Abstract
The evolutionarily conserved Six1-Eya1 transcription complex is central to mammalian organogenesis, and deletion of these genes in mice results in developmental anomalies of multiple organs that recapitulate human branchio-oto-renal (BOR) and DiGeorge syndromes. Here, we report that both Six1 and Eya1 are strongly expressed in the peri-cloacal mesenchyme (PCM) surrounding the cloaca, the terminal end of hindgut dilation. Six1 and Eya1 are absent from the intra-cloacal mesenchyme (ICM), a cell mass that divides the cloaca into dorsal hindgut and ventral urogenital sinus. Deletion of either or both Six1 and Eya1 genes results in a spectrum of genitourinary tract defects including persistent cloaca - hypoplastic perineum tissue between external urogenital and anorectal tracts; hypospadias - ectopic ventral positioning of the urethral orifice; and hypoplastic genitalia. Analyses of critical signaling molecules indicate normal expression of Shh in the cloaca and cloaca-derived endodermal epithelia. Using a Cre/loxP genetic fate mapping strategy, we demonstrate that Six1-positive PCM progenitors give rise to the most caudal structures of the body plan including the urogenital and anorectal complex, and the perineum region. Thus, Six1 and Eya1 are key regulators of both upper and lower urinary tract morphogenesis. Results from this study uncover essential roles of the PCM progenitors during genitourinary tract formation.
INTRODUCTION
Understanding morphogenesis remains a major challenge in developmental biology. At the most caudal end of a developing mammal, the embryonic cloaca undergoes morphological changes to form two separate structures: the dorsal anorectal and the ventral urogenital tracts. Caudal cloaca endodermal epithelial cells make direct contact with ectodermal epithelia to form the cloacal membrane (CM), where there is no intervening intra-embryonic mesoderm; a similar situation is observed at the rostral extremity of the gut tube in the oropharyngeal membrane. Mesodermal progenitors from the caudal part of the primitive streak pass around the side of the CM to form the peri-cloacal mesenchyme (PCM). The intra-cloacal mesenchyme (ICM) forms a cell mass that is known as the urorectal septum. Asymmetric growth and patterning of the cloacal mesoderm result in the division of the cloacal cavity and formation of the genital tubercle (GT). Malformations of the urogenital and anorectal structures are among the most common forms of congenital human birth anomalies. However, the molecular embryology of these caudal structures remains poorly understood.
Patterning of the cloacal mesoderm and morphogenesis of caudal structures likely depends on the coordinated actions of intrinsic transcriptional regulators and extrinsic signaling molecules. Recent studies have identified key extrinsic signals including sonic hedgehog (Shh) (Haraguchi et al., 2001; Haraguchi et al., 2007; Lin et al., 2009; Miyagawa et al., 2009a; Perriton et al., 2002; Petiot et al., 2005; Seifert et al., 2009a; Seifert et al., 2009c), fibroblast growth factors (Fgfs) (Haraguchi et al., 2000; Petiot et al., 2005; Yucel et al., 2004), bone morphogenetic proteins (Bmps) (Morgan et al., 2003; Suzuki et al., 2003; Wu et al., 2009), Wnts (Lin et al., 2008; Miyagawa et al., 2009a; Nakata et al., 2009; Yamaguchi et al., 1999), and ephrins (Dravis et al., 2004). However, our understanding of the intrinsic transcriptional mechanisms underlying these key developmental processes remains limited (Lin et al., 2008; Mo et al., 2001; Morgan et al., 2003; Scott et al., 2005). We, as well as others, have shown previously that Six1 and Eya1 transcription factors are critical regulators of mammalian organogenesis (Li et al., 2003; Oliver et al., 1995; Xu et al., 1999; Xu et al., 1997; Xu et al., 2003). Mouse deletions of Six1 and Eya1 recapitulate the most common features found in human branchio-oto-renal syndrome (BOR) and DiGeorge/22q11 deletion/velo-cardio-facial syndromes (Guo et al., 2011; Li et al., 2003; Ruf et al., 2004; Xu et al., 1999). Here, we report functional characterization of the Six1 and Eya1 transcription factors, and genetic fate mapping of Six1-positive progenitor cells during genitourinary tract development. Results from these studies show that 1) Six1 and Eya1 transcription factors are critical intrinsic regulators of PCM; 2) cells of the ICM do not express either Six1 or Eya1, suggesting that ICM is molecularly distinct from PCM; 3) deletion of Six1 and/or Eya1 causes a spectrum of genitourinary tract defects including persistent cloaca, hypospadias and hypoplastic genitalia; and 4) the Six1-positive PCM but not ICM is the major source of progenitors of caudal structures of the body plan including the urogenital and anorectal complexes, and the perineum region. Together, our findings identify an essential set of transcription regulators in the PCM progenitor cells and begin to shed light on cloacal morphogenesis and human congenital urogenital/anorectal anomalies.
MATERIALS AND METHODS
Mice
Six1Cre-hpAP/+ mice were generated using a strategy identical to that used to create the Six1lacZ mutant (Li et al., 2003). Briefly, a partial Six1 coding sequence was replaced with the Cre-hpAP (human placenta alkaline phosphatase linked to an internal ribosomal entry site (IRES)) dicistronic reporter and a PGK-neo selection gene by homologous recombination in mouse 129sv-ES cells. The resulting Six1Cre-hpAP/+ allele is Six1 null. Four independent ES cell clones were identified by genomic Southern blot analyses using both 5' and 3' external probes. Two homologous recombinant clones were used for generating chimeras and the mutant allele was transmitted through the germline to obtain Six1Cre-hpAP/+ heterozygous mutants. Homozygous mutants from these two lines of ES cells showed identical phenotypes and results from one line are presented in this article. The Six1lacZ, Eya1, R26RZ/+ mice have been described previously (Li et al., 2003; Soriano, 1999; Xu et al., 1999).
Histology analyses, in situ hybridization, X-gal and AP staining
Embryos for histology and in situ hybridization analyses were dissected in cold PBS and fixed with 4% paraformaldehyde (PFA). Hematoxylin and Eosin (H&E) staining and in situ hybridization were performed as described (Guo et al., 2011). To visualize hpAP activity of Six1Cre-hpAP/+ mice, staged heterozygous embryos were fixed with PFA, heated at 70°C to inactivate endogenous AP activity and then stained with BM-purple (Roche).β-galactosidase activity of Six1Cre-hpAP/+;R26RZ/+ embryos was detected with previously described methods (Li et al., 2003; Li et al., 2002).
Cell proliferation and cell death analyses
Phospho-histone H3 Ser10 antibody (Upstate 06-570) was utilized to label proliferating cells as previously reported (Guo et al., 2011). Sections were counterstained with DAPI to visualize tissue structure. Lysotracker® (Invitrogen) staining of apoptotic cells was performed according to the manufacturer’s protocol. Briefly, freshly dissected embryos were stained with 5 µM Lysotracker® dye in pre-warmed PBS at 37°C for 0.5 h. Genital structures were dissected, fixed with PFA and sectioned before imaging. Terminal deoxynucleartidyle transferase nick end labeling (TUNEL) assay was performed on frozen sections using the Cell Death Detection kit (Roche).
