Abstract
Objective
To characterize GFP-expressing cells in the striatum of Cb6-Tg(Gad1-EGFP)G42Zjh/J mice, in which the Gad1 (also referred to as GAD67) promoter drives GFP expression (Gad1-GFP mouse).
Background
GFP-expressing cells of the GAD1-GFP mouse have been described to be a population of parvalbumin-positive basket interneurons residing in the cerebral cortex and the cerebellum. However, the cells in the dorsal striatum of these mice have not been characterized.
Methods
Using a combination of immunohistochemistry, electrophysiology, DiI labeling, and retrograde tracing, we investigated the phenotypes of GFP-expressing cells in the GAD1-GFP mice.
Results
A small number of striatal neurons express GFP in these mice. In the mature striatum, these cells are preferentially located in the lateral striatum with a strong expression in the lateral striatal streak. The GAD1-GFP positive neurons are distinct from the standard fast-spiking and low-threshold-spiking GAD-67 expressing striatal interneurons and appear to be a subset of medium spiny neurons. These neurons are generally colocalized with striosomal markers such as dynorphin, mu-opioid receptors, as well as CB1 and calretinin-immunopositive fibers. Striatal Gad1-GFP neurons can be separated into two groups based on the shape of the somata and patterns of action potential firing. Retrograde labeling indicated that a proportion of these cells are projection neurons.
Conclusions
The examination of GAD1-GFP cells in these mice revealed 2 subpopulations of ventral striosomal striatal medium spiny neurons, based on morphology, patch-matrix segregation and membrane properties.
Keywords: Basal Ganglia, Patch, GABA, Medium Spiny Neuron, BAC Transgenic
Introduction
The generation of Bacterial Artificial Chromosome (BAC) transgenic mice in which BACs containing specific promoters drive the expression of enhanced green fluorescent protein (GFP) has allowed the examination of morphology, protein expression, pharmacology and physiology of specific subsets of neurons. Since BAC clones have the ability to carry several hundred kilobases of DNA, they are able to include the entire transcription unit and all of the associated regulatory elements for a gene allowing for a high rate of success in reproducing the expression pattern of a gene of interest [1]. However, unexpected information about neuronal subtypes has also been revealed by these mice.
In this study, characterization of GFP-expressing cells in the striatum of transgenic mice in which a BAC containing the glutamic acid decarboxylase 1 (GAD1 also referred to as GAD67) promoter drives the expression of GFP [2] was performed. These transgenic mice were first described by Chattopadhyaya et al [2] in which their G42 line selectively expresses GFP in the calcium-binding protein parvalbumin-expressing subclass of basket interneurons in the cortex and the cerebellum [2–4]. More recently, GAD1-GFP positive cells, including SNAP-25 or 5-HT immunoreactive neurons, were observed in the taste bud and horizontal cells of the retina, respectively [5,6].
The striatum is the primary input site of the basal ganglia, a subcortical brain region with crucial roles in action control and learning and memory. Cytoarchitecturally, the striatum appears to be a homogeneous structure composed mainly of one neuronal type, the medium spiny neuron (MSN). However, numerous Golgi, intracellular injection, and immunohistochemical studies have revealed a mosaic organization based on neuroanatomical markers and patterns of afferent, efferent, and intrinsic connections. There is general agreement that there are six morphologically distinct types of neurons that can be divided by cell body size (large vs medium) as well as the presence or absence of spines [7].
The most frequently and well-studied striatal neuronal type is the MSN that makes up ~95% of the total neuronal number and is the main projection neuron from the striatum [8]. The morphological features of this cell type are a medium-sized perikaryon and the possession of many dendritic spines [9,10]. The remaining cells of the striatum, the aspiny intrinsic neurons, are characterized by the size of their cell bodies and mainly aspiny or sparsely spiny dendrites [9–11]. These cells are immunoreactive for either choline acetyltransferase, or a combination of glutamate decarboxylse (GAD) coexpressed with either somatostatin, neuropeptide Y, or parvalbumin [12–17].
MSNs can be subdivided by their expression of either the D1 or D2 dopamine receptor as well as their output pathways; those that project to the substantia nigra pars reticulata (direct pathway) and to the globus pallidus (indirect pathway). Another division is the segregation of MSNs into striosome (aka patch) and matrix compartments. Striosomes have been identified as regions of high mu-opioid receptor (μOR) binding and immunoreactivity [18,19]. Striosomes are enriched in enkephalin- and substance P-like immunoreactivity as well as a calretinin-rich fiber plexus [20–22]. This is complementary to a matrix marked by immunoreactivity for acetylcholinesterase, and somatostatin- and calbindin-positive processes [13,14,18, 23,24]. Dynorphin, a class of opioid peptide that exerts effects via the kappa opioid receptor, appears to be expressed slightly higher in ventral striatal areas and distinctly higher in striosomal neurons [21].
The patch/striosome and matrix also differ in their cortical innervation. The prelimbic cortex preferentially innervates striosomes [13], whereas other prefrontal, motor, and sensory cortical areas provide inputs to the striatal matrix [25,26]. The orbitofrontal cortex has been shown to project to both striosomes and matrix [27,28]. In addition, the two striatal compartments give rise to separate projection systems to the substantia nigra; striosomal neurons project to the pars compacta whereas matrix neurons project to the pars reticulata [13,14].
Our original intent was to use the GAD1-GFP to identify and study the parvalbumin-expressing GABAergic interneurons in the striatum. Upon investigation of these mice, however, we found that a small number of striatal neurons express GFP, were spiny, and were mainly localized to the ventral lateral region of the dorsal striatum and the lateral striatal streak. Further characterization of these cells has produced unexpected results.
Materials and Methods
Mice
All procedures involving animals were in full compliance with the NIH guidelines for the Care and Use of Laboratory Animals, and approved by the Animal Care and Use Committee, Division of Intramural Clinical and Biological Research of NIAAA. GAD1-GFP mice were originally created by Z. Josh Huang in which the bacterial artificial chromosome (BAC) clone 56I19 containing the mouse Gad1 gene (and 60 kb of upstream and downstream sequence) was modified by insertion of an enhanced green fluorescent protein (GFP) cDNA and phosphoglycerate kinase polyadenylation sequence in the first coding exon at the translation initiation site of the Gad1 gene [2]. We obtained the mice from the Jackson Laboratory (Bar Harbor, ME). In previous studies, these GAD1-GFP (line G42) mice were found to selectively express enhanced green fluorescent protein (GFP) in the calcium-binding protein parvalbumin (PV)-expressing subclass of basket interneurons (soma, dendrites, and axons) and also in putative presynaptic boutons in brain regions including cortex and the cerebellum [2–4]. Expression of GFP was not observed in other interneuron classes expressing markers such as somatostatin (SOM), cholecystokinin (CCK), calretinin (CR), and VIP. The expression of GFP is detected as early as embryonic day 14 (E14) with high expression levels throughout postnatal development [2,3].
