Abstract
Previous studies described functional roles for Rho GDP dissociation inhibitor 2 (RhoGDI2) in bladder, gastric and breast cancers. However, only limited expression and no functional analyses have been done for RhoGDI2 in ovarian cancer. We determined RhoGDI2 protein expression and function in ovarian cancer. First, protein gel blot analysis was performed to determine the expression levels of RhoGDI2 in ovarian cells lines. RhoGDI2 but not RhoGDI1 protein expression levels varied widely in ovarian carcinoma cell lines, with elevated levels seen in Ras-transformed ovarian epithelial cells. Next, immunohistochemistry was performed to detect RhoGDI2 expression in patient samples of ovarian cysts and ovarian cancer with known histological subtype, stage, grade and outcome. RhoGDI2 protein was significantly overexpressed in high-grade compared with low-grade ovarian cancers, correlated with histological subtype, and did not correlate with stage of ovarian cancer nor between carcinomas and benign cysts. Unexpectedly, stable suppression of RhoGDI2 protein expression in HeyA8 ovarian cancer cells increased anchorage-independent growth and Matrigel invasion in vitro and in tail-vein lung colony metastatic growth in vivo. Finally, we found that RhoGDI2 stably-associated preferentially with Rac1 and suppression of RhoGDI2 expression resulted in decreased Rac1 activity and Rac-associated JNK and p38 mitogenactivated protein kinase signaling. RhoGDI2 antagonizes the invasive and metastatic phenotype of HeyA8 ovarian cancer cells. In summary, our results suggest significant cell context differences in RhoGDI2 function in cancer cell growth.
Key words: guanine nucleotide dissociation inhibitor 2, Rho small GTPase, ovarian cancer, Rac, metastasis
Introduction
Ras homologous (Rho) GTPases comprise a major branch of the Ras superfamily of small GTPases, with Rac1, Cdc42 and RhoA being the best characterized.1–3 Rho GTPases are important regulators of actin cytoskeleton organization, cell polarity and migration, cell cycle progression and gene expression. Like Ras, their aberrant function has been associated with human oncogenesis and other diseases.1–3 However, unlike the frequent direct mutational activation of Ras in cancer, Rho GTPases are not directly mutated, and instead, their functions are deregulated indirectly through the altered expression and activity of Rho regulatory proteins. To date, one of the best characterized mechanisms involve inappropriate activation of guanine nucleotide exchange factors (RhoGEFs) that promote formation of the activated, GTP-bound form of Rho GTPases, and loss of expression of GTPase activating proteins (RhoGAPs) that accelerate GTP hydrolysis and formation of inactive GDP-bound Rho GTPases. The involvement of a third class of regulators, Rho GDP dissociation inhibitors (RhoGDIs), is less well-understood and has recently gained more attention as possible therapeutic targets for cancer treatment.4,5
The three human RhoGDI isoforms, RhoGDI1 (also called GDI, GDIα), RhoGDI2 (LyGDI/D4GDI/GDIβ), and RhoGDI3 (GDIγ), are generally considered to function as negative regulators of Rho GTPase activity through three distinct biochemical mechanisms.6 First, RhoGDIs bind and mask the C-terminal isoprenyl group attached posttranslationally to the C-terminal membrane targeting sequence of Rho family small GTPases. RhoGDI binding of Rho GTPases forms in an inactive complex in the cytosol, since the isoprenoid lipid modification is essential for membrane association, proper subcellular localization and biological activity. Second, RhoGDIs inhibit RhoGEF-mediated GDP-GTP exchange, favoring the inactive GDP-bound form. Third, they interact with the GTP-bound form to inhibit GTP hydrolysis, RhoGAP-catalyzed GTPase activity, and prevent interactions with downstream effector targets. However, RhoGDIs can also act as positive regulators via targeting Rho GTPases to subcellular membranes where they interact with different effectors or protect Rho GTPases from caspase degradation. Finally, the different tissue expression patterns, subcellular localization and interactions with specific Rho GTPase family members suggest distinct functions for the three RhoGDIs.
Whereas RhoGDI1 is expressed ubiquitously and RhoGDI2 is expressed preferentially in normal hematopoietic tissue, the altered expression of RhoGDI1 and/or RhoGDI2 in a variety of cancers has been reported in reference 4 and 5. However, the precise roles of RhoGDI1 and RhoGDI2 in cancer cell growth are complex, with sometimes opposing roles and possible tumor type distinct functions described. A role for RhoGDI2 as a metastasis suppressor has been established in bladder cancer. Applying gene array analyses, Theodorescu and colleagues reported that decreased RhoGDI2 but not RhoGDI1 gene/protein expression was associated with a more invasive variant of the HRAS mutation positive T24 bladder cancer cell line.7 Ectopic restoration of RhoGDI2 expression in the invasive T24 variant decreased metastasis as determined by tail-vein lung tumor colonization in mice. Finally, immunohistochemical (IHC) analysis of 51 bladder tumors found that RhoGDI2 overexpression correlated with shorter time to disease-specific death.8
In contrast to the findings with bladder cancer, a tumor promotion role for RhoGDI2 overexpression was described in one study of gastric cancer. RhoGDI2 protein expression was found to be higher in gastric tumor tissue when compared with normal gastric tissue and the expression level of RhoGDI2 protein correlated with lymph node metastasis.9 Ectopic overexpression of RhoGDI2 in poorly invasive gastric carcinoma cell lines significantly increased Matrigel invasiveness in vitro. Conversely, depletion of endogenous RhoGDI2 in RhoGDI2 overexpressing gastric carcinoma cells suppressed invasion in vitro. Forced expression of RhoGDI2 in these cells lines increased tumor growth, angiogenesis and lung metastasis in mice. Thus, in contrast to bladder carcinoma cells, RhoGDI2 overexpression promoted rather than inhibited invasion and metastasis in breast and gastric tumor cell lines.10
More complex, sometimes opposing, expression changes and roles for RhoGDIs in breast cancer have been described. Similar to bladder cancer, one study found reduced expression of RhoGDI1 and RhoGDI3 but not RhoGDI2 in breast tumor tissues when compared with normal and that loss of expression correlated with nodal and distant metastasis and poor prognosis.11 In contrast, a second study using IHC staining of 71 patient tumors found a biphasic pattern of increased RhoGDI2 expression with breast hyperplasia, but decreased expression with progression and lymph node metastasis.12 In a third study, expression of RhoGDI2 was not associated with either disease-free or overall survival in two large breast cancer cohorts, although they found that RNAi suppression of RhoGDI2 expression enhanced MDA-MB-231 breast cancer cell invasion in vitro.10 Finally, a fourth study found high RhoGDI2 expression in tumor but not benign breast tumor cell lines and that the knockdown of RhoGDI2 in MDA-MB-231 cells resulted in decreased motility and Matrigel invasion in vitro.13 These contrasting observations prevent establishment of a clear pattern for RhoGDI2 expression changes in tumor progression and of a functional role for RhoGDI2 in breast cancer malignant growth.
