Abstract
Due to their excellent specificity for a single epitope, monoclonal antibodies (mAbs) present a means of influencing the function of cells at the molecular level. In particular they show great promise in the treatment of cancer because they can inhibit cancer cell proliferation, tumor angiogenesis, invasiveness and malignant spread of cancerous cells. Many mAbs are in various stages of testing and 11 are currently marketed in the US or Europe for the treatment of cancers that express particular antigens such as human epidermal growth factor receptor-2, CD20, epidermal growth factor receptor and vascular endothelial growth factor. Strategies to conjugate mAbs to toxins, radioactive isotopes and chemotherapeutic drugs to improve efficacy are under intense investigation and numerous immunoconjugates have been studied in the clinical setting. However, the molecules have limitations, and so nanomaterials (NMs), which potentially offer more flexibility of design and functionality in providing platforms for binding of multiple therapeutic agents in a single structure, are being examined as an alternative. Studies utilizing mAb-targeted NMs have shown that they exhibit focused targeting, improved pharmacokinetics and improved “passive” drug delivery via leaky vasculature. Nevertheless, before they can be utilized to treat cancer, potential NM toxicity must be thoroughly investigated. Thus, rigorous testing of NM-mAb conjugates in both in vitro and in vivo systems is underway to determine how NM-mAb conjugates will interact with cells and tissues of the body. In this review, we discuss the broad range of nanomaterials that are under investigation as potential platforms for the presentation of mAbs either as single therapeutics or in combination with other drugs and their advantages and limitations in specifically targeting cancer.
Key words: cancer, immunotherapy, nanomaterial, nanomedicine
Introduction
With the development of a technique to produce monoclonal antibodies (mAbs) in 1975, cancer cell-specific treatment became possible.1 Thirty five years later, mAb-mediated therapies have gained widespread acceptance, due in part to the successful development of antibody-based cancer therapies. A total of 27 mAb therapeutics, 11 of which are cancer treatments, are marketed in the US or Europe, and global sales of mAbs in 2010 were over $40 billion. Scientists are now able to evaluate the potential of mAbs as immunotherapeutic drugs, while also relating their physical properties, mechanisms of action, and how the characteristics of target antigens determine efficacy, to improve the clinical value of mAb-based therapies. Toward this end, mAbs are being developed as targeted vehicles, combining the actions of mAbs with chemotherapy and radiotherapy.2–4 An extension of this is the study of antibody-modified nanomaterials (NMs), which offer the promise of selective drug delivery to tumor cells, including internalization and intracellular therapeutic agent release within targeted cells.2
Although the last 50 years has seen remarkable progress in the prevention, detection and treatment of cancer, the most common methods (i.e., radiation, surgery and chemotherapy) often result in serious side effects.5–7 Additional deficits of current cancer therapies include non-specific systemic distribution, non-specific suppression of rapidly dividing cell types, inadequate drug concentrations at target tissues (i.e., tumors or cancerous cells), multi-drug resistance and a limited ability to monitor therapeutic responses.5–9
Two major goals in the development of improved anti-cancer therapies are greater targeting selectivity and better delivery efficiency.10 An ideal anti-cancer therapeutic would be one that can be selectively concentrated in cancer cells while exerting minimal effects on normal tissues.10 To achieve this, scientists are exploring biological molecules such as mAbs designed to target receptors on cancer cells or ligands relevant to cancer pathways that will facilitate delivery of cytotoxins, radioactive isotopes or chemotherapeutic drugs. The mAbs approved by the US Food and Drug Administration for cancer treatment are listed in Table 1. The approach of conjugating bio-active anti-cancer molecules to mAbs has some limitations, e.g., low drug to mAb conjugation ratios. Increasingly, researchers are examining NMs to overcome some of the shortcomings of immunoconjugates.
Table 1.
Monoclonal antibodies approved by the US food and drug administration for the treatment of cancer
| Year | International non-proprietary name/Trade name | Target | Indication |
| 1997 | Rituximab/Rituxan | CD20 | B-cell lymphoma |
| 1998 | Trastuzumab/Herceptin | HER2 | Breast cancer |
| 2001 | Alemtuzumab/Campath | CD52 | Chronic lymphocytic leukemia |
| 2002 | Ibritumomab tiuxetan/Zevalin | CD20 | B-cell lymphoma |
| 2003 | Tositumomab/Bexxar | CD20 | B-cell lymphoma |
| 2004 | Bevacizumab/Avastin | VEGF | Colon, lung, breast and renal cancer |
| 2004 | Cetuximab/Erbitux | EGFR | Colon; lung cancer |
| 2006 | Panitumumab/Vectibix | EGFR | Colon cancer |
| 2009 | Ofatumumab/Arzerra | CD20 | Chronic lymphocytic leukemia |
| 2011 | Ipilimumab/Yervoy | CTLA-4 | Melanoma |
Note: The immunoconjugate gemtuzumab ozogamicin (Mylotarg®) was approved by the US Food and Drug Administration in 2000 and withdrawn from marketing in 2010. Catumaxomab (Removab®) was approved in the European Union in 2009.
In this review, we discuss the limitations of immunoconjugates as treatments for cancer, the advantages that NMs confer compared to immunoconjugates and the classes of NMs that have been used together with mAbs for targeted treatment of cancer. We also explain methods of functionalization for several types of nanomaterials with mAbs and present results from studies that have used mAbs as novel targeting agents for NMs.
Challenges to Conjugating a Drug/Toxin/Isotope to mAbs
A number of factors must be considered when designing methods to conjugate drugs and other therapeutic molecules to mAbs. The chemotherapeutic agents may be antimetabolites, alkylating agents, intercalating drugs or microtubule inhibiting drugs. Most of these agents possess complex chemical structures designed to act on their target so that they inhibit crucial biological functions and ultimately cause cell death.11 Some of these drugs cannot be chemically modified appropriately for conjugation with cancer-targeting mAbs.11 Thus, in designing drug antibody conjugates consideration must be given to the relationship between biological activity and the structure of the anti-cancer drugs and cancer-targeting mAbs. Other factors that should be carefully considered when designing drug-antibody conjugates include the potential for toxicity to non-target cells and tissues, the stability of the conjugates, both in vitro and in vivo, and retention of specificity and activity of mAbs after conjugation.
