Abstract
