Abstract
The total syntheses of two alkaloids isolated from a marine sponge of the Leucetta sp. have been accomplished in 6 and 7 steps starting from a 4,5-diiodoimidazole derivative. Grignard mediated halogen-metal exchange was used to install the benzyl side chain. C2 substitution was accomplished via lithiation followed by quenching with trisyl azide which provided isonaamine C after hydrogenation. Isonaamidine E was then prepared from isonaamine C via introduction of the hydantoin ring by reaction with an activated parabanic acid.
Keywords: Leucetta; 2-aminoimidazole; 4,5-diiodoimidazole; azidation; methylparabanic acid
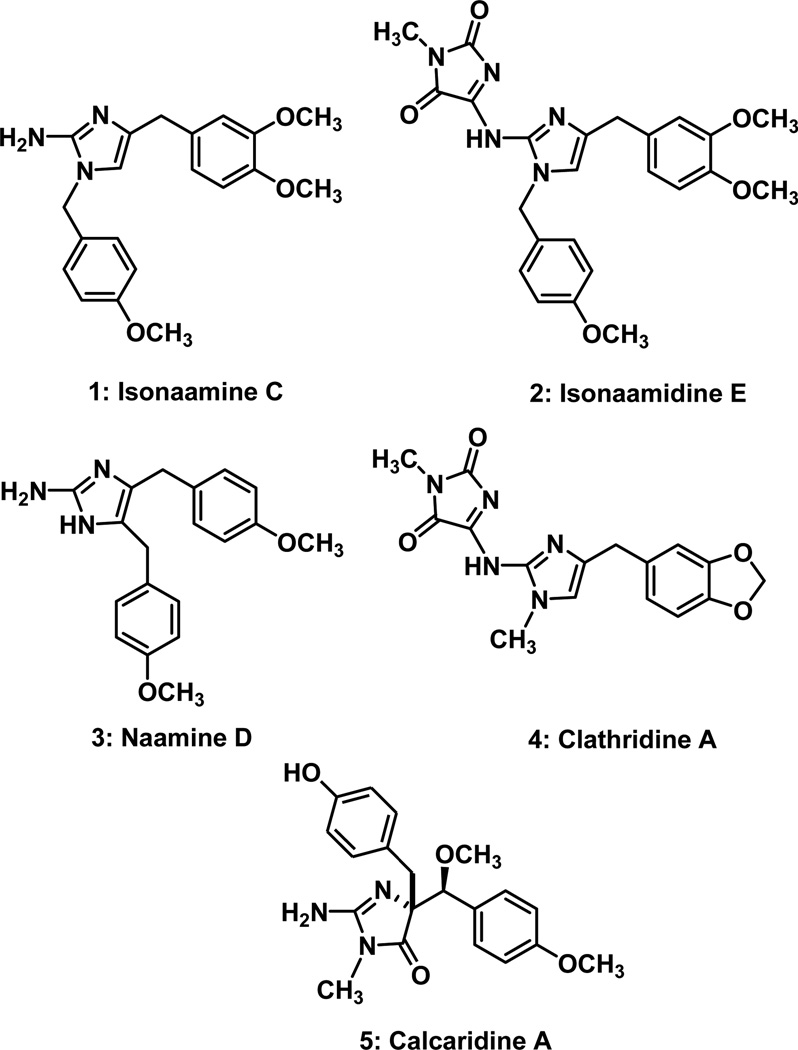
Marine sponges of the Leucetta and Clathrina genera produce a number of imidazole-containing alkaloids that are characterized by a 2-amino or N-imidazolidinedionyl group at C2, and varying benzyl substitution at C4 and C5 on the imidazole.1; 2 Isonaamine C (1)3 and isonaamidine E (2) isolated by Wright in 2002,4 among others, are representative examples of this class of natural products (Figure 1).5 Calcaridine A (5)6; 7 is an example of the more highly oxidized members of this family. Many of these alkaloids have been reported to exhibit varying biological activity including those of interest in this manuscript. Both isonaamine C (1) and isonaamidine E (2) were found to be cytotoxic against stomach (HM02) and liver (HepG2 and Huh7) cancer cell lines with GI50 values in the range of 1.3 to 7.0 µg/mL.4
Figure 1.
Examples of Leucetta alkaloids
A robust, efficient, and adaptable synthetic strategy developed by our lab to access this family of natural products centers around the functionalization of 4,5-diiodoimidazoles via halogen-metal exchange of Grignard reagents to prepare the benzyl substituted framework.2 C2 substitution using n-BuLi and TsN3 or TrisN3 to ultimately lead to the 2-amino substituted target following reduction has been a very effective method as evidenced by the successes in our own lab7–9 and in numerous other examples. This manuscript will detail the application of this approach in the total syntheses of isonaamine C (1) and isonaamidine E (2).
The sequential functionalization of 4,5-dihaloimidazoles with Grignard reagents has been well documented, and follows the order of C5, then C4.10–12 However, C4 functionalization of N-benzyl substituted derivatives leading to C4 benzyl substitution has not been demonstrated before, and the occurrence of competitive lateral metalation of the N-benzyl side chain is a possible complication.13; 14 Therefore, the application of this synthetic strategy to isonaamine C provides the first example of selective C4 metalation in the presence of an N-benzyl substitutuent. Going forward, 4,5-diiodoimidazole (6) was treated with NaH, then 4-methoxybenzyl chloride to give the PMB-protected imidazole 7. Treatment of 7 with EtMgBr induced a halogen-metal exchange at C5 selectively which when quenched with water resulted in an overall reduction leading to the 4-iodoimidazole intermediate 8 (Scheme 1).
