Abstract
Background
Dietary components effective in weight maintenance efforts have not been adequately identified.
Objective
To determine impact of changes in dietary consumption on weight loss and maintenance during the Weight Loss Maintenance (WLM) clinical trial.
Design
WLM was a randomized controlled trial. Successful weight loss participants who completed Phase I of the trial and lost 4kg were randomized to one of three maintenance intervention arms in Phase II and followed for an additional 30 months.
Participants/setting
The multicenter trial was conducted from 2003–2007. This substudy included 828 successful weight loss participants.
Methods
Dietary Measures
The Block Food Frequency Questionnaire (FFQ) was used to assess nutrient intake levels and food group servings. Carbohydrates, proteins, fats, dietary fiber and fruit/vegetable and dairy servings were utilized as predictor variables.
Data collection
The FFQ was collected on all participants at study entry (beginning of Phase I). Those randomized to Phase II completed the FFQ at three additional time points; randomization (beginning of Phase II), 12 and 30 months.
Intervention
The main intervention focused on long term maintenance of weight loss using the Dietary Approaches to Hypertension (DASH) diet. This substudy examined whether changes to specific dietary variables were associated with weight loss and maintenance.
Statistical analyses performed
Linear regression models that adjusted for change in total energy examined the relationship between changes in dietary intake and weight for each time period. Site, age, race, sex, and a race-sex interaction were included as covariates.
Results
Participants who substituted protein for fat lost, on average, 0.33 kg per 6-months during Phase I (p<0.0001) and 0.07 kg per 6-months during Phase II (p<0.0001) per 1% increase in protein. Increased intake of fruits and vegetables was associated with weight loss in Phases I and II: 0.29 kg per 6-months (p<0.0001) and 0.04 kg per 6-months (p=0.0062), respectively, per 1-serving increase. Substitution of carbohydrates for fat and protein for carbohydrates were associated with weight loss during both phases. Increasing dairy intake was associated with significant weight loss during Phase II (−0.17 kg per 6-months per 1-serving increase, p=0.0002), but not in Phase I. Dietary fiber revealed no significant findings.
Conclusion
Increasing fruits, vegetables, and low-fat dairy may help achieve weight loss and maintenance.
Keywords: Weight Loss Maintenance Trial, dietary intakes, food frequency questionnaire
INTRODUCTION
Obesity is a major contributor to soaring health-care costs. The rising prevalence of obesity was reported to be responsible for almost $40 billion of increased medical spending in 2006, while the overall medical costs of obesity accounted for an estimated $147 billion per year in 2008 (1). Using prevalence data from the National Health and Nutrition Examination Survey (NHANES), Wang et al. (2) predict that, should current trends continue, within 15 years 80% of all adults in the United States will be either overweight or obese.
Many weight loss interventions are aimed at changing behaviors and lifestyles. These interventions include: prolonged continuous intervention contacts (3–5), self-monitoring (6–7), accountability (8–9), motivational interviewing (10–13), frequent self-weighing (14) and regular physical activity (15–17). The American Dietetic Association (ADA) has noted that achieving a negative energy balance is the most important factor affecting amount and rate of weight loss over time (18). Recommended strategies to achieve negative energy balance include: calorie counting, modifying macronutrient composition and/or energy density, and may include meal replacements or low calorie diets. Reducing dietary fat and/or carbohydrates is a practical way to create a calorie deficit of 500 to 1000 kilocalories (kcal) per day, and should result in a weight loss of one to two pounds per week (18). Hill notes that even small reductions in energy intake (~100 kcal), coupled with increased physical activity, can help reduce weight gain (19) and may have a greater likelihood of being sustained in the long term.
The United States Department of Agriculture’s (USDA) MyPyramid helps people interpret the USDA’s Dietary Guidelines for Americans, and essentially promotes a diet high in fruits and vegetables and low in fat (20). The guidelines are jointly issued and updated every five years by the USDA and the U.S. Department of Health and Human Services. The general message of MyPyramid is that good dietary habits can promote health and reduce risk for major chronic disease; the guidelines recommend the Dietary Approaches to Stop Hypertension (DASH) diet as a model of healthy eating (20). The DASH diet promotes fruit and vegetable intake, intake of low-fat dairy, focus on whole grains, reduced intake of meat, and is low in fat at approximately 27–28% of calories (21). The DASH diet was shown to be effective for weight loss in the PREMIER Study, which preceded the Weight Loss Maintenance (WLM) trial and was conducted by the same investigative team, and included both African Americans (AA) and Caucasians who were overweight and hypertensive (22).
Despite the recommendations by USDA and the proven outcomes associated with the DASH diet, the research examining diet composition for the management of obesity remains mixed. Previous studies have found that low fat diets promote short-term weight loss (23), however, some studies suggest that low carbohydrate, high protein, and high fat diets may also result in substantial weight loss (24). However, evidence for type of diet on long-term weight maintenance remains debatable (25–26). A substantial body of literature exists suggesting that weight loss can be achieved by varying the macronutrient distribution and composition of dietary factors. Specifically, in a systematic review by Abete et al. (27), it was concluded that there are numerous dietary strategies focused on macronutrient distribution many of which have good weight loss outcomes. However, the challenge remains to find the appropriate approach for weight maintenance and relapse prevention tailored to the individual.
Therefore, the purpose of this current substudy is threefold: 1) to provide additional evidence to the literature on macronutrient composition specific to a diverse population of at-risk patients with cardiovascular disease, 2) to determine which changes in consumption of macronutrients, fruits, vegetables, low-fat dairy and dietary fiber were associated with weight loss during an intensive behavioral weight loss phase (i.e., Phase I of the WLM trial) and 3) to determine which changes in macronutrients, fruit, vegetable, low-fat dairy and dietary fiber consumption were associated with maintenance of weight loss over a 30 month period (i.e., WLM trial Phase II).
STUDY METHODS
The WLM study was funded by the National Heart, Lung, and Blood Institute (NHLBI) and included 1,032 participants from four different sites (Pennington Biomedical Research Center, Baton Rouge, LA; Kaiser Permanente Center for Health Research, Portland, OR; Johns Hopkins University, Baltimore, MD; and Duke University Medical Center, Durham, NC). The study was approved by Institutional Review Boards at each participating site and by a protocol review committee appointed by the NHLBI. All participants provided written informed consent, and a data and safety monitoring board provided trial oversight. Enrollment occurred from August 2003–July 2004 and randomization, February–December 2004. Data collection was completed in June 2007.