When conjugated to mAbs, anti-cancer drugs are most often coupled to either a carboxylic or amino group. The steps involved in conjugation of chemotherapeutic agents to mAbs often give rise to a neutral linkage and so the solubility of the antibody may decrease, resulting in aggregation and precipitation.11 Also, careful attention should be given to the number of drug residues conjugated to each mAb. Ideally, the ratio of drug residues per mAb should be maximized, while still retaining an acceptable level of activity and specificity of the mAb. This factor places a limit on the amount of therapeutic agent that can be bound to antibody. The complexity of immunoconjugate development is one reason mAb-targeted NM drug delivery agents are currently under intense investigation.12
Nanomaterials
“Nano” derives from the Greek noun nanos, meaning dwarf.13 A nanometer (nm) is one billionth of a meter; the width of a DNA molecule is approximately 2.5 nm, the width of cell membranes in the range of 6–10 nm, and the dimensions of proteins range from 1.0–20 nm. Although nature has worked on the nano-scale for millennia, it has been only in the last several decades that NMs have come to play an increasingly important role in commercial development. Indeed, we may expect to see many revolutionary breakthroughs with a potential major impact on the overall world economy from advances in nanotechnology. All told, nanotechnologies are estimated to have affected $251 billion of the global economy in 2009, and this value is estimated to grow to $2.4 trillion by 2015.14
The small size and corresponding large specific surface area of nano-sized materials confers specific properties to them.15,16 The importance of the type of NM and its surface area becomes evident when considering that surface atoms or molecules play a dominant role in determining bulk properties; the ratio of surface to total atoms or molecules increases exponentially with decreasing particle size.17 Increased surface reactivity predicts that NMs exhibit greater biologic activity per given mass compared with larger particles. It is for this reason that NMs display significantly different properties when compared to the same bulk material and it is primarily this factor that opens up the opportunity for their novel utilization and application.15,18
Medical Applications of Nanomaterials
Perhaps the greatest promise that nanotechnology presents is in its many potential applications in the field of medicine. The development of nano-scale medical technologies, which have been designated “nanomedicines” by the National Institutes of Health, show extraordinary promise and have already begun to change the way diseases are treated or prevented.16 Advances in nanotechnology have provided ways to design cell-type specific delivery systems that can deliver therapeutic drugs and biological molecules more effectively.19–21 Through precise engineering of atoms and molecules, nanomedicine promises to produce new molecular assemblies on the scale of individual cells and cellular organelles that could aid in the development of NM-based “personalized medicines” appropriate for an individual's particular disease and genome.22,23
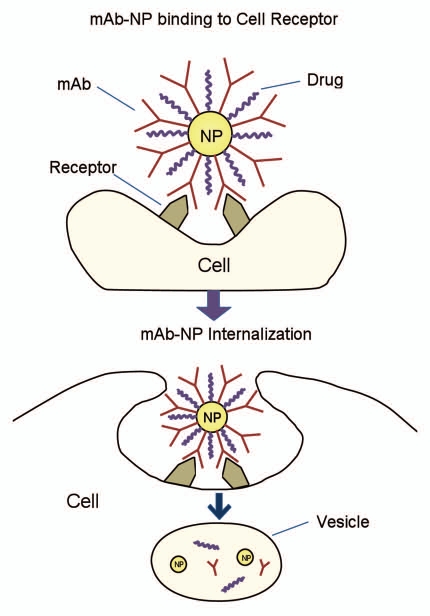
Nanomaterial-based drug delivery systems can be developed using a variety of different nanomaterials including organic nano-assemblies (e.g., polymeric nanoparticles), lipid systems (e.g., liposomes, emulsions), dendrimers, carbon nanostructures (e.g., nanotubes, fullerenes) and self-assembled micelles (Table 2), although inorganic nanostructures made of silicon, silver and gold NMs can also be used.24 Through the precise engineering of atoms and molecules, nanomedicine promises to yield new molecular assemblies on the scale of individual cells, organelles or even smaller components, providing targeted personalized medicines. The basis of nanoparticle (NP)-based, mAb-directed drug delivery is illustrated in Figure 1. The concept is that NP form a platform to which other biomolecules can be simultaneously linked while remaining minimally affected, i.e., with retention of their original biological properties.
Table 2.
Types of nanomaterials utilized in the treatment of cancer, their advantages and limitations
| Class of nanomaterial | Advantages | Limitations | Materials often utilized to construct nanomaterial |
| Liposomes | Amphiphilic, generally biocompatible, protect drugs from degradation | Large size, limited stability | Phosphatidylethanolamine, phosphatidylcholine, dioleoylphosphatidylethanolamine |
| Polymeric | Easily modified, some are biodegradable | Large scale uniform production is difficult, some exhibit cytotoxicity | PEG, PMLA, PGA, PLGA, block copolymers |
| Micelles | Easily modified, generally small size, biocompatible | Limited stability | Polyoxyethylene, polyoxypropylene, phosphatidylethanolamine |
| Metals | Unique optical properties, easily functionalized | Not biodegradable, tend to agglomerate when exposed to physiological environment | Gold, silver, platinum, copper |
| Non-metals | Stable and resistant to environmental changes, some possess unique physical properties | Generally not biodegradable, some have exhibited cytotoxic effects | Silica, carbon |
Figure 1.
Cellular uptake of mAb conjugated nanoparticle. mAb conjugated nanoparticles can be recognized by receptors on the cell membrane. Thus the nanoconjugates are internalized and trafficked along intracellular retrograde transport pathways.
Antibody-Nanomaterial Linkers
Factors such as attaching or encapsulating a molecule, drug or mAb to a NM and selection of an appropriate linker that facilitates attachment and release of the payload at the correct tissue or sub-cellular site are major determinants of potential NM-mediated efficacy. Therefore, the choice of linker or conjugation method is exceedingly important in the engineering of antibody-targeted NM-based drug delivery systems.25 In general, many of the linkers and conjugation techniques that are used to construct such systems have been adapted from those that are used to create immunoconjugates.25
New conjugation approaches to linking therapeutic agents to mAb-targeted NMs is a rapidly advancing field. However, three common types of linkers are most often employed, and each type can be categorized by their mechanism of cleavage. Hydrazone linkers are susceptible to an acidic pH,25–27 disulfide linkers cleave when exposed to a reducing environment25,28 and peptide linkers are cleaved in the presence of proteases.26,29 All three types of linkers are intended to exploit a biochemical process or internal environment in cancer cells. For example, mAb-targeted NM drug carriers that are meant to release their payloads in the lysosome should be designed using hydrazone linkers that respond to the low pH of lysosomic vesicles.25,30
Another method of conjugation that has been used for linking nucleic acids such as siRNA to NMs capitalizes on the strong binding affinity between avidin/streptavidin and biotin. This type of conjugation was made possible by studies that demonstrated that the effectiveness of siRNA mediated gene silencing is not inhibited when the sense strand is biotinylated and conjugated to avidin or streptavidin. In particular, Xia and colleagues31,32 have shown the potential of avidin/biotin linker technology by demonstrating uptake across the blood brain barrier of biotinylated siRNA linked to a mAb (with avidin bound to it) designed against the insulin receptor.
Utilization of Antibody-Targeted Nanomaterial-Based Drug Delivery
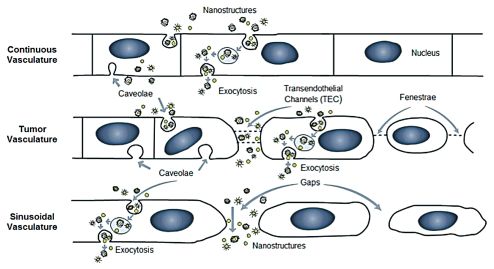
NMs show a high drug loading efficiency while additionally being able to protect the therapeutic agent from premature release and degradation12 and, since NMs can be functionalized with multiple therapeutic agents, they may be able to circumvent multidrug resistance in cancers.12 Malignant cancers have an increased level of angiogenesis and leaky vasculature with pore sizes that range from 200–600 nm in diameter,33 and therefore NM-based drug delivery systems that preferentially accumulate in tumors may allow for more effective “passive” drug delivery.34 This NM facilitated delivery of bioactive mAbs and drugs to tumors has great potential due to the well-known enhanced permeability and retention (EPR) effect.36,37 This mechanism, due to the combination of “leaky” tumor vasculature and poor tumor drainage via the lymphatics, provides a distinct advantage for nanoconjugates. However, it should also be noted that, even within an individual, the range of tumor permeability can be highly variable and some cases, e.g., a large tumor with a necrotic center, may not exhibit an EPR effect. This may suggest that there will be roles for combined therapies, e.g., immunoconjugate plus nanoconjugate, to provide the greatest efficacy. Of course, multiple therapeutic approaches to cancer treatment have been the rule, and as nanoconjugates are tested and proven, they will be added to the therapeutic “arsenal” available to oncologists. Figure 2 shows the range of vascular types and the typical pore sizes of each and provides a notional guide to the potential for NM mediated mAb and drug delivery to tumors. Thus, to design effective NP-based drug delivery, mechanism of escape from the vasculature (extravasation) and into the target tissue is an important consideration.
Figure 2.
Possible mechanisms of nanostructure extravasation. In the case of a continuous vascular endothelial barrier, nanostructures may be internalized by endothelial cells, e.g., via caveolae, transported via trans-cellular transport mechanisms and escape into the extracellular space via exocytosis. It is generally accepted that extravasation through tight gap junctions is limited to molecules or particles smaller than 10 nm.33,112 In the case of tumor vasculature, nanostructures may also escape the vasculature via transendothelial channels (TEC) and fenestrae. Molecules or particles up to 100 nm diameter may escape the vasculature via this route.1 Where the vasculature is sinusoidal/discontinuous (e.g., liver, spleen), nanostructures and large molecules may readily escape the vasculature.
Although both mAbs and NM have been available for three to four decades, only recently have resources to underpin studies in linking the technologies for the development of therapeutics become more available. Balanced against the advantages of NM noted above, e.g., protecting the therapeutic agent from degradation,12 there are disadvantages that will need to be considered in approaches to nanoconjugate design. The increased size of nanoconjugates compared with immunoconjugates renders them potentially more likely to be cleared by the reticulo-endothelial system (RES) or inhibits their extravasation and access to target tissues.33 Orientation of conjugated mAbs in the complex also affects this clearance mechanism. Thus, Fc-directed conjugation is a strategy that can reduce RES-mediated clearance.35 Another factor that will require attention in nanoconjugate design is the inclusion of surface chemistries that inhibit NM aggregation. Thus, there are multiple challenges to the development of successful nanoconjugates and researchers are beginning to address these questions.