Only limited evaluation of RhoGDI expression and function in ovarian cancer has been described. In one study, RhoGDI1 protein expression was identified via proteomic analyses to be overexpressed in three invasive ovarian tumors when compared with three low malignant potential ovarian tumors,14 consistent with a role for RhoGDI1 in promotion of invasion and metastasis. In a gene array study of six serous cystadenocarcoma and one cystadenoma, upregulation of RHOGDI2 transcription was associated with carcinoma when compared with the benign adenoma tissue.15 However, no analysis of protein expression was done. In addition, in a microarray study to identify gene expression changes associated with paclitaxel resistance in ovarian cancers, RhoGDI2 overexpression correlated with resistance.16 Their immunohistochemical analyses of serous ovarian cancer tissues from patients who received paclitaxel-based chemotherapy found that RhoGDI2 protein overexpression was not correlated with stage or histological grade, but was observed more frequently in non-responders (four of five cases) than in responders (two of 16 cases). They concluded that RhoGDI2 expression may be a predictive marker of paclitaxel resistance not only in paclitaxel-resistant cell lines, but also in patient samples.
Presently, only limited analyses of RhoGDI1 and RhoGDI2 protein expression and function in ovarian cancer has been done. In the current study, we found that RhoGDI2 but not RhoGDI1 protein expression varied widely in a panel ovarian cancer cell lines and ovarian tumors, and additionally, was elevated in Ras-transformed human ovarian surface epithelial cells, suggesting that RhoGDI2 overexpression may promote tumor growth. RhoGDI2 was significantly overexpressed in high-grade compared with low-grade ovarian cancers. However, RhoGDI2 levels did not correlate with the stage of ovarian cancer and was expressed in both carcinomas and benign cysts. Surprisingly, interfering RNA suppression of RhoGDI2 in the HeyA8 ovarian carcinoma cell line increased Matrigel invasion and increased lung colonization in a tail-vein lung metastatic assay. Our results support an invasion and metastasis suppression role for RhoGD2.
Results
Variable expression of RhoGDI2 protein in ovarian carcinoma cell lines and tumor tissue.
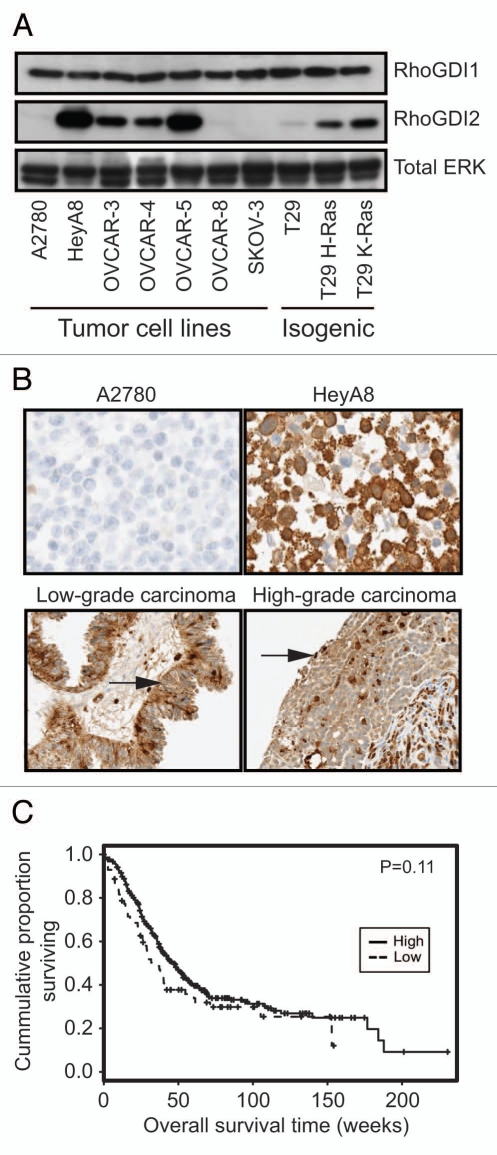
Although previous studies suggested that RhoGDI2 protein overexpression correlated with ovarian tumor progression,15 these analyses evaluated only gene transcription and no analyses of a functional contribution to ovarian tumor growth were addressed. Therefore, we first performed protein gel blot analysis and evaluated the expression of RhoGDI2 protein in a panel of human ovarian carcinoma cell lines and additionally matched pairs of immortalized human ovarian surface epithelial cells (T29) and their transformed counterparts with ectopicallyexpressed mutationally-activate Ras (designated T29 H-Ras, T29 K-Ras) (Fig. 1A). Very low or undetectable RhoGDI2 expression was seen in the untransformed T29 cells as well as three ovarian tumor cell lines (A2780, OVCAR-8 and SKOV-3). In contrast, we found high RhoGDI2 levels in four ovarian carcinoma cells lines (HeyA8, OVCAR-3, OVCAR-4 and OVCAR-5). We also found elevated RhoGDI2 expression in both T29 H-Ras and T29 K-Ras cells, suggesting a role for RhoGDI2 in transformation. In contrast, RhoGDI1 expression levels were equivalent for all cell lines. RhoGDI2 overexpression did not correlate with the invasive ability of cells through Matrigel or anchorage-independent growth in soft agar (data not shown). These data suggest that RhoGDI2 overexpression may be associated with the transformed and tumorigenic growth of ovarian cancer cells.