Quantitative PCR
GT tissue of e13.5 embryos was microdissected out and snap frozen on dry ice/ethanol. RNA was prepared and reverse transcribed according to manufacturer’s protocols (Qiagen RNeasy mini and Stratagene Accuscript™ High Fidelity 1st strand cDNA synthesis). Relative gene expression levels were normalized to a GAPDH internal control, analyzed using the SYBR Green method on an ABI-7500 detector (Applied BioSystems). The following oligos were used: AR F: CCT TGG ATG GAG AAC TAC TCC G; AR R: TCC GTA GTG ACA GCC AGA AGC T; Dusp6 F: CTC GGA TCA CTG GAG CCA AAA C; Dusp6 R: TCT GCA TGA GGT ACG CCA CTG T; GAPDH F: TTG TCT CCT GCG ACT TCA AC; GAPDH R: GTC ATA CCA GGA AAT GAG CTT G; Hoxa13 F: CCC AAA GAG CAG ACG CAG CCT; Hoxa13 R: GTG TAA GGC ACG CGC TTC TTT C; Msx1 F: AGG ACT CCT CAA GCT GCC AGA A; Msx1 R: CGG TTG GTC TTG TGC TTG CGT A; Msx2 F: AAG ACG GAG CAC CGT GGA TAC A; Msx2 R: CGG TTG GTC TTG TGT TTC CTC AG; Shh F: GGA TGA GGA AAA CAC GGG AGC A; Shh R: TCA TCC CAG CCC TCG GTC ACT; Grem1 F: AGG TGC TTG AGT CCA GCC AAG A; Grem1 R: TCC TCG TGG ATG GTC TGC TTC A.
RESULTS
Six1 and Eya1 are expressed in the PCM progenitor cells
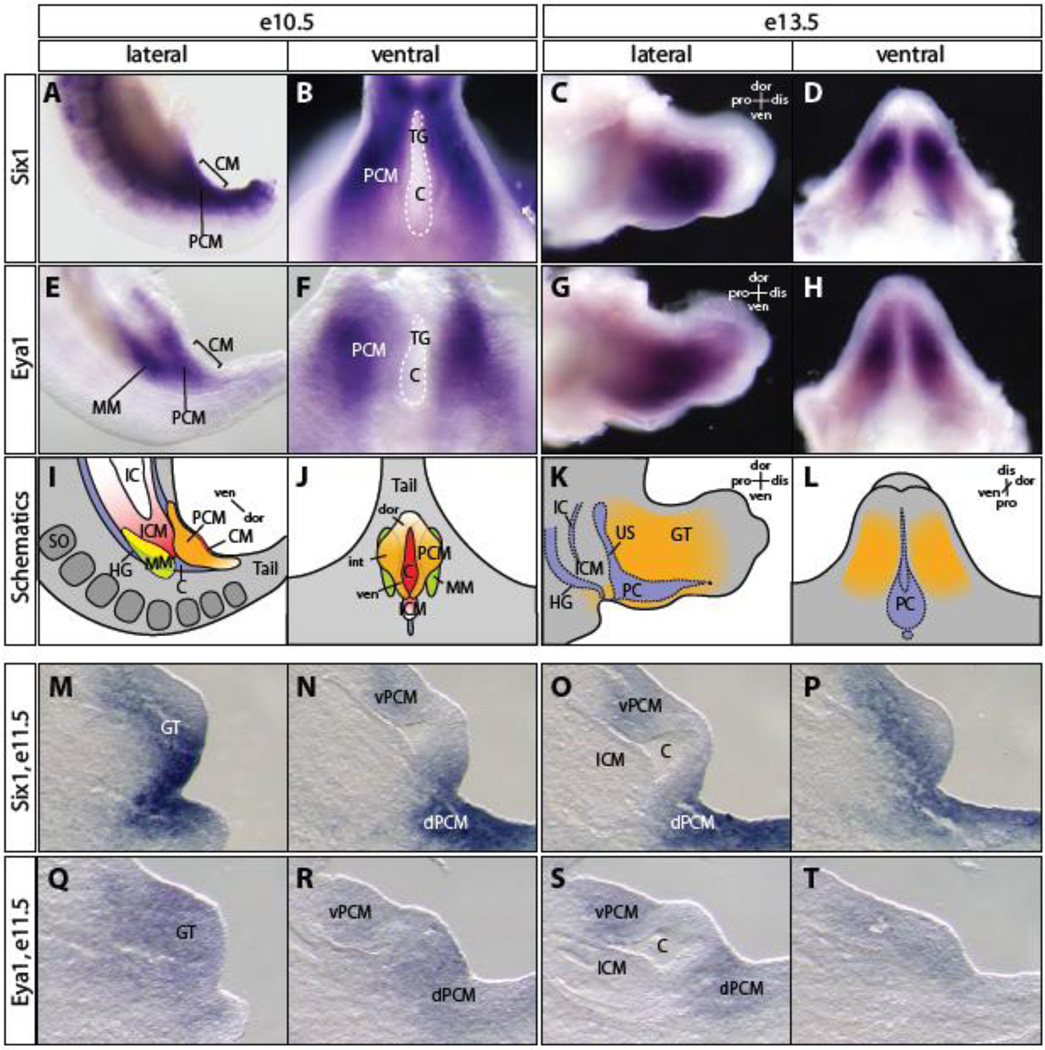
The renal and genitourinary systems are descendents of the intermediate mesoderm. The juxtaposition and intimate relationship of the renal and genitourinary mesoderm with the cloacal endoderm suggests that they are part of a common developmental entity. Therefore, the molecular programs underlying development of these structures might be integrated. We and others have shown that Six1 and Eya1 are critical regulators of early stages of renal development (Li et al., 2003; Xu et al., 1999; Xu et al., 2003). To investigate the possibility that Six1 and Eya1 might also be involved in the formation of the genital system, we first examined whether Six1 and Eya1 are expressed in mesodermal progenitor cells surrounding the cloaca (Figure 1). As reported previously (Li et al., 2003; Oliver et al., 1995; Xu et al., 1997), both Six1 and Eya1 were expressed in the intermediate mesoderm progenitors, including the metanephric mesenchyme (MM), at embryonic day 10.5 (e10.5). Six1 was also expressed in the somites of the paraxial mesoderm. In addition, both Six1 and Eya1 were expressed in the PCM cells surrounding the cloaca at e10.5 and in the developing GT at e13.5 (Figure 1A-L).
Figure 1. Spatiotemporal expression patterns of Six1 and Eya1 during genitourinary tract development.
(A-H) whole mount in situ hybridization using Six1 (A-D) and Eya1 (E-H) specific probes revealed their broad expression pattern in peri-cloacal mesenchyme (PCM) cells surrounding cloaca and metanepharic mesenchyme (MM) at e10.5 (A, B, E and F) and genital mesenchymal cells at e13.5 (C, D, G and H). (I-L) Schematic representation of Six1 and Eya1 expression patterns in PCM (orange), MM (green) and intra-cloacal mesenchyme (ICM, pink) at e10.5 (I, J) and genital mesenchyme (orange) at e13.5 (K, L). Cloaca membrane (CM) is red. (M-T) Section in situ hybridization of e12 and e11.75 serial sagittal sections showed Six1 (M-P) and Eya1 (Q-T) expression in the PCM but not ICM cells. C, cloaca; dis, distal; dor, dorsal; dPCM, dorsal PCM; GT, genital tubercle; HG, hindgut; IC, intraembryonic coelom; int, intermediate; iPCM, intermediate PCM; PC, phallic cloaca; pro, proximal; SO, somite; TG, tail gut; US, urogenital sinus; ven, ventral; vPCM, ventral PCM.
