Abstract
Motor learning in the vestibulo-ocular reflex (VOR) and eyeblink conditioning use similar neural circuitry, and they may use similar cellular plasticity mechanisms. Classically conditioned eyeblink responses undergo extinction after prolonged exposure to the conditioned stimulus in the absence of the unconditioned stimulus. We investigated the possibility that a process similar to extinction may reverse learned changes in the VOR. We induced a learned alteration of the VOR response in rhesus monkeys using magnifying or miniaturizing goggles, which caused head movements to be accompanied by visual image motion. After learning, head movements in the absence of visual stimulation caused a loss of the learned eye movement response. When the learned gain was low, this reversal of learning occurred only when head movements were delivered, and not when the head was held stationary in the absence of visual input, suggesting that this reversal is mediated by an active, extinction-like process.
It is often adaptive to retain memories over a long period of time. When environmental circumstances change, however, old memories may no longer be useful, and in some cases may be maladaptive. Therefore, an ideal learning system should have a mechanism for suppressing old memories. Old memories can be abolished or suppressed through passive forgetting or through an active process such as extinction, the reduction of a conditioned response that occurs when the learned association between a cue and reinforcement is degraded.
Some cerebellum-dependent forms of learning have been shown to exhibit extinction, and a mechanism by which the cerebellar circuitry could support the extinction of classically conditioned eyeblink responses has been proposed (Medina et al. 2002). The present study investigated whether extinction could be a universal feature of cerebellum-dependent learning. More specifically, we examined whether learned changes in the amplitude, or gain, of the vestibulo-ocular reflex (VOR), a well-studied cerebellum-dependent motor learning paradigm, exhibit a process of reversal analogous to extinction.
The VOR is a reflexive eye movement that stabilizes images on the retina by using vestibular signals to drive compensatory eye movements in the opposite direction from head movements. This reflex is calibrated by a form of motor learning that depends on the cerebellum (Robinson 1976). When head movements are consistently paired with unwanted image motion, motor learning occurs, producing a change in the gain of the reflex that reduces image motion (Gonshor and Melvill-Jones 1973; Ito et al. 1974; Miles and Fuller 1974; Gauthier and Robinson 1975). In the laboratory, changes in VOR gain can be induced using magnifying or miniaturizing goggles that increase or decrease the VOR gain, respectively, by causing image motion during head movements.
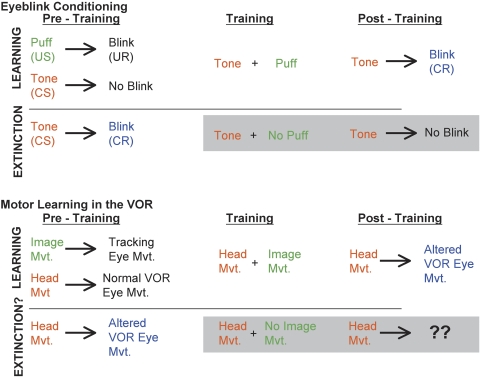
At the behavioral level, motor learning in the VOR is analogous in many ways to eyeblink conditioning (Fig. 1). In each paradigm, the pairing of two stimuli during training causes a learned change in the response to one of the stimuli, when that stimulus is subsequently presented alone. In eyeblink conditioning, the pairing of a tone (conditioned stimulus; CS) with a puff of air to the eye (unconditioned stimulus; US) causes a change in the response to the tone alone. In the VOR, pairing of a head movement (vestibular stimulus; CS) with visual image motion (US) causes a change in the response to the head movement alone.
Figure 1.
Comparison between eyeblink conditioning and motor learning in the VOR. Before training in the eyeblink conditioning paradigm, an air puff to the eye (unconditioned stimulus; US) elicits a reflexive blink (unconditioned response; UR). When a tone (conditioned stimulus; CS) is paired with the air puff during training, the animal learns to blink (conditioned response; CR) in response to the tone alone. During extinction training, the tone is presented repeatedly without the air puff, leading to the eventual extinction of the learned response. Before training in the VOR, image movement across the retina elicits a tracking eye movement response, and head movement elicits the normal VOR eye movement response. During training, head movements (CS) are paired with image movements (US) and these two stimuli together elicit an altered eye movement response which is approximately the sum of the eye movement response to the image movement and the eye movement response to the head movement. Following training, head movement elicits this altered eye movement response in the absence of the visual stimulus (CR). In the second phase of training, the head movement is presented repeatedly in the absence of image movement. We tested whether this would lead to an extinction-like change in the eye movement response to the head movement.
In both paradigms, the learned response to the CS resembles the response observed when the CS and US are presented together during training. More specifically, the air puff elicits a reflexive eyeblink (unconditioned response; UR), but the tone elicits no blink response before training. During training, the combined presentation of tone and air puff elicits a blink, and as learning progresses the tone alone comes to elicit a blink (conditioned response; CR). By analogy, in the VOR, image motion on the retina elicits a tracking eye movement (UR) to stabilize the image. The head movement can also elicit an eye movement response before training, which is the VOR. When the head movement is paired with image motion on the retina during training, the combined stimulus elicits an eye movement response that is approximately equal to the sum of the normal response to the head movement and the tracking response to the image motion. Over time, the head movement alone comes to elicit an altered eye movement response (CR), similar to that elicited in the presence of combined head and image motion.