Transgenic mice (Tg(GAD65_3e/gfp3.3)#15) expressing GFP under the GAD65 promoter on C6CBA background were generated as described [29] and maintained in house by homozygous breeding. Briefly, a genomic clone containing 5.5kb upstream region and the first six exons of the mouse GAD65 gene was isolated from a mouse λ phase genomic library, and the GFP marker gene without its own translation start site was fused in frame to the first or third exon of the GAD65 gene. Transgenic mice were derived by standard pronuclear injection of CBA/C57BL6F2 fertilized eggs. The expression of GFP is almost exclusively in GABAergic neurons in many brain regions including the neocortex and hippocampus.
In our studies, genotyping for GFP in both mouse lines was either performed at P0–P2 by use of an ultraviolet light source and goggles fitted with a UV filter. For adult GAD1-GFP mice, genotyping for the GFP gene was performed by PCR using the following primers: forward 5' AAGTTCATCTGCACCACCG; reverse 5' TGCTCAGGTACTGGTTGTCG.
Immunohistochemistry
Mice were briefly anesthetized with isofluorane (Baxter) and perfused intracardially with 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS). Brains were then removed and immersion fixed overnight in 4% PFA. Brains were sectioned in PBS at a thickness of 40μm on a Vibratome Series 1000 (Leica, Buffalo Grove, IL). Sections were blocked in 5% BSA, 0.2% triton X-100 in 1× PBS for 2 hours. Sections were then incubated overnight at 4°C in primary antibodies diluted in 0.2% Triton X-100 in PBS. Table 1 lists the concentrations and sources for the primary antibodies used. After several washes in 0.2% Triton X-100 in PBS, sections were incubated in secondary antibodies (Alexa 488, 568; Invitrogen, Carlsbad, CA) overnight at 4°C. After several washes in PBS, sections were mounted and coverslipped in Fluoromount (Southernbiotech, Birmingham, AL).
Table 1.
Primary antibody concentrations
| ANTIBODY | SPECIES | SOURCE | DILUTION |
|---|---|---|---|
| Calbindin | Rabbit | Millipore (Billerica, MA) | 1:1000 |
| Calretinin | Rabbit | Millipore | 1:1000 |
| Darpp-32 | Rabbit | Cell Signaling (Davers, MA) | 1:500 |
| Dynorphin | Rabbit | EMD Chemicals (Gibbstown, NJ) | 1:200 |
| GFP | Chicken | Abcam (Cambridge, MA) | 1:2000 |
| μ-opiod receptor | Rabbit | Millipore | 1:500 |
| Neuropeptide Y | Rabbit | Millipore | 1:1000 |
| Parvalbumin | Rabbit | Swant (Bellinzona, Switzerland) | 1:1000 |
| PanGAD | Rabbit | Sigma (St. Louis, MO) | 1:200 |
| Somatostatin | Rabbit | Zymed (San Francisco, CA) | 1:1000 |
Retrograde labeling
GAD1-GFP mice at least P60 were placed into a stereotaxic instrument (Kopf, Tujunga, CA) fitted with an isofluorane rebreather system (Euthanex, Palmer, PA). A 0.1% solution of the retrograde neuronal tract tracer Alexa Fluor 555-conjugated cholera toxin B subunit (Invitrogen, Eugene, OR) was made in PBS. A 50nL syringe (Hamilton, Reno, NV) was used to pressure inject 300nL of the tracer solution at a rate of 50nL/minute into the substantia nigra. The coordinates for targeting the substantia nigra were (in mm relative to Bregma) AP=−3.20, ML=+1.45, and DV=−5.0. Animals were sacrificed 10 days later and immunohistochemical methods were used to reveal retrogradely labeled neurons in the dorsal striatum.
DiI labeling
Sections 200μm thick were obtained from 4% PFA-fixed GAD1-GFP brains. Areas of the striatum enriched with GFP cells were visualized under a dissecting microscope (MVX10, Olympus, Center Valley, PA). A small amount of 1,1'-Dioctadecul-3,3,3',3'-tetramethylindocarbocyanine iodide (DiI, 3mg/100μL, Invitrogen), dried into a crystalline form, was transferred to the slice via a glass micropipette similar to that used for patch-clamp recording but with the tip fire-polished closed. The sections were incubated in PBS at 4°C for 2 weeks. Sections were mounted and coverslipped in Fluoromount.
Golgi Impregnation
Golgi impregnation protocol was previously described [30]. Briefly, adult male Sprague Dawley rats were perfused intracardially first by 0.1M phosphate buffer, then by a mixture of 2% glutaraldehyde and 4% PFA in 0.1M PB. Coronal sections of 150μm thick were obtained using a vibrating microtome and then incubated in 1% osmium tetroxide for 30 minutes followed by 3.5% potassium dichromate overnight. Sections were developed in 1% silver nitrate for 4–6 hours.
Image acquisition and analysis
Images of the dorsal striatum were captured on an inverted microscope (Axiovert 200, Zeiss, Thornwood, NY) with either a 10× (Plan-Neofluar 10×/0.31, Zeiss) or 40× (40×/1.30, Zeiss) objective. Montages of individual sections were constructed to include a full view of the dorsal striatum. At least 3 coronal sections through the striatum of each animal (N) was analyzed for a given immunohistochemical experiment. Using Image J (National Institutes of Health, Bethesda, MD), immunopositive cells were counted. Data were presented as mean ± SEM.
Z-stack images (512 × 512; x-y scaling, 0.14 μm/pixel) of individual cells and dendrites were captured on a confocal microscope (LSM510 Meta, Zeiss) with a 20× objective (numerical aperture, 0.8; z spacing of 1.3 μm) or 63× water-immersion objective (numerical aperture, 1.2; z spacing of 0.45 μm), respectively. Cell body length, width and area and spine density were measured in DiI labeled cells that were also either GFP positive or negative using Image J (National Institutes of Health, Bethesda, MD). Cell body “length” was determined to be the longest length of the cell and the “width” was then measured at a 90-degree angle to the “length”. Data ware compared between different groups using one-way analysis of variance (ANOVA), post hoc Tukey's Multiple Comparison Test and expressed as mean ± SEM.