With this brief overview of the potential strengths and weaknesses of NM mediated mAb and drug delivery, we describe in the following text several common types of NMs and provide some examples of mAb-targeted NM-based drug delivery systems.
Liposomes
One of the most highly investigated classes of NM-based drug delivery carriers are liposomes.36,37 Liposomes are artificial vesicles composed of a phospholipid monolayer that varies in size from 50–1,000 nm and can be loaded with a variety of water-soluble drugs in their inner aqueous compartment. Water insoluble drugs may also be incorporated if the liposome possesses a hydrophobic compartment (liposomes usually, but not by definition, contain a core of aqueous solution).36 Some examples of molecules utilized in the production of liposomes include phosphatidylethanolamine, phosphatidylcholine, dioleoylphosphatidylethanolamine and cholesterol.
Liposomes have several advantages compared with other drug delivery systems. Specifically, they are not usually immunogenic and drugs encapsulated into liposomes are protected from degradation by external media.23 However, the majority of liposome formulations rely on non-specific “passive” targeting, making them less than ideal for delivering drugs that are designed to act on a particular tissue type. Also, liposomes have been shown to be toxic to cells if administered in the large concentrations that are often necessary when relying on passive targeting methods.38 Furthermore, liposomes are often large (up to 1,000 nm) making them a poor choice for targeting peripheral tissues.39,40
However, an effective strategy is the use of mAb-targeted “stealth” immunoliposomes (SIL), which utilize a coating of poly-ethylene glycol (PEG) to facilitate circulation of liposomes for days as stable constructs, ultimately providing a higher degree of passive targeting to tumor tissue. MCC-465, a SIL encapsulating doxorubicin (DXR) (a chemotherapeutic drug) was evaluated in a Phase 1 clinical study.41 The stealth liposome utilized the IgG1 F(ab)2 fragment of the human mAb GAH, which positively reacts to greater than 90% of cancerous stomach tissues but negatively to normal tissues.41 Although no antitumor response was observed in patients treated with the MCC-465, stable disease was observed in 55% of those treated.41
Cheng et al.42 studied SIL-DXR conjugates targeted against the B-cell antigen CD19, via a murine IgG1 mAb HD37, the Fab fragment of the same mAb, and a Fv single chain fragment of HD37.42 Their results indicated that the stealth liposomes targeted with the HD37 Fab fragment showed the greatest efficacy (2.5-fold increase) in prolonging the lifespan of mice implanted with tumors compared with mice treated with stealth liposomes loaded with DXR alone.42 SIL-DXR targeted with the whole and single chain Fv fragment HD37 mAbs only doubled the life expectancy of treated mice compared with mice administered SIL-DXR alone.42 The authors suggested that the Fv single chain mAb was cleared due to increased liver uptake, while the whole mAb was cleared rapidly from circulation due to Fc-mediated uptake in the liver and spleen.42
Several in vitro studies have utilized mAb-targeted liposomes (immunoliposomes). Kirpotin et al. demonstrated internalization of immunoliposomes bearing trastuzumab (Herceptin®), which targets human epidermal growth factor receptor (HER-2). Their results indicate that internalization was due to the specificity of the mAb coupled to the liposomes because control liposomes lacking trastuzumab showed little internalization by HER-2+ cells. The authors also found that immunoliposomes appeared to induce a high anti-proliferative effect that was superior to that of trastuzumab only.
A study with liposomes bearing the AR-3 mAb showed that these liposomes were highly effective as an anti-tumor agent and induced less systemic toxicity compared to the free drug alone.44 Huwyler et al. utilized liposomes conjugated with a mAb targeting the transferrin (TF) receptor to deliver chemotherapeutic drugs across the blood-brain barrier and into brain cancer cells.45 Xu et al.46 utilized a similar approach by using anti-TF mAbs to target liposomes encapsulating plasmids containing the p53 tumor suppressor gene to cancer cells. Delivery of these plasmids resulted in the sensitization of the transfected cancer cells to ionizing radiation.
Cetuximab (Erbitux®) immunoliposomes (ILs) for delivery of boron compounds as a means to administer targeted radiation therapy to epidermal growth factor receptor (EGFR)-positive glioma cells was recently reported by Pan et al. These studies used immunoliposomes based primarily on cholesterol and, like several others, have also used PEG as a stabilizing agent. The boron payload delivery was greatly improved as boron uptake into the target cells was increased 8-fold in the cetuximab-targeted treatment compared with the non-targeted liposome treatment.
Polymeric Nanomaterials
Nano-sized natural and synthetic organic polymers are also attractive as potential drug delivery systems48–50 because polymeric NM constructs may be conjugated to therapeutic agents by means of chemical linkers that are stable in blood, but labile in the acidic or enzymatic conditions typical of diseased tissues such as tumors.51 Most often, polymeric NMs are spherical in shape and have a size distribution varying from 1–1,000 nm.52 Depending on the properties of the polymer used, the means of encapsulation of therapeutic agents varies; for example, the majority of positively charged cationic polymers have been found to be useful in the encapsulation of plasmids.52 Nucleic acids can also be captured and incorporated into the matrix of polymeric NMs, and they can be adsorbed or conjugated to the surface of polymeric NMs using certain chemical modifications and linkers.48,52–54
Polymers used as drug conjugates can be divided into two groups: natural and synthetic. Examples of natural polymers include chitosan and heparin.52 Among synthetic polymers, N-(2-hydroxypropyl)-methacrylamide copolymer (HPMA), polystyrene-maleic anhydride copolymer, polyethylene glycol (PEG), poly-l-glutamic acid, (PGA), polylactic acid (PLA), poly(β-L-malic acid) (PMLA), are some of the most widely utilized.52
Albumin, a natural protein, has been complexed with paclitaxel to form a polymer nanoconjugate with a particle size of 130 nm.55 This formulation, termed ABI-007, has advanced to clinical studies and is used in the treatment of non-hematologic malignancies56 and metastatic breast cancer.53 Another approach used albumin with trastuzumab to target overexpressing HER-2 breast cancer cells.57 The payload, DXR, has dose limiting side effects because of its non-specific actions and high toxicity in all fast-proliferating tissues. However, by including trastuzumab, tumor cell targeting appeared to be improved, suggesting that further study of this approach is warranted.
Wang et al.58 used a streptavidin NP derived from bacterial proteins and showed an increase of payload in HER-2 over-expressing cells when treated with a NP-trastuzumab-labeled molecule. Both a fluorescent label (Lissamine) and a radio label (99mTc) were used to demonstrate cellular uptake. Interestingly, the results suggested that these NP do not require a stabilization agent because of their surface chemistry.
Results for a mAb-targeted synthetic polymer NP (immunopolymer NP) were reported by Fujita et al.59 These investigators conjugated two different mAbs (OX26, a mouse anti-TF receptor mAb; 2C5, a mouse anti-nucleosome mAb) onto PLA to construct a dual-targeted immunopolymer NP.59 Antisense oligonucleotides to vascular protein laminin-8 mRNA, a vascular basement membrane component that is overexpressed in some human brain tumors were also conjugated to the immunopolymer NP. Results from in vitro and in vivo fluorescent detection studies showed that tumor targeting for immunopolymer NPs that utilized both the OX26 and 2C5 mAbs more readily accumulated in target tissues, suggesting improved efficacy for tandem configuration of antibodies than for single configurations carried by the immunopolymer NPs.59
Another study that utilized PLA immunopolymers to treat cancer was conducted by Huang et al. In this study, an anti-vascular endothelial growth factor (VEGF) mAb was conjugated to PLA NPs loaded with the chemotherapeutic agent 5-FU. Nude mice with human gastric carcinoma xenografts were then treated with the 5-FU-loaded immunopolymer NPs. Results from these in vivo experiments led the investigators to conclude that 5-FU loaded immunopolymer NPs can increase the tumor inhibitory rate of 5-FU and induce apoptosis by inhibiting tumor angiogenesis (via the action of the anti-VEGF mAb) with fewer side effects compared to 5-FU treatment alone.