Figure 1.
Variable levels of RhoGDI2 but not RhoGDI1 protein expression in human ovarian cell lines. (A) Western blot analyses of RhoGDI1 and RhoGDI2 protein expression. Cell lysates were subjected to immunoblotting analysis using antibodies specific for RhoGDI1, RhoGDI2, or the loading control (ERK1 and ERK2). T29 is a nontransformed, immortalized human ovarian surface epithelial cell line that was then stablytransfected with expression vectors encoding activated, transforming mutant H-Ras(12V) or K-Ras(12V), and designated T29 H-Ras and T29 K-Ras, respectively. Results shown are representative of three independent experiments. (B) Validation of anti-RhoGDI2 antibody for IHC analysis. Negative (top left) and positive (top right) control paraffin-embedded ovarian cancer cell lines that express low (A2780) or high (HeyA8) levels of RhoGDI2. A borderline tumor (serous tumor of low malignant potential, bottom right) demonstrated moderate diffuse cytoplasmic staining and a high-grade serous carcinoma (bottom left) demonstrated strong but patchy staining of RhoGDI2. Arrows indicate cancer cells that stain positive for RhoGDI2. (C) IHC analysis of RhoGDI2 protein expression in ovarian cancer patient-derived tissue. Kaplan-Meier survival curves of low and high RhoGDI2 expression groups from a total number of 388 ovarian cancer patient tissues. “Low” expression groups are defined as staining intensity*percent ≤20 percent (73 samples) and “High” as staining intensity*percent > 20 percent (315 samples).
To determine whether overexpression of RhoGDI2 can be correlated with specific pathological phenotypes in patientderived ovarian cancer tissues, we assessed the expression of RhoGDI2 protein in previously described and validated ovarian tissue microarrays containing 388 cases of surgically removed human ovarian cancer tissues with known clinical history.17–19 We first validated the RhoGDI2 antibody for IHC using cell lines that were formalin-fixed and paraffin embedded. In agreement with our protein gel blot data (Fig. 1A), we found that the anti-RhoGDI2 antibody stained negative in A2780 cells and positive in HeyA8 cells (Fig. 1B). RhoGDI2 staining was seen primarily in the cytoplasm of ovarian tumor cells, with staining also observed in the nucleus.
RhoGDI2 was significantly overexpressed in high-grade (344 patients) compared with low-grade ovarian cancer (43 patients), although expression did not correlate with stage of ovarian cancer (Fig. 1B and Table 1). The association between expression of RhoGDI2 and histological subtype was statistically significant (Table 1). In addition, there was no difference in expression between carcinomas from 70 patients and benign cysts from 32 patients (Table 1). Low expression of RhoGDI2 was proportionally more frequent in clear cell carcinoma compared with serous and transitional cell carcinomas; mucinous compared with serous, poorly differentiated, malignant mixed mullerian tumor (MMMT), and mixed type carcinomas; endometrioid compared with serous and mixed type carcinomas (Table 2). Although not statistically significant, there was a trend for ovarian cancer patients with high levels of RhoGDI2 to have a decreased overall survival (Fig. 1C).
Table 1.
Correlations between expression of RhoGDI2 and histological subtype, grade, stage and tumor verses non-tumor ovarian patient tissues
| Characteristics | No. of patients (%) | |||
| High RhoGDI2 | Low RhoGDI2 | Total no. | P* | |
| Histological subtype | 0.0002379 | |||
| Clear-cell | 7 (58.3%) | 5 (41.7%) | 12 | |
| Serous | 177 (84.3%) | 33 (15.7%) | 210 | |
| Mucinous | 2 (28.6%) | 5 (71.4%) | 7 | |
| Endometrioid | 24 (64.9%) | 13 (35.1%) | 37 | |
| Poorly differentiated | 11 (78.6%) | 3 (21.4%) | 14 | |
| MMMT | 15 (83.3%) | 3 (16.7%) | 18 | |
| Mixed type | 71 (86.6%) | 11 (13.4%) | 82 | |
| Transitional cell | 7 (100%) | 0 (0%) | 7 | |
| Grade | 0.038 | |||
| Low | 15 (71.4%) | 6 (28.6%) | 21 | |
| Intermediate | 14 (63.6%) | 8 (36.4%) | 22 | |
| High | 286 (83.1%) | 58 (16.9%) | 344 | |
| Stage | 0.1072 | |||
| I | 16 (64%) | 9 (36%) | 25 | |
| II | 11 (73.3%) | 4 (26.7%) | 15 | |
| III | 24 (85.7%) | 4 (14.3%) | 28 | |
| IV | 263 (82.4%) | 56 (17.5%) | 319 | |
| Tumor verses non tumor tissues | 0.4287 | |||
| Benign Cysts | 31 (96.9%) | 1 (3.1%) | 32 | |
| Tumor | 64 (91.4%) | 6 (8.6%) | 70 | |
p-values were calculated by Fisher's exact test or chi-square test to test for association between RhoGDI2 expression and each clinical attribute. “Low” and “High” expression of RhoGDI2 are defined as Intensity *Percent ≤20 (73 cases) and Intensity *Percent >20 (315 cases). For example, the p-value of 0.4287 implies that the association between the expression of RhoGDI2 (low/high) and tumor/non tumor is not statistically significant.