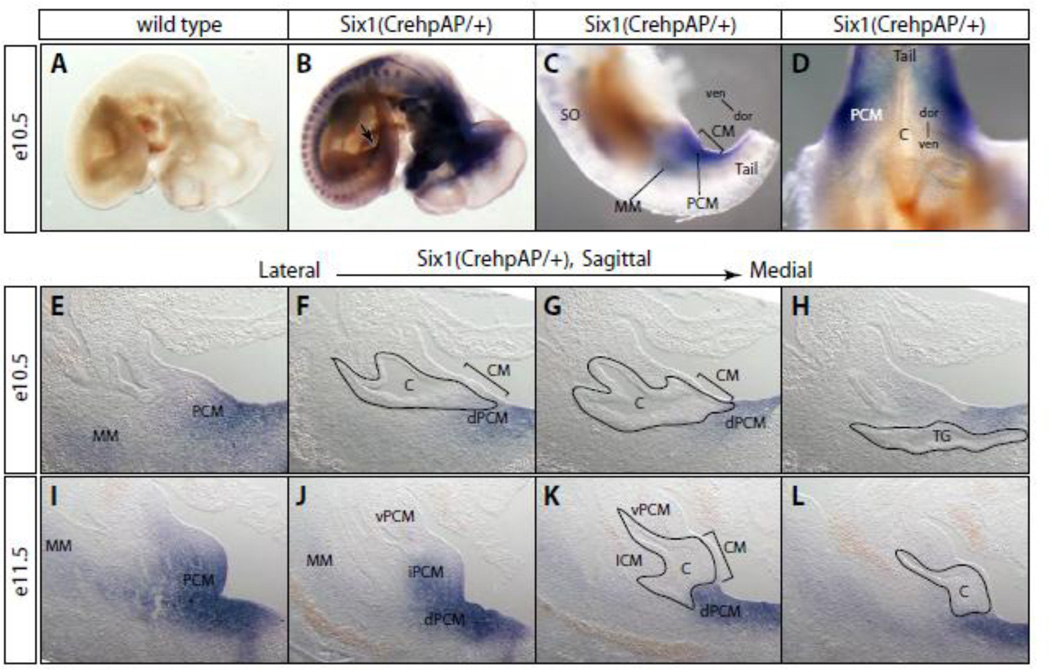
Mesenchymal cells surrounding cloaca can be defined by their geometric positions along body axes. The dorsal PCM (dPCM), which is also known as tail gut mesenchyme, caps the most caudal end of the cloacal cavity adjacent to the dorsal cloaca membrane (dCM). The intermediate PCM (iPCM) forms bilateral genital folds along the sides of cloaca. The ventral PCM (vPCM) cells form the infraumbilical ventral wall at this stage. To determine the spatiotemporal expression pattern of Six1 and Eya1 during cloaca morphogenesis, we performed section in situ hybridization experiments on serial sagittal sections at e11.5 (Figure 1M-T). At this stage, genital protrusion becomes apparent as a consequence of the rapid increase of the number of iPCM and vPCM cells. As expected from the whole mount in situ results at e10.5, Six1 and Eya1 were continuously expressed in the PCM at e11.5 (Figure 1M-T). Six1 appeared to have stronger expression in the dPCM than the vPCM (Figure 1MP). However, neither Six1 nor Eya1 was detected in the ICM at any stage analyzed.
At e13.5, asymmetric growth of PCM cells along the dorsoventral axis has successfully relocated the CM to the ventral side of the GT, and the dorsoventral axis of CM is now elongated into the proximodistal axis of GT. The dorsoventral axis of PCM along CM is reversed to become the ventrodorsal axis of GT. Both Six1 and Eya1 were continuously expressed in a sub-population of the GT mesenchyme (Figure 1C, D, G and H).
Six1 and Eya1 mutants exhibit urogenital and anorectal defects
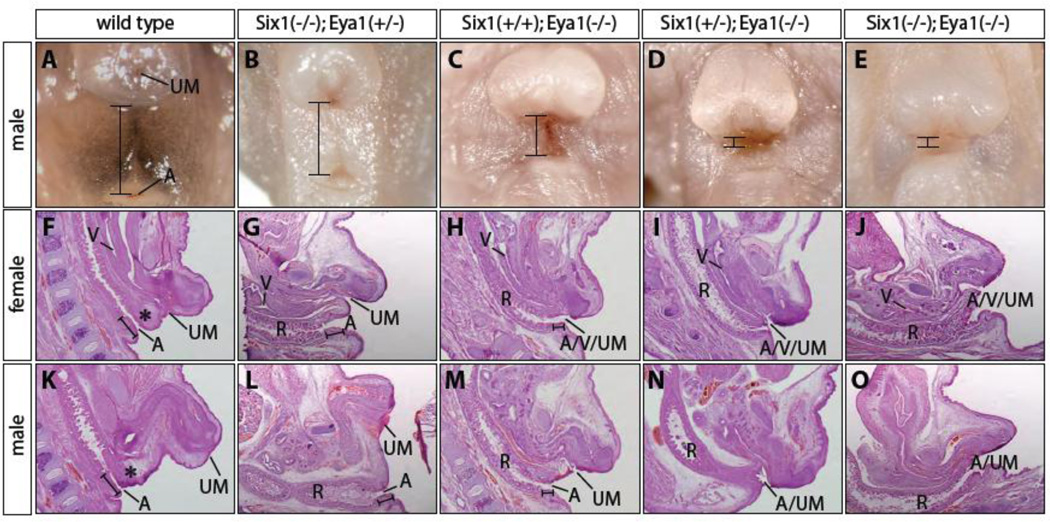
To determine the potential roles of Six1 and Eya1 transcription factors in the formation of the caudal structures, we examined e17.5 and newborn single and compound Six1;Eya1 mouse gene-deletion mutants (Figure 2). Less than 30% of Six1−/− mutants had hypospadias phenotype, where urinary meatus (UM) was displaced at the ventral and proximal region of the genitalia (data not shown). Loss of one copy of Eya1 increased penetrance of the Six1−/− hypospadias phenotype to 100% (Figure 2B). All Eya1 mutants had severe hypospadias phenotype and hypoplastic genitalia (Figure 2C). Additional loss of one or both copies of Six1 gene increased severity of genital phenotype of Eya1 null mutants (Figures 2D and E), an observation that is consistent with synergistic relationship between Six1 and Eya1 during renal and cardiac development (Guo et al., 2011; Li et al., 2003; Sajithlal et al., 2005). The perineum, which separates the base of genitalia and anus, was hypoplastic in the Eya1 mutant and was completely absent from the Six1+/−;Eya1−/− and double null mutants (Figures 2C, D and E). The anogenital distance was reduced by 50% in Eya1−/− mutants and almost nonexistent in the Six1+/−;Eya1−/− and double null mutants (Figures 2C, D and E, bracket). Thus, while Eya1 seems to play predominant roles, Six1 and Eya1 synergistically regulate lower urinary tract development.
Figure 2. Genitourinary tract phenotypes of Six1 and Eya1 mutants.
(A-E) Gross defects of urogenital and anorectal complex of newborn male pups. Brackets indicate anogenital distance, which is reduced in the Six1+/+;Eya1−/− (C), Six1+/−;Eya1−/− (D) and Six1−/−;Eya1−/− (E) mutants. (F-O) H&E histological analyses of newborn female pups (F-J) and male pups (KO). Urogenital and anorectal systems were separated by perineum (black asterisk), which was hypoplastic (G, H, L and M) or completely absent (I, J, N and O). The severe Six1+/−;Eya1−/− and Six1−/−;Eya1−/− mutant phenotype (I, J, N and O) resembles persistent cloaca defect, in which the urinary meatus (UM), vagina (V) and anus (A) share a common opening. Brackets indicate anal channel. R, rectum.
To confirm these gross observations, we performed histological analysis of sagittal sections of both male and female mutants (Figure 2F-O). At e17.5, a dense population of stromal cells was clearly visible in the perineum of both male and female control embryos (Figures 2F and K, black asterisks). This tissue, however, was hypoplastic in the Eya1 mutant, Six1;Eya1 compound mutants, and was completely absent from double null mutants (Figures 2H, M, I, N, J and O). In female mutants, rectum, vagina and urinary tract opened to a common channel, which resembles human persistent cloaca anomaly (Figures 2H, I and J). A similar cloaca phenotype was found in the male compound mutants (Figures 2N and O). In addition, anal channel was shorter in the mutants compared to controls (Figures 2L, M, N andO). Together, these findings demonstrate the essential roles of Six1 and Eya1 in the PCM cells and normal development of urogenital and anorectal structures.
Proliferation and survival defects of the mutant genital tubercle
We have shown previously that Six1 and Eya1 are required for proliferation and survival of both renal and cardiac progenitor cells (Guo et al., 2011; Li et al., 2003; Xu et al., 1999). To determine if a similar mechanism underlies GT development, we examined expression of phospho-histone H3 (p-HH3), which is enriched in the G2/M phases and is indicative of cell proliferation. We also investigated the extent of cell death using both LysoTracker® and TUNEL assays (Figure 3).