In 2006, Nobs et al. reported results for a construct combining trastuzumab with PLA as a NP base. A fluorescent label was used to facilitate tracking to effectively follow the conjugates into the specific tissues. A 10-fold increase of payload delivery to targeted tissues versus control (irrelevant IgG-PLA) was observed.
In 2009, Kos et al. developed an immunopolymer NP by conjugating an anti-cytokeratin mAb to cysteine protease inhibitor (cystatin)-loaded poly(lactic-co-glycolic acid) (PLGA) NPs.62 Cytokeratin is expressed on the invasive breast cancer cell line MCF-10A neoT, but not present on the surface of the colorectal cancer cell line CACO-2.62 Furthermore, binding of the anti-cytokeratin mAb reduced the cellular secretion of plasmin, a key extracellular enzyme involved in cell adhesion, invasion and signaling of breast tumor cells.62 Cystatin is known to inactivate cathepsin B, which was shown to be involved in an important tumor promoting factor for progression to malignant disease.62 Results from in vitro studies indicated that the cystatin-loaded PLGA immunopolymer NPs specifically bound to breast cancer cells expressing cytokeratin, inhibited plasmin generation, were readily internalized, and promoted cell adhesion (preventing malignant spread of cancerous cells) through cystatin inhibiting cathepsin B.62
More recently, PLGA was used in a formulation containing tetraiodothyroacetic acid, which binds an integrin receptor (the αVβ3 sub-type) to inhibit tumor cell growth in vitro.63 This form did not enter cells and had higher potency and a larger anti-proliferative effect compared to the free inhibitor. When used in combination with reversitrol and cetuximab, the effects were additive. This is a good example of a nanostructure having increased potency because it is precluded from cellular uptake, a scenario that is opposite from many others that require cell entry for efficacy.
Micelles
Micelles are colloidal dispersions that generally vary in size from 5–100 nm.23 Some of the common molecules used in the construction of micelles include polyoxyethylene, polyoxypropylene and phosphatidyl ethanolamine (PE). The functional properties of micelles are based on the action of detergents or amphiphilic polymers, which assemble to form nanosized structures in an aqueous environment that consist of a shell and core structure (similar to the plasma membrane of a cell). Unlike liposomes, micelles do not possess a hydrophilic core. Therefore, the core region serves as a place for the entrapment of hydrophobic drugs, whereas the hydrophilic shell serves to stabilize the hydrophobic core and allows the structure to be water-soluble, a factor that allows micelles to be stable in the blood and allows them to be administered intravenously.23,52 Hence, micelles are especially attractive for the potential delivery of hydrophobic chemotherapeutic agents that have limited efficacy due to poor solubility in the blood.23
Like polymeric NMs, therapeutic agents may be incorporated into micelles in two distinct ways: physical incorporation or through chemical covalent attachment.64,65 Due to their small size, micelles demonstrate spontaneous penetration into bodily structures with leaky vasculature (tumors and infarcts).66–69 However, unless micelles are conjugated to a particular targeting ligand, their uptake will be mediated via non-specific mechanisms.66
Roby et al. encapsulated the poorly soluble photosensitizing anti-cancer drug meso-tetraphenylporphine (TPP), an agent that brings about the formation of cytotoxic products when irradiated with light of a suitable wavelength, into immunomicelles.70 The immunomicelles were constructed from polyethylene glycol/phosphatidyl ethanolamine conjugate (PEG-PE) bearing the anti-nucleosome 2C5 mAb.70 In vitro experiments with the immunomicelle revealed that encapsulation and targeted delivery of TPP to cancer cells resulted in significantly improved anti-cancer effects of the drug under photo dynamic therapy conditions.70
Liao et al. synthesized immunomicelles conjugated with poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-PCL) that was used to attach cetuximab. These immunomicelles were loaded with DXR and superparamagnetic iron oxide (SPIO), both of which have been shown to be cytotoxic to cancer cells, as well as other cells of the body. Due to their hydrophobic nature, micelles are a good carrier for these two substances. With the addition of cell-specific targeting, these investigators were able to increase the amount of these toxic substances specifically delivered into the cancerous cells. The ability to carry drugs and MRI visible particles allows micelles to be used in imaging-guided cancer chemotherapy.72
Metal Nanomaterials
Metal NMs often exhibit surface-plasmon coupling that provides them with unique optical properties; thus, they may be readily monitored when administered in biological systems.73,74 For example, Raman spectroscopy can be used to detect and track most noble-metal NMs in a biological system.74 Due to these special optical properties, noble-metal NMs (gold in particular) have been used extensively in detecting nucleic acids and proteins. Oligonucleotide and siRNA-functionalized noble-metal NMs have also been developed for use in assays for the detection of biomarkers for diseases.75,76 Furthermore, noble-metal NMs have been extensively investigated as an alternative to liposome and polymer-mediated gene delivery.75,76
Metal NMs are relatively easy to prepare, in general have low toxicity, and they have a readily modified surface.76,77 For example, gold NPs that were functionalized using quaternary ammonium chains may have up to eight times higher transfection efficiency into mammalian cells compared with polymer NMs.75 Metal NMs can also be conjugated with polymers to improve their pharmacokinetic properties, e.g., half-life in the circulatory system. Systemic delivery of plasmid DNA has been demonstrated with polyethylene glycol (PEG)-modified gold nanoparticles.75 Surface functionalization of gold NMs using PEG has also been demonstrated to result in rapid cellular uptake and internalization.78 However, there still remain several concerns regarding the use of noble-metal NMs in vivo. Primarily, researchers are concerned over how noble-metal NMs will be eliminated from the body after they've carried out their therapeutic role. Because they are not biodegradable like most liposomes and polymers, the long-term effect of metal NMs on cellular functions will need to be carefully studied.79
Melancon et al. utilized gold nanorods (GNRs) targeted to cancer cells using anti-HER2 trastuzumab, which selectively binds to cells that overexpress HER2 on their plasma membrane.80 The GNRs were also functionalized with PEG to facilitate a greater degree of stability and circulation half-life.80 Results from this study demonstrated successful tumor accumulation of functionalized GNRs within HER2 overexpressing breast tumors in tumor-bearing nude mice and support the notions that GNRs can be used for molecular imaging or drug delivery into tumors.80
Gold NPs functionalized with anti-EGFR cetuximab were also used in a study conducted by Curley et al. The investigators treated pancreatic and colorectal cancer cells expressing EGFR with cetuximab functionalized gold NPs, allowing the nanoconjugates to be taken up by the cells. The cells were then treated with noninvasive shortwave radiofrequency (RF) energy. Exposure of cells to the RF field caused internalized gold NPs to produce heat that ultimately resulted in nearly 100% cell death in cells that had internalized the cetuximab functionalized gold NPs. Breast cancer cells that did not express EGFR that were treated with the cetuximab functionalized gold NPs showed no cell death when exposed to RF.
Another study utilizing gold NPs functionalized with cetuximab was conducted by Patra et al.82 but instead of using RF to induce cell death, gemcitabine (a chemotherapeutic agent) was also conjugated to the surface of the gold NPs. In vitro experiments conducted on pancreatic cancer cells expressing variable amounts of EGFR and in vivo orthotopic pancreatic cancer studies were conducted. Results demonstrated that administration of the cetuximab targeted, gemcitabine functionalized gold NPs resulted in significant inhibition of pancreatic tumor cell proliferation in cells highly expressing EGFR compared to cells or mice treated with gemcitabine alone.
Non-Metal Nanomaterials
There are several different types of non-metal NMs; those composed of carbon are some of the most extensively studied. Of the many different “pure” carbon-based constructs that have been investigated, fullerenes, which consist of carbon atoms arranged in a spherical shape known as a truncated icosahedron, and carbon nanotubes (NTs) (also technically considered a type of fullerene), which are carbon cylinders composed of rings of carbon atoms, have been most frequently studied in biological systems.83,84 Addition of chemical modifications to carbon fullerenes and NTs allows them to be functionalized so that they can be linked to a wide variety of active molecules, including peptides, proteins, nucleic acids and therapeutic agents.84–87 However, carbon NMs are completely insoluble in most solvents and, therefore, must be conjugated with biological molecules and polymers before they can be effectively used for drug delivery applications in vivo.52 Also, like noble-metal NMs, carbon NMs (without special functionalization) are generally not biodegradable and consequently pose potential health concerns.88–90
Another popular type of non-metal NM is quantum dots (QDs). QDs are nanocrystals measuring approximately 2–10 nm that can be made to fluoresce when stimulated by a particular wavelength of light.52,79,91 Their structure consists of an inorganic core (the size of which determines the color/wavelength of emitted light), an inorganic shell and an aqueous organic coating to which biomolecules such as PEG are conjugated.92 As with the other NMs described, QDs can also be functionalized with mAbs to facilitate cancer targeting; however, many QD formulations include highly toxic metals such as cadmium or lead, making them totally unsuitable as therapeutics.