The purpose of the WLM study was to examine strategies for maintenance of weight loss after an initial 6 month weight loss phase (Phase I) and during a 30-month weight loss maintenance phase (Phase II). During Phase I of WLM, participants were instructed in the basics of the DASH diet. They were specifically asked to increase consumption of fruits and vegetables, low-fat dairy and whole grains, along with other typical strategies for weight loss. Participants were encouraged to continue these dietary habits in Phase II.
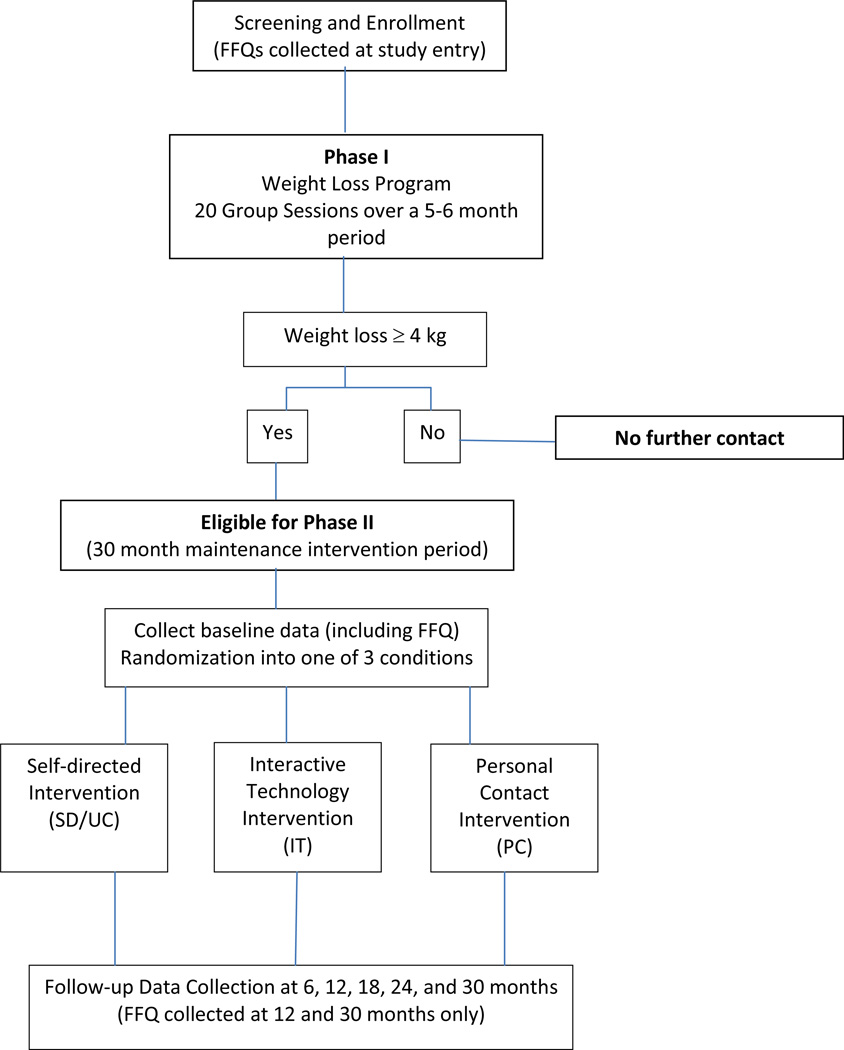
Data used in this substudy were collected at four time points: 1) entry into the trial (prior to Phase I), 2) baseline (prior to randomized into Phase II), 3) 12months post-randomization, and 4) 30 months post-randomization. Data collection included calibrated height and weight and dietary intake information in the form of the Food Frequency Questionnaire (FFQ) (28). A total of 1,685 participants completed Phase I of the trial. The 1,032 participants who lost a minimum of 4 kg (i.e., successful Phase I participants) were randomized to the weight maintenance phase (Phase II) of the WLM study. Figure 1 presents the study design and helps to further clarify the design of the WLM trial. Additional details of the WLM trial have been reported elsewhere (3, 8, 29–30). Of the participants in Phases I and II, 828 completed the FFQ at all four time points (study entry, at baseline prior to Phase II randomization, and at 12 and 30 months post randomization) and are included in the current analysis. The effect of specific maintenance condition on macronutrient intake was not a focus of this substudy; rather the goal of this study was to examine changes in dietary consumption across time. Therefore, all participants, regardless of randomization to study treatment assignment, were collapsed into a single group for analysis.
Figure 1. Weight Loss Maintenance Trial study design.
Note that food frequency questionnaires (FFQs)were collected at 4 time points: study entry, randomization into Phase II, and 12 and 30 months following Phase II randomization. Individuals not randomized into Phase II did not complete any further data collection, hence no FFQ was available for unsuccessful Phase I participants.
Dietary intake was assessed using the Block FFQ (28). The 100-item National Cancer Institute Block FFQ was created using data from NHANES II to measure relative nutrient intake as well as to measure absolute nutrient values. The FFQ has been extensively validated (28). The Block FFQ was used to identify foods that significantly contribute to caloric intake of a large sample of the population and to identify 17 macro-and micronutrients in the national diet (28, 31). The WLM study employed the NutritionQuest Data-on-Demand System (32). Due to budgetary considerations, the FFQ was chosen to estimate usual eating patterns of WLM participants. In Spring 2003, a training session for WLM staff on administering and reviewing the FFQ was conducted by the lead interventionist at each site. Subsequent new staff members were trained by the clinic coordinator. WLM staff filled in participant IDs, gender and age prior to giving the form to participants. Verbal and written instructions were given for the FFQ along with the serving size photo and a pencil for proper completion. Participants were asked to complete the scannable form at home and return it at the next clinic visit. During screening and prior to study entry into Phase I, FFQs were received and reviewed by the interventionist who conducted the screening visit. After randomization into Phase II, FFQs administered at baseline, 12- and 30-month visits, were reviewed for completeness by WLM clinic staff with participants so that any blank answers could be resolved. FFQ booklets were photocopied and stored in the coordinator’s office and the batches of the originals were mailed periodically to NutritionQuest for scanning, analysis, and calculation of nutrient intakes. The WLM Coordinating Center received the FFQ data from Block. Where needed, data queries were sent to the study coordinator with detailed instructions for resolving data queries.