Electrophysiology
Adult male and female GAD1-GFP mice were briefly anesthetized with isofluorane (Baxter) and decapitated. Brains were rapidly removed and placed in ice-cold cutting solution containing in mM: sucrose, 194; NaCl, 30; KCl, 4.5; MgCl2, 1; NaHCO3, 26; NaH2PO4, 1.2; glucose, 10, aerated with a mixture of 95%O2/5% CO2 gas. Coronal brain slices at a thickness of 300 μm containing the striatum and overlying cortex were obtained using a Vibrating Blade Microtome (Leica VT1200S). Slices were equilibrated for 1 hour in artificial cerebral spinal fluid (aCSF) containing in mM: NaCl, 124; KCl, 4.5; MgCl2, 1; NaHCO3, 26; NaH2PO4, 1.2; glucose, 10; CaCl2, 2, continuously aerated with a mixture of 95%O2/5% CO2 gas at a temperature of 33°C. Slices were then transferred to room temperature until experimental use. One hemisphere of a slice was transferred to a recording chamber on the stage of an upright microscope (Axioskop 2, Zeiss). Slices were stabilized by an overlying platinum weight and continuously perfused with solution maintained at a temperature of 28–32°C with the temperature not varying more than 1°C during a given experiment (Automatic Temperature Controller, Warner Instruments, Hamden, CT). GFP-expressing cells were identified under fluorescence illumination and DIC using a 40× water immersion objective (numerical aperture, 0.8). Real-time images were displayed on a video monitor, which aided the navigation and placement of the recording pipettes. Patch pipettes were pulled from borosilicate glass capillaries (1.5 mm outer diameter, 0.86 mm inner diameter; World Precision Instruments, Sarasota, FL) and filled with internal solution. For voltage clamp recordings of EPSCs and IPSCs a CsCl-based internal solution containing in mM: CsCl, 150; HEPES, 10; MgCl2, 2; Na-GTP, 0.3; Mg-ATP, 3; BAPTA-4K, 0.2 was used. For current clamp recordings a K-gluconate-based internal solution containing in mM: K-gluconate, 126; KCl, 4; HEPES, 10; Na-GTP, 0.3; Mg-ATP, 4; phosphocreatine, 10; pH 7.2 was used.
When filled with internal solution, the patch pipettes had resistances of 2–4 MΩ. Recordings were made using a Multiclamp 700A amplifier (Molecular Devices, Foster City, CA). Membrane currents were filtered at 2kHz, digitized using Clampex v9.2 and analyzed with Clampfit v9.2 (Molecular Devices) or MiniAnalysis (Synaptosoft v6.0.7, Decatur, GA). Statistical analysis was performed using SigmaStat 3.0 (SPSS Inc., Chicago, IL) or GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). Data are reported as mean ± standard error of the mean (SEM).
RESULTS
The GFP-expressing cells of the BAC-GAD1 (GAD1-GFP) mouse were originally described to be a population of parvalbumin-positive basket interneurons in the cerebral cortex [2] and cerebellum [3], and more recently, include cells immunoreactive for SNAP-25 in the taste bud [5] and 5-HT in horizontal cells of the retina [6]. However the characterization of these GAD1-GFP cells residing in other brain regions, in particular the striatum, was not explored. Therefore using a combination of immunohistochemistry, electrophysiology, and retrograde labeling we sought to detail the characteristics of GAD1-GFP cells in the dorsal striatum.
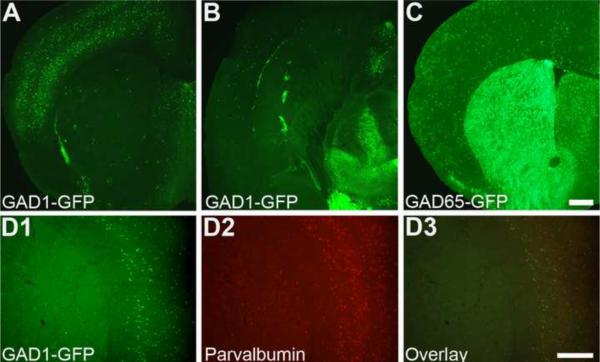
Using epifluorescence microscopy, we ascertained the distribution pattern and morphology of GAD1-GFP cells in the striatum. In both sections oriented in the coronal (Figure 1A) and horizontal (Figure 1B) plane through the striatum, the number of GAD1-GFP cells was quite sparse in comparison to that in the adjacent cortex. This was in stark contrast to the distribution of GFP-expressing cells in the striatum of GAD65-GFP mice (Figure 1C) in which GFP is expressed in cells expressing the 65kDa isoform of glutamic acid decarboxylase that is prevalent in MSNs [29,31]. Similar to Chattopadhyaya and colleagues [2], a large majority of GAD1-GFP positive cells in the cortex coexpressed parvalbumin (Figure 1D; 892 of 1121; 6 animals).
Figure 1. The distribution of GFP+ cells is limited in the striatum of GAD1-GFP mice.
Coronal (A) and horizontal (B) sections obtained from a P50 GAD1-GFP mouse demonstrating the sparse localization of GFP-expressing cells in the striatum. Note the heavy, patch-like localization in the ventral portion of the dorsal striatum, especially along the lateral striatal streak. (C) Coronal section obtained from a GAD65-GFP mouse reveals a heavy distribution of GFP-expressing presumably MSNs in the dorsal striatum. (D) A large majority of GAD1-GFP cells in the cortex (D1) coexpressed parvalbumin (D2–3). Scale bars in (A) and (B) = 500μm.
It was striking that although the appearance of GAD1-GFP cells was sparse throughout the striatum, they were in high density in the subcallosal streak and in what appeared to be clusters/patches within the striatum (Fig 1A–B). To determine if GAD1-GFP-positive neurons are mainly expressed in the patch/striosome compartment within the striatum, immunohistochemistry was performed with antibodies raised against dynorphin and μOR; two peptides with more prevalent expression in striatal patches (Figure 2A–B; [18,19,21,32]), and calbindin, whose expression is preferentially located in the matrix (Figure 2C; [24]). A high proportion of GAD1-GFP cells coexpressed dynorphin A (99.3 ± 0.8%; N = 3) or μOR (93.6 ± 1.4%; N = 3), with a concomitant low percentage of coexpression with calbindin (13.4 ± 0.9%; N = 3; Figure 2D). A low-magnification image containing the striatum shows that GAD1-GFP cells cluster in areas rich in μOR (Figure 2E). Calretinin-immunoreactive fibers have been shown to cluster in areas corresponding to striosomes in the medial, central and ventral areas of the dorsal striatum [22,33]. GAD1-GFP cells were located in areas that were rich with calretininimmunoreactive fibers (Figure 2F). Together, these findings suggest that GAD1-GFP cells are preferentially located within striosomes in the dorsal striatum.