Other QD variants may prove to have low toxicity and thus provide another class of biomedically useful NP. Nurunnabi et al. used QDs with PEG surface modifications to attach trastuzumab. These were loaded into micelles that ranged from 130–150 nm in size. The authors reported 77.3% shrinkage in initial tumor size and inhibition of tumor growth compared with the control (saline). They also studied the QDs in nude tumor-bearing mice for simultaneous tumor suppression and image therapy and found that the NM distributed uniformly throughout the entire body, including the tumors. With the natural fluorescent properties of the QD, they were able to obtain better images for diagnosis of tumors in early stages. A limitation of using fluorescence imaging is the shallow tissue penetration of this technology, which limits tumor detection to those that are very close to the skin.
McDevitt et al. reported some of the first carbon NM-based chemotherapeutic drug delivery carriers. These investigators functionalized carbon NTs with radio-labeled chimeric anti-CD20 rituximab or humanized anti-CD33 lintuzumab. In vitro experiments revealed that rituximab functionalized carbon NTs effectively targeted to CD20+ Daudi cells, while lintuzumab functionalized carbon NTs effectively targeted CD33+ HL60 cells. Additional in vivo studies with rituximab functionalized carbon NTs confirmed the specificity of the nanocarriers to tumor cells versus normal cells.94
Ma et al. employed a nanoconjugate derived from silica-coated iron NPs decorated with QDs to target cancer cells expressing carcino-embryonic antigen (CEA), an antigen not usually expressed in normal adult cells, with anti-CEA mAbs. Through the combination of magnetic and fluorescent NMs, the investigators were able to easily monitor the distribution of the nanoconjugates using luminescence and application of an external magnetic field. In vitro testing of the mAb-targeted nanoconjugates with human lung adenocarcinoma SPCA-1 cells, human leukemic K562 cells and human embryonic lung fibroblasts MRC-5 cells confirmed the specificity of the nanoconjugates to CEA. Additional experiments revealed that mAb-targeted nanoconjugates were able to detect cancer cells in pleural effusion from lung cancer patients, suggesting that they may be utilized in the detection and monitoring of lung cancer.
Nanomaterial Photothermal Ablation
mAbs have recently been conjugated to NM that have the propensity to generate heat when excited by specifically tuned input energy. Carpin et al. reported use of silica-gold nanoshells that were conjugated with trastuzumab and effectively used to kill trastuzumab-sensitive and trastuzumab-resistant breast cancer cells by means of thermal ablation. By using heat to treat the target cancer cells, drug resistance is no longer a constraint. Nanoshell binding was confirmed using two-photon laser scanning microscopy, and cells were then subjected to treatment with an 808-nm NIR diode laser. Cell viability assays revealed that both drug resistant and drug sensitive HER2+ cells were killed. There was a clear reduction in cell viability in the treated area, and adjacent cells were unaffected. These data suggest that immuno-conjugated gold nanoshell-mediated photothermal ablation may provide an effective alternative for treating drug resistant forms of breast cancer.
In 2009, Kikumori et al. reported use of immunoliposomes targeted via anti-HER2 mAbs to deliver magnetite nanoparticles (HMLs), which generated heat in response to an alternating magnetic field (AMF), to two groups of nude mice. One group of mice had previously received a cancer cell line that overexpressed HER2 at a high level. The other received a cancer cell line that overexpressed HER2 at a low level. The HMLs were then injected into the respective cancer nodules in the nude mice, and the mice were exposed to AMF. The HMLs were found to bind and accumulate only in the HER2 high overexpression nodules, where an increase in temperature was observed (40°C compared with body temperature of 38°C). Tumor regression was observed for up to 10 weeks following hyperthermia treatment. Localization of the HML at sufficient concentration thus appears to be necessary to evoke a meaningful response to this treatment regime. Nonetheless, both studies indicate that these novel approaches may pave the way for effective alternative cancer treatments that incorporate targeting via mAbs.
Escape from the Endosome or Lysosome After Cellular Uptake
Although some mAbs have been shown to be effective in allowing cell-specific uptake of targeted-nanoconjugates, some chemotherapeutic drugs may still be rendered ineffective if they are unable to escape the cell's endosomes and degradation in lysosomes.98 Targeting of specific intracellular compartment proteins by mAbs99 is a strategy that may prove useful in directing drug-loaded nanostructures away from lysosomal degradation. A novel approach to endosomal escape involves the modification of mAb targeted-NMs with cell penetrating peptides (CPPs).98 CPPs are class of proteins and peptides that can facilitate NM uptake and endosomal escape, therefore preventing the destruction of pH sensitive chemotherapeutic drugs in lysosomes. Over the last two decades, several proteins and peptides have been found to traverse the cellular membrane and endosome, delivering their cargo molecules into the cytoplasm or nucleus. One of the most widely used CPPs is transactivating transcriptional activator (TAT), which is derived from the human immunodeficiency virus type 1 (HIV-1).100 Other CPPs include Antennapedia, a transcription factor of Drosophila and VP22, a herpes virus protein.101,102 Studies of these proteins showed that their translocation across the plasma membrane is mediated via short sequences within these proteins of fewer than 20 amino acids that are rich in basic residues.23 Since their discovery, CPPs have been utilized for intracellular delivery of various cargoes, including nucleic acids and chemotherapeutic drugs.
Liu et al.103 co-functionalized a nanocarrier with trastuzumab and CPP TAT for the delivery of a radioactively labeled morpholino, which is a type of antisense molecule used to specifically downregulate the expression of a particular gene. All three components of the nanocarrier were added using streptavidin and biotin technology. It should be noted that this technology is very useful for proof-of-principle studies. However, as streptavidin is highly immunogenic, it cannot be considered for therapeutic use. Nevertheless, evaluation of trastuzumab and TAT functionalized nanocarriers in vitro studies utilizing SUM190 (HER2+), SUM149 (HER2−) and SK-BR-3 (HER2+) cells lines confirmed that trastuzumab facilitated specific binding to the HER2+ target cells, TAT improves cellular delivery, and the morpholino provided the specific retention of the radioactivity in the target cell nucleus.103 Also, results indicated that the morpholino was able to effectively survive entrapment of the endosome and its mRNA-binding ability was preserved. These results suggest that future mAb-targeted nanoconjugates that are utilized to deliver siRNAs, antisense oligonucleotides or morpholinos may benefit from the addition of CPPs to help facilitate endosomal escape and effective gene regulation.103
Challenges to the Future Application of mAb-Targeted NMs for the Treatment of Cancer
Although many studies have shown the potential of mAb-targeted NMs, there are still numerous hurdles that must be overcome before they may be more universally implemented. Indeed, one of the primary concerns is the potential acute and chronic toxic effects that a non-biodegradable NM may cause. Further complications stem from the fact that each mAb-targeted NM and each configuration of a NM has unique physical and chemical properties that must be individually characterized before being used in medical applications. For example, in vitro and in vivo studies of silica nanoparticles (NP) have shown that they are relatively non-toxic and possess great promise as drug delivery devices, while studies conducted on equivalent doses of silica NWs provide evidence that both supports and refutes (depending upon the biological system in which they were tested) the suitability of NWs for drug delivery purposes.104–106 Another important point is the potential effects of NM size, shape, and surface properties on drug pharmacokinetic parameters such as half-life and clearance characteristics. It is generally accepted that small NM (perhaps 2–10 nm), may be more readily dispersed through the circulatory system and extravasated to tissues, reaching and being internalized by its target cells. However, the majority of studies have also found that small NMs tend to be more toxic.89,107,108
Further considerations include the stability of NM in vivo and the development of methods for more effective encapsulation or conjugation of mAbs, chemotherapeutic drugs, and biological molecules to the surface of NM. For example, the large surface area of NM, which accounts for their potential as drug delivery agents, can lead to agglomeration in vivo. In turn, the potential of NM to agglomerate determines the effective particle size and hence clearance kinetics.