Table 2.
p-values for pairwise association between different histological subtypes
| Histological type | Transitional cell | Mixed type | MMMT | Poorly differentiated | Endometrioid | Mucinous | Serous |
| Clear-cell | 0.106 | 0.029 | 0.209 | 0.400 | 0.738 | 0.349 | 0.035 |
| Serous | 0.598 | 0.717 | 1 | 0.476 | 0.010 | 0.002 | |
| Mucinous | 0.020 | 0.001 | 0.016 | 0.055 | 0.103 | ||
| Endometrioid | 0.085 | 0.012 | 0.212 | 0.503 | |||
| Poorly differentiated | 0.521 | 0.423 | 1 | ||||
| MMMT | 0.534 | 0.713 | |||||
| Mixed type | 0.59 |
p-values were calculated by Fisher's exact test.
Suppression of RhoGDI2 expression enhances HeyA8 ovarian tumor cell growth properties in vitro and in vivo.
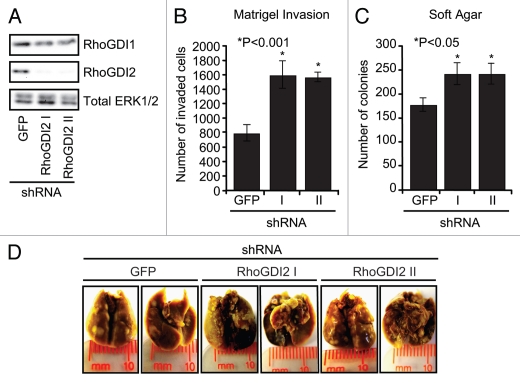
To determine if RhoGDI2 overexpression contributed to the growth and invasive phenotype of ovarian cancer cells, we elected to study HeyA8 cells, which exhibited very high levels of RhoGDI2 protein expression (Fig. 1A). Our rationale to focus on the HeyA8 cell line was based on the fact that this line was derived originally from a woman with advanced, therapy-refractory ovarian cancers and represents the extremes of growth seen in clinical disease. Finally, HeyA8 cells are commonly used in the mouse tail-vein lung colonization metastasis assay because of the cells ability to invade through the vasculature, attach and form lung colonies.20,21 HeyA8 cells were stably infected with retrovirus vectors expressing either control off-target shRNA (GFP) or two independent shRNAs directed against RhoGDI2 (designated RhoGDI2 I and RhoGDI II). Three days after infection, cells were selected with puromycin to establish mass populations of stably-infected cells, and we performed blot analyses to verify suppression of expression of RhoGDI2. The GFP shRNA offtarget control construct had no effect on RhoGDI2 expression, whereas both RhoGDI2 I and RhoGDI2 II shRNA-expressing cells showed ∼90% decreased steady-state protein expression levels (Fig. 2A). In addition, RhoGDI1 expression levels did not change after knockdown of RhoGDI2, indicating no compensatory increase.
Figure 2.
Stable suppression of RhoGDI2 in HeyA8 cells increases Matrigel invasion, anchorage-independent growth in soft agar and lung colonization in the tail-vein lung mouse metastasis assay. (A) Stably shRNA suppression of RhoGDI2 protein expression. HeyA8 cells were stably infected with either control GFP shRNA or two independent shRNA sequences against human RhoGDI2 (designated RhoGDI2 I and RhoGDI2 II) followed by selection with puromycin. Puromycin-resistant cells were analyzed for RhoGDI1, RhoGDI2 and the total protein loading control, ERK1/2. (B) Effect of knockdown of RhoGDI2 on the Matrigel invasive ability of HeyA8 cells. 20,000 cells were plated in Matrigel chamber and assayed after 24 h. *p < 0.001 (two-tailed Student's t test). Bars indicate the mean of triplicate samples + SEM (C) RhoGDI2 requirement for anchorage-independent growth. Cells were suspended in soft agar supplemented with growth medium and colonies and monitored for three weeks for the appearance of proliferating colonies of cells. Colonies were stained with MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and quantified using Image J software and scanned images of soft agar plates are shown. *p < 0.05. Data shown are representative of three independent experiments. (D) Tail-vein lung metastasis assay to determine role of RhoGDI2 in HeyA8 lung colonization. 1.5 x 106 cells of either RhoGDI2 knockdown (I and II) or control (GFP) cells were injected into the mouse tail-vein. After six weeks, mice were euthanized. Lungs were inflated, fixed, stained with Bouin's fixative, and then destained with ethanol.
Stable suppression of RhoGDI2 expression did not cause a detectable alteration in cell morphology nor growth rate in vitro (data not shown). However, when the cells were evaluated for invasion through Matrigel, surprisingly, HeyA8 RhoGDI2 knockdown cells displayed an approximately 2-fold increase in invasion when compared with control cells (Fig. 2B). Additionally, RhoGDI2 suppression significantly increased HeyA8 anchorageindependent growth as measured by colony formation frequency in soft agar, when compared with control cells (Fig. 2C and D). Thus, unexpectedly, although RhoGDI2 expression was elevated in HeyA8 cells, these results support a tumor and invasion suppressor and not an oncogene function for RhoGDI2 in ovarian tumor cells.
To determine whether downregulation of RhoGDI2 altered the metastatic growth properties of HeyA8 cells, cells were injected intravenously into the tail veins of nude mice and lung colonization and tumor formation were measured. In this experimental metastasis assay, we observed a significant increase in the number of lung nodules in mice injected with HeyA8 RhoGDI2 knockdown cells compared with control cells (Fig. 2E and Table 3). Metastatic nodules were not observed in other organs in the mice. These results demonstrate that targeted disruption of RhoGDI2 increased the experimental metastasis activity of HeyA8 cells in nude mice.