Thus, at the behavioral level, there are clear parallels between the two learning paradigms. One difference is that the CS for eyeblink conditioning (tone) initially elicits no response, whereas the CS for motor learning in the VOR (head movement) does initially elicit a response; in many other respects, however, the two paradigms are very similar.
The neural circuits mediating eyeblink conditioning and motor learning in the VOR are similar as well (for review, see Raymond et al. 1996). Both forms of learning depend on the cerebellum. In both systems, sensory information about the CS (tone or head movement) is carried to the cerebellum by mossy fibers, and information about the US (air puff or image motion) comes to the cerebellum via the climbing fibers. These similarities at the circuit level have led to the idea that these two forms of motor learning may use similar neural mechanisms for the acquisition of learning. Indeed, both paradigms appear to invoke learning-related changes in both the cerebellar cortex and the deep cerebellar nuclei. (The vestibular nucleus serves as the deep cerebellar nucleus for the VOR.)
In this study, we investigate whether the parallels between eyeblink conditioning and motor learning in the VOR extend to include the reversal of learned behavioral changes. In eyeblink conditioning, when the tone (CS) is presented repeatedly to a trained animal in the absence of the air puff (US), the learned blink response is extinguished. An analogous process in the VOR would correspond to a loss of the altered eye movement response (a return toward the normal VOR response) caused by repeated presentation of head movements in the absence of image motion. Here we report evidence for such an extinction-like process in the VOR.
RESULTS
Head Movements in the Absence of Visual Stimulation Reverse Learned Changes in VOR Gain
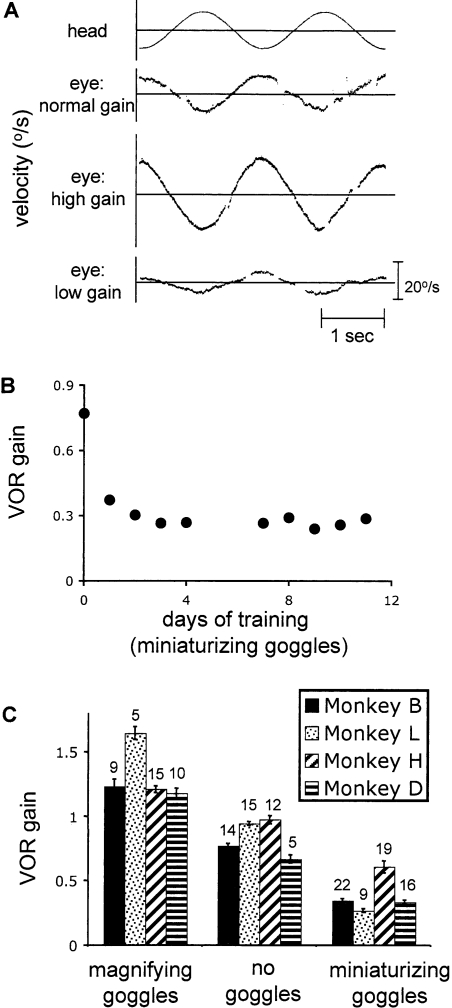
We first induced motor learning in the VOR to alter the eye movement response to head motion. We then presented head movements in the dark to look for evidence of a reversal of learning that could reflect an extinction-like process. We induced motor learning using 2.2× magnifying or 0.25× miniaturizing goggles, which the animals wore in their home cages for several days. This training paradigm induced large changes in the eye movements elicited by head movements. Figure 2A shows an example of a normal eye movement response to head movements in an untrained monkey and examples of altered eye movement responses following training with magnifying or miniaturizing goggles. The head movement stimulus used to measure the VOR was rotation about an earth vertical axis, with a sinusoidal velocity profile (0.5 Hz, ±10°/sec peak). The eye movement response was characterized by measuring the VOR gain, which is defined as the ratio of the amplitude of the eye movement to the amplitude of the head movement. VOR gain was measured in the dark to distinguish eye movements driven by the VOR from visually driven eye movements. In an untrained animal, the head movement stimulus (Fig. 2A, top trace) evoked an eye movement response in the opposite direction to the head movement and with approximately equal amplitude, that is, the VOR had a gain close to 1 (normal gain). Training with magnifying goggles increased the gain of the VOR so that head motion elicited a larger eye movement response (high gain), and training with miniaturizing goggles decreased the gain (low gain).
Figure 2.
Learned changes in VOR gain following training with goggles. (A) Example traces of eye velocity during head movements in the dark. The top trace shows the sinusoidal head velocity profile used to measure the VOR (± 10°/sec, 0.5 Hz). The bottom three traces show the eye velocity responses to the head movements before training (eye normal gain), following training with magnifying goggles (eye high gain), and following training with miniaturizing goggles (eye low gain). The sharp discontinuities in the traces are saccades, which were removed for analysis. (B) Example time course, showing the VOR gain as a function of days of training with miniaturizing goggles. (C) Average VOR gain at the start of the experiments following training with magnifying goggles (left), miniaturizing goggles (right), or no goggles (middle). Each bar represents the average gain for one monkey. Numbers above the bars are the number of experiments for each monkey in each condition, and error bars represent standard errors.