The Statistical Analysis System (SAS) (version 9.1, 2002, SAS Institute, Cary, NC) was used for data analysis. Descriptive statistics, including frequencies, means and percentages, were used to summarize the data; these included the total number of participants, those completing the FFQ, age, ethnicity, educational level and household income. Changes in weight, body mass index (BMI) and dietary intake were defined as the difference between the measurements taken at the start and end of each measurement time period. Change in dietary intake represents an individual's average change in daily dietary intake over the time period. Least-squares mean changes in weight and dietary intake were calculated for each time period; these means were derived from linear regression models that were adjusted for site, age, physical activity, race, sex, and a race-sex interaction.
Linear regression models were used to examine the relationship between changes in dietary intake and changes in weight during the 6 month pre-randomization phase (Phase I) and the 30 month post-randomization phase (Phase II) of the WLM study. Because of the different lengths of the two study phases, weight change within Phase II was summarized by its average 6-month weight change (i.e., total Phase II weight change divided by 5) to facilitate comparisons between the two phases. Changes in intake of macronutrients were examined in a multivariate nutrient density model in which changes in the macronutrient densities were included as predictors of weight loss and change in total energy was included as a covariate (33). Two separate regression models were fit to the data: one with Δ % carbohydrate and Δ % protein as macronutrient predictors, and a second with Δ % fat and Δ % protein as macronutrient predictors. Because total energy was included as a covariate, the model describes the effect of substituting 1% of a macronutrient for an isocaloric amount of another macronutrient (the macronutrient absent from the model), while intake of the third macronutrient (the other macronutrient predictor in the model) is held constant.
Changes in intake of different food groups were examined in a standard multivariate model in which changes in absolute intake (servings per day) of different food groups were included as predictors, and change in total energy was included as a covariate (33); three separate regression models (one for each food group of interest) were used in order to estimate the three (non-independent) effects of increasing intake of recommended food groups. All models also adjusted for the effects of age, race, sex, race-by-sex interaction, and change in minutes of moderate to vigorous physical activity (MVPA) per week. Physical activity was measured using accelerometers (30) Participants were instructed to wear a calibrated, triaxial accelerometer (RT3, Stayhealthy Inc, Monrovia, CA) for at least 10 hours per day for at least 4 days, including 1 weekend day. Accelerometry results that comprised at least 1 weekday and 1 weekend day were used to estimate total weekly minutes of MVPA. This reflects both leisure time physical activity and daily activity patterns, and provides a measure of total MVPA-related energy expenditure. While actual physical activity measures are not addressed in this paper, it is important that the dietary changes be adjusted for change in physical activity, potentially a confounding factor.
RESULTS
The majority of the 1,032 participants randomized to the weight maintenance phase of the study were non-African American (Non-AA; 62%), female (63%), with at least a college education (61%), or with a household income level of at least $60,000 (57%). Of these, 828 (80.2%) completed a FFQ at all four time points and were included in this analysis. Demographic characteristics were similar between those completing versus not completing FFQs at all four time points (Table 1). When comparing the percentage of completed FFQs in the 1032 participants of Phase II, the group with the lowest percentage completed for all four time points was African American (AA) women. Within each race-sex group, those that completed the FFQ at all four time points, and thus were included in the analysis, were similar to the overall WLM sample in terms of age, education level and income. In terms of weight loss, the participants with all four FFQs completed lost more weight initially (Phase I) than those not completing all four FFQs and lost more weight overall. However, the Phase II weight loss was similar among those with all four FFQs versus those without all FFQs completed (data not shown).
Table 1.
Descriptive characteristics of participants randomized to weight maintenance phase (Phase II) of WLM (n = 1032) compared with those WLM participants completing the food frequency questionnaires (FFQ) at all four time points (n=828)
| All Men | All Women | FFQ | Men w/FFQ | Women w/FFQ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | AA | Non-AA | AA | Non-AA | Total | AA | Non-AA | AA | Non-AA | |
| Number of participants | 1032 | 121 | 257 | 267 | 387 | 828 | 98 | 213 | 197 | 320 |
| Completed FFQ, % | ||||||||||
| Baseline | 98.9 | 98.4 | 99.2 | 98.1 | 99.5 | |||||
| 6 months | 97.6 | 96.7 | 97.3 | 95.5 | 99.5 | |||||
| 12 months | 91.7 | 92.6 | 93.4 | 87.6 | 93.0 | |||||
| 30 months | 84.9 | 86.8 | 90.3 | 79.8 | 84.2 | |||||
| All four time points | 80.2 | 81.0 | 82.9 | 73.8 | 82.7 | |||||
| Baseline age, mean, y | 55.6 | 53.1 | 53.1 | 57.8 | 56.7 | 56.0 | 53.6 | 57.9 | 53.4 | 57.0 |
| Baseline age range, y | 28–83 | 31–77 | 33–83 | 28–74 | 37–78 | |||||
| Hispanic, % | 1.0 | 0.0 | 2.3 | 0.0 | 1.0 | 1.0 | 0.0 | 2.3 | 0.0 | 1.0 |
| Education Level, % | ||||||||||
| Some college or less | 38.7 | 30.6 | 28.0 | 44.9 | 43.9 | 37.7 | 29.6 | 27.2 | 44.2 | 43.1 |
| College degree | 21.9 | 32.2 | 19.5 | 19.9 | 21.7 | 22.5 | 31.6 | 19.7 | 22.8 | 21.3 |
| Post-college | 39.4 | 37.2 | 52.5 | 35.2 | 34.4 | 39.9 | 38.8 | 53.1 | 33.0 | 35.6 |
| Annual household income, % | ||||||||||
| <$30,000 | 7.7 | 2.5 | 3.9 | 14.1 | 7.4 | 7.3 | 3.1 | 3.8 | 13.2 | 7.2 |
| $30,000–$59,000 | 34.9 | 23.6 | 24.9 | 44.0 | 38.8 | 35.4 | 23.5 | 23.5 | 45.7 | 40.6 |
| $60,000–$89,999 | 31.6 | 39.2 | 31.1 | 29.9 | 30.8 | 31.3 | 38.8 | 32.4 | 28.4 | 30.0 |
| ≥$90,000 | 25.8 | 34.7 | 40.1 | 12.1 | 23.1 | 26.1 | 34.7 | 40.4 | 12.7 | 22.2 |
AA = African American
Non-AA = Non-African American or Caucasian
Change in dietary components
All participants in this substudy experienced a minimum 4 kg weight loss during Phase I and were randomized to weight maintenance (i.e., Phase II). From baseline to six months (Phase I weight loss period), participants experienced significant decreases in weight (8.4 ± 0.1 kg) and changes in all dietary components (Table 2). Energy intake at baseline was 2,031 ± 953 kcal and decreased on average by 377 ± 30 kcal, with a corresponding 8.1 ± 0.3% decrease in fat, over the six month intervention. Significant increases from baseline to six months were seen in percent kcal from carbohydrate (8.4 ± 0.3%) and protein (0.9 ± 0.1%), daily dairy servings (0.4 ± 0.03) and daily fruit and vegetable servings (3.5 ± 0.1).