Figure 2. GAD1-GFP cells are associated with patches in the ventral part of the dorsal striatum.
A high percentage of GFP+ cells in GAD1-GFP mice express dynorphin (A, D) and mu-opiate receptor (μOR, B, D). Most GAD1-GFP cells do not coexpress calbindin and in fact cluster within areas low in calbindin immunoreactivity (C,D). GAD1-GFP cells cluster within areas rich in μOR (E) and calretinin fibers (F). Data expressed as mean ± SEM.
High magnification images of GAD1-GFP-positive cells in the dorsomedial striatum showed spiny varicosities arising from the dendrites (Fig 3A). This surprising result suggested that, unlike their counterparts in the cortex and cerebellum, these cells were either a newly identified subset of spiny parvalbumin-expressing GABAergic neuron or a subset of MSN. To determine if GAD1-GFP cells are phenotypically similar to striatal GABAergic interneurons, immunohistochemistry using antibodies raised against the interneuron markers parvalbumin (Figure 3B), calbindin (Figure 3C), calretinin (Figure 3D), somatostatin (Figure 3E) and neuropeptide-Y (NPY; Figure 3F) was performed. Quantification of the percentage of GAD1-GFP cells that also expressed parvalbumin (5.2 ± 1.8%; N = 5), calbindin (13.4 ± 0.9%; N = 3), calretinin (1.3 ± 0.8%; N = 5), somatostatin (0 ± 0%; N = 3) and NPY (6.3 ± 1.3%; N = 4), revealed that a very low proportion of GAD1-GFP cells express markers for GABAergic interneurons (Figure 3G). This finding suggests that a majority of GAD1-GFP cells in the striatum do not express markers for, and are therefore unlikely to be, a subset of striatal GABAergic interneurons.
Figure 3. Most GAD1-GFP cells do not express markers for GABAergic interneurons in the dorsal striatum.
(A) Magnified image of a GAD1-GFP cell in the ventral part of the dorsal striatum. GAD1-GFP cells (B1–F1) do not express markers for GABAergic interneurons such as parvalbumin (B2), calbindin (C2), calretinin (D2), somatostatin (E2) and neuropeptide Y (NPY, F2) as seen in the overlay (B3–F3). Scale bar in F3 = 50 μm. (G) Quantification of numbers of GAD1-GFP cells that coexpress a given GABAergic interneuron marker. Data expressed as mean ± SEM.
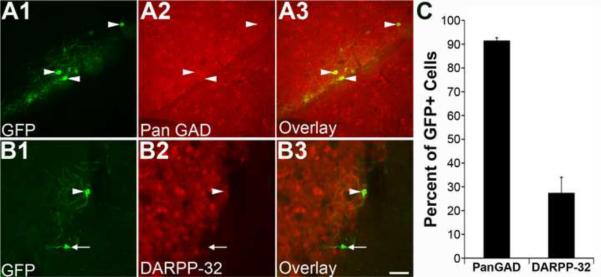
Next, we examined whether GAD1-GFP cells were a subset of MSN. Since most if not all, striatal MSNs utilize GABA as a neurotransmitter and therefore express GAD, we performed immunohistochemistry with an antibody that recognizes both GAD1 and GAD2 (panGAD; Figure 4B). A majority of GAD1-GFP cells were immunoresponsive to the panGAD antibody (91.4 ± 1.4%; N = 3; Figure 4A3, C), suggesting that these cells are GABAergic. The regional expression of the dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32) correlates with the distribution of dopamine-innervated neurons and is expressed in the majority of MSNs in the dorsal striatum [34–38]. Immunohistochemistry performed with an antibody raised against DARPP-32 (Figure 4B), revealed that less than a third of GAD1-GFP cells coexpressed DARPP-32 (27.3 – 6.7%; N = 4; Figure 4C). Since it has been shown that DARPP-32 negative medium sized cells could not be distinguished from their DARPP-32 counterparts by morphological criteria [38], it is possible that GAD1-GFP cells are either a subset of GABAergic interneuron that can not be classified by coexpression of calcium binding proteins or peptidergic neurotransmitters or a subset of projecting MSNs that do not use DARPP-32-mediated signal transduction cascade to influence their physiology similar to typical MSNs.
Figure 4. A high proportion of GAD1-GFP cells express markers for medium spiny neurons.
(A) Immunostaining with an antibody raised against all forms of GAD (A2) shows overlap in expression with a high percentage of GFP+ cells in GAD1-GFP mice (A3,C). (B) Approximately 1/3 of GFP+ cells express low levels of DARPP-32 (B,C). Arrowheads denote GAD1-GFP cells that coexpressed Pan GAD (A) or DARPP-32 (B). Arrow in B is an example of a GAD1-GFP cell that does not express DARPP-32. Data expressed as mean ± SEM.
To determine whether GAD1-GFP cells project out of the dorsal striatum, we used retrograde tracer injections into the substantia nigra, one of the major targets of the dorsal striatum (Figure 5A). We then counted the number of GAD1-GFP cells (Figure 5B1; circles in Figure 5C), cells that have taken up the red fluorescent tracer (Figure 5B2; dots in Figure 5C) and GAD1-GFP cells that have taken up the tracer (Figure 5B3; red stars in Figure 5C) in the dorsal striatum. In our studies, ~50% of the GAD1-GFP cells counted in the striatum took up the retrograde tracer and therefore project to the substantia nigra. However this may be an underestimate due to the size and placement of the retrograde tracer injection. Furthermore, we were unsuccessful in identifying cells projecting to the globus pallidus by retrograde tracing, as our pallidal dye injections in the mice invariably spread into striatum. Thus, it is possible that some of the GAD1-GFP neurons not identified as direct pathway (SN-projecting) neurons, may project to the globus pallidus as part of the indirect pathway. Nevertheless, the observation that a proportion of GAD1-GFP cells have taken up the tracer suggests that a proportion of GAD1-GFP cells project out of the dorsal striatum into the substantia nigra.
Figure 5. A proportion of GAD1-GFP cells in the dorsal striatum project to the substantia nigra.
(A) Photomicrograph demonstrating the injection site of the tracer solution in the substantia nigra. (B1) GAD1-GFP cells located in the ventral portion of the dorsal striatum. (B2) Cells in the same area that have taken up the retrograde tracer and therefore project to the substantia nigra. (B3) An overlay of the two channels showing that some of the GAD1-GFP cells project to the substantia nigra. (C) Map of the location of tracer-positive cells (dots), GAD1-GFP cells (circles), and GAD1-GFP cells that are tracer-positive (red stars) in rostral (C1), middle (C2), and caudal (C3) sections.
