol. 2011;48:860–866. doi: 10.1016/j.molimm.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Pan H, Chen K, Chu L, Kinderman F, Apostol I, Huang G. Methionine oxidation in human IgG2 Fc decreases binding affinities to protein A and FcRn. Protein Sci. 2009;18:424–433. doi: 10.1002/pro.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han M, Phan D, Nightlinger N, Taylor L, Jankhah S, Woodruff B, et al. Optimization of CE-SDS method for antibody separation based on multi-users experimental practices. Chromatographia. 2006;64:335–342. [Google Scholar]
- 57.Rustandi RR, Washabaugh MW, Wang Y. Applications of CE SDS gel in development of biopharmaceutical antibody-based products. Electrophoresis. 2008;29:3612–3620. doi: 10.1002/elps.200700958. [DOI] [PubMed] [Google Scholar]
- 58.Ha S, Ou Y, Vlasak J, Li Y, Wang S, Vo K, et al. Isolation and characterization of IgG1 with asymmetrical Fc glycosylation. Glycobiology. 2011;21:1087–1096. doi: 10.1093/glycob/cwr047. [DOI] [PubMed] [Google Scholar]
- 59.Huhn C, Selman MHJ, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics. 2009;9:882–913. doi: 10.1002/pmic.200800715. [DOI] [PubMed] [Google Scholar]
- 60.Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21:11–16. doi: 10.1021/bp040016j. [DOI] [PubMed] [Google Scholar]
- 61.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364:8–18. doi: 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 62.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li H, Miele RG, Mitchell TI, Gerngross TU. N-linked glycan characterization of heterologous proteins. Methods Mol Biol. 2007;389:139–150. doi: 10.1007/978-1-59745-456-8_10. [DOI] [PubMed] [Google Scholar]
- 64.Gong B, Cukan M, Fisher R, Li H, Stadheim TA, Gerngross T. Characterization of N-linked glycosylation on recombinant glycoproteins produced in Pichia pastoris using ESI-MS and MALDI-TOF. In: Packer NH, Karlsson NG, editors. Methods in Molecular Biology, Glycomics: Methods and Protocols. Vol. 534. Humana Press; 2009. pp. 213–223. [DOI] [PubMed] [Google Scholar]
- 65.Zhang N, Liu L, Dumitru CD, Cummings NRH, Cukan M, Jiang Y, et al. Glycoengineered Pichia produced anti-HER2 is comparable to trastuzumab in preclinical study. mAbs. 2011;3:289–298. doi: 10.4161/mabs.3.3.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma S, Nashabeh W. Carbohydrate analysis of a chimeric recombinant monoclonal antibody by capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1999;71:5185–5192. doi: 10.1021/ac990376z. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Salas-Solano O, Gennaro LA. Investigation of sample preparation artifacts formed during the enzymatic release of N-linked glycans prior to analysis by capillary electrophoresis. Anal Chem. 2009;81:6823–6829. doi: 10.1021/ac9010588. [DOI] [PubMed] [Google Scholar]
- 68.Duman JG, Miele RG, Liang H, Grella DK, Sim KL, Castellino KJ, et al. O-mannosylation of Pichia pastoris cellular and recombinant proteins. Biotechnol Appl Biochem. 1998;28:39–45. [PubMed] [Google Scholar]
- 69.Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, et al. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J Biol Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- 70.Sasaki T, Yamada H, Matsumura K, Shimizu T, Kobata A, Endo T. Detection of O-mannosyl glycans in rabbit skeletal muscle alpha-dystroglycan. Biochim Biophys Acta. 1998;1425:599–606. doi: 10.1016/s0304-4165(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 71.Smalheiser NR, Haslam SM, Sutton-Smith M, Morris HR, Dell A. Structural analysis of sequences O-linked to mannose reveals a novel Lewis X structure in cranin (dystroglycan) purified from sheep brain. J Biol Chem. 1998;273:23698–23703. doi: 10.1074/jbc.273.37.23698. [DOI] [PubMed] [Google Scholar]
- 72.Lommel M, Strahl S. Protein O-mannosylation: conserved from bacteria to humans. Glycobiology. 2009;19:816–828. doi: 10.1093/glycob/cwp066. [DOI] [PubMed] [Google Scholar]
- 73.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 74.Kim H, Yamaguchi Y, Masuda K, Matsunaga C, Yamamoto K, Irimura T, et al. O-glycosylation in hinge region of mouse immunoglobin G2b. J Biol Chem. 1994;269:12345–12350. [PubMed] [Google Scholar]
- 75.Arnold JN, Radcliffe CM, Wormald MR, Royle L, Harvey DJ, Crispin M, et al. The glycosylation of human serum IgD and IgE and the accessibility of identified oligomannose structures for interaction with mannan-binding lectin. J Immunol. 2004;173:6831–6840. doi: 10.4049/jimmunol.173.11.6831. [DOI] [PubMed] [Google Scholar]
- 76.Martinez T, Pace D, Brady L, Gerhart M, Balland A. Characterization of a novel modification on IgG2 light chain evidence for the presence of O-linked mannosylation. J Chrom A. 2007;1156:183–187. doi: 10.1016/j.chroma.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 77.Girrbach V, Strahl S. Members of the evolutionarily conserved PMT family of protein O-mannosyltransferases form distinct protein complexes among themselves. J Biol Chem. 2003;278:12554–12562. doi: 10.1074/jbc.M212582200. [DOI] [PubMed] [Google Scholar]
- 78.Kuroda K, Kobayashi K, Kitagawa Y, Nakagawa T, Tsumura H, Komeda T, et al. Efficient antibody production upon suppression of O mannosylation in the yeast Ogataea minuta. Appl Environ Microbiol. 2008;74:446–453. doi: 10.1128/AEM.02106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stadheim TA, Li H, Kett W, Burnina IN, Gerngross TU. Use of high-performance anion exchange chromatography with pulsed amperometric detection for O-glycan determination in yeast. Nat Protocols. 2008;3:1026–1031. doi: 10.1038/nprot.2008.76. [DOI] [PubMed] [Google Scholar]
- 80.Wang S, Ionescu R, Peekhaus N, Leung J, Ha S, Vlasak J. Separation of post-translational modifications in monoclonal antibodies by exploiting subtle conformational changes under mildly acidic conditions. J Chrom A. 2010;1217:6496–6502. doi: 10.1016/j.chroma.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 81.Ionescu R, Vlasak J, Price C, Kirchmeier M. Contribution of variable domains to the stability of humanized IgG1 monoclonal antibodies. J Pharm Sci. 2008;97:1414–1426. doi: 10.1002/jps.21104. [DOI] [PubMed] [Google Scholar]
- 82.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki E, Niwa R, Saji S, Muta M, Hirose M, Iida S, et al. A nonfucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clin Cancer Res. 2007;13:1875–1882. doi: 10.1158/1078-0432.CCR-06-1335. [DOI] [PubMed] [Google Scholar]