In all four animals tested, training with goggles effectively induced motor learning in the VOR. Most of the learned changes in VOR gain occurred in the first day of training with goggles, and the gain reached an asymptote within ∼3 d (see example in Fig. 2B). The average VOR gain for each of four monkeys with normal gain (no goggles) or after at least 24 h of training with magnifying goggles or miniaturizing goggles is plotted in Figure 2C.
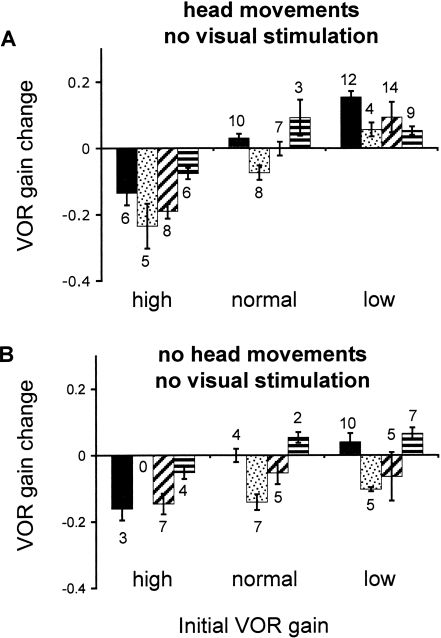
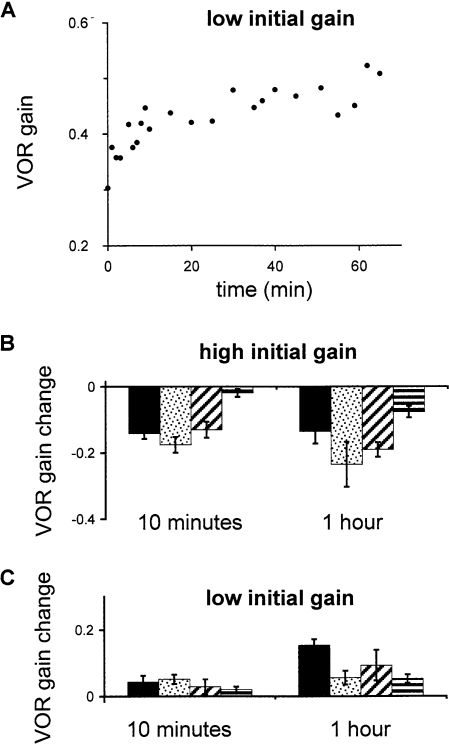
After the animals had worn the goggles for at least 24 h, they were subjected to 1 h of head movements (0.5 Hz, ±10°/sec) in the absence of a visual stimulus. The animals were placed in a completely dark room to avoid any visual stimulation that might cause adaptive changes in VOR gain. When the learned VOR gain was high following training with magnifying goggles, head movements in the dark caused the gain to decrease. This effect was highly significant (p < 10-7, n = 25) and was consistent in all four animals (Fig. 3A, high initial gain). After training with miniaturizing goggles, head movements in the dark caused an increase in VOR gain (p < 10-5, n = 39), which was also consistent in all four animals (Fig. 3A, low initial gain). Thus, 1 h of head movements in the absence of visual stimulation caused a loss of the learned changes that had been acquired during one or more days of training with magnifying or miniaturizing goggles. Head movements in the dark did not have any consistent effect on normal VOR gains (p = 0.1, n = 28; Fig. 3A, normal initial gain), confirming that changes induced by head movements in the dark depended on previous learning and could not be explained by nonspecific factors such as a change in arousal during the course of the hour. The effect of head movements in the dark on VOR gain did not depend on the number of days of training with goggles (linear regression; p = 0.33, n = 25 for magnifying goggles; p = 0.11, n = 39 for miniaturizing goggles; data not shown); therefore, data from all days were averaged. The VOR gain changes following head movements in the dark were significantly different between both high and low initial gain and the normal gain control (p < 10-7 and p < 10-4, respectively).
Figure 3.
Reversal of learned changes in VOR gain. (A) Change in VOR gain after 1 h of head movements in the dark (post minus pre). When the initial gain was high (left) or low (right), the VOR gain returned toward normal. (B) Change in VOR gain after 1 h of sitting stationary in the dark.