Table 2.
Initial variables [mean (SDa)] and adjusted weight change and dietary changes [mean (SEb)], overall and by intervention (Phase I) and maintenance (Phase II) (n = 828).
| Visit | Initial c | Intervention (Phase I) changed |
Maintenance (Phase II) changed |
Overall change d |
|---|---|---|---|---|
| Weight (kg) | 98.6 (16.9) | −8.4 (0.1)*** | 4.4 (0.2)*** | −4.0 (0.2)*** |
| % change | −8.6 (0.1)*** | 5.0 (0.2)*** | −4.1 (0.2)*** | |
| BMI (kg/m2) | 34.0 (5.0) | −2.9 (0.1)*** | 1.5 (0.1)*** | −1.4 (0.1)*** |
| Energy intake (kcal/d) | 2028 (1116) | −376.6 (30.2)*** | 51.5 (24.9)* | −325.1 (33.1)*** |
| Percent energy from carbohydrate | 46.9 (8.8) | 8.4 (0.3)*** | −3.7 (0.4)***+ | 4.7 (0.4)*** |
| Percent energy from fat | 38.5 (7.6) | −8.1 (0.3)*** + | 3.5 (0.3)*** | −4.6 (0.3)*** |
| Percent energy from protein | 15.2 (3.4) | 0.9 (0.1)***ʃ | −0.5 (0.1)*** | 0.4 (0.1)**ʄ |
| Fruit and vegetable servings, number | 5.2 (3.0) | 3.5 (0.1)*** + | −1.9 (0.2)*** | 1.6 (0.12)*** |
| Dairy servings, number | 1.1 (0.9) | 0.4 (0.03)*** | −0.2 (0.03)*** | 0.2 (0.03)*** |
| Dietary fiber (g) | 18.9 (10.8) | 3.7 (0.3)*** | −2.3 (0.4)*** | 1.5 (0.3)**‡ |
SD = Standard Deviation
SE = Standard Error
Adjusted mean (SD) given for initial measures.
Change estimates are mean (SE). Least-squares mean (SE) adjusted for site, age, race, sex, and race-by-sex interaction.
P<0.05,
P<0.01,
P<0.0001
Race difference, P<0.05
Sex difference, P<0.01
Race and Sex interaction, P<0.01;
P<0.05
During the 30 month Phase II maintenance period, participants overall regained approximately 52% of weight lost during the intervention. Even after weight regain, however, participants maintained an average of 4.0 ± 0.2 kg weight loss, corresponding to a −4.1% change, compared to their initial weight prior to Phase I. Dietary changes during Phase II were moderate compared to those during the Phase I initial intervention; however, compared to baseline eating habits prior to entry into Phase I, participants were still consuming fewer kcal, less fat, more carbohydrate, more protein, more dairy, and more fruits and vegetables.
Several observations were made regarding sex and race differences. Overall during the entire period of both phases of the intervention AA males decreased their percentage of protein intake (P=0.03), whereas other groups increased. Males increased fiber intake more than women (P=0.01). During Phase I, it was noted that AAs did not decrease the percentage of energy from fat compared to non-AAs (P=0.03). AA men had the lowest increase in percentage of energy protein (P=0.004). AAs did not increase fruit and vegetable intake as much as non-AAs (P=0.04). During Phase II, it was observed that that AAs did not decrease the percentage of energy from carbohydrate as much as non-AAs (P=0.03).
Effects on weight loss of specific dietary changes
In both the weight loss and weight maintenance phases of the WLM substudy, substitution of carbohydrates for fat, substitution of protein for fat, substitution of protein for carbohydrates, and increasing intake of fruits and vegetables were associated with significant six-month weight loss (Table 3). Increasing dairy intake was associated with significantly less weight regain in Phase II, but not significant weight loss in Phase I. Increasing intake of dietary fiber was not associated with weight loss during either phase of the trial. Substitution of protein for fat was associated with the largest six-month weight changes during the intensive intervention, Phase I, at −0.33 kg per six months (per +1% increase in protein) and resulted in a loss of −0.07 kg per six months during weight maintenance (Phase II). Even after adjusting for total energy intake and physical activity, all three macronutrient changes were associated with larger six-month weight changes during the intervention compared to the weight maintenance phase. Of the three food group changes examined, increasing consumption of fruits and vegetables was associated with the largest six-month weight change during Phase I (−0.29 kg per six months, per +1 serving), while increasing consumption of dairy products was associated with the largest six-month weight change over Phase II (−0.17 kg per six months, per +1 serving).
Table 3.