Compared to presumptive MSNs labeled with DiI (DiI cells; Figure 6A), there appeared to be two populations of GAD1-GFP based on the shape of their cell body (Figure 6B) and location within the dorsal striatum. Ovoid shaped GAD1-GFP cells (Figure 6B1) tended to be located within patches within the ventral portion of the dorsal striatum and the lateral striatal streak, whereas elongated GAD1-GFP cells (Figure 6B2) were preferentially located within the lateral striatal streak. The existence of elongated cells located along the lateral striatal streak were also found using golgi staining methods in adult rat dorsal striatum (Figure 6C), suggesting that this cell type exists in other species and is not a phenomenon specific to the GAD1-GFP transgenic mouse line.
Figure 6. GAD1-GFP cells are spiny and can be divided into two populations based on somatic shape.
(A) Example of an MSN labeled with DiI. (B1–2) Two examples of GFP+ cells observed in the ventral striatum. Note the difference in cell body shape and spine density on the dendrites. Insets in A–B are enlarged images of a dendritic segment from each cell. (C) Golgi-stained dorsal striatum of an adult Sprague-Dawley rat reveals the presence of an elongated cell within the lateral striatal streak (arrow). CC: corpus callosum. (D) Examples of the cell body length and width measurements that were taken to determine cell body shape in two cells located in the lateral striatal streak. (E) The ratio of cell body width/length determined for DiI labeled cells and GFP+ cells. Two populations of GFP+ cells, those with a ratio of >0.7 and those <0.7 were unveiled by this analysis. (F) The ratio of width of the cell body to the length of elongated GFP+ cells was significantly different than DiI and ovoid GFP+ cells. (G) The average width and length of the cell body was determined for DiI cells and for the two populations of GFP+ cells. The average length was not significantly different between the three groups. However the width of the elongated cells was significantly different. (H) The average area of the cell body was not significantly different between the three groups. (I) Ovoid GFP+ cells had a significantly lower average spine density than DiI and elongated GFP+ cells. Data was compared between different groups using one-way analysis of variance (ANOVA), post hoc Tukey's Multiple Comparison Test and expressed as mean ± SEM. *: p<0.05 compared to DiI cells; *◆: p<0.05 compared to DiI cells and ovoid cells; *●: p<0.05 compared to DiI cells and elongated cells.
To more closely examine the somatic shapes of the GAD1-GFP neurons, measurements of the length and width of the cell body were made in which the length was determined to be the largest measurement of distance that traversed the center of the cell body and the width was determined as the largest measurement that was perpendicular to the length (Figure 6D). Analysis of the ratio of cell body width/length revealed that on average this ratio was lower in GAD1-GFP cells compared to DiI cells (DiI cells: 0.9 ± 0.01; GAD1-GFP cells: 0.7 ± 0.04; Students t-test p = 0.03; Figure 6E). However, upon closer examination of this ratio of individual cells it appeared as though there was a group of GAD1-GFP cells whose average ratio was similar to that of DiI cells and a second group of GAD1-GFP cells whose ratio was lower (Figure 6E). Therefore, GAD1-GFP cells were characterized as ovoid (Figure 6B1) if their width/length ratio was above 0.7 and characterized as elongated (Figure 6B2) if their width/length ratio was below 0.7. On average, the width/length ratio of ovoid GAD1-GFP cells was similar to DiI cells (DiI cells: 0.9 ± 0.01; ovoid GAD1-GFP cells: 0.9 ± 0.02; Figure 6F). The average width/length ratio of elongated GAD1-GFP cells was significantly smaller than that of DiI and ovoid GAD1-GFP cells (elongated GAD1-GFP cells: 0.6 ± 0.03; ANOVA p = 0.0005; Figure 6F). This decrease in width/length ratio was due to the width of elongated GAD1-GFP cells being significantly smaller than that of DiI and ovoid GFP cells (DiI cells: 14.2 ± 2.3 μm; ovoid GAD1-GFP cells: 12.4 ± 0.8 μm; elongated GAD1-GFP cells: 8.6 ± 0.7 μm; ANOVA p = 0.014; Figure 6G), since the length did not significantly differ between the three cell types (DiI cells: 16.0 ± 2.7 μm; ovoid GAD1-GFP cells: 13.7 ± 0.8 μm; elongated GAD1-GFP cells: 15.2 ± 0.9 μm; ANOVA p = 0.50; Figure 6G). The average area of the cell body did not differ between the three cell types (DiI cells: 185.7 ± 32.4 μm2; ovoid GAD1-GFP cells: 153.1 ± 15.3 μm2; elongated GAD1-GFP cells: 118.8 ± 15.3 μm2; ANOVA p = 0.10; Figure 6H).
What was striking from looking at images of the three cell types (Figure 6A–B insets) was the difference in spine density. Therefore spines were counted along stretches of secondary and tertiary dendrites. Spines were only counted in DiI-filled cells that were either negative (referred to as DiI/GFP− cells) or positive for GFP (referred to as either DiI/Ovoid GFP+ or DiI/Elong GFP+) in order to bypass any potential differences in observing spines between GFP− and DiI-labeling. The spine density of DiI-labeled cells was similar to that reported in MSNs examined in mice (DiI cells: 0.78 ± 0.03 spines/μm; [39–41]). In fact compared to DiI cells, ovoid GAD1-GFP cells had a significantly decreased spine density, while elongated GAD1-GFP cells had a significantly increased spine density (ovoid GAD1-GFP cells: 0.37 ± 0.02 spines/μm; elongated GAD1-GFP cells: 1.0 ± 0.04 spines/μm; ANOVA p < 0.0001; Figure 6I).
To determine if the different MSN and GAD1-GFP-positive neurons have different intrinsic membrane properties, we performed whole-cell patch clamp electrophysiology in GFP negative cells that were presumably MSNs (Figure 7A), ovoid GAD1-GFP (Figure 7B) and elongated GAD1-GFP (Figure 7C) cells. In agreement with the measurement of cell body areas (Figure 6H) suggesting similar somatic membrane areas, there were no differences in membrane capacitance (data not shown). However the input resistance of ovoid GAD1-GFP cells (127.9 ± 7.9 MΩ) was significantly larger than GFP− cells (103.6 ± 9.2 MΩ) and elongated GAD1-GFP cells (73.7 ± 5.1 MΩ; ANOVA; p = 0.042; Figure 7D). The input resistance of elongated GAD1-GFP cells was significantly smaller than the other two cell types (ANOVA; p = 0.021; Figure 7D). Overall, the resting membrane potential of elongated GAD1-GFP cells (−59.5 ± 2.5 mV) was more depolarized than GFP− (−63.1 ± 1.4 mV) and ovoid GAD1-GFP cells (−64.8 ± 1.4 mV; ANOVA p = 0.047; Figure 7E). We did not observe any spontaneous firing in any of the cell types examined.