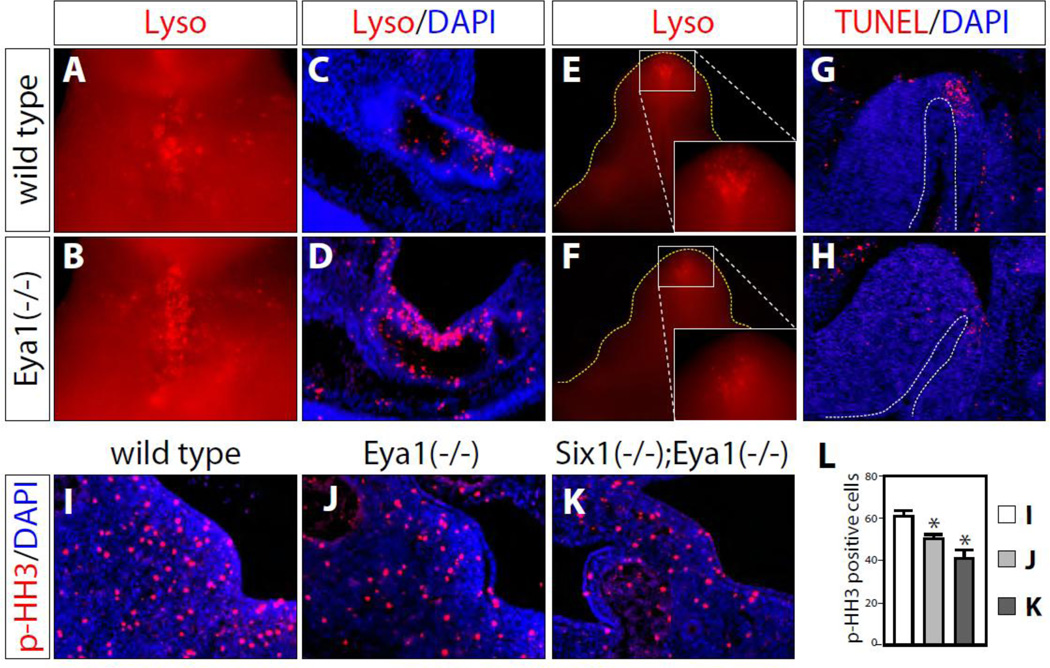
Figure 3. Proliferation and apoptosis defects.
(A-D) LysoTracker® stained apoptotic cells of e10.5 wild type control (A and C) and Eya1 mutant (B and D). A and B, wholemount ventral views; C and D, sagittal sections. More apoptotic cells were detected in Eya1 mutant embryos (B and D). (E-H) At e13.5, Eya1 mutants (F and H) had less apoptotic cells than wild type controls (E and G) based on both LysoTracker® staining (E and F) and TUNEL staining (G and H). E and F, ventral views; G and H, sagittal sections. (I-L) Less phospho-histone H3 (p-HH3) positive (red) mesenchyme cells in e11.0 Eya1 mutant (J) and Six1−/−;Eya1−/− compound mutant GT (K) than littermate wild type controls (I). L, Quantification of these results presented in I-K (* p<0.05, n=3).
At e10.5, compared with littermate controls, Eya1 mutants had many more apoptotic cells labeled by whole mount LysoTracker® red staining (Figure 3A-B). Sections of stained e10.5 embryos showed that cell death was primarily localized at the cloacal epithelium (Figure 3C-D). At e11.5, with the growth of GT, less cell death was detected in wild type GT. However, we observed an increase in apoptotic cells in Eya1 mutants and even more so in Six1+/−; Eya1−/− compound mutants (data not shown). At e13.5, apoptosis was found at the bilateral regions near distal urethral epithelial region (dUE) in the wild type GTs at e13.5 as reported previously (Haraguchi et al., 2001; Sasaki et al., 2004) (Figures 3E and 3G). Interestingly, Eya1 mutants exhibited less apoptosis based on whole mount LysoTracker® red staining at this stage (Figure 3F). This finding was confirmed by TUNEL assay (Figure 3G-H).
At e11.0, p-HH3 staining of serial sagittal sections detected many proliferating cells in the genital mesenchyme of wild type controls, Eya1 null and Six1−/−; Eya1−/− compound mutants (Figure 3I-L). The number of p-HH3 positive cells was significantly lower in Eya1 null mutants (Figure 3J, p=0.0264, n=4) than in the wild type littermate controls (Figures 3I and L), and it was further reduced in Six1−/−; Eya1−/− double null mutants (Figures 3K and L, p=0.006, n=3). Thus, similar to other organs, Six1 and Eya1 are required for progenitor cell proliferation (Guo et al., 2011; Li et al., 2003; Xu et al., 1999). Collectively, these data suggest that the Six1/Eya1 transcription complex controls homeostasis of GT mesenchymal progenitor cell proliferation and survival.
Bmp signaling is aberrantly up regulated in mutants
To investigate whether Six1/Eya1 transcription complex is involved in actions of signaling pathways, we examined expression pattern of candidate genes involved in Shh, Bmp and Fgf signaling pathways. Shh is expressed in the endodermal progenitor cells of urogenital and anorectal tracts. Shh deletion results in persistent cloaca and genital agenesis phenotypes (Haraguchi et al., 2001; Mo et al., 2001; Perriton et al., 2002), and inactivation of Shh at later developmental stages causes hypoplastic genitalia and hypospadias defects (Lin et al., 2009; Miyagawa et al., 2009a; Seifert et al., 2009a). A recent study demonstrated that Shh signaling regulates the cell cycle kinetics of GT mesenchyme and therefore growth of genitalia (Seifert et al., 2009c). A similar level of Shh was observed in the Eya1 mutant and Six1;Eya1 compound mutants with the stage-matched littermate controls in the urethral plate, bladder and hindgut at e11.5 and e13.5 (Figure 4A-D and 4O, and data not shown). However, the mutant urethral plate labeled by Shh appeared to be shorter than that of controls, which was consistent with the grossly smaller genitalia of the mutants at later developmental stages (Figure 2A-D).
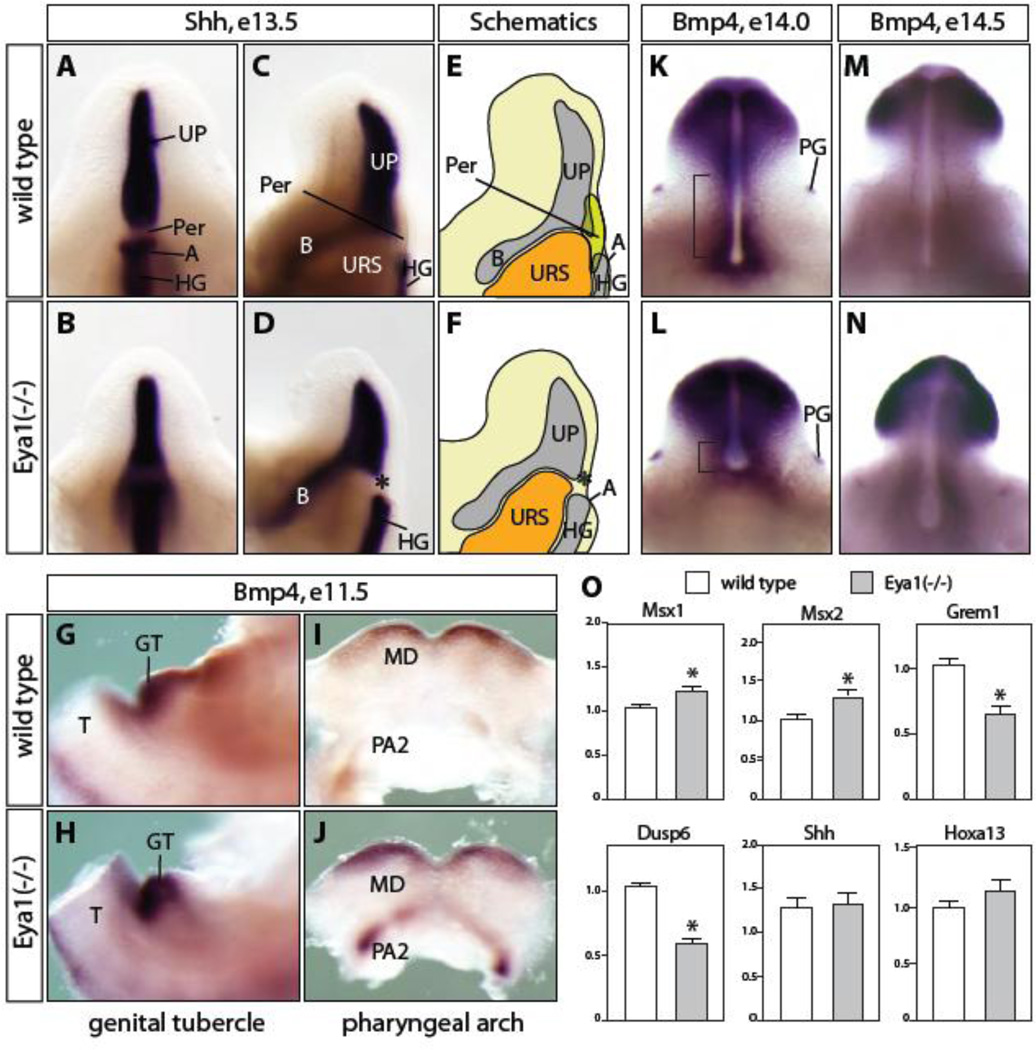
Figure 4. Gene expression analyses of Shh, Fgf and Bmp signaling pathways.