Average weight change associated with changes in nutrient and food intake (adjusted for site, age, race, sex, race-by-sex interaction, change in total energy intake, and change in amount of physical activity), by study phase (n = 828)
| Phase I (weight loss) | Phase II (weight maintenance) | |||||
|---|---|---|---|---|---|---|
| Effect on weight loss of: | Est. β | (95% CI) | p value | Est. β | 95% CI) | p value |
| Macronutrients | ||||||
| +1% carbohydrates, sub. for fat a | −0.15 a | (−0.19, −0.11) | <0.0001 | −0.03 a | (−0.04, −0.02) | <0.0001 |
| +1% protein, sub. for fat a | −0.33 a | (−0.45, −0.22) | <0.0001 | −0.07 a | (−0.10, −0.05) | <0.0001 |
| +1% protein, sub. for carb. b | −0.18 b | (−0.29, −0.07) | 0.0011 | −0.05 b | (−0.07, −0.02) | 0.0003 |
| Different food groups | ||||||
| +1 fruit/vegetable serving c | −0.29 c | (−0.43, −0.15) | <0.0001 | −0.04 c | (−0.07, −0.01) | 0.0062 |
| +1 dairy serving c | −0.18 c | (−0.58, 0.21) | 0.3619 | −0.17 c | (−0.26, −0.08) | 0.0002 |
| +1g dietary fiber c | 0.01 c | (−0.07, −0.08) | 0.8521 | −0.01 c | (−0.03, 0.004) | 0.1645 |
Mean weight change associated with substituting 1% of macronutrient for isocaloric amount of fat while intake of third macronutrient is held constant
Mean weight change associated with substituting 1% of macronutrient for isocaloric amount of carbohydrates while intake of third macronutrient is held constant
Mean weight change associated with substituting 1 unit of food group for isocaloric amount of other food choices
DISCUSSION
This study reports dietary changes in diverse overweight, at-risk patients with cardiovascular disease who successfully lost weight during an intensive weight loss program and maintained a portion of their weight loss long-term. A recent position paper by the American Dietetic Association (ADA) states that the most important factor influencing weight loss over time is creating a state of negative energy balance (18). One recommended strategy for achieving a reduction in energy balance is modifying macronutrient composition and/or energy density. The WLM trial promoted use of the DASH diet to create negative energy balance during both weight loss and weight maintenance phases. Weight loss was clinically relevant (34–35), and similar to previous weight loss studies. However, weight regain is a common problem and remains a challenge in weight loss interventions (36–37). Weight regain experienced in this study population was comparable with other studies. However, despite weight regain, even modest amounts of weight loss result in clinically significant benefits. Modest weight loss improves cardiovascular risk factors (34, 38–40), decreases systolic blood pressure (41), and promotes a reduction in incident diabetes (35).
Thirty-eight percent of the study population was minority. Minorities are generally underrepresented in weight loss literature, usually experiencing less weight loss (42). Race effects are typically not revealed in studies due to lack of statistical power to show effect. However, this substudy was sufficiently powered to demonstrate significant race effects with regards to dietary composition (e.g., protein, fat, carbohydrates, fruits and vegetables). Although these results should be interpreted cautiously due to the limitations of using multiple comparisons within each regression model, the findings remain unique. Notably, to date, there are no other studies of dietary composition examining race effects. These results suggest that culturally-specific, tailored dietary recommendations, based on preferences for more satiating foods, may prove beneficial in weight loss promotion among AAs.
In addition to a reduction in kcal intake, participants decreased fat intake while increasing protein, dairy, and fruits and vegetables, suggesting that participants were adherent to recommendations of the DASH diet, albeit not to the exact levels promoted. The Dietary Guidelines for Americans 2010 (20) recommends a range of carbohydrate consumption at 45–65% of energy and DASH recommends 55%. In this study, participants were initially at the lower end of daily carbohydrate intake (e.g., 46.9%), met the DASH recommendations in Phase I (55.3%), and ended Phase II at approximately 51%.
The pattern of fat intake varied across the study phases, but resulted in a net deficit suggesting a total decrease in fat over time. Specifically, Phase I resulted in a decrease of fat intake, but increased during Phase II. Nutrition intervention studies have focused on reducing dietary fat; however, long-term results have not always yielded positive results (27, 43–44). Traditional recommendations for macronutrient distribution include decreased dietary fat intake, with favorable short-term weight loss. However, low reported satiety (45–46) and participant inability to sustain adherence long-term has made maintenance of weight loss problematic. Results from this substudy seem to mirror similar issues in the literature; during the short-term, the ability to reduce dietary fat is more pronounced, however, sustaining fat reduction wanes over time.
Similarly, increased energy from protein was observed, yet levels did not reach the DASH dietary pattern for protein (18% of energy). As previously mentioned, there has been an increase in intervention studies focused on decreasing hunger and increasing satiety in order to facilitate weight maintenance and improve long-term adherence (47–49). Many of these diets have had success with weight loss promotion through use of a moderately high protein diet. In this substudy, protein levels were lower than those promoted in these alternative diets. It is plausible that participants experienced difficulty with dietary adherence long-term due to reductions in dietary fat and protein. Future research should systematically assess observed levels of satiety and subjective ratings of fullness to determine whether dietary composition impacts hunger levels.
Gender differences have been observed in the literature regarding fruit and vegetable intakes. In a study involving increased fiber and associated weight loss in women, researchers observed higher fruit and vegetable consumption (50). A recent weight loss study in men by Collins and colleagues (51) found that men reported some positive dietary changes during weight loss, however, significant increases in vegetables and decreases in alcohol were not observed. de Oliveria et al. (50) and Collins et al. (51) studies suggest that the impact of sex differences in dietary changes during weight loss merits additional research. In this substudy, sex effects were analyzed and outcomes interpreted with caution since each model contained multiple comparisons. However, a few sex differences were revealed. AA men had lower increases in protein intake across the study duration, and men, in general, increased fiber more than women. de Oliveria et al. (50) found that women increased dietary fiber through consumption of fruits and vegetables, whereas Collins et al. (51) reported that men did not significantly increase fruit and vegetable intake during the weight loss program. This current substudy reported increased fruit and vegetable intake regardless of sex, yet a significantly higher intake of fiber in men compared to women. Therefore, observations from this substudy suggest that increasing servings of fruits and vegetables regardless of sex has an important role in successful weight loss; while additional dietary fiber intake through sources other than fruits and vegetables may be a preference among men. An examination of gender preferences with regards to diet composition merits further investigation and may elucidate methods to optimize weight loss outcomes based on sex.