Scheme 1.
Substitution of 4,5-diiodoimidazole
Treatment of 8 with another round of EtMgBr followed by 3,4-dimethoxybenzaldehyde (9) led to a mixture of alcohol 10 and the corresponding ketone 12, which is thought to form via an Oppenauer-type oxidation as a result of coordination through the magnesium alkoxide salt and the benzaldehyde 9 (Scheme 2).15–17 However, it was found that the formation of the ketone by-product could be minimized by the addition of the prepared Grignard reagent to a dilute solution of the aldehyde at room temperature. In addition, the crude mixture of products isolated from the Grignard reaction could be treated with NaBH4 in MeOH to provide 10 in 73% yield over the two steps.
Scheme 2.
Grignard reaction leading to 10 and 12
Reduction of 10 with Et3SiH and TFA7–9 led to 11 allowing access to the necessary bis-benzyl substituted framework of isonaamine C and isonaamidine E. The 2-amino substituent was introduced by treatment of 11 with n-BuLi and quenched with TrisN3 to give 13 which was reduced via hydrogenation on Pd/C resulting in isonaamine C (1) in 86% yield. The hydantoin ring of isonaamidine E was introduced following the literature procedure involving reaction of 1-methylparabanic acid and BSA leading to the activated acid 14.8; 9; 18; 19 Upon treatment of 1 with 14, isonaamidine E (2) was isolated in 51% yield from 1 (Scheme 3). In both cases, the spectroscopic data for compounds 1 and 2 agree with reported values.4
Scheme 3.
Total synthesis of isonaamine C and isonaamidine E
In conclusion, isonaamine C (27% overall) and isonaamidine E (14% overall) have been synthesized in 6 and 7 steps respectively from 7 following a general synthetic route developed by us to access this family of natural products. Central to this approach is the application of the chemoselective reactivity of 4,5-dihaloimidazole substitution that has allowed for the controlled elaboration of imidazoles, including those having N-benzyl substituents, leading to the desired benzyl substituted core of the target alkaloids. To date a number of Leucetta and Clathrina alkaloids have been synthesized following this strategy attesting not only to its versatile and efficient nature, but also its potential applicability in medicinal chemistry projects.
Supplementary Material
Acknowledgments
This work was supported by the Robert A. Welch Foundation (Y-1362) and in part by the NIH (GM066503). The NSF (CHE-0840509, CHE-0234811) is thanked for partial funding of the purchases of the NMR spectrometers used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Experimental procedures and copies of 1H and 13C NMR data for all new compounds are available.
References and Notes
- 1.Sullivan JD, Giles RL, Looper RE. Curr Bioact Comp. 2009;5:39–78. [Google Scholar]
- 2.Koswatta PB, Lovely CJ. Nat Prod Rep. 2011;28:511–528. doi: 10.1039/c0np00001a. [DOI] [PubMed] [Google Scholar]
- 3.Ermolat'ev DS, Alifanov VL, Rybakov VB, Babaev EV, Van der Eycken EV. Synthesis. 2008:2083–2088. [Google Scholar]
- 4.Gross H, Kehraus S, Koenig GM, Woerheide G, Wright AD. J Nat Prod. 2002;65:1190–1193. doi: 10.1021/np020050c. [DOI] [PubMed] [Google Scholar]
- 5.The nomenclature for the natural products in this manuscript follows that of the original isolation paper (see ref 4).
- 6.Edrada RA, Stessman CC, Crews P. J Nat Prod. 2003;66:939–942. doi: 10.1021/np020503d. [DOI] [PubMed] [Google Scholar]
- 7.Koswatta PB, Sivappa R, Dias HVR, Lovely CJ. Org Lett. 2008;10:5055–5058. doi: 10.1021/ol802018r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koswatta PB, Lovely CJ. Tetrahedron Lett. 2009;50:4998–5000. [Google Scholar]
- 9.Koswatta PB, Lovely CJ. Tetrahedron Lett. 2010;51:164–166. doi: 10.1016/j.tetlet.2009.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carver DS, Lindell SD, Saville-Stones EA. Tetrahedron. 1997;53:14481–14496. [Google Scholar]
- 11.Dehmel F, Abarbri M, Knochel P. Synlett. 2000;3:345–346. doi: 10.1021/jo000235t. [DOI] [PubMed] [Google Scholar]
- 12.Knochel P, Dohle W, Gommermann N, Kneisel FF, Kopp F, Korn T, Sapountzis I, Vu VA. Angew Chem , Int Ed. 2003;42:4302–4320. doi: 10.1002/anie.200300579. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick DJ, Ngochindo RI. J Chem Soc, Perkin Trans 1. 1984:481–486. [Google Scholar]
- 14.Moreno-Mañas M, Bassa J, Lladó N, Pleixats R. J Heterocycl Chem. 1990;27:673–678. [Google Scholar]
- 15.Byrne B, Karras M. Tetrahedron Lett. 1987;28:769–772. [Google Scholar]
- 16.Kloetzing RJ, Krasovskiy A, Knochel P. Chem Eur J. 2006;13:215–227. doi: 10.1002/chem.200600738. [DOI] [PubMed] [Google Scholar]
- 17.Hofslokken NU, Skattebol L. Acta Chem Scand. 1999;53:258–262. [Google Scholar]
- 18.Aberle NS, Lessene G, Watson KG. Org Lett. 2006;8:419–421. doi: 10.1021/ol052568o. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S, Kawasaki I, Yamashita M, Ohta S. Heterocycles. 2003;60:583–598. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.