(A-D) Shh expression in Eya1 mutants (B, D) remained at a similar level to wild type control (A, C) in the urethral plate (UP) and hindgut (HG) at e13.5. A cell mass at the perineum region (Per) was not observed in the mutants (asterisk in D and F). Shh expression in the bladder epithelium was similar to the wild type control, although it appeared weaker in controls than mutants on the sagittal views. (E and F) Schematic representations of C and D, respectively. (G-J) Bmp4 expression was enhanced in the GT mesenchyme region (H) and the mandible domain of the first pharyngeal arch (MD) and the second pharyngeal arch (PA2) of e11.5 Eya1 mutant (J). (K-N) Bmp4 expression domain was reduced in Eya1 mutants at e14.0 (bracket in K, L) and e14.5 (M, N), while stayed the same in preputial glands (PG) of Eya1 mutants (L). (O) Quantitative PCR analyses for Msx1, Msx2, Grem1, Dusp6, Shh and Hoxa13 expression in e13.5 Eya1 mutants and wild type littermate control (n=4). A, anus; B, bladder; T, tail; URS, urorectal septum.
Unlike Shh, Bmp4 is expressed in the PCM and GT mesenchymal cells during genitourinary tract development (Lin et al., 2008; Lin et al., 2009; Suzuki et al., 2003), which is similar to the expression pattern of Six1 and Eya1. Bmp4 is regulated by the activities of both Shh and canonical Wnt/β-catenin signaling pathways (Lin et al., 2008; Lin et al., 2009; Miyagawa et al., 2009a). Exogenous Bmp4 suppresses cell proliferation and promotes apoptosis in GT organ culture (Suzuki et al., 2003). Conditional deletion of Bmp receptor 1a (Bmpr1a) in the surface ectoderm of GT results in increased expression of Fgf8 in the distal urethral plate endoderm and confers an overall growth advantage to the genitalia (Suzuki et al., 2003). Thus, Bmp4 is a critical component of the signaling network controlling mesenchymal cell proliferation and survival during GT development. We have reported previously that Bmp4 was ectopically up-regulated and Fgf8 was down regulated in the Six1;Eya1 compound mutants during cardiovascular development (Guo et al., 2011) (data not shown). In the Eya1 mutants, Bmp4 expression in the GT mesenchyme was increased at e11.5 (Figure 4G and H). Consistently, Bmp4 expression was expanded in mandible component of the first pharyngeal arch and significantly increased in the second pharyngeal arch (Figure 4I and J). At late stages of GT development (e14.0 and e14.5 Figure 4K-N), mutants were significantly smaller than the wild type controls. Expression of Bmp4 was maintained in the distal GT region and mesenchymal cells surrounding urethral plate (Figure 4K-N). Real time quantitative PCR (rt-qPCR) analyses, however, did not detect any significant increases of Bmp4 expression in the micro-dissected Eya1−/− and Six1+/−;Eya1−/− GTs at these stages (data not shown). Since expression of Grem1, a Bmp antagonist, is Six1-dependent during renal development (Nie et al., 2011), we examined its expression level in the micro-dissected e13.5 GT tissue (Figure 4O). We found that Grem1 was significantly lower in Eya1−/− and Six1+/−;Eya1−/− mutants based on quantitative rt-qPCR analyses (Figure 4O and data not shown). Consistently, expression levels of Bmp4 downstream target genes, Msx1 and Msx2 (Alappat et al., 2003; Hayashi et al., 2006; Suzuki et al., 2003), were significantly up regulated in Eya1−/− and Six1+/−;Eya1−/− mutants, suggesting enhanced Bmp signaling in the absence of Eya1 and Six1 (Figure 4O, n=4, and data not shown). Expression of Fgf8, which is inversely correlated with Bmp signaling during genital development (Suzuki et al., 2003), did not show any statistically significant difference (data not shown). However, a downstream effector of Fgf signaling, dual specificity protein phosphatase 6 (Dusp6) (Seifert et al., 2009b), was dramatically reduced in Eya1−/− and Six1+/−;Eya1−/− mutants (p<0.0001) (Figure 4O and data not shown). Collectively, Eya1 and Six1 mutants had augmented Bmp but attenuated Fgf signaling activities.
Inactivation of Hoxa13 in mice leads to hypospadias and reduced AR expression (Morgan et al., 2003), and AR is essential for GT development and masculinization (Miyagawa et al., 2009b; Yucel et al., 2004). No significant difference was observed for Hoxa13 and AR expression in Eya1 null mutants based on rt-qPCR analyses of microdissected external GT tissues (Figure 4O and data not shown).
Fate mapping of the Six1-positive progenitor cells
Since Six1 and Eya1 are present in the PCM but absent from the ICM (Figure 1M-T), the perineal agenesis and cloacal phenotypes suggested that the PCM, as opposed to the ICM, contains the progenitors of tissue between the anus and genitalia. To examine this possibility directly, we generated a Cre-expressing mouse line by replacing the Six1 coding region with a Cre-hpAP dicistronic reporter (data not shown). With this approach, expression of Cre site-specific DNA recombinase and a second cistron of human placental alkaline phosphatase (hpAP) are under control of the endogenous Six1 promoter and enhancer and, therefore, are expected to show spatiotemporal expression patterns identical to that of the native Six1 gene. Four independent homologous recombinant mouse embryonic stem (ES) cell clones were identified, and two of them were used to generate the Six1Cre-hpAP heterozygous mutant lines. The heterozygous mutants displayed no apparent phenotype while the homozygous mutants had phenotypes identical to previous reports (Laclef et al., 2003; Li et al., 2003; Ozaki et al., 2004).
We first analyzed the hpAP staining pattern of the Six1Cre-hpAP/+ heterozygous embryos to confirm that the Cre-hpAP reporter fully recapitulated endogenous Six1 gene expression pattern. Strong hpAP activity was detected in the craniofacial regions including nasal, otic and pituitary placodes at e10.5 (Figure 5B). In the trunk region, both somites and dorsal root ganglia stained positive for hpAP. Muscle progenitor cells migrating into the limb bud were labeled as well. We also observed hpAP activity in the MM of renal progenitor cells. Similar to the Six1-specific in situ hybridization results (Figure 1A and B), hpAP activity was strongly detected in PCM progenitor cells surrounding the cloaca (Figure 5C and D). Staining of serial histological sections confirmed the PCM-specific expression pattern in both e10.5 and e11.5 embryos (Figure 5E-L). Expression in the dPCM was observed at e10.5 and increased at e11.5. At e11.5, hpAP activity was also detected in the iPCM and vPCM, but not the ICM (Figure 5J and K). Overall, the hpAP staining pattern in the PCM suggested the possibility that Six1 is asymmetrically localized along the dorsoventral axis with a strong dorsal expression and a weak ventral expression (Figure 1M-P).