Table 3.
Comparison of the tumor colonization ability of RhoGDI2 knockdown and control GFP cells in the tail-vein lung metastasis assay
| HeyA8 | Average tumor size (mm) | Tumor number p* |
| GFP | 0.6 × 0.6 | 0, 15, 8, 11, 13, 0, 15, 0, 0, 0 |
| RhoGDI2 I | 0.8 × 0.7 | 18, 16, 20, 10, 17, 2, 0, 15, 0, 22 ≤ 0.05 |
| RhoGDI2 II | 1.1 × 1.3 | 63, 10, 41, 56, 37, 0, 29, 72, 53, 49 ≤ 0.001 |
p-values are representative to two independent experiments.
RhoGDI2 function is associated with regulation of Rac activity and signaling.
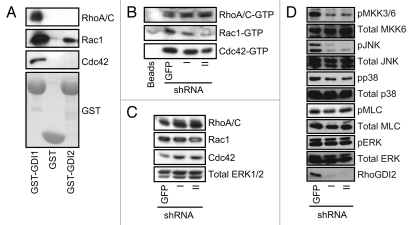
We next evaluated a mechanism by which RhoGDI2 may control invasion. Since the best characterized activity of RhoGDI2 is to modulate Rho GTPase activity, we determined which Rho GTPases associate with RhoGDI2 in HeyA8 cells. Whole cell extracts from HeyA8 cells were incubated with either recombinant glutathione S-transferase (GST) alone or GST fusion proteins containing full length RhoGDI2 or RhoGDI1, resolved on SDS-PAGE and then subjected to protein gel blot analysis probing for the classically studied Rho GTPases, Rac1, Cdc42 and RhoA/C. In agreement with previous study in breast cancer,22 we found that all three Rho GTPases were associated with RhoGDI1. In contrast, we detected only Rac1 association with RhoGDI2.
Next, to determine if stable knockdown of RhoGDI2 expression changed the activity level of Rac1, Cdc42 or RhoA/C, cell lysates were subjected to pull down analyses to measure the level of active GTP-bound GTPases in cells. Cell lysates from control GFP and RhoGDI2 silenced cells were incubated with GST fusion proteins containing the Rho-GTP binding domains (RBD) of the RhoA/C effector Rhotekin (Rhotekin-RBD) or the Rac1/Cdc42 effector PAK1 (GST-PAK-RBD), or control GST-beads alone and subjected to SDS-PAGE followed by protein gel blot analysis to detect Rac1, Cdc42 and RhoA/C (Fig. 3A). Unexpectedly, Rac1-GTP expression levels decreased rather than increased after knockdown of RhoGDI2, whereas the levels of GTP-bound RhoA/C or Cdc42 did not change (Fig. 3B). Total Rac1, RhoA and Cdc42 expression levels were not changed between control and RhoGDI2 knockdown cells (Fig. 3C). Thus, suppression of RhoGDI2 expression was associated with a reduction and not an increase in the activity of Rac1.
Figure 3.
RhoGDI2 binds and regulates Rac1 activity, localization and downstream signaling in HeyA8 cells. (A) HeyA8 cell lysates were incubated with either GST alone, GST-GDI1 or GST-GDI2 beads and then subjected to protein gel blot analysis probing for RhoA/C, Rac1 or Cdc42. (B) RhoGDI2 suppression and effect on steady-state Rho GTPase activity. To determine if knockdown of RhoGDI2 affects levels of GTP-bound Rho GTPases, pull down analyses were done on cell lysates GST-PAK-RBD and then subjected to protein gel blot analysis. (C) western blot analysis of total input lysates were used for pull downs. (D) RhoGDI2 suppression and effect on Rho GTPase-associated signaling activity. western blot analysis was performed with HeyA8 cells stably infected with shRNA targeting GFP or RhoGDI2 and then examined for phosphorylated MMK3/6 (pMMK3/6), total MKK6, pJNK, total JNK, pp38, total p38, pMLC, total MLC, pERK or RhoGDI2. Blot analysis for total ERK1/2 was done to verify equivalent loading of total cellular protein. Data shown are representative of three independent experiments.
Activated Rac1 has been shown to stimulate activation of the p38 and JNK mitogen-activated protein kinase (MAPK) cascades.23 Since we found that Rac1 activity was altered on RhoGDI2 suppression, we determined if Rac-associated signaling was altered. Western blot analysis was done to monitor the levels of phosphorylated and activated MKK3/MKK6, upstream activators of p38 or JNK (Fig. 3D). We found that knockdown of RhoGDI2 decreased phospho-MKK3/6, phospho-JNK and phospho-p38 levels. This reduced activity is consistent with RhoGDI2 suppression-associated loss of Rac1 activation.
Discussion
Although RhoGDI2 expression has been documented to be altered in a variety of human cancers, divergent conclusions have been made regarding association with tumor progression and functional significance.4,5 RhoGDI2 expression is decreased in advanced bladder cancer and exhibited invasion and metastasis suppression activity.7,8,24 In contrast, RhoGDI2 expression was elevated in gastric cancer and exhibited tumor promoting activities.9 To date, there has been very limited analyses of RhoGDI2 protein expression in ovarian cancer and no studies of functional relevance.15,16,25 Our RhoGDI2 shRNA suppression studies in the HeyA8 ovarian carcinoma cell line demonstrated that suppression of RhoGDI2 expression was associated with increased anchorageindependent growth, Matrigel invasion and lung colony metastatic tumor formation. However, our IHC analyses found that RhoGDI2 was significantly overexpressed in high-grade compared with low-grade ovarian cancers, that RhoGDI2 expression correlated with histological subtype of cancer, and no statistically significant association with survival. In summary, our mechanistic studies suggest that RhoGDI2 may function as a tumor suppressor in HeyA8 ovarian cancer cells and RhoGDI2 expression is higher in more advanced ovarian cancer patient tissues.