Figure 7. Intrinsic membrane properties differ in GAD1-GFP cells versus MSNs.
(A) Example of a GFP negative (GFP−), presumably MSN cell, filled internally with Alexa, 568. (B1–2) Example of an ovoid GFP+ cell (B1) filled internally with Alexa 568 (B2). (C) Example of an elongated GFP+ cell (elong). (D) The input resistance was significantly higher in ovoid cells and significantly lower in elong cells compared to GFP− cells. (E) The resting membrane potential of elong cells was more depolarized than the other cell types. (F–H) Representative traces recorded from a GFP− cell (F), an ovoid GFP+ cell (G) and an elongated GFP+ cell (H). (H) Approximately 70% of elongated GFP+ cells recorded displayed rebound spikes. (I) Summary of action potential firing frequency in GFP−, ovoid, and elong cells in response to increasing depolarizing current injections. (J) The membrane potential threshold to elicit action potentials (AP) was lower in elong cells compared to GFP− and ovoid cells. (K) The frequency of APs was higher in elong cells compared to GFP− and ovoid cells. (L) The average halfwidth of action potentials was significantly smaller in elong cells compared to the other two groups. Data was compared between different groups using one-way analysis of variance (ANOVA), post hoc Tukey's Multiple Comparison Test and expressed as mean ± SEM. *◆: p<0.05 compared to GFP-negative cells and ovoid cells; *●: p<0.05 compared to GFP-negative cells and elongated cells.
To compare excitability and action potential firing, 250-ms current injections were delivered from the 0 current level to drive membrane potentials to hyperpolarizing and depolarizing levels in GFP negative (Figure 7F), ovoid GAD1-GFP (Figure 7G) and elongated GAD1-GFP (Figure 7H) cells. At hyperpolarized membrane potentials, none of the neuronal types displayed depolarization sag while action potentials elicited at depolarized membrane potentials showed little to no accommodation, consistent with the known membrane properties of MSNs [42,43]. In approximately 70% of elongated GAD1-GFP cells recorded, rebound action potentials after hyperpolarizing current pulses were observed (Figure 7H), a property that was never observed in GFP− cells or ovoid GAD1-GFP cells. The summary of the current-AP frequency relationship with increasing depolarizing current injections shows that it takes less current to elicit an action potential in elongated GAD1-GFP cells compared to GFP− and ovoid GAD1-GFP cells (Figure 7I). In fact, the current needed to elicit an action potential using a 250-ms duration pulse was significantly smaller in elongated GAD1-GFP cells (40.1 ± 8.1 pA) compared to GFP− (120.0 ± 23.1 pA) and ovoid GAD1-GFP cells (92.9 ± 13.4 pA; ANOVA p = 0.046). This corresponded with a more hyperpolarized mean action potential membrane threshold in elongated GAD1-GFP cells (−51.1 ± 2.1 mV) compared to GFP− (−47.4 ± 1.3 mV) and ovoid GAD1-GFP cells (−48.2 ± 0.7 mV; ANOVA p = 0.019; Figure 7J). The average action potential frequency was significantly greater in elongated GAD1-GFP cells (16.5 ± 2.4 Hz) compared to GFP− (28.9 ± 1.9 Hz) and ovoid GAD1-GFP cells at most membrane potentials (29.2 ± 1.8 Hz; ANOVA p = 0.0058; Figure 7K), but the peak action potential firing was similar in all cell types (Figure 7I). Thus, the main difference between neuronal subtypes was a leftward shift in the voltage/AP frequency curve for the elongated GAD1-GFP neurons in comparison to the other subtypes. The halfwidth of the elicited action potentials was smaller in elongated GAD1-GFP cells (1.9 ± 0.1 ms) compared to GFP− (2.4 ± 0.1 ms) and ovoid GAD1-GFP cells (2.5 ± 0.1 ms; ANOVA p = 0.04; Figure 7L). These findings indicated increased neuronal excitability in elongated GAD1-GFP cells compared to the other two cell types.
Whole-cell patch clamp recording was also used to measure GABAergic and glutamatergic synaptic transmission onto neurons in dorsal striatal slices. To isolate sIPSCs, APV and DNQX were added to the perfusate to block NMDA- and AMPA-mediated currents, respectively. GABAA receptor-mediated sIPSCs recorded from GFP negative cells, that were presumed to be MSNs (GFP−; Figure 8A), were indistinguishable from those recorded from ovoid GAD1-GFP cells (ovoid; Figure 8B). However, those recorded from elongated GAD1-GFP cells (elong; Figure 8C) displayed longer interevent intervals (IEIs) compared to GFP negative cells, suggesting a decrease in GABA release frequency or decreased number of GABAergic synapses (GFP−: 0.43 ± 0.08 s; ovoid: 0.58 ± 0.11 s; elong: 0.84 ± 0.17 s; Figure 8D,E; one-way ANOVA, p = 0.039). The sIPSCs recorded from elongated GAD1-GFP cells (38.0 ± 4.3 pA) displayed decreased amplitude compared to GFP− (48.8 ± 6.3 pA) and ovoid GAD1-GFP cells (47.1 ± 3.8 pA; Figure 8F –G; one-way ANOVA, p = 0.047). No difference in sIPSC area and rise or decay time across the three neuronal subtypes was observed (Figure 8H–I). Differences in amplitude suggest postsynaptic differences that are likely due to a decrease in receptor number and not a change in receptor subunit composition since event kinetic characteristics such as decay and rise time were unchanged.
Figure 8. GAD1-GFP cells receive GABAergic inputs.
Representative traces of sIPSCs recorded from GFP-negative presumed MSNs (GFP−; A), ovoid GFP+ (B), and elongated GFP+ cells (C). The lower panels are expanded time-base portions of the traces shown above. (D–E) The average (D) and cumulative distribution (E) of the interevent interval (IEI) between sIPSCs measured in cells from the ventral area of the dorsal striatum. The average (F) and cumulative distribution (G) of the amplitude (amp) of individual sIPSCs demonstrates a significant decrease in amplitude in elongated GFP+ cells compared to GFP negative and ovoid GFP+ cells. (H) The average area measured for individual sIPSCs. (I) The average rise and decay time of sIPSCs was not significantly different between all three groups. Data was compared between different groups using one-way analysis of variance (ANOVA), post hoc Tukey's Multiple Comparison Test and expressed as mean ± SEM. *: p<0.05 compared to GFP-negative cells.