Figure 5. A detailed Six1 gene expression analysis using the hpAP knockin reporter.
(A-D) Whole mount hpAP staining of wild type (A) and Six1Cre-hpAP heterozygous embryos (BD). (E-L) Serial sagittal cryostat sections through cloaca of the hpAP stained heterozygous embryos at e10.5 (E-H) and e11.5 (I-L). (C-H) hpAP activity was observed in the PCM cells surrounding cloaca at e10.5 (C-H) and e11.5 (I-L). See Figure 1 legend for more abbreviations.
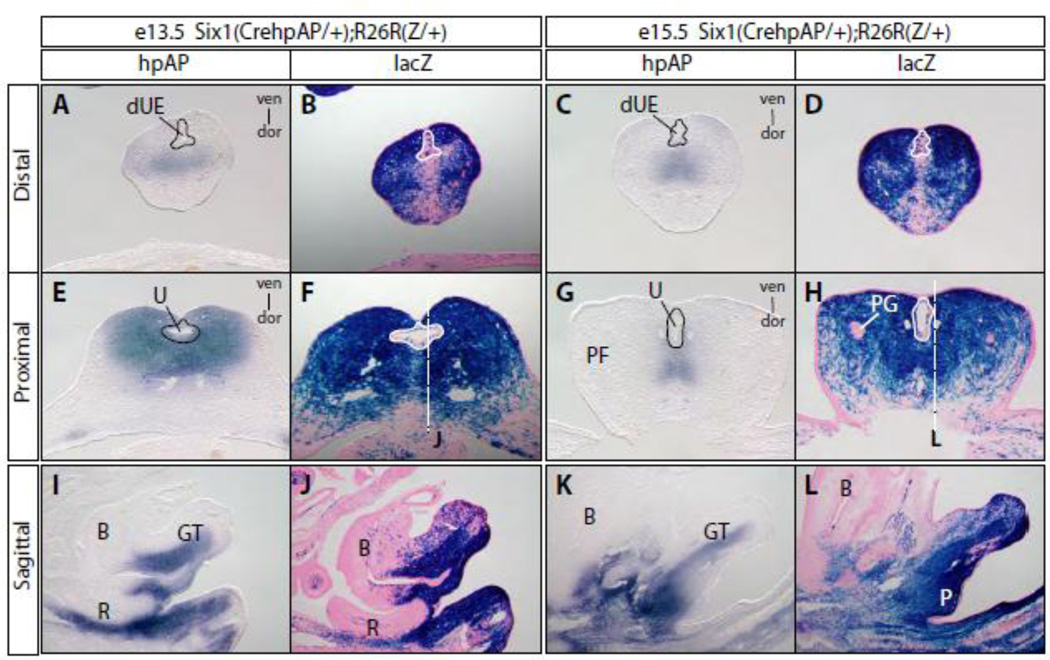
To determine the fate of Six1-positive progenitors during genitourinary tract development, we analyzed the Six1Cre-hpAP/+ and Rosa26-lacZ reporter (R26RZ/+) double heterozygous embryos, in which Six1 lineages were permanently labeled by the lacZ reporter. Serial cross and sagittal sections of GT from double heterozygous embryos at e13.5 and e15.5 were stained for hpAP and β-gal activities, which are surrogates of Six1 gene expression and Six1 lineages, respectively (Figure 6). In the distal GT, hpAP activity was restricted to the center of the GT and dorsal to the developing urethra. In the proximal region, however, hpAP activity was detected broadly at e13.5, but became restricted at e15.5 (Figure 6E and G). The hpAP staining pattern at e15.5 suggested that the stained cells belonged to the glans tissue. Staining of sagittal sections revealed restricted hpAP activity at the perineum, bladder neck region, mesenchyme surrounding the rectum and at the tail (Figure 6I, K). Interestingly, hpAP staining was not found in the dorsal half of the GT at either e13.5 or e15.5 (Figure 6I and K), or in the preputial fold at e15.5, or the GT ectoderm. This restricted expression pattern of Six1, as indicated by hpAP activity, suggests a potential role of Six1 in pattern formation of the GT at late developmental stages.
Figure 6. Genetic fate mapping of Six1-positive progenitors.
Adjacent cross sections (A-H) and sagittal sections (I-L) from the Six1Cre-hpAP and Rosa26-lacZ reporter (R26RZ/+) double heterozygous embryos at both e13.5 and e15.5 were stained for hpAP and β-galactosidase (β-gal) activities. B, bladder; dUE, distal urethral epithelia; P, perineum; PF, preputial fold; PG, preputial gland; U: urethral. See Figure 1 legend for more abbreviations.
X-gal staining of sections adjacent to those stained with hpAP showed that the majority, if not all, of GT mesenchyme belonged to the Six1 lineage (Figure 6 B, D, F, H, J and L). Specifically, we found all of the perineum stromal tissue was stained with X-gal, consistent with the perineum agenesis phenotype found in the Six1 and Eya1 compound mutants (Figure 2). The bulk of the preputial fold mesenchyme and to a lesser extent, the dorsal GT mesenchyme, was labeled with X-gal (Figure 6J and L). We also observed scattered X-gal positive cells within the urethral plate, perineum, bladder urothelium and rectal epithelium (Figure 6F, H, J and L). However, we did not detect any X-gal positive cells in the preputial glands and GT ectoderm at e15.5 (Figure 6H). Taken together, these genetic fate mapping data support the conclusion that Six1-positive PCM, but not ICM, is the major source of progenitors of genitourinary tissue including stromal components of the perineum, preputial fold and glans.
DISCUSSION
In this report, we provide initial functional evidence that the evolutionarily conserved Six1 and Eya1 transcription factors are essential for normal development of the genitourinary tract. We show that Six1 and Eya1 are critical intrinsic regulators of the PCM progenitors. The restricted expression patterns of Six1 and Eya1 in the PCM suggest that cells of the ICM are molecularly distinct from those in the PCM. The genetic fate mapping studies demonstrate for the first time that the PCM is the major source of progenitors of the genitourinary tract, and that PCM cells are also required for complete separation of cloaca into urogenital and anorectal tracts.