Head Movements Are Required for Reversal of Learned Decreases But Not Increases in VOR Gain
The observation that head movements in the dark can reverse the previously acquired change in VOR gain could reflect either an active, extinction-like process requiring exposure to head movements in the absence of a visual stimulus or a passive process requiring only the absence of visual stimuli. To distinguish between these possibilities, we compared the changes induced by head movements in the dark with the changes induced by 1 h of sitting in the dark with no visual stimuli or head movements. In animals with a normal VOR gain, there was no consistent effect of 1 h of sitting stationary in the dark (p = 0.1, n = 18; Fig. 3B, normal gain). When the learned VOR gain was high, the gain passively decreased toward normal after 1 h (p < 10-4, n = 14; Fig. 3B, high initial gain). The amplitude of this change was not significantly different from the change observed when the animals with learned high gains underwent 1 h of head movements in the dark (p = 0.26; Fig. 3A). Therefore, exposure to head movements did not appear to produce any loss of the learned high-gain response beyond that which occurred passively in the absence of a visual stimulus. In contrast, when the learned VOR gain was low, 1 h of sitting stationary in the dark did not induce a consistent change in the VOR gain (p = 0.45, n = 27; Fig. 3B, low initial gain), and the change following 1 h of head movements in the dark was significantly greater than the change following 1 h of sitting stationary in the dark (p < 0.005). These results suggest that head movements are required to drive a learned low VOR gain back toward normal, but head movements are not required to drive a learned high VOR gain back toward normal.
Confirmation of the Lack of Visual Stimuli
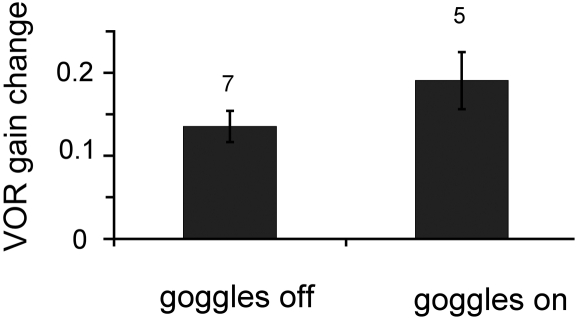
The interpretation of our results depends critically on the room being completely dark so that the effect of exposure to head movements in the absence of any visual cues could be assessed. Therefore, every care was taken to ensure that there was no stray light in the room. In addition, in one monkey we compared results when the miniaturizing goggles were on versus off during the 1-h period of head movements in the dark. With the goggles on, any stray light would provide visual stimuli that should either maintain the learned low gain or drive additional changes in the learned direction (i.e., a further decrease in gain). In contrast, the gain consistently increased toward pretraining gain even when the monkey was wearing the miniaturizing goggles in the darkened room (Fig. 4). There was no significant difference between the results obtained when the goggles were on versus off (p = 0.81, n = 7 for goggles off; n = 5 for goggles on). Therefore, visual stimulation from stray light cannot have been the cause of the changes in the VOR shown in Figure 3A.
Figure 4.
Confirmation of the lack of visual stimuli during head rotations in the dark. After training with miniaturizing goggles in Monkey B, the change in VOR gain after 1 h of head movements in the dark was similar when the goggles were removed during the experiments (left) and when they remained on during the experiments (right), suggesting that the effects could not be explained by stray visual stimulation.
Time Course of Reversal
The time course of the change toward normal during head movements in the dark is illustrated in Figure 5. The VOR gain changed most rapidly during the first few minutes of head movements, and the rate of change generally slowed as the hour progressed. Figure 5A shows an example time course for one experiment when the initial gain was low. Figure 5, B and C, compare the average changes for each monkey after 10 min (left) and the full hour (right) of head movements in the dark. The change was not complete after 10 min (significantly greater change after 1 h than after 10 min; p < 0.05, n = 25 for magnifying goggles; p < 10-3, n = 39 for miniaturizing goggles), showing that the reversal of learned changes in gain is a gradual process.
Figure 5.
Time course of the reversal of learned changes in VOR gain. (A) Example time course of changes in VOR gain in Monkey B during head rotations in the dark after the gain had been lowered through training with miniaturizing goggles. Most of the change occurred during the first few minutes of head movements, but changes continued throughout the hour. (B) Change in VOR gain after 10 min of head movements in the dark (left) compared with the change after the full hour of head movements in the dark when the initial gain was high. (C) Same as B, when initial trained gain was low.
DISCUSSION
The purpose of motor learning in the VOR is to calibrate the eye movement so that it effectively stabilizes images on the retina. The presence of retinal image motion during head movements indicates an improperly calibrated VOR, which is corrected by appropriate adjustment of the VOR. Therefore, retinal image motion is generally considered to be the error signal that drives learned changes in the VOR (e.g., Ito 1982). This study, however, demonstrates a situation in which retinal image motion is not necessary to drive changes in the VOR. Following several days of training, the learned VOR gain reverted toward normal after only 1 h of head movements in the absence of visual input.
Passive Decay
After the acquisition of an increased VOR gain, the VOR gain decreased when the animal sat stationary in the dark. This decrease seems to reflect a decay of the recently acquired increase in gain, because animals with a normal, untrained VOR gain exhibited no consistent gain change after sitting stationary in the dark for 1 h. This passive decay of the learned increase in VOR gain could reflect a process of forgetting or it could reflect the loss of an arousal-dependent expression of the increased VOR response. In contrast, when the learned VOR gain was low, sitting stationary in the dark had no effect on the learned gain. Thus, at least part of the learned increase in gain is labile and subject to short-term, passive reversal, whereas the learned decrease in gain is more stable.