Dietary fiber intake significantly increased most likely secondary to increases in fruits and vegetables. Study participants reported a 20% increase in dietary fiber from study start. The ADA’s position statement on health implications for dietary fiber suggests whole foods, high in dietary fiber, assist with weight loss (52). The NHLBI has sponsored several studies on the DASH eating pattern proven to be an effective weight loss diet (53–55). However, dietary fiber per se was not associated with weight loss in the current substudy. This population reported dietary intakes of 24 g/day exceeding the reported 15 g/day previously reported but falling short of the recommended 25 g/day for women and 38 g/day for men (20). Slavin (56) reported that epidemiologic studies support that dietary fiber prevents obesity; fiber intake is inversely associated with body fat, BMI, and weight. However, results from clinical intervention studies are mixed (57–59). General findings suggest that increases in dietary fiber reduce caloric intake resulting in decreased body weight, yet mechanisms of action remain undetermined. While this needs further investigation, it is unknown if specific aspects of DASH or the dietary pattern as a whole contributes to weight loss, but may provide a framework and macronutrient profile for sustaining calorie reduction.
The increase in total number of dairy servings was associated with weight loss in Phase I and significantly associated with less regain in Phase II. Adding low-fat dairy calories, while eliminating products such as sugar sweetened beverages, provides individuals with a better quality diet (60). Zemel et al. (61) found that increasing dietary calcium intake from approximately 400 to 1,200 mg per day during energy restriction resulted in 26% to 28% increase in weight and fat loss, respectively. Another study determined effects of dietary calcium on body weight and fat loss, secondary to energy-restricted diets (500 kcal/d) comparing effects of supplemental calcium carbonate and dairy calcium and found that 400 to 1,300 mg of calcium per day significantly augmented weight and fat loss secondary to caloric restriction (62). Zemel (63) noted that a high-calcium, high dairy diet enhanced efficacy of energy restricted diets in weight control. Although, Zemel’s research suggests that dietary calcium plays an important role in weight loss, other evidence is less conclusive. In a systematic literature review, Lanou and Barnard (64) evaluated 49 trials examining effects of dairy product or calcium intake on body weight. Forty-one found non-significant results suggesting that the majority of clinical trials are not supportive of dairy products or calcium supplementation on weight management. Additionally, ADA’s practice guidelines for Adult Weight Management encourage low fat dairy foods for weight loss; citing research suggesting that calcium intake lower than recommended levels is associated with increased body weight. However, the effects of levels of calcium at or above that recommended for weight loss remains unclear (18).
LIMITATIONS
Because weight loss during Phase I was required for entry into Phase II, there was not comparable dietary information for people who did not lose enough weight to continue into Phase II. Therefore, this could be construed as participation bias since only those who lost weight initially were only eligible for inclusion; hence these results have limitation regarding external validity. It is possible that there were Phase I participants who had similar changes to their diets as those who continued in Phase II, but because they did not lose weight initially, follow-up data was not available for them to be included. In addition, teasing out causal determinants of weight regain in Phase II is challenging. Even though on average participants regained only half the weight lost in Phase I, this is still a significant regain.
Bias may also be associated with self-administered FFQs. Although strategies were in place to assure proper completion, inherent bias exists when individuals are relied upon to complete such an instrument. An additional limitation is that 19.2% of participants were excluded from analysis due to missing FFQ data for at least one time point. Although no systematic differences were found between those with complete versus incomplete FFQ data, exclusion of these participants may limit generalizability of findings.
CONCLUSIONS AND IMPLICATIONS
A great deal of interest surrounds the question of what dietary changes may be most effective in weight loss and maintenance efforts. The results of this substudy indicate that increases in fruits, vegetables, and low-fat dairy, as part of a calorie controlled diet, helps both achieve and maintain weight loss. All of these dietary components contribute to a reduction of total daily kcal intake. Fruits and vegetables, an important component of healthy weight loss programs provide few calories, but considerable amounts of fiber, vitamins, and minerals. This study systematically looked at an at-risk, diverse population, and found interesting race and gender effects. This needs to be further researched so that tailored approaches to diet can be established based on demographic and self-reported food preferences. Further research examining dietary components with current self-reported food preferences might further clarify how individuals maximize weight loss over time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Catherine M. Champagne, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808-4124, Phone: 225-763-2553, Fax: 225-763-3045, catherine.champagne@pbrc.edu.
Stephanie T Broyles, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808-4124, Phone: 225-763-2760, Fax: 225-763-3009, stephanie.broyles@pbrc.edu.
Laura D. Moran, Baton Rouge Clinic, 7373 Perkins Road, Baton Rouge, LA 70808, Phone: 225-246-9413, Fax: 225-246-9159, Lollyd1515@hotmail.com.
Katherine C. Cash, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808-4124, Phone: 225-763-3110 Fax: 225-763-3045, katherine.cash@pbrc.edu.
Erma J. Levy, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808-4124, Phone: 225-763-3167, Fax: 225-763-3045, erma.levy@pbrc.edu.
Pao-Hwa Lin, Department of Medicine, Nephrology Division, Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC, Phone: 919-660-6685, Fax: 919-660-8802, pao.hwa.lin@duke.edu.
Bryan C. Batch, Department of Medicine, Division of Endocrinology, Metabolism and Nutrition, Duke University Medical Center Box 3031, Durham, NC 27710, Phone: 919-681-2168, Fax: 919-681-9846, bryan.batch@duke.edu.
Lillian F. Lien, Division of Endocrinology, Metabolism, and Nutrition, Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27710, Phone: 919-684-9036, Fax: 919-681-7796, lillian.lien@duke.edu.
Kristine L. Funk, Kaiser Permanente Center for Health Research, 3800 N. Interstate Ave., Portland, OR 97227-1110, Phone: 503-335-2443, Fax: 503-335-2428, kristine.funk@kpchr.org.
Arlene Dalcin, Johns Hopkins ProHealth, 1849 Gwynn Oak Avenue Suite 3, Baltimore, MD 21207, Phone: 410-265-1109, Fax: 410-281-1134, adalcin1@jhmi.edu.
Catherine Loria, National Heart, Lung and Blood Institute, National Institutes of Health, Division of Cardiovascular Services, 6701 Rockledge Drive, Ste. 10018, MSC 7936, Bethesda, MD 20892-7936, Phone: 301-435-0702, Fax: 301-480-5158, loriac@mail.nih.gov.