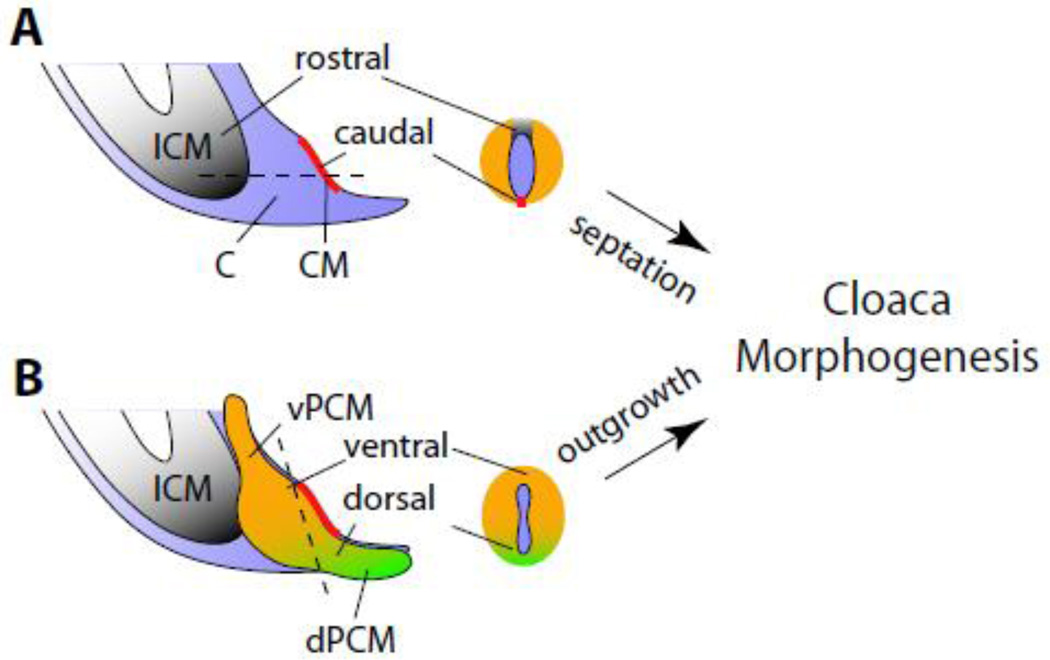
The embryological theory of mammalian cloaca morphogenesis is an ongoing debate. Theories of cloaca transformation are largely based on histological and 3-dimensional reconstruction analyses of normal and abnormal embryos (Hynes and Fraher, 2004; Kluth et al., 1995; Paidas et al., 1999; Penington and Hutson, 2003; Rathke, 1832; Retterer, 1890; Tourneux, 1888; van der Putte, 1986; van der Putte, 2005). Although the current studies were not designed to test the existing models, results from our functional characterization and genetic fate mapping analyses suggest an alternative model: patterning along the dorsoventral and rostrocaudal axes controls cloaca morphogenesis (Figure 7).
Figure 7. Pattern formation of caudal mesoderm during cloacal morphogenesis and genital tubercle development.
Schematic representations of mammalian midline sagittal view of the cloaca region without PCM (A) and with PCM (B). Sections through cloaca (as indicated by dash lines) are also included. We propose that rostrocaudal patterning of the peri-cloacal mesenchyme (PCM, orange) leads to cloaca septation, and dorsoventral patterning of PCM results in the outgrowth of genital tubercle. Processes of rostrocaudal and dorsoventral patterning are linked by mechanisms that are likely dependant on the cloaca membrane (CM, red) and dorsal peri-cloacal mesenchymes (dPCM, green). C, cloaca; ICM, intra-cloacal mesenchymes; vPCM, ventral peri-cloacal mesenchyme See Figure 1 legend for more abbreviations.
Along the rostrocaudal axis, the rostral-localized ICM expands rapidly between e10.5 and e12.5. On the other hand, the CM at the caudal cloaca lacks intra-embryonic mesoderm and eventually breaks open to form the anal and urinary orifices. This asymmetry leads to formation of the dorsal anorectal tract and ventral urogenital tract. Our observation that ICM lacks expression of Six1 and Eya1 is consistent with the idea that ICM is molecularly distinct from the PCM. This does not imply, however, that the ICM is an anatomically distinct structure from the PCM. In addition to the potential role of ICM in cloaca morphogenesis, the persistent cloaca phenotype of Six1 and Eya1 compound mutants suggests that a complete separation of urogenital and anorectal tracts also depends on the PCM. This finding is consistent with the genetic fate mapping data, which demonstrated that the Six1-positive PCM contributes to the genitourinary tract, including the perineum stromal tissue between the anus and the base of the genitalia (Figure 6).
Along the dorsoventral axis, proliferation and expansion of the vPCM and iPCM lead to the external protrusion and formation of the GT. This morphogenetic event coincides with increased levels of apoptosis of the dPCM and the tail gut, which are likely important for the ventral shift of cloaca and the CM (Qi et al., 2000a; Qi et al., 2000b; Sasaki et al., 2004), and therefore patterning along the rostrocaudal axis. Previous studies using chick embryos demonstrated that the tail bud-derived mesenchyme promotes urinary tract segmentation during renal development (Brenner-Anantharam et al., 2007). Interestingly, DiI-labeled tail bud mesenchyme contributes extensively to mesenchyme surrounding the chick cloaca. Therefore, it is tempting to speculate that dPCM is important for both upper and lower urinary tract development. The dPCM is apparently expanded in the Sd mutants (Kluth et al., 1995; Nakata et al., 2009), Skt knockout (Suda et al., 2010), as well as retinoid-induced teratogenic mouse mutants (Liu et al., 2003; Nakata et al., 2009). This dorsoventral growth/patterning defect is accompanied by shortening of the dorsal CM. In these mutants, the ICM or urorectal septum fails to reach the surface and therefore is unable to divide the cloaca completely. Consequently, the dorsoventral patterning defect is coupled with abnormal development of the rostrocaudal axis in these mutants. We speculate that both CM and dPCM are involved in patterning the cloaca along these axes.
Our model predicts that genes asymmetrically expressed in the PCM might be important for cloaca and GT morphogenesis. Interestingly, Six1 appeared to be highly enriched in the dPCM (Figures 1 and 5), which suggests that Six1-Eya1 transcription complex is directly involved in asymmetric patterning of PCM. In a preliminary gene expression array analysis, we have identified a putative Six1 target gene, which is expressed only in the dPCM but not iPCM or vPCM (unpublished data). Six1 and Eya1 functionally interact with Shh signaling pathway and both Six1 and Eya1 are down regulated in Shh mutants (unpublished preliminary observations). Although Shh is ubiquitously expressed in cloacal endoderm epithelial cells and their derivatives, Shh signaling activity seems to be asymmetrically localized based on a Gli-reporter activity (Haraguchi et al., 2007; Lin et al., 2009). Integration of Shh and canonical Wnt/β-catenin signaling pathways is critical for growth and patterning of cloaca and genitalia (Lin et al., 2008; Lin et al., 2009; Miyagawa et al., 2009a). Therefore, in addition to evidence presented here suggests that Six1 and Eya1 are involved in coordinating expression of genes in Fgf and Bmp signaling pathways (Figure 4), it is most likely that Six1-Eya1 transcription complex might be a converging point of multiple signaling pathways during lower urinary tract development. Independent to regulating gene expression, Eya1 controls cell polarity, cell fate and Notch signaling in lung development (El-Hashash et al., 2011); and regulates actin cytoskeleton, cell shape and mobility through its intrinsic phosphatase activity (Pandey et al., 2010). Future studies will be key to understand how Six1-Eya1 complex regulates growth and morphogenesis of anorectal and urogenital complex.
Highlight.
Peri-cloaca mesenchyme (PCM) is the major source of progenitors for caudal body structures.
Six1 and Eya1 are critical transcription regulators for PCM progenitors.
PCM progenitors are molecularly distinct from the intra-cloaca mesenchyme.
Loss of murine Six1 and Eya1 cause genitourinary tract defects and aberrant Bmp4 signaling.
Six1/Eya1 mutants are murine models for human hypospadias and persistent cloaca defects.