These results confirm and extend a previous study of the forgetting of motor learning in the VOR (Miles and Eighmy 1980). In that study, animals wore magnifying or miniaturizing goggles for 2 wk and then sat with their heads immobilized in the dark for a period of several days. Daily measurements examined the decay of learning. As in our study, Miles and Eighmy reported passive decay of the learned increase in VOR gain produced by long-term training with magnifying goggles, but no passive decay of the learned decrease in VOR gain produced by training with miniaturizing goggles. Moreover, the decay of the increase in gain they observed 1 d after training was similar in magnitude to the decay we saw after just 1 h, suggesting that the majority of passive decay occurs quickly in the absence of visual input.
Extinction-Like Return Toward Normal VOR Gain
After the acquisition of either a learned increase or decrease in VOR gain, head movements in the absence of visual stimulation induced changes back toward normal gain (Fig. 3). Because the absence of visual stimuli alone caused a similar decay of a learned high gain, it appears that no additional extinction-like process was induced by the head movements. This does not necessarily mean that an increase in VOR gain cannot undergo extinction, but we were unable to detect such a phenomenon with the paradigms used in the current study. In contrast, when the learned VOR gain was low, changes back toward normal gain occurred only during head movements in the dark. Sitting stationary in the dark did not cause the learned low gains to return toward normal. Thus, the reversal of the learned decrease in VOR gain required an active process, which is driven by head movements and which is similar to the extinction of a classically conditioned response.
Head movements in the absence of visual input have been reported to produce decreases in VOR gain through habituation (e.g., Jeannerod et al. 1976; Schmid and Jeannerod 1985). However, habituation cannot explain the observation that learned low gains increased during head movements in the dark (Fig. 3A), because habituation always results in a decreased gain. The gain increase we observe is more akin to extinction. It should be noted, however, that the stimulus used to produce extinction of a classically conditioned response is usually identical to the conditioned stimulus used in the acquisition of learning. In the current study, we used somewhat different head movement stimuli for the acquisition of learning (natural head movements in the home cage) and the reversal of learning (0.5 Hz sinusoidal head movements). Nevertheless, natural head movements contain many different frequencies including 0.5 Hz (Armand and Minor 2001), and therefore, the sinusoidal head movements can be thought of as a subset of the original head movement stimuli used for the acquisition of learning. Despite this procedural difference between the current experiments and more standard extinction paradigms, it is clear that the presentation of head movements alone drives a reversal of the learned behavioral changes that does not occur in the absence of the head movements. The anatomical and behavioral similarities between eyeblink conditioning and VOR adaptation (Raymond et al. 1996) suggest that this reversal of learning in the VOR may share neural mechanisms with extinction of classically conditioned eyeblink responses.
Candidate Neural Mechanisms for the Extinction-Like Process in the VOR Circuit
There are two main hypotheses concerning which neural signals trigger changes in the VOR circuit (Ito 1972, 1982; Miles and Lisberger 1981), and evidence to suggest that both could operate in parallel (Raymond and Lisberger 1998; Boyden et al. 2003; Ke and Raymond 2004). One hypothesis is that plasticity is triggered in the VOR circuit by instructive signals carried by the simple spike outputs of Purkinje cells (Miles and Lisberger 1981). Purkinje cells exhibit different responses in the presence of visual/vestibular stimuli that induce learned increases versus decreases in VOR gain, and it has been suggested that these different neural responses could trigger the different changes that must occur in the VOR circuit under such conditions. As learning progresses, Purkinje cells also exhibit learning related changes in their responses during performance of the VOR, that is, during head movements in the dark. Before learning, Purkinje cells show little response to head movements in the dark, but after learning has been induced, the Purkinje cells exhibit robust responses to head movements in the dark, which are similar to those that were present during the specific visual/vestibular stimulus used to induce learning. More specifically, during visual/vestibular stimuli that increase VOR gain and during head movements in the dark in animals with a learned high VOR gain, Purkinje cells increase their firing during contraversive head movements. During visual/vestibular stimuli that drive decreases in VOR gain and during head movements in the dark in animals with a learned low VOR gain, the Purkinje cells increase their firing during ipsiversive head movements (Dufosse et al. 1978; Lisberger and Fuchs 1978; Miles et al. 1980a,b; Watanabe 1984; Lisberger et al. 1994; Partsalis et al. 1995).
Therefore, if the Purkinje cell responses during head movements in the dark were to trigger changes in the VOR circuit, these changes should continue in the learned direction (Lisberger 1996). In other words, head movements in the dark following a learned increase in VOR gain should cause a further increase in gain, and head movements in the dark following a learned decrease in gain should induce a further decrease. This predicted change is the opposite of what was observed (Fig. 3A), suggesting that Purkinje cells do not provide the instructive signals that trigger the extinction-like return to normal VOR gain.