Conclusions
Due to their ability to specifically bind to a particular biological target, mAbs present a means of influencing the function of cells at the molecular level. In particular, mAbs show great promise in the treatment of cancer because the specificity of binding allows them to inhibit cancer cell proliferation, tumor angiogenesis and malignant spread of cancerous cells. Strategies to conjugate antibodies to toxins, radioactive isotopes and chemotherapeutic drugs to increase efficacy are under intense investigation. However, direct conjugation of toxins, radioactive isotopes and chemotherapeutic drugs to mAbs is limited to only a few molecules per antibody and may inhibit the effectiveness of both the chemotherapeutic agent and the antibody.
Increasingly, researchers are investigating nanomaterials to overcome the limitations of immunoconjugates. Some of the advantages of NMs include a large surface to volume ratio, which allows for excellent drug loading efficiency. Furthermore, in some cases, NMs can be designed in such a way as to protect the therapeutic agent from premature release and degradation. Additionally, since NMs can be functionalized with multiple therapeutic agents, they may be able to circumvent multidrug resistance in tumors. Finally, because solid tumors have an increased level of angiogenesis and leaky vasculature, mAb-targeted nanoconjugates may preferentially accumulate in tumors allowing for more effective “passive” drug delivery.
Indeed, many mAb-targeted NM platforms have been investigated, including those derived from liposomes, micelles, polymers, metals and non-metals. Results from both in vitro and in vivo studies have generally been promising. However, several challenges still remain before mAb-targeted NMs can be effectively utilized as drug delivery platforms to treat cancer in humans. First, the molecular characterization of the multitude of different tumors must continue to be undertaken in order to effectively pinpoint cancer-specific targets for future mAbs. Also, new methods of conjugation of mAbs and chemotherapeutic agents to NMs must continue to be developed in order to facilitate coupling that maintains the potency of drug delivery system components and promotes the timely release of chemotherapeutic agents. Furthermore, there is a need for expanded investigation of the use of mAbs to target NMs. In fact, although there are nearly a dozen anti-cancer mAbs currently approved in the US and Europe, researchers continue to mainly focus on only a few products (trastuzumab, rituximab and cetuximab in particular) for the targeting of NMs.61,82,109–111 Researchers should also continue to focus on the development of “smart drugs” that are designed to bypass the known challenges that cell-specific delivery of personalized medicines present.
NMs face several challenges before they can be utilized to treat cancer. One of the most pressing problems lies with ascertaining the potential toxic effects a NM may have on the system in which it is used. Therefore, the future of nanomedicine depends on effectively characterizing the influence of physicochemical factors such as NM size, shape, surface characteristics, solubility, stability and the ability to be functionalized, have on NM toxicity. To achieve this, rigorous testing of NM in both in vitro and in vivo systems should be conducted in order to determine how NM will interact with cells and tissues of the body.
Acknowledgments
This work was supported by the University of Idaho Blue Ribbon BANTech initiative and by the National Science Foundation under award number CBET-0709468.
Abbreviations
- AMF
alternating magnetic field
- CDP
cyclodextrin containing polymer
- CEA
carcino-embryonic antigen
- CPP
cell penetrating peptide
- DXR
doxorubicin
- EGFR
epidermal growth factor receptor
- EPR
enhanced permeability and retention
- Fc
constant domain of antibody
- FDA
Food and Drug Administration
- Fv
single chain antigen-binding fragment of antibody
- GNR
gold nanorods
- HER
human epidermal growth factor receptor
- HIV
human immunodeficiency virus
- HPMA
N-(2-hydroxypropyl)-methacrylamide copolymer
- IgG
immunoglobulin G
- iv
intravenous
- mAb
monoclonal antibody
- MDR
multi-drug resistance
- MRI
magnetic resonance imaging
- NM
nanomaterials
- NP
nanoparticle
- NT
nanotube
- PCL
poly ε-caprolactone
- PE
phosphatidyl ethanolamine
- PEG
poly-ethylene glycol
- PGA
poly-l-glutamic acid
- PLA
polylactic acid
- PLGA
poly lactic-co-glycosidic acid
- PMLA
poly(β-L-malic acid) (PMLA)
- QD
quantum dot
- RES
reticulo endothelial system
- RF
radiofrequency
- SIL
“stealth” immunoliposomes
- siRNA
short interfering RNA
- SPIO
supraparamagnetic iron oxide
- TAT
transactivating transcriptional activator
- Tf
transferrin
- TPP
meso-tetraphenylporphine
- VEGF
vascular endothelial growth factor
References
- 1.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 2.Majidi J, Barar J, Baradaran B, Abdolalizadeh J, Omidi Y. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies. 2009;18:81–100. doi: 10.3233/HAB-2009-0204. [DOI] [PubMed] [Google Scholar]
- 3.Natarajan A, Xiong CY, Gruettner C, DeNardo GL, DeNardo SJ. Development of multivalent radioimmunonanoparticles for cancer imaging and therapy. Cancer Biother Radiopharm. 2008;23:82–91. doi: 10.1089/cbr.2007.0410. [DOI] [PubMed] [Google Scholar]
- 4.Sachdeva MS. Drug targeting systems for cancer chemotherapy. Expert Opin Investig Drugs. 1998;7:1849–1864. doi: 10.1517/13543784.7.11.1849. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5:1909–1917. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- 6.Stewart Bak P. World Cancer Report. World Health Organization; 2003. [Google Scholar]
- 7.Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15–21. doi: 10.1111/j.1349-7006.2003.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabr-Milane LS, van Vlerken LE, Yadav S, Amiji MM. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat Rev. 2008;34:592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Wang X, Wei D, Cai M, Li G. [Internalization of antibody-targeted immunonanoparticles into human hepatoma cells and its reversal effect on MDR] Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:431–434. [PubMed] [Google Scholar]
- 10.Wang X, Yang L, Chen ZG, Shin DM. Application of nanotechnology in cancer therapy and imaging. CA Cancer J Clin. 2008;58:97–110. doi: 10.3322/CA.2007.0003. [DOI] [PubMed] [Google Scholar]
- 11.Pietersz GA, Kanellos J, Smyth MJ, Zalcberg J, McKenzie IF. The use of monoclonal antibody conjugates for the diagnosis and treatment of cancer. Immunol Cell Biol. 1987;65:111–125. doi: 10.1038/icb.1987.13. [DOI] [PubMed] [Google Scholar]
- 12.Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res. 2006;23:1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 13.Walker B, Jr, Mouton CP. Nanotechnology and nanomedicine: a primer. J Natl Med Assoc. 2006;98:1985–1988. [PMC free article] [PubMed] [Google Scholar]
- 14.National Nanotechnology Initiative. 2010. www.nano.gov/
- 15.Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150:552–558. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 17.Poole CP. Introduction to Nanotechnology. Hoboken, New Jersey: John Wiley and Sons; 2003. [Google Scholar]
- 18.Hassan M. Nanotechnology. Science. 2004;304:1732–1734. doi: 10.1126/science.304.5678.1732. [DOI] [PubMed] [Google Scholar]
- 19.Webster TJ. Nanomedicine: what's in a definition? Int J Nanomedicine. 2006;1:115–116. doi: 10.2147/nano.2006.1.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 21.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain KK. Role of nanobiotechnology in developing personalized medicine for cancer. Technol Cancer Res Treat. 2005;4:645–650. doi: 10.1177/153303460500400608. [DOI] [PubMed] [Google Scholar]
- 23.Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007;9:128–147. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Silva MN. Nanotechnology and nanomedicine: a new horizon for medical diagnostics and treatment. Arch Soc Esp Oftalmol. 2007;82:331–334. [PubMed] [Google Scholar]