Valerie H. Myers, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808-4124, Phone: 225-763-3085, Fax: 225-763-3045, valerie.myers@pbrc.edu.
REFERENCES
- 1.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obesey estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 3.Brantley P, Appel L, Hollis J, Stevens V, Ard J, Champagne C, Elmer P, Harsha D, Myers V, Proschan M, William V, Svetkey L. Design considerations and rationale of a multi-center trial to sustain weight loss: the Weight Loss Maintenance Trial. Clin Trials. 2008;5:546–556. doi: 10.1177/1740774508096315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150:255–262. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- 5.Wylie-Rosett J, Herman WH, Goldberg RB. Lifestyle intervention to prevent diabetes: intensive and cost effective. Curr Opin Lipidol. 2006;17:37–44. doi: 10.1097/01.mol.0000203890.27267.eb. [DOI] [PubMed] [Google Scholar]
- 6.Burke LE, Sereika SM, Music E, Warziski M, Styn MA, Stone A. Using instrumented paper diaries to document self-monitoring patterns in weight loss. Contemp Clin Trials. 2008;29:182–193. doi: 10.1016/j.cct.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke LE, Styn MA, Glanz K, Ewing LJ, Elci OU, Conroy MB, Sereika SM, Acharya SD, Music E, Keating AL, Sevick MA. SMART trial: A randomized clinical trial of self-monitoring in behavioral weight management-design and baseline findings. Contemp Clin Trials. 2009;30:540–551. doi: 10.1016/j.cct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens VJ, Funk KL, Brantley PJ, Erlinger TP, Myers VH, Champagne CM, Bauck A, Samuel-Hodge CD, Hollis JF. Design and implementation of an interactive website to support long-term maintenance of weight loss. J Med Internet Res. 2008;10:e1. doi: 10.2196/jmir.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones N, Furlanetto DL, Jackson JA, Kinn S. An investigation of obese adults' views of the outcomes of dietary treatment. J Hum Nutr Diet. 2007;20:486–494. doi: 10.1111/j.1365-277X.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 10.Webber KH, Tate DF, Quintiliani LM. Motivational interviewing in internet groups: a pilot study for weight loss. J Am Diet Assoc. 2008;108:1029–1032. doi: 10.1016/j.jada.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008;70:31–39. doi: 10.1016/j.pec.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Hoy MK, Winters BL, Chlebowski RT, Papoutsakis C, Shapiro A, Lubin MP, Thomson CA, Grosvener MB, Copeland T, Falk E, Day K, Blackburn GL. Implementing a low-fat eating plan in the Women's Intervention Nutrition Study. J Am Diet Assoc. 2009;109:688–696. doi: 10.1016/j.jada.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiMarco ID, Klein DA, Clark VL, Wilson GT. The use of motivational interviewing techniques to enhance the efficacy of guided self-help behavioral weight loss treatment. Eat Behav. 2009;10:134–136. doi: 10.1016/j.eatbeh.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 14.VanWormer JJ, Martinez AM, Martinson BC, Crain AL, Benson GA, Cosentino DL, Pronk NP. Self-weighing promotes weight loss for obese adults. Am J Prev Med. 2009;36:70–73. doi: 10.1016/j.amepre.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Macfarlane DJ, Thomas N. Exercise and diet in weight management: updating what works. Br J Sports Med. 2010 Dec;44(16):1197–1201. doi: 10.1136/bjsm.2009.065235. Epub 2009 Oct 20. [DOI] [PubMed] [Google Scholar]
- 16.Andersen RE, Jakicic JM. Interpreting the physical activity guidelines for health and weight management. J Phys Act Health. 2009;6:651–656. doi: 10.1123/jpah.6.5.651. [DOI] [PubMed] [Google Scholar]
- 17.Mekary RA, Feskanich D, Malspeis S, Hu FB, Willett WC, Field AE. Physical activity patterns and prevention of weight gain in premenopausal women. Int J Obes (Lond) 2009;33:1039–1047. doi: 10.1038/ijo.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seagle HM, Strain GW, Makris A, Reeves RS. Position of the American Dietetic Association: weight management. J Am Diet Assoc. 2009;109:330–346. doi: 10.1016/j.jada.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Hill JO. Can a small-changes approach help address the obesity epidemic? A report of the Joint Task Force of the American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. Am J Clin Nutr. 2009;89:477–484. doi: 10.3945/ajcn.2008.26566. [DOI] [PubMed] [Google Scholar]
- 20.Dietary Guidelines for Americans, 2010. 7th Edition. Washington, DC: U.S. Government Printing Office; 2010. Dec, US Department of Agriculture and U.S. Department of Health and Human Services. [Google Scholar]
- 21.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 22.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 23.Astrup A, Grunwald GK, Melanson EL, Saris WH, Hill JO. The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int J Obes Relat Metab Disord. 2000;24:1545–1552. doi: 10.1038/sj.ijo.0801453. [DOI] [PubMed] [Google Scholar]
- 24.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [Erratum, JAMA. 2007;298:178.] [DOI] [PubMed] [Google Scholar]
- 25.Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systematic review of randomized controlled trials of low-carbohydrate, vs low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009;10:36–50. doi: 10.1111/j.1467-789X.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 26.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunešová M, Pihlsgård M, Stender S, Holst C, Saris WH, Astrup A. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abete I, Astrup A, Martínez JA, Thorsdottir I, Zulet MA. Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr Rev. 2010;68(4):214–231. doi: 10.1111/j.1753-4887.2010.00280.x. [DOI] [PubMed] [Google Scholar]
- 28.Harlan LC, Block G. Use of adjustment factors with a brief food frequency questionnaire to obtain nutrient values. Epidemiology. 1990;1:224–231. doi: 10.1097/00001648-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, Champagne CM, Dalcin A, Erlinger TP, Funk K, Laferriere D, Lin PH, Loria CM, Samuel-Hodge C, Vollmer WM, Svetkey LP. Weight Loss Maintenance Trial Research Group. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, Vollmer VM, Gullion CM, Funk K, Smith P, Samuel-Hodge C, Myers V, Lien LF, Laferriere D, Kennedy B, Jerome GJ, Heinith F, Harsha DW, Evans P, Erlinger TP, Dalcin AT, Coughlin J, Charleston J, Champagne CM, Bauck A, Ard JD, Aicher K. Weight Loss Maintenance Collaborative Research Group. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 31.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 32.NutritionQuest. [accessed May 26 2011];Assessment Tools. 2009 http://www.nutritionquest.com/