ACKNOWLEDGEMENTS
We would like to thank Rosalyn Adam for comments on the manuscript. Y.S. is an American Urological Association Foundation Research Scholar. This work was supported by American Urological Association Foundation Research Scholar award (Y.S.) and a grant from the NIH (1R01DE019823, XL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alappat S, Zhang ZY, Chen YP. Msx homeobox gene family and craniofacial development. Cell Res. 2003;13:429–442. doi: 10.1038/sj.cr.7290185. [DOI] [PubMed] [Google Scholar]
- Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development. 2007;134:1967–1975. doi: 10.1242/dev.004234. [DOI] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- El-Hashash AH, Turcatel G, Al Alam D, Buckley S, Tokumitsu H, Bellusci S, Warburton D. Eya1 controls cell polarity, spindle orientation, cell fate and Notch signaling in distal embryonic lung epithelium. Development. 2011;138:1395–1407. doi: 10.1242/dev.058479. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guo C, Sun Y, Zhou B, Adam RM, Pu WT, Morrow BE, Moon A, Li X. A Tbx1-Six1/Eya1-Fgf8 Genetic Pathway Controls Mammalian Cardiovascular and Craniofacial Morphogenesis. Journal of Clinical Investigation. 2011 doi: 10.1172/JCI44630. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134:525–533. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, Yamada G. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127:2471–2479. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Nakamura S, Nishida W, Sobue K. Bone morphogenetic protein-induced MSX1 and MSX2 inhibit myocardin-dependent smooth muscle gene transcription. Mol Cell Biol. 2006;26:9456–9470. doi: 10.1128/MCB.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes PJ, Fraher JP. The development of the male genitourinary system. I. The origin of the urorectal septum and the formation of the perineum. BRJ Plast Surg. 2004;57:27–36. doi: 10.1016/j.bjps.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Kluth D, Hillen M, Lambrecht W. The principles of normal and abnormal hindgut development. J Pediatr Surg. 1995;30:1143–1147. doi: 10.1016/0022-3468(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Laclef C, Hamard G, Demignon J, Souil E, Houbron C, Maire P. Altered myogenesis in Six1-deficient mice. Development. 2003;130:2239–2252. doi: 10.1242/dev.00440. [DOI] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–1183. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- Lin C, Yin Y, Long F, Ma L. Tissue-specific requirements of {beta}-catenin in external genitalia development. Development. 2008;135:2815–2825. doi: 10.1242/dev.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136:3959–3967. doi: 10.1242/dev.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sugiyama F, Yagami K, Ohkawa H. Sharing of the same embryogenic pathway in anorectal malformations and anterior sacral myelomeningocele formation. Pediatr Surg Int. 2003;19:152–156. doi: 10.1007/s00383-002-0908-y. [DOI] [PubMed] [Google Scholar]
- Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, Nakahara C, Suzuki K, Matsumaru D, Kaneko T, Matsuo I, Yang L, Taketo MM, Iguchi T, Evans SM, Yamada G. Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development. 2009a;136:3969–3978. doi: 10.1242/dev.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, Taketo MM, Nakagata N, Matsumoto T, Takeyama K, Kato S, Yamada G. Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol Endocrinol. 2009b;23:871–880. doi: 10.1210/me.2008-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo R, Kim JH, Zhang J, Chiang C, Hui CC, Kim PC. Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol. 2001;159:765–774. doi: 10.1016/S0002-9440(10)61747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EA, Nguyen SB, Scott V, Stadler HS. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130:3095–3109. doi: 10.1242/dev.00530. [DOI] [PubMed] [Google Scholar]
- Nakata M, Takada Y, Hishiki T, Saito T, Terui K, Sato Y, Koseki H, Yoshida H. Induction of Wnt5a-expressing mesenchymal cells adjacent to the cloacal plate is an essential process for its proximodistal elongation and subsequent anorectal development. Pediatr Res. 2009;66:149–154. doi: 10.1203/PDR.0b013e3181aa304a. [DOI] [PubMed] [Google Scholar]
- Nie X, Xu J, El-Hashash A, Xu PX. Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis. Dev Biol. 2011;352:141–151. doi: 10.1016/j.ydbio.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, Zipursky SL, Gruss P. Homeobox genes and connective tissue patterning. Development. 1995;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131:551–562. doi: 10.1242/dev.00943. [DOI] [PubMed] [Google Scholar]
- Paidas CN, Morreale RF, Holoski KM, Lund RE, Hutchins GM. Septation and differentiation of the embryonic human cloaca. J Pediatr Surg. 1999;34:877–884. doi: 10.1016/s0022-3468(99)90391-3. [DOI] [PubMed] [Google Scholar]
- Pandey RN, Rani R, Yeo EJ, Spencer M, Hu S, Lang RA, Hegde RS. The Eyes Absent phosphatase-transactivator proteins promote proliferation, transformation, migration, and invasion of tumor cells. Oncogene. 2010;29:3715–3722. doi: 10.1038/onc.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington EC, Hutson JM. The absence of lateral fusion in cloacal partition. J Pediatr Surg. 2003;38:1287–1295. doi: 10.1016/s0022-3468(03)00384-1. [DOI] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- Qi BQ, Beasley SW, Williams AK, Fizelle F. Apoptosis during regression of the tailgut and septation of the cloaca. J Pediatr Surg. 2000a;35:1556–1561. doi: 10.1053/jpsu.2000.18309. [DOI] [PubMed] [Google Scholar]
- Qi BQ, Williams A, Beasley S, Frizelle F. Clarification of the process of separation of the cloaca into rectum and urogenital sinus in the rat embryo. J Pediatr Surg. 2000b;35:1810–1816. doi: 10.1053/jpsu.2000.19265. [DOI] [PubMed] [Google Scholar]
- Rathke H. Abhandlungen zur Bildungs- und Entwicklungsgeschichte der Tiere. Leipzig. 1832 [Google Scholar]
- Retterer E. Sur l'origine et l'evolution de la region anogenitale des mammiferes. Journal of Anatomy (Paris) 1890;26:126–216. [Google Scholar]
- Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM, Jr., Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, Hildebrandt F. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101:8090–8095. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajithlal G, Zou D, Silvius D, Xu PX. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol. 2005;284:323–336. doi: 10.1016/j.ydbio.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki C, Yamaguchi K, Akita K. Spatiotemporal distribution of apoptosis during normal cloacal development in mice. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:761–767. doi: 10.1002/ar.a.20062. [DOI] [PubMed] [Google Scholar]
- Scott V, Morgan EA, Stadler HS. Genitourinary functions of Hoxa13 and Hoxd13. J Biochem. 2005;137:671–676. doi: 10.1093/jb/mvi086. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Bouldin CM, Choi KS, Harfe BD, Cohn MJ. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development. 2009a;136:3949–3957. doi: 10.1242/dev.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Yamaguchi T, Cohn MJ. Functional and phylogenetic analysis shows that Fgf8 is a marker of genital induction in mammals but is not required for external genital development. Development. 2009b;136:2643–2651. doi: 10.1242/dev.036830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Zheng Z, Ormerod BK, Cohn MJ. Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat Commun. 2009c;1:1–9. doi: 10.1038/ncomms1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Suda H, Lee KJ, Semba K, Kyushima F, Ando T, Araki M, Araki K, Inomata Y, Yamamura KI. The Skt gene, required for anorectal development, is a candidate for a molecular marker of the cloacal plate. Pediatr Surg Int. 2010 doi: 10.1007/s00383-010-2785-0. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, Crenshaw EB, 3rd, Yamada G. Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling [corrected] Development. 2003;130:6209–6220. doi: 10.1242/dev.00846. [DOI] [PubMed] [Google Scholar]
- Tourneux F. Sur les premiers developpements du cloaque du tubercle genitale et de l'anus chez l'embryon de mouton. Journal of Anatomy (Paris) 1888;24:503–517. [Google Scholar]
- van der Putte SC. Normal and abnormal development of the anorectum. J Pediatr Surg. 1986;21:434–440. doi: 10.1016/s0022-3468(86)80515-2. [DOI] [PubMed] [Google Scholar]
- van der Putte SC. The devlopment of the perineum in the human. A comprehensive histological study with a special reference to the role of the stromal components. Adv Anat Embryol Cell Biol. 2005;177:1–131. [PubMed] [Google Scholar]
- Wu X, Ferrara C, Shapiro E, Grishina I. Bmp7 expression and null phenotype in the urogenital system suggest a role in re-organization of the urethral epithelium. Gene Expr Patterns. 2009;9:224–230. doi: 10.1016/j.gep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1- deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu PX, Woo I, Her H, Beier DR, Maas RL. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997;124:219–231. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130:3085–3094. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yucel S, Liu W, Cordero D, Donjacour A, Cunha G, Baskin LS. Anatomical studies of the fibroblast growth factor-10 mutant, Sonic Hedge Hog mutant and androgen receptor mutant mouse genital tubercle. Adv Exp Med Biol. 2004;545:123–148. doi: 10.1007/978-1-4419-8995-6_8. [DOI] [PubMed] [Google Scholar]