A second hypothesis about the neural mechanisms mediating motor learning in the VOR contends that climbing fibers provide the neural instructive signal guiding plasticity in the VOR circuit (Ito 1972, 1982). Climbing fibers respond strongly to image motion (Maekawa and Simpson 1973; Simpson and Alley 1974), suggesting that they might be suited to trigger the changes induced when head movements are paired with image motion. New evidence, however, suggests that climbing fibers can also respond during head movements in the dark, hence they may be well poised to induce the extinction-like reversal of learned changes in VOR gain. When rabbits with a normal, untrained VOR gain undergo head movements in the dark, climbing fibers show an increase in firing rate during contraversive head movements, as they do in the presence of visual-vestibular stimuli that cause a decrease in VOR gain (Simpson et al. 2002). Following gain decreases, some climbing fibers exhibit responses during head movements in the dark like those normally seen in the presence of visual/vestibular stimuli that increase VOR gain (increased firing rate during ipsiversive head movements; Belton et al. 2002), which could potentially explain the increase in VOR gain observed when animals with a low learned gain underwent head movements in the dark (Fig. 3A, low initial gain).
Climbing fiber activity also has been implicated in the extinction of eyeblink conditioning. Pharmacological manipulations that block inhibition of climbing fibers prevent the extinction that normally would be induced by presentations of the CS alone, and manipulations that inhibit climbing fibers cause extinction during continued CS-US pairings (Medina et al. 2002). Therefore, it has been hypothesized that inhibition of climbing fiber activity is the neural signal that triggers extinction in this paradigm.
Comparison of Different Motor Learning Paradigms
The results presented here show that following several days of motor learning, VOR gain changes can be reversed substantially over a very short timescale in the absence of visual input. This reversal requires head movements when the initial learning results in a low VOR gain, but not when the learned gain is high, which suggests that at least two distinct processes govern the reversal of motor learning in the VOR.
The current results are consistent with previous reports that learned decreases in VOR gain are more difficult to reverse than learned increases (Miles and Eighmy 1980; Boyden and Raymond 2003; Kuki et al. 2004). Boyden and Raymond proposed that the difference in reversal properties could reflect the contribution of different neural plasticity mechanisms to the acquisition of these two forms of motor learning in the VOR. The idea of different mechanisms for learned increases and decreases in gain is also supported by reports of different pharmacological sensitivities of gain increases and decreases (Li et al. 1995; Boyden et al. 2003).
Thus, within the VOR circuit, different mechanisms seem to contribute to the storage and reversal of motor memories for increases versus decreases in gain. Nevertheless, we find similarities between the reversal of a learned low VOR gain and extinction in eyeblink conditioning, which suggests that some mechanisms for the reversal of memory may be shared between the different regions of the cerebellum contributing to these behaviors.
METHODS
The subjects in this experiment were one female and three male rhesus monkeys (Macaca mulatta; 6-15 kg). Monkey L seemed to have impaired vision in one eye. The eye movements reported for that monkey were recorded in the eye with apparently normal vision. Using sterile procedures described previously (Lisberger et al. 1994; Raymond and Lisberger 1996), animals were implanted with an eye coil for measuring vertical and horizontal eye movements and a head holder for restraining the head. During experiments, animals were seated in a primate chair to which their head holder was secured. Head movement stimuli were delivered using a servo-controlled turntable (Carco) that rotated the animal, the primate chair, and a set of magnetic field coils (CNC Engineering) together about a vertical axis. All surgical and behavioral procedures conformed to guidelines established by the U.S. Department of Health and Human Services (National Institutes of Health) Guide for the Care and Use of Laboratory Animals (1996) as approved by Stanford University.
VOR Gain Training
Animals were fitted with either 2.2× magnifying or 0.25× miniaturizing goggles, which they wore in their home cages for up to 4 wk. The first experiments were conducted after the monkey had been wearing the goggles for at least 24 h. Following each day's experiment (which lasted <2 h), animals were returned to the home cage with the goggles on until the next experiment. Experiments were most often separated by 24 h and were never separated by <18 h. After the animals had worn goggles for a period of several weeks, the goggles were removed and the animals were allowed at least 1 wk to recover in their home cages before more experiments were conducted. After removal of the goggles, the VOR gain returned to normal in ∼3 d.
Testing Procedures
During an experiment, the animal was seated in a primate chair and its head was restrained using the implanted head holder. The chair was secured to the turntable. Once the animal's head was fixed, the goggles were removed and the eye coil was calibrated by having the monkey fixate a small visual target at a number of locations. Within 10 min of the goggles being removed, the lights were turned off. At no time during the setup were the animals allowed to experience combined visual and vestibular stimulation when they were not wearing goggles. All experiments were conducted in a room specially designed to keep all light out, and this was checked periodically by the experimenters by adapting to the darkness in the room for 15 min and searching for stray light. To further control for possible light leaks into the room, some experiments on Monkey B were conducted while the goggles remained on throughout the hour of head movements in the dark (Fig. 4).
In some experiments, animals were rotated at 0.5 Hz, ±10°/sec for 1 h. During this period, the VOR was recorded at various time points without disrupting the continuous rotation of the animal. In a subset of these experiments on Monkeys B and D, the period of uninterrupted 0.5-Hz head movements was both preceded and followed by 1 min each of 0.5-Hz, 1-Hz, 2-Hz, and 5-Hz head movements for the purpose of measuring changes in VOR gain at different frequencies.