- 25.Polakis P. Arming antibodies for cancer therapy. Curr Opin Pharmacol. 2005;5:382–387. doi: 10.1016/j.coph.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko T, Willner D, Monkovic I, Knipe JO, Braslawsky GR, Greenfield RS, et al. New hydrazone derivatives of adriamycin and their immunoconjugates—a correlation between acid stability and cytotoxicity. Bioconjug Chem. 1991;2:133–141. doi: 10.1021/bc00009a001. [DOI] [PubMed] [Google Scholar]
- 28.Hamann PR, Hinman LM, Beyer CF, Lindh D, Upeslacis J, Flowers DA, et al. An anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Choice of linker. Bioconjug Chem. 2002;13:40–46. doi: 10.1021/bc0100206. [DOI] [PubMed] [Google Scholar]
- 29.Sanderson RJ, Hering MA, James SF, Sun MM, Doronina SO, Siadak AW, et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res. 2005;11:843–852. [PubMed] [Google Scholar]
- 30.Ulbrich K, Etrych T, Chytil P, Jelinkova M, Rihova B. Antibody-targeted polymer-doxorubicin conjugates with pH-controlled activation. J Drug Target. 2004;12:477–489. doi: 10.1080/10611860400011869. [DOI] [PubMed] [Google Scholar]
- 31.Xia CF, Boado RJ, Pardridge WM. Antibody-mediated targeting of siRNA via the human insulin receptor using avidin-biotin technology. Mol Pharm. 2009;6:747–751. doi: 10.1021/mp800194y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia CF, Zhang Y, Boado RJ, Pardridge WM. Intravenous siRNA of brain cancer with receptor targeting and avidin-biotin technology. Pharm Res. 2007;24:2309–2316. doi: 10.1007/s11095-007-9460-8. [DOI] [PubMed] [Google Scholar]
- 33.Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res. 2010;2:14. doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenwald RB. PEG drugs: an overview. J Control Release. 2001;74:159–171. doi: 10.1016/s0168-3659(01)00331-5. [DOI] [PubMed] [Google Scholar]
- 35.John R, Rezaeipoor R, Adie SG, Chaney EJ, Oldenburg AL, Marjanovic M, et al. In vivo magnetomotive optical molecular imaging using targeted magnetic nanoprobes. Proc Natl Acad Sci USA. 2010;107:8085–8090. doi: 10.1073/pnas.0913679107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 37.Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A. Liposomal encapsulated anti-cancer drugs. Anticancer Drugs. 2005;16:691–707. doi: 10.1097/01.cad.0000167902.53039.5a. [DOI] [PubMed] [Google Scholar]
- 38.Zhong YQ, Wei J, Fu YR, Shao J, Liang YW, Lin YH, et al. [Toxicity of cationic liposome Lipofectamine 2000 in human pancreatic cancer Capan-2 cells] Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1981–1984. [PubMed] [Google Scholar]
- 39.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 40.Munn LL. Aberrant vascular architecture in tumors and its importance in drug-based therapies. Drug Discov Today. 2003;8:396–403. doi: 10.1016/s1359-6446(03)02686-2. [DOI] [PubMed] [Google Scholar]
- 41.Matsumura Y, Gotoh M, Muro K, Yamada Y, Shirao K, Shimada Y, et al. Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Ann Oncol. 2004;15:517–525. doi: 10.1093/annonc/mdh092. [DOI] [PubMed] [Google Scholar]
- 42.Cheng WW, Allen TM. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: a comparison of whole monoclonal antibody, Fab' fragments and single chain Fv. J Control Release. 2008;126:50–58. doi: 10.1016/j.jconrel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Kirpotin D, Park JW, Hong K, Zalipsky S, Li WL, Carter P, et al. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 1997;36:66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- 44.Crosasso P, Brusa P, Dosio F, Arpicco S, Pacchioni D, Schuber F, et al. Antitumoral activity of liposomes and immunoliposomes containing 5-fluorouridine prodrugs. J Pharm Sci. 1997;86:832–839. doi: 10.1021/js9604467. [DOI] [PubMed] [Google Scholar]
- 45.Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA. 1996;93:14164–14169. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L, Pirollo KF, Tang WH, Rait A, Chang EH. Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther. 1999;10:2941–2952. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- 47.Pan X, Wu G, Yang W, Barth RF, Tjarks W, Lee RJ. Synthesis of cetuximab-immunoliposomes via a cholesterol-based membrane anchor for targeting of EGFR. Bioconjug Chem. 2007;18:101–108. doi: 10.1021/bc060174r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawat M, Singh D, Saraf S. Nanocarriers: promising vehicle for bioactive drugs. Biol Pharm Bull. 2006;29:1790–1798. doi: 10.1248/bpb.29.1790. [DOI] [PubMed] [Google Scholar]
- 49.Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17:1263–1268. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 50.Li C. Poly(L-glutamic acid)—anticancer drug conjugates. Adv Drug Deliv Rev. 2002;54:695–713. doi: 10.1016/s0169-409x(02)00045-5. [DOI] [PubMed] [Google Scholar]
- 51.Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Br J Cancer. 2008;99:392–397. doi: 10.1038/sj.bjc.6604483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 53.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 54.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 55.Gradishar WJ. Albumin-bound nanoparticle paclitaxel. Clin Adv Hematol Oncol. 2005;3:348–349. [PubMed] [Google Scholar]
- 56.Nyman DW, Campbell KJ, Hersh E, Long K, Richardson K, Trieu V, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23:7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 57.Anhorn MG, Wagner S, Kreuter J, Langer K, von Briesen H. Specific targeting of HER2 overexpressing breast cancer cells with doxorubicin-loaded trastuzumab-modified human serum albumin nanoparticles. Bioconjug Chem. 2008;19:2321–2331. doi: 10.1021/bc8002452. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Liu X, Chen L, Cheng D, Rusckowski M, Hnatowich DJ. Tumor delivery of antisense oligomer using trastuzumab within a streptavidin nanoparticle. Eur J Nucl Med Mol Imaging. 2009;36:1977–1986. doi: 10.1007/s00259-009-1201-2. [DOI] [PubMed] [Google Scholar]
- 59.Fujita M, Lee BS, Khazenzon NM, Penichet ML, Wawrowsky KA, Patil R, et al. Brain tumor tandem targeting using a combination of monoclonal antibodies attached to biopoly(beta-L-malic acid) J Control Release. 2007;122:356–363. doi: 10.1016/j.jconrel.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang KH, Liu JH, Wang LY, Zhu ZH, Chen QK, Min J, et al. [Study of the anti-tumor effect of anti-vascular endothelial growth factor McAb 5-fluorouracil loaded polylactic acid nanoparticles] Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10:482–485. [PubMed] [Google Scholar]
- 61.Nobs L, Buchegger F, Gurny R, Allemann E. Biodegradable nanoparticles for direct or two-step tumor immunotargeting. Bioconjug Chem. 2006;17:139–145. doi: 10.1021/bc050137k. [DOI] [PubMed] [Google Scholar]
- 62.Kos J, Obermajer N, Doljak B, Kocbek P, Kristl J. Inactivation of harmful tumour-associated proteolysis by nanoparticulate system. Int J Pharm. 2009;381:106–112. doi: 10.1016/j.ijpharm.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 63.Lin HY, Landersdorfer CB, London D, Meng R, Lim CU, Lin C, et al. Pharmacodynamic modeling of anti-cancer activity of tetraiodothyroacetic acid in a perfused cell culture system. PLoS Comput Biol. 2011;7:1001073. doi: 10.1371/journal.pcbi.1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Batrakova EV, Dorodnych TY, Klinskii EY, Kliushnenkova EN, Shemchukova OB, Goncharova ON. Anthracycline antibiotics non-covalently incorporated into the block copolymer micelles: in vivo evaluation of anti-cancer activity. Br J Cancer. 1996;74:1545–1552. doi: 10.1038/bjc.1996.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakanishi T, Fukushima S, Okamoto K, Suzuki M, Matsumura Y, Yokoyama M, et al. Development of the polymer micelle carrier system for doxorubicin. J Control Release. 2001;74:295–302. doi: 10.1016/s0168-3659(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 66.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 67.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 68.Maeda H. SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv Drug Deliv Rev. 2001;46:169–185. doi: 10.1016/s0169-409x(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 69.Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74:47–61. doi: 10.1016/s0168-3659(01)00309-1. [DOI] [PubMed] [Google Scholar]