To isolate sEPSCs, picrotoxin (50 μM) was added to the aCSF and bath-perfused over the slice. Electrophysiological assessment of glutamatergic synaptic transmission in whole-cell recordings (Figure 9A–C) revealed an increased frequency of glutamatergic sEPSCs (observed as decreased interevent interval, IEI) in elongated GAD1-GFP cells (0.73 ± 0.10 s) compared to GFP negative (GFP−; 1.87 ± 0.51 s) and ovoid GAD1-GFP cells (2.95 ± 0.71 s; Figure 9D –E; one-way ANOVA, p = 0.048). This decreased interevent interval could result from increased presynaptic release of glutamate or increased number of glutamatergic synapses, as suggested by the observed increase in spine density (Figure 6H). Elongated GAD1-GFP cells also displayed sEPSCs of larger amplitude (GFP−: 27.5 ± 2.4 pA; ovoid: 29.3 ± 2.8 pA; elong: 42.3 ± 3.5 pA; Figure 9F –G; one-way ANOVA p = 0.0052) and faster rise time (GFP−: 2.2 ± 0.2 ms; ovoid: 2.2 ± 0.1 ms; elong: 1.7 ± 0.1 ms; Figure 9I; one-way ANOVA, p = 0.022) compared to the GFP-negative and ovoid GAD1-GFP cells. However there were no observed differences in sEPSC area (GFP−: 136.1 ± 30.7; ovoid: 157.0 ± 28.8; elong: 183.5 ± 26.6; one-way ANOVA, p = 0.57) and decay time (GFP−: 4.9 ± 0.9 ms; ovoid: 5.2 ± 0.7 ms; elong: 4.7 ± 0.6 ms; one-way ANOVA, p = 0.86) between the three cell groups examined (Figure 9HI). This suggests that postsynaptic differences in elongated GAD1-GFP cells are likely due to either an increase in receptor number or alterations in receptor subunit composition, or both.
Figure 9. GAD1-GFP cells receive glutamatergic inputs.
Representative traces of sEPSCs recorded from GFP-negative (GFP−; A), ovoid GFP+ (B), and elongated GFP+ (C) cells in the ventral area of the dorsal stratum. (D–E) The average (D) and cumulative distribution (E) of the interevent interval (IEI) between sEPSCs measured in cells from the ventral area of the dorsal striatum reveal a significant decrease in IEI in elongated GFP+ cells compared to GFP− and ovoid GFP+ cells. The average (F) and cumulative distribution (G) of the amplitude (amp) of individual sEPSCs demonstrates a significant increase in amplitude in elongated GFP+ cells compared to GFP negative and ovoid GFP+ cells. (H) The average area of individual sEPSCs was similar across cell types. (I) The average rise time of sEPSCs measured in elongated GFP+ cells was significantly faster than the other two cell groups examined. The average decay time was similar across cell types examined. Data was compared between different groups using one-way analysis of variance (ANOVA), post hoc Tukey's Multiple Comparison and expressed as mean ± SEM. *◆: p<0.05 compared to GFP-negative cells and ovoid cells.
CONCLUSIONS
Here we demonstrate that unlike GAD1-GFP+ cells expressed in the cortex and the cerebellum, the large majority of GAD1-GFP+ cells expressed in the striatum of the BAC-GAD1 mouse line designed by Huang and colleagues do not express the calcium-binding protein parvalbumin. Further immunohistochemical and retrograde tracing analysis suggests that striatal GAD1-GFP+ cells are a subset of MSNs, since a majority of striatal GAD1-GFP+ cells do not express markers for GABAergic interneurons, express GABA, and a proportion of these neurons project to one of the output nuclei, the substantia nigra. However one of the markers commonly used for MSNs, DARPP-32 [34–38], was detectable in less than 30% of GAD1-GFP+ cells. Therefore it is possible that GAD1-GFP cells are either a subset of spiny GABAergic interneuron that cannot be classified by coexpression of calcium binding proteins or peptidergic neurotransmitters, or a subset of projecting MSNs that do not use DARPP-32-mediated signal transduction cascade to influence their physiology like typical MSNs, or both.
Our current study identifies two new populations of neurons, a majority of which are located within patches of the ventral area of the dorsal striatum or in the lateral striatal streak as seen by coexpression with dynorphin and μOR and association with calretinin-rich fibers, peptides with more prevalent expression in striatal patches [18,21,22,32]. These two populations are distinguishable by the shape of their cell bodies, ovoid versus elongated. The elongated cells are preferentially located within the lateral striatal streak whereas ovoid GFP+ cells were found in both the patches of the ventral portion of the dorsal striatum and the lateral striatal streak. Morphologically, compared to MSNs, ovoid GAD1-GFP cells have similar sized cell bodies and fewer spines. On the other hand, elongated GAD1-GFP cells differ from MSNs in that their cell bodies are not ovoid in shape and they have more spines.
Electrophysiologically, the input resistance of ovoid GFP+ cells was significantly higher than MSNs. Examination of GABAergic and glutamatergic neurotransmission found no differences between ovoid GAD1-GFP cells and GFP negative cells. Compared to MSNs and ovoid GAD1-GFP cells, elongated GFP+ cells have a lower input resistance, a more depolarized resting membrane potential, more hyperpolarized AP threshold, and increased AP frequency at most membrane potentials. Elongated GAD1-GFP cells also displayed sIPSCs of smaller amplitude and larger interevent interval than their GFP negative and ovoid GAD1-GFP cell counterparts. In regards to their glutamatergic transmission, elongated GAD1-GFP cells displayed sEPSCs of larger amplitude, faster rise time, and shorter interevent intervals.
Acknowledgments
Acknowledgement
This work was supported by the NIAAA Division of Intramural Clinical and Biological Research. The authors greatly appreciate Dr. Ariel Y. Deutch for his generosity in providing images of golgi-impregnated rat sections of the striatum and Guoxiang Luo for genotyping.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DisclosuresAuthors have nothing to disclose.