Altered RhoGDI2 expression has been observed for a variety of cancer types including ovarian cancers. For ovarian cancer, several studies suggest that RhoGDI2 overexpression is associated with tumor progression, with increased expression in cancerous tissue. In our analyses of a panel of ovarian tumor cell lines, we found widely variable levels, with very high levels in a subset of cell lines. Since we found that RhoGDI2 expression levels were increased in Ras-transformed immortalized human ovarian surface epithelial cells, we speculated that RhoGDI2 may function as positive mediator of ovarian tumor cell growth. Therefore, we were surprised when we found that shRNA suppression of RhoGDI2 expression enhanced growth, invasion and metastasis of the RhoGDI2-overexpressing HeyA8 tumor cell line. Thus, similar to bladder cancer,7 RhoGDI2 may function as an invasion and metastasis suppressor. Further study with other cell lines will be needed to determine if this RhoGDI2 function can be generalized to other ovarian tumor cells.
Our analysis of 388 patient ovarian tumors represents the most comprehensive evaluation of RhoGDI2 protein expression for this cancer. While we found that RhoGDI2 protein expression was strong in high-grade ovarian cancer patient tissues when compared with low-grade patient tissues, no statistically significant correlation was seen with overall survival. Thus, in contrast to similar analyses of bladder and gastric cancers,7,9 we cannot conclude that RhoGDI2 expression provides a clear prognostic or diagnostic marker for ovarian cancer. However, since there is evidence that RhoGDI2 expression may influence sensitivity to paclitaxol and other chemotherapeutic agents, it remains possible that RhoGDI2 expression may still provide an informative marker for drug response.
The best known function of RhoGDI2 is that of a negative regulator of Rho GTPase function, and unlike RhoGDI1, RhoGDI2 appears to preferentially regulate Rac1 and not RhoA or Cdc42.6 Consistent with this Rho GTPase selectivity, we found that RhoGDI2 associated preferentially with Rac1 and that sustained suppression of RhoGDI2 expression in HeyA8 ovarian tumor cells resulted in decreased Rac1 but not RhoA nor Cdc42 activity, and was correlated with decreased activities of the JNK and p38 MAPK Rac-associated signaling pathways. Since p38 and JNK activation has been shown previously to inhibit ovarian cancer metastasis,23 this suggests that the reduced activity of these Rac-associated signaling pathways contributes to the enhanced growth properties of RhoGDI2-depleted HeyA8 cells. Our observations contrast with those made in MDA-MB-231 breast carcinoma cells, where knockdown of RhoGDI2 resulted in Rac1 activation, JNK and p38 activation, and decreased tumorigenic and metastatic growth.22 In contrast, results from the study of RhoGDI2 function in bladder cancer are more consistent with our observations. Schwartz and colleagues found that RhoGDI2 preferentially bound and activated Rac1, and that Rac1 activation antagonized metastasis.24 As in our studies, they also reported that suppression of RhoGDI2 expression was associated with altered subcellular localization and reduced Rac-GTP levels. Since RhoGDIs can modulate Rho GTPase interaction with RhoGEFs and RhoGAPs, perhaps in ovarian as well as bladder tumor cells, RhoGDI2 may activate Rac through altered regulation of GDP/GTP cycling. Finally, that Rac activation can antagonize tumor invasion and metastasis is also in agreement with other studies. For example, loss of the Tiam1 Rac-specific RhoGEF was seen to enhance skin carcinoma formation.26
In summary, our observations support the role of RhoGDI2 as an invasion and metastasis suppressor in ovarian cancer. Clearly, the seemingly contrasting functions of RhoGDI2 reported in other cancers reveal the complex nature of RhoGDI2 function, where it can activate or inactivate Rac function in difference cellular settings. The functions of RhoGDI2 in cancer may also involve functions independent of Rho GTPase regulation. One limitation of our studies was our analyses of mechanism in one ovarian carcinoma cell line. Clearly, further analyses of RhoGDI2 function in additional ovarian carcinoma cell lines and in mouse models of ovarian cancer will be needed to better elucidate the significance and importance of RhoGDI2 as a diagnostic or therapeutic target for cancer treatment.
Materials and Methods
Cell lines.
OVCAR-3, OVCAR-4, OVCAR-5, OVCAR-8 and SKOV-3 ovarian cancer cells were obtained originally from American Type Culture Collection. A2780 ovarian cancer cell line was established and characterized by Dr. T. Fojo of Cancer Therapeutics Branch at NCI. The HeyA8 ovarian cancer cell line was established and characterized by Dr. G. Mills at MD Anderson Cancer Center. Human ovarian surface epithelial cells immortalized by SV40 T/t antigen and telomerase catalytic hTERT subunit expression (T29) and T29 cells stably expressing mutationally activated H-Ras(12V) (T29 H-Ras) or K-Ras(12V) (T29 K-Ras) were established and characterized as we have described previously in reference 27. All cell lines were obtained directly from a cell bank or laboratory that established the cell lines and passaged for fewer than six months.
Protein expression and activation analyses.
The phospho-specific and total JNK, MKK3/6, MLC and p38 antibodies were obtained from Cell Signaling. ERK1/2, RhoGDI1, RhoGDI2 [Ly-GDI (C20)], and RhoA/C (119) were obtained from Santa Cruz Biotechnology. Rac1 antibody was purchased from Upstate and Cdc42 antibody from BD Transduction. All antibodies were used for protein gel blot analyses as we have described previously in reference 28.