- 33.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 34.Stevens VJ, Obarzanek E, Cook NR, Lee I, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, Millstone M, Raczynski J, Brewer A, Singh B, Cohen J for the Trials of Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, Phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 35.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J for the Diabetes Prevention Program Research Group. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatr Clin North Am. 2005;28(1):151–170. doi: 10.1016/j.psc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Dansinger ML, Tabsioni A, Wong JB, Chung M, Balk EA. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147(1):41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 38.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM for the Diabetes Prevention Program Research Group. Reduction of the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, Superko HR, Fortmann SP, Albers JJ, Vranizan KM, Ellsworth NM, Terry RB, Haskell WL. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319(18):1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 40.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 41.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 42.Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. Am J Clin Nutr. 1991;53(6S):S1631–S1638. doi: 10.1093/ajcn/53.6.1631S. [DOI] [PubMed] [Google Scholar]
- 43.Anton SD, Han H, York E, Martin CK, Ravussin E, Williamson DA. Effect of calorie restriction on subjective ratings of appetite. J Hum Nutr Diet. 2009;22:141–147. doi: 10.1111/j.1365-277X.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clifton PM, Keogh JB, Noakes M. Long-term effects of a high-protein weight-loss diet. Am J Clin Nutr. 2008;87:23–29. doi: 10.1093/ajcn/87.1.23. [DOI] [PubMed] [Google Scholar]
- 45.Radulian G, Rusu E, Dragomir A, Posea M. Metabolic effects of low glycaemic index diets. Nutr J. 2009;8:5. doi: 10.1186/1475-2891-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Astrup A. Dietary management of obesity. J Parenter Enter Nutr. 2008;32:575–577. doi: 10.1177/0148607108321707. [DOI] [PubMed] [Google Scholar]
- 47.Soenen S, Westerterp-Plantenga MS. Proteins and satiety: implications for weight management. Curr Opin Clin Nutr Metab Care. 2008;11:747–751. doi: 10.1097/MCO.0b013e328311a8c4. [DOI] [PubMed] [Google Scholar]
- 48.Parra D, Ramel A, Bandarra N, Kiely M, Martinez JA, Thorsdottir I. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite. 2008;51:676–680. doi: 10.1016/j.appet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr. 2008;87 Suppl:S1558–S1561. doi: 10.1093/ajcn/87.5.1558S. [DOI] [PubMed] [Google Scholar]
- 50.de Oliveira MC, Sichieri R, Venturim Mozzer R. A low-energy-dense diet adding fruit reduces weight and energy intake in women. Appetite. 2008;51:291–295. doi: 10.1016/j.appet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Collins CE, Morgan PJ, Warren JM, Lubans DR, Callister R. Men participating in a weight-loss intervention are able to implement key dietary messages, but not those relating to vegetables or alcohol: the Self-Help, Exercise and Diet using Internet Technology (SHED-IT) study. Public Health Nutr. 2011;14(1):168–175. doi: 10.1017/S1368980010001916. [DOI] [PubMed] [Google Scholar]
- 52.Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2008;108:1716–1731. doi: 10.1016/j.jada.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Washington, DC: U.S. Government Printing Office; 2006. U.S. Department of Health and Human Services NIH, National Heart, Lung, and Blood Institute. Your Guide to Lowering Your Blood Pressure with DASH. [Google Scholar]
- 54.Miller ER, Erlinger TP, Young DR, Jehn M, Charleston J, Rhodes D, Wasan SK, Appel LJ. Results of the Diet, Exercise, and Weight Loss Intervention Trial (DEW-IT) Hypertension. 2002;40:612. doi: 10.1161/01.hyp.0000037217.96002.8e. [DOI] [PubMed] [Google Scholar]
- 55.Ledikwe JH, Rolls BJ, Smiciklas-Wright H, Mitchell DC, Ard JD, Champagne C, Karanja N, Lin P, Stevens VJ, Appel LJ. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am J Clin Nutr. 2007;85(5):1212–1221. doi: 10.1093/ajcn/85.5.1212. [DOI] [PubMed] [Google Scholar]
- 56.Slavin JL. Dietary fiber and body weight. Nutrition. 2005;721(3):411–418. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Te Morenga LA, Levers MT, Williams SM, Brown RC, Mann J. Comparison of high protein and high fiber weight-loss diets in women with risk factors for the metabolic syndrome: a randomized trial. Nutr J. 2011 Apr 28;10:40. doi: 10.1186/1475-2891-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saquib N, Rock CL, Natarajan L, Flatt SW, Newman VA, Thomson CA, Caan BJ, Pierce JP. Does a healthy diet help weight management among overweight and obese people? Health Educ Behav. 2009 Jun;36(3):518–531. doi: 10.1177/1090198108314617. Epub 2009 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M, Stumvoll M, Stampfer MJ. Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008 Jul 17;359(3):229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 60.Ledikwe JH, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Low-energy-density diets are associated with high diet quality in adults in the United States. J Am Diet Assoc. 2006;106:1172–1180. doi: 10.1016/j.jada.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Zemel MB, Donnelly JE, Smith BK, Sullivan DK, Richards J, Morgan-Hanusa D, Mayo MS, Sun X, Cook-Wiens G, Bailey BW, Van Walleghen EL, Washburn RA. Effects of dairy intake on weight maintenance. Nutr Metab. 2008;5:28. doi: 10.1186/1743-7075-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zemel MB, Thompson W, Milstead A, Morris K, Campbell P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes Res. 2004;12:582–590. doi: 10.1038/oby.2004.67. [DOI] [PubMed] [Google Scholar]
- 63.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr. 2005;24:537S–546S. doi: 10.1080/07315724.2005.10719502. [DOI] [PubMed] [Google Scholar]
- 64.Lanou AJ, Barnard ND. Dairy and weight loss hypothesis: an evaluation of the clinical trials. Nutr Rev. 2008;66(5):272–279. doi: 10.1111/j.1753-4887.2008.00032.x. [DOI] [PubMed] [Google Scholar]