References Need to be [numbered]
- [1].Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- [2].Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24(43):9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ango F, Di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119(2):257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- [4].Brennaman LH, Maness PF. Developmental regulation of GABAergic interneuron branching and synaptic development in the prefrontal cortex by soluble neural cell adhesion molecule. Mol Cell Neurosci. 2008;37(4):781–793. doi: 10.1016/j.mcn.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27(40):10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huckfeldt RM, Schubert T, Morgan JL, Godinho L, Di Cristo G, Huang ZJ, Wong ROL. Transient neuritis of retinal horizontal cells exhibit columnar tiling via homotypic interactions. Nat Neurosci. 2008;12:35–43. doi: 10.1038/nn.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10(10):3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- [9].Dimova R, Vuillet J, Seite R. Study of the rat neostriatum using a combined Golgi-electron microscope technique and serial sections. Neuroscience. 1980;5:1581–1596. doi: 10.1016/0306-4522(80)90022-6. [DOI] [PubMed] [Google Scholar]
- [10].Chang HT, Wilson CJ, Kitai ST. A golgi study of rat neostriatal neurons: light microscopic analysis. J Comp Neurol. 1982;208:107–126. doi: 10.1002/cne.902080202. [DOI] [PubMed] [Google Scholar]
- [11].Bolam JP, Wainer BH, Smith AD. The section-Golgi impregnantion procedure-3. Combination of Golgi-impregnation with enzyme histochemistry and electron microscopy to characterize acetylcholinesterase-containing neurons in the rat neostriatum. Neuroscience. 1984a;12:687–709. doi: 10.1016/0306-4522(84)90164-7. [DOI] [PubMed] [Google Scholar]
- [12].Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combinaion of choline acetyltransferase immunohistochemistry, Golgiimpregnation and electron microscopy. Neuroscience. 1984b;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- [13].Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- [14].Gerfen CR. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985;236:454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- [15].Chesselet MF, Graybiel AM. Striatal neurons expressing somatostatin-like immunoreactivity: Evidence for a peptidergic interneuronal system in the cat. Neuroscience. 1986;17:547–571. doi: 10.1016/0306-4522(86)90030-8. [DOI] [PubMed] [Google Scholar]
- [16].Kita H, Kitai ST. Glutamate decarboxylase immunoreactive neurons in rat neostriatum: Their morphological types and populations. Brain Res. 1988;447:346–352. doi: 10.1016/0006-8993(88)91138-9. [DOI] [PubMed] [Google Scholar]
- [17].Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- [18].Herkenham M, Pert CB. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat sriatum. Nature. 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- [19].Pert CB, Kuhar MJ, Snyder SH. Opiate receptor: autoradiographic localization in rat brain. PNAS. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Graybiel AM, Ragsdale CW, Yoneoka ES, Elde RD. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience. 1981;6:377–397. doi: 10.1016/0306-4522(81)90131-7. [DOI] [PubMed] [Google Scholar]
- [21].Gerfen CR, Young WS. Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- [22].Hiroi N. Compartmental organization of calretinin in the rat striatum. Neurosci Lett. 1995;197(3):223–226. doi: 10.1016/0304-3940(95)11942-p. [DOI] [PubMed] [Google Scholar]
- [23].Graybiel AM, Ragsdale CW. Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. PNAS. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jimenez-Castellanos J, Graybiel AM. Compartmental origins of striatal efferent proections in the cat. Neuroscience. 1989;32:297–321. doi: 10.1016/0306-4522(89)90080-8. [DOI] [PubMed] [Google Scholar]
- [25].Herkenham M, Edley SM, Stuart J. Cell clusters in the nucleus accumbens of the rat, and the mosaic relationship of opiate receptors, acetylcholinesterase and subcortical afferent terminations. Neuroscience. 1984;11:561–593. doi: 10.1016/0306-4522(84)90045-9. [DOI] [PubMed] [Google Scholar]
- [26].Wright AK, Arbuthnott GW. The pattern of innervation of the corpus striatum by the substantia nigra. Neuroscience. 1981;6:2063–2067. doi: 10.1016/0306-4522(81)90044-0. [DOI] [PubMed] [Google Scholar]
- [27].Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci. 1995;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- [29].López-Bendito G, Sturgess K, Erdélyi F, Szabo G, Molnár Z, Paulsen O. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb Cortex. 2004;14(10):1122–1133. doi: 10.1093/cercor/bhh072. [DOI] [PubMed] [Google Scholar]
- [30].Kusnoor SV, Parris J, Muly EC, Morgan JI, Deutch AY. Extracellular role for Cerebellin 1: modulation of dendritic spine density and synapses in striatal medium spiny neurons. J Comp Neurol. 2010;518:2525–2537. doi: 10.1002/cne.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Marchis S, Temoney S, Erdelyi F, Bovetti S, Bovolin P, Szabo G, Puche AC. GABAergic phenotypic differentiation of a subpopulation of subventricular derived migrating progenitors. Eur J Neurosci. 2004;20(5):1308–1317. doi: 10.1111/j.1460-9568.2004.03584.x. [DOI] [PubMed] [Google Scholar]
- [32].Graybiel AL, Chesselet M-F. Compartmental distribution of striatal cell bodies expressing [Met]enkephalin-like immunoreactivity. PNAS. 1984;81:7980–7984. doi: 10.1073/pnas.81.24.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Davis MI, Puhl HL., 3rd Nr4a1-eGFP is a marker of striosome-matrix architecture, development and activity in the extended striatum. PLoS One. 2011;6(1):e16619. doi: 10.1371/journal.pone.0016619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular distribution in the rat brain. J Neurosci. 1984;4:84–98. doi: 10.1523/JNEUROSCI.04-01-00084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ouimet CC, Miller PE, Hemmings HC, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-rgulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunohistochemical localization. J Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hemmings HC, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci. 1986;6:1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Anderson KD, Reiner A. Immunohistochemical localization of DARPP-32 in striatal projection neurons and striatal interneurons: implications for the localization of D1-like dopamine receptors on different types of striatal neurons. Brain Res. 1991;568:235–243. doi: 10.1016/0006-8993(91)91403-n. [DOI] [PubMed] [Google Scholar]
- [38].Ouimet CC, Langley-Gullion KC, Greengard P. Quantitative immunohistochemistry of DARPP-32-expressing neurons in the rat caudatoputamen. Brain Res. 1998;808:8–12. doi: 10.1016/s0006-8993(98)00724-0. [DOI] [PubMed] [Google Scholar]
- [39].Day M, Wang Z, Ding J, An C, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9(2):251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- [40].Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59(4):621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31(5):1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparations preserving cortical inputs. J Neurphsyiol. 1989;62(5):1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- [43].Tepper JM, Sharpe NA, Koos TZ, Trent F. Postnatal development of the rat neostriatum: electophysiological, light- and electron-microscopic studies. Dev Neurosci. 1998;20:125–145. doi: 10.1159/000017308. [DOI] [PubMed] [Google Scholar]