IHC staining was performed in the Bond Autostainer (Leica Microsystems, Inc.). Briefly, slides were dewaxed in Bond Dewax solution and hydrated in Bond Wash solution. Antigen retrieval was performed for 30 min at 100°C in Bond-Epitope Retrieval solution 1, pH 6.0. Slides were incubated with primary antibody (1:700) for 1 h. Detection was performed using the Bond Intense R Detection Refine System supplied with DakoCytomation LSAB + System-HRP (DakoCytomation, Inc.). Stained slides were dehydrated and coverslipped. Stained slides were digitally scanned at 20x using Aperio ScanScope CS (Aperio Technologies), and the scanned images were analyzed with ImageScope (Aperio Technologies) for the positive cell quantification.
We scored the tumor cells in each core for intensity of staining on a 4 point scale (0–3), with 1 being weak staining, and 3 being intense staining. The percentage of tumor cells that stained was also noted. The scores were only counted when the staining was present in the tumor cells and not the background stroma. Fisher's exact test or chi-square test, as appropriate was performed to evaluate the association of RhoGDI2 expression with clinical factors. Two-tailed p-values are reported for Fisher's exact test. The Kaplan-Meier method was used to estimate the probability of overall survival. Comparison of the overall survival between patients with “Low” and “High” RhoGDI2 expressions was performed using the log rank test. “Low” and “High” expression of RhoGDI2 was defined as Intensity*Percent ≤20 and Intensity*Percent >20, which was found in 73 and 315 patient samples. Results were considered statistically significant at p < 0.05. All statistical analyses were performed using the R package (www.r-project.org).
Plasmids and creation of stable cell lines.
Infectious retroviruses were generated by co-transfection of pSuper-Retro-Puro shRNA plasmids directed against either green fluorescent protein (GFP; off-target control) or the RhoGDI2 sequences 5′-GCG AGG CAC GTA CCA CA-3′ (designated RhoGDI2 I) and 5′-GGC CTG AAA TAC GTT CAG C-3′ (designated RhoGDI2 II) together with the pCL-10A1 packaging plasmid. Infectious virus supernatants were used for stable infection of HeyA8 ovarian cancer cells, followed by selection of puromycinresistant cells, with multiple drug-resistant colonies then pooled together to establish mass populations of stably-infected cells with stable suppression of RhoGDI2 expression, as verified by protein gel blot analyses.
Tail-vein lung colonization metastasis assay.
Female athymic nude mice (4–6 weeks old) were injected via lateral tail vein, with 106 cells that were trypsinized and suspended in phosphate-buffered saline. After six weeks, the mice were sacrificed by CO2 exposure, and the lungs were removed and kept in Bouin's fixative. Tumor nodules on the lung surface appeared as white tissue patches and were counted and measured under a dissecting microscope.
Pull down Rho GTPase activation assay.
Pull down analyses to measure levels of activated GTP-bound Rho GTPases were done as previously described in reference 29. Briefly, the GTP-bound Rho GTPase levels in lysates were measured using a glutathione S-transferase (GST) fusion protein containing the isolated Rho-GTP binding domain (RBD) derived from the RhoA/C effector, Rhotekin (GST-Rhotekin-RBD provided by K. Burridge, University of North Carolina—Chapel Hill) or from the Rac and Cdc42 effector, the PAK1 serine/threonine kinase, GST-PAK-RBD (Cytoskeleton, Inc.). Immunoblot analyses were performed using antibodies against Rac1, Cdc42 and RhoA/C for detection of GTP-bound Rho GTPases and for total cell lysate protein levels.
In vitro binding of RhoGDIs to Rho GTPases.
Cells were lysed with 50 mm Tris, pH 7.5, 150 mm NaCl, 1 mm dithiothreitol, 5 mm MgCl2, 0.5% Triton X-100, and a protease inhibitor mixture tablet (Invitrogen). The lysates were cleared by centrifugation at 12,000 rpm for 10 min at 4°C and 200 µg of total protein extracts were used per condition. Fifteen µg of either bacterially expressed GST, GST-RhoGDI1 or GST-RhoGDI2 (E. Boulter, University of North Carolina—Chapel Hill) were immobilized on glutathione Sepharose™ 4B beads (Amersham Biosciences), and then were incubated with lysates and placed on a rotator at 4°C for 1 h. The beads were washed three times with lysis buffer, and the affinity-precipitated proteins were eluted in protein sample buffer and analyzed by SDS-PAGE and protein gel blotting with either RhoGDI1 or RhoGDI2 specific antibodies.
Acknowledgements
We thank Lanika DeGraffenreid for assistance in figure preparation, Etienne Boulter for providing reagents and useful discussions, and Courtney Boyd and members of the UNC Translational Pathology Laboratory for technical assistance. Our studies were supporte by National Institutes of Health grants R01CA129610 and U19-CA67771 (to C.J.D.) and a Susan G. Komen Breast Cancer postdoctoral fellowship (to C.O.).