In some experiments, the VOR was measured by delivering a 0.5-Hz head movement for 1 min before and after 1 h of sitting stationary in the dark. A subset of these experiments on Monkeys B and D included 1-min tests at each of 0.5 Hz, 1 Hz, 2 Hz, and 5 Hz before and after the hour of sitting stationary in the dark. Therefore, the animals received at most 5 min of head movements before the hour of sitting in the dark. Experiments in which the animal experienced 1 h of head movements in the dark and experiments in which the animal experienced 1 h of sitting stationary in the dark were usually conducted on alternate days.
To keep the animals alert, they were rewarded with a drop of juice every 1.5-2.5 sec for keeping their eyes within 15° of straight ahead gaze in the dark. If the eye movement traces showed the characteristic irregular eye velocity drift and lack of spontaneous saccades suggesting that an animal was beginning to fall asleep, it was wakened by experimenters using auditory stimulation.
VOR Measurements and Data Analysis
Voltages related to eye and head velocity were recorded on-line at a sampling rate of 500 Hz per channel. Eye velocity traces were edited off-line to remove saccades (see Lisberger et al. 1994; Raymond and Lisberger 1996). Sine wave cycles were aligned on zero crossings of the head velocity and averaged. Only cycles in which the monkey maintained its gaze within 15° of straight ahead were included in the averages. Most averages included at least 10 cycles, but a few included fewer than 10 cycles. Averaged eye and head velocity were analyzed using Fourier analysis. The gain of the VOR was calculated as the ratio of the amplitude of the fundamental component of the eye velocity to the amplitude of the fundamental component of the head velocity. Gain changes are reported as the difference between the gain measured after 1 h of rotating or sitting in the dark and the gain measured before the 1-h period.
Except where noted, statistical significance was assessed using a Student's T-test. The use of monkeys in this study necessitated a relatively small number of experimental subjects; therefore, replications from all four monkeys were pooled to calculate significance across conditions. This method treated each measurement as equal and weighted the contribution from each monkey by the number of measurements in that monkey. The plots in Figures 2, 3, 4, 5 show data from each monkey individually so that the reader can assess monkey-to-monkey variability. With four monkeys, statistical tests that treat the average of all replications from a given monkey as one data point (n = 4 for each experimental condition) are not accurate. Nevertheless, T-tests done in this manner yielded quantitatively similar results.
Acknowledgments
We thank K. Oh and P. Louderback for excellent technical assistance and M. Ke, A. Bristol, and R. Kimpo for helpful comments on the manuscript. This work was supported by an HHMI Predoctoral Fellowship (M.R.C.), NIH Training Grant 5T32MH200160-05 (G.W.M.), a NDSEG Predoctoral Fellowship (R.J.S.), and NIH R01DC04154 (J.L.R.).
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.82304
References
- Armand, M. and Minor, L.B. 2001. Relationship between time- and frequency-domain analyses of angular head movements in the squirrel monkey. J. Comput. Neurosci. 11: 217-239. [DOI] [PubMed] [Google Scholar]
- Belton, T., Suh, M., and Simpson, J.I. 2002. The nonvisual complex spike signal in the flocculus responds to challenges to the vestibulo-ocular reflex gain. Ann. NY Acad. Sci. 978: 503-504. [DOI] [PubMed] [Google Scholar]
- Boyden, E.S. and Raymond, J.L. 2003. Active reversal of motor memories reveals rules governing memory encoding. Neuron 39: 1031-1042. [DOI] [PubMed] [Google Scholar]
- Boyden, E.S., Chatila, T., and Raymond, J.L. 2003. Motor memories in the vestibulo-ocular reflex of CamKIV knockout mice. Program No. 73.18. Soc. Neurosci. Abstracts.
- Dufosse, M., Ito, M., Jastreboff, P.J., and Miyashita, Y. 1978. A neuronal correlate in rabbit's cerebellum to adaptive modification of the vestibulo-ocular reflex. Brain Res. 150: 611-616. [DOI] [PubMed] [Google Scholar]
- Gauthier, G.M. and Robinson, D.A. 1975. Adaptation of the human vestibulo-ocular reflex to magnifying lenses. Brain Res. 92: 331-335. [DOI] [PubMed] [Google Scholar]
- Gonshor, A. and Melvill-Jones, G. 1973. Changes of human vestibulo-ocular response induced by vision-reversal during head rotation. J. Physiol. (Lond) 234: 102-103. [PubMed] [Google Scholar]
- Ito, M. 1972. Neural design of the cerebellar motor control system. Brain Res. 40: 81-84. [DOI] [PubMed] [Google Scholar]
- ____. 1982. Cerebellar control of the vestibulo-ocular reflex-around the flocculus hypothesis. Annu. Rev. Neurosci. 5: 275-298. [DOI] [PubMed] [Google Scholar]
- Ito, M., Shiida, T., Yagi, N., and Yamamoto, M. 1974. The cerebellar modification of rabbit's horizontal vestibulo-ocular reflex induced by sustained head rotation combined with visual stimulation. Proc. Jpn. Acad. 50: 85-89. [Google Scholar]
- Jeannerod, M., Magnin, M., Schmid, R., and Stefanelli, M. 1976. Vestibular habituation to angular velocity steps in the cat. Biol. Cybern. 22: 39-48. [DOI] [PubMed] [Google Scholar]
- Ke, M.C. and Raymond, J.L. 2004. Neural instructive signals guiding motor learning in the VOR. Soc. Neurosci. Abstracts (in press).