- 70.Roby A, Erdogan S, Torchilin VP. Solubilization of poorly soluble PDT agent, meso-tetraphenylporphin, in plain or immunotargeted PEG-PE micelles results in dramatically improved cancer cell killing in vitro. Eur J Pharm Biopharm. 2006;62:235–240. doi: 10.1016/j.ejpb.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao C, Sun Q, Liang B, Shen J, Shuai X. Targeting EGFR-overexpressing tumor cells using Cetuximab-immunomicelles loaded with doxorubicin and superparamagnetic iron oxide. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2010.08.005. In press. [DOI] [PubMed] [Google Scholar]
- 72.Sharma P, Brown S, Walter G, Santra S, Moudgil B. Nanoparticles for bioimaging. Adv Colloid Interface Sci. 2006;123–126:471–485. doi: 10.1016/j.cis.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 73.Shim SY, Woo JR, Nam EJ, Hong HJ, Mook-Jung I, Kim YH, et al. Stepwise silver-staining-based immunosorbent assay for amyloid-beta autoantibody detection. Nanomedicine. 2008;3:485–493. doi: 10.2217/17435889.3.4.485. [DOI] [PubMed] [Google Scholar]
- 74.Roca M, Haes AJ. Probing cells with noble metal nanoparticle aggregates. Nanomedicine. 2008;3:555–565. doi: 10.2217/17435889.3.4.555. [DOI] [PubMed] [Google Scholar]
- 75.Kawano T, Yamagata M, Takahashi H, Niidome Y, Yamada S, Katayama Y, et al. Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulses. J Control Release. 2006;111:382–389. doi: 10.1016/j.jconrel.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 76.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 77.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 78.Shenoy D, Fu W, Li J, Crasto C, Jones G, DiMarzio C, et al. Surface functionalization of gold nanoparticles using hetero-bifunctional poly(ethylene glycol) spacer for intracellular tracking and delivery. Int J Nanomedicine. 2006;1:51–57. doi: 10.2147/nano.2006.1.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bawarski WE, Chidlowsky E, Bharali DJ, Mousa SA. Emerging nanopharmaceuticals. Nanomedicine. 2008;4:273–382. doi: 10.1016/j.nano.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, Elliot AM, et al. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol Cancer Ther. 2008;7:1730–1739. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Curley SA, Cherukuri P, Briggs K, Patra CR, Upton M, Dolson E, et al. Noninvasive radiofrequency field-induced hyperthermic cytotoxicity in human cancer cells using cetuximab-targeted gold nanoparticles. J Exp Ther Oncol. 2008;7:313–326. [PubMed] [Google Scholar]
- 82.Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008;68:1970–1978. doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
- 83.Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 84.Ashcroft JM, Tsyboulski DA, Hartman KB, Zakharian TY, Marks JW, Weisman RB, et al. Fullerene (C60) immunoconjugates: interaction of water-soluble C60 derivatives with the murine anti-gp240 melanoma antibody. Chem Commun. 2006:3004–3006. doi: 10.1039/b601717g. [DOI] [PubMed] [Google Scholar]
- 85.Pastorin G, Wu W, Wieckowski S, Briand JP, Kostarelos K, Prato M, et al. Double functionalization of carbon nanotubes for multimodal drug delivery. Chem Commun. 2006:1182–1184. doi: 10.1039/b516309a. [DOI] [PubMed] [Google Scholar]
- 86.Wu W, Wieckowski S, Pastorin G, Benincasa M, Klumpp C, Briand JP, et al. Targeted delivery of amphotericin B to cells by using functionalized carbon nanotubes. Angew Chem Int Ed Engl. 2005;44:6358–6362. doi: 10.1002/anie.200501613. [DOI] [PubMed] [Google Scholar]
- 87.Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res. 2008;41:60–68. doi: 10.1021/ar700089b. [DOI] [PubMed] [Google Scholar]
- 88.Yang H, Liu C, Yang D, Zhang H, Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2009;29:69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- 89.Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 90.Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66:1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 91.Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. Noninvasive imaging of quantum dots in mice. Bioconjug Chem. 2004;15:79–86. doi: 10.1021/bc034153y. [DOI] [PubMed] [Google Scholar]
- 93.Nurunnabi M, Cho KJ, Choi JS, Huh KM, Lee YK. Targeted near-IR QDs-loaded micelles for cancer therapy and imaging. Biomaterials. 2010;31:5436–5444. doi: 10.1016/j.biomaterials.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 94.McDevitt MR, Chattopadhyay D, Kappel BJ, Jaggi JS, Schiffman SR, Antczak C, et al. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J Nucl Med. 2007;48:1180–1189. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 95.Ma J, Fan Q, Wang L, Jia N, Gu Z, Shen H. Synthesis of magnetic and fluorescent bifunctional nanocomposites and their applications in detection of lung cancer cells in humans. Talanta. 2010;81:1162–1169. doi: 10.1016/j.talanta.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 96.Carpin LB, Bickford LR, Agollah G, Yu TK, Schiff R, Li Y, et al. Immunoconjugated gold nanoshell-mediated photothermal ablation of trastuzumab-resistant breast cancer cells. Breast Cancer Res Treat. 2010;125:27–34. doi: 10.1007/s10549-010-0811-5. [DOI] [PubMed] [Google Scholar]
- 97.Kikumori T, Kobayashi T, Sawaki M, Imai T. Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Res Treat. 2009;113:435–441. doi: 10.1007/s10549-008-9948-x. [DOI] [PubMed] [Google Scholar]
- 98.Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev. 2005;57:637–651. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 99.Marschall AL, Frenzel A, Schirrmann T, Schungel M, Dubel S. Targeting antibodies to the cytoplasm. MAbs. 2011;3:3–16. doi: 10.4161/mabs.3.1.14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 101.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 102.Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci USA. 1991;88:1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu X, Wang Y, Nakamura K, Kubo A, Hnatowich DJ. Cell studies of a three-component antisense MORF/tat/Herceptin nanoparticle designed for improved tumor delivery. Cancer Gene Ther. 2008;15:126–132. doi: 10.1038/sj.cgt.7701111. [DOI] [PubMed] [Google Scholar]
- 104.Yang H, Wu Q, Tang M, Liu X, Deng H, Kong L, et al. In vitro study of silica nanoparticle-induced cytotoxicity based on real-time cell electronic sensing system. J Nanosci Nanotechnol. 10:561–568. doi: 10.1166/jnn.2010.1735. [DOI] [PubMed] [Google Scholar]
- 105.Kneuer C, Sameti M, Bakowsky U, Schiestel T, Schirra H, Schmidt H, et al. A nonviral DNA delivery system based on surface modified silica-nanoparticles can efficiently transfect cells in vitro. Bioconjug Chem. 2000;11:926–932. doi: 10.1021/bc0000637. [DOI] [PubMed] [Google Scholar]
- 106.Julien DC, Richardson CC, Beaux MF, 2nd, McIlroy DN, Hill RA. In vitro proliferating cell models to study cytotoxicity of silica nanowires. Nanomedicine. 2010;6:84–92. doi: 10.1016/j.nano.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 107.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ye Y, Liu J, Xu J, Sun L, Chen M, Lan M. Nano-SiO2 induces apoptosis via activation of p53 and Bax mediated by oxidative stress in human hepatic cell line. Toxicol In Vitro. 24:751–758. doi: 10.1016/j.tiv.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 109.Chen TJ, Cheng TH, Chen CY, Hsu SC, Cheng TL, Liu GC, et al. Targeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem. 2009;14:253–260. doi: 10.1007/s00775-008-0445-9. [DOI] [PubMed] [Google Scholar]
- 110.Chiu SJ, Ueno NT, Lee RJ. Tumor-targeted gene delivery via anti-HER2 antibody (trastuzumab, Herceptin) conjugated polyethylenimine. J Control Release. 2004;97:357–369. doi: 10.1016/j.jconrel.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 111.Cirstoiu-Hapca A, Bossy-Nobs L, Buchegger F, Gurny R, Delie F. Differential tumor cell targeting of anti-HER2 (Herceptin) and anti-CD20 (Mabthera) coupled nanoparticles. Int J Pharm. 2007;331:190–196. doi: 10.1016/j.ijpharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 112.Choi D, McIlroy D, Nagler J, Aston E, Hrdlicka P, Gustin K, et al. One-dimensional silica structures and their applications to the biological sciences. In: Kumar SSR, editor. Nanostructured Oxides for Life Sciences. Vol. 3. Wiley-VCH; 2009. pp. 83–108. [Google Scholar]