Abbreviations
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- IHC
immunohistochemical
- MAPK
mitogen-activated protein kinase
- RBD
Rho-GTP binding domain
- Rho
Ras homologous
- RhoGDI2
Rho GDP dissociation inhibitor 2
- RhoGEF
Rho guanine nucleotide exchange factors
- RhoGAP
Rho GTPase activating proteins
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28:5–14. doi: 10.1007/s10555-008-9166-3. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 4.Cho HJ, Baek KE, Yoo J. RhoGDI2 as a therapeutic target in cancer. Expert Opin Ther Targets. 2010;14:67–75. doi: 10.1517/14728220903449251. [DOI] [PubMed] [Google Scholar]
- 5.Harding MA, Theodorescu D. RhoGDI signaling provides targets for cancer therapy. Eur J Cancer. 2010;46:1252–1259. doi: 10.1016/j.ejca.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Gildea JJ, Seraj MJ, Oxford G, Harding MA, Hampton GM, Moskaluk CA, et al. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002;62:6418–6423. [PubMed] [Google Scholar]
- 8.Theodorescu D, Sapinoso LM, Conaway MR, Oxford G, Hampton GM, Frierson HF., Jr Reduced expression of metastasis suppressor RhoGDI2 is associated with decreased survival for patients with bladder cancer. Clin Cancer Res. 2004;10:3800–3806. doi: 10.1158/1078-0432.CCR-03-0653. [DOI] [PubMed] [Google Scholar]
- 9.Cho HJ, Baek KE, Park SM, Kim IK, Choi YL, Nam IK, et al. RhoGDI2 expression is associated with tumor growth and malignant progression of gastric cancer. Clin Cancer Res. 2009;15:2612–2619. doi: 10.1158/1078-0432.CCR-08-2192. [DOI] [PubMed] [Google Scholar]
- 10.Schunke D, Span P, Ronneburg H, Dittmer A, Vetter M, Holzhausen HJ, et al. Cyclooxygenase-2 is a target gene of rho GDP dissociation inhibitor beta in breast cancer cells. Cancer Res. 2007;67:10694–10702. doi: 10.1158/0008-5472.CAN-07-1621. [DOI] [PubMed] [Google Scholar]
- 11.Jiang WG, Watkins G, Lane J, Cunnick GH, Douglas-Jones A, Mokbel K, et al. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin Cancer Res. 2003;9:6432–6440. [PubMed] [Google Scholar]
- 12.Hu LD, Zou HF, Zhan SX, Cao KM. Biphasic expression of RhoGDI2 in the progression of breast cancer and its negative relation with lymph node metastasis. Oncol Rep. 2007;17:1383–1389. [PubMed] [Google Scholar]
- 13.Zhang Y, Zhang B. D4-GDI, a Rho GTPase regulator, promotes breast cancer cell invasiveness. Cancer Res. 2006;66:5592–5598. doi: 10.1158/0008-5472.CAN-05-4004. [DOI] [PubMed] [Google Scholar]
- 14.Jones MB, Krutzsch H, Shu H, Zhao Y, Liotta LA, Kohn EC al. Proteomic and identification of new biomarkers and therapeutic targets for invasive ovarian cancer. Proteomics. 2002;2:76–84. doi: 10.1002/1615-9861(200201)2:1<76::AID-PROT76>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Tapper J, Kettunen E, El-Rifai W, Seppala M, Andersson LC, Knuutila S. Changes in gene expression during progression of ovarian carcinoma. Cancer Genet Cytogenet. 2001;128:1–6. doi: 10.1016/S0165-4608(01)00386-7. [DOI] [PubMed] [Google Scholar]
- 16.Goto T, Takano M, Sakamoto M, Kondo A, Hirata J, Kita T, et al. Gene expression profiles with cDNA microarray reveal RhoGDI as a predictive marker for paclitaxel resistance in ovarian cancers. Oncol Rep. 2006;15:1265–1271. [PubMed] [Google Scholar]
- 17.Rosen DG, Cai KQ, Luthra R, Liu J. Immunohistochemical staining of hMLH1 and hMSH2 reflects microsatellite instability status in ovarian carcinoma. Mod Pathol. 2006;19:1414–1420. doi: 10.1038/modpathol.3800672. [DOI] [PubMed] [Google Scholar]
- 18.Rosen DG, Mercado-Uribe I, Yang G, Bast RC, Jr, Amin HM, Lai R, et al. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer. 2006;107:2730–2740. doi: 10.1002/cncr.22293. [DOI] [PubMed] [Google Scholar]
- 19.Rosen DG, Yang G, Deavers MT, Malpica A, Kavanagh JJ, Mills GB, et al. Cyclin E expression is correlated with tumor progression and predicts a poor prognosis in patients with ovarian carcinoma. Cancer. 2006;106:1925–1932. doi: 10.1002/cncr.21767. [DOI] [PubMed] [Google Scholar]
- 20.Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 21.Cheng KW, Lu Y, Mills GB. Assay of Rab25 function in ovarian and breast cancers. Methods Enzymol. 2005;403:202–215. doi: 10.1016/S0076-6879(05)03017-X. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Rivera Rosado LA, Moon SY, Zhang B. Silencing of D4-GDI inhibits growth and invasive behavior in MDA-MB-231 cells by activation of Rac-dependent p38 and JNK signaling. J Biol Chem. 2009;284:12956–12965. doi: 10.1074/jbc.M807845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickson JA, Huo D, Vander Griend DJ, Lin A, Rinker-Schaeffer CW, Yamada SD. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 2006;66:2264–2270. doi: 10.1158/0008-5472.CAN-05-3676. [DOI] [PubMed] [Google Scholar]
- 24.Moissoglu K, McRoberts KS, Meier JA, Theodorescu D, Schwartz MA. Rho GDP dissociation inhibitor 2 suppresses metastasis via unconventional regulation of RhoGTPases. Cancer Res. 2009;69:2838–2844. doi: 10.1158/0008-5472.CAN-08-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhen H, Yang S, Wu H, Wang S, Lv J, Ma L, et al. LyGDI is a promising biomarker for ovarian cancer. Int J Gynecol Cancer. 20:316–322. doi: 10.1111/IGC.0b013e3181d0b02d. In press. [DOI] [PubMed] [Google Scholar]
- 26.Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature. 2002;417:867–871. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–1663. doi: 10.1158/0008-5472.CAN-03-3380. [DOI] [PubMed] [Google Scholar]
- 28.Stevens EV, Nishizuka S, Antony S, Reimers M, Varma S, Young L, et al. Predicting cisplatin and trabectedin drug sensitivity in ovarian and colon cancers. Mol Cancer Ther. 2008;7:10–18. doi: 10.1158/1535-7163.MCT-07-0192. [DOI] [PubMed] [Google Scholar]
- 29.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–483. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]