- Kuki, Y., Hirata, Y., Blazquez, P.M., Heiney, S.A., and Highstein, S.M. 2004. Memory retention of vestibuloocular reflex motor learning in squirrel monkeys. Neuroreport 15: 1007-1011. [DOI] [PubMed] [Google Scholar]
- Li, J., Smith, S.S., and McElligott, J.G. 1995. Cerebellar nitric oxide is necessary for vestibulo-ocular reflex adaptation, a sensorimotor model of learning. J. Neurophysiol. 74: 489-494. [DOI] [PubMed] [Google Scholar]
- Lisberger, S.G. 1996. Motor learning and memory in the vestibulo-ocular reflex: The dark side. Ann. NY Acad. Sci. 781: 525-531. [DOI] [PubMed] [Google Scholar]
- Lisberger, S.G. and Fuchs, A.F. 1978. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J. Neurophysiol. 41: 733-763. [DOI] [PubMed] [Google Scholar]
- Lisberger, S.G., Pavelko, T.A., Bronte-Stewart, H.M., and Stone, L.S. 1994. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J. Neurophysiol. 72: 954-973. [DOI] [PubMed] [Google Scholar]
- Maekawa, K. and Simpson, J.L. 1973. Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J. Neurophysiol. 36: 649-666. [DOI] [PubMed] [Google Scholar]
- Medina, J.F., Nores, W.L., and Mauk, M.D. 2002. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature 416: 330-333. [DOI] [PubMed] [Google Scholar]
- Miles, F.A. and Eighmy, B.B. 1980. Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J. Neurophysiol. 43: 1406-1425. [DOI] [PubMed] [Google Scholar]
- Miles, F.A. and Fuller, J.H. 1974. Adaptive plasticity in the vestibulo-ocular responses of the rhesus monkey. Brain Res. 80: 512-516. [DOI] [PubMed] [Google Scholar]
- Miles, F.A. and Lisberger, S.G. 1981. Plasticity in the vestibulo-ocular reflex: A new hypothesis. Annu. Rev. Neurosci. 4: 273-299. [DOI] [PubMed] [Google Scholar]
- Miles, F.A., Fuller, J.H., Braitman, D.J., and Dow, B.M. 1980a. Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J. Neurophysiol. 43: 1437-1476. [DOI] [PubMed] [Google Scholar]
- Miles, F.A., Braitman, D.J., and Dow, B.M. 1980b. Long-term adaptive changes in primate vestibuloocular reflex. IV. Electrophysiological observations in flocculus of adapted monkeys. J. Neurophysiol. 43: 1477-1493. [DOI] [PubMed] [Google Scholar]
- Partsalis, A.M., Zhang, Y., and Highstein, S.M. 1995. Dorsal Y group in the squirrel monkey. II. Contribution of the cerebellar flocculus to neuronal responses in normal and adapted animals. J. Neurophysiol. 73: 632-650. [DOI] [PubMed] [Google Scholar]
- Raymond, J.L. and Lisberger, S.G. 1996. Behavioral analysis of signals that guide learned changes in the amplitude and dynamics of the vestibulo-ocular reflex. J. Neurosci. 16: 7791-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 1998. Neural learning rules for the vestibulo-ocular reflex. J. Neurosci. 18: 9112-9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, J.L., Lisberger, S.G., and Mauk, M.D. 1996. The cerebellum: A neuronal learning machine? Science 272: 1126-1131. [DOI] [PubMed] [Google Scholar]
- Robinson, D.A. 1976. Adaptive gain control of vestibuloocular reflex by the cerebellum. J. Neurophysiol. 39: 954-969. [DOI] [PubMed] [Google Scholar]
- Schmid, R. and Jeannerod, M. 1985. Vestibular habituation: An adaptive process? In Adaptive mechanisms in gaze control (eds. A. Berthoz and G. Melvill-Jones), Vol. 1, pp. 113-122. Elsevier, New York. [PubMed] [Google Scholar]
- Simpson, J.I. and Alley, K.E. 1974. Visual climbing fiber input to rabbit vestibulo-cerebellum: A source of direction-specific information. Brain Res. 82: 302-308. [DOI] [PubMed] [Google Scholar]
- Simpson, J.I., Belton, T., Suh, M., and Winkelman, B. 2002. Complex spike activity in the flocculus signals more than the eye can see. Ann. NY Acad. Sci. 978: 232-236. [DOI] [PubMed] [Google Scholar]
- Watanabe, E. 1984. Neuronal events correlated with long-term adaptation of the horizontal vestibulo-ocular reflex in the primate flocculus. Brain Res. 297: 169-174. [DOI] [PubMed] [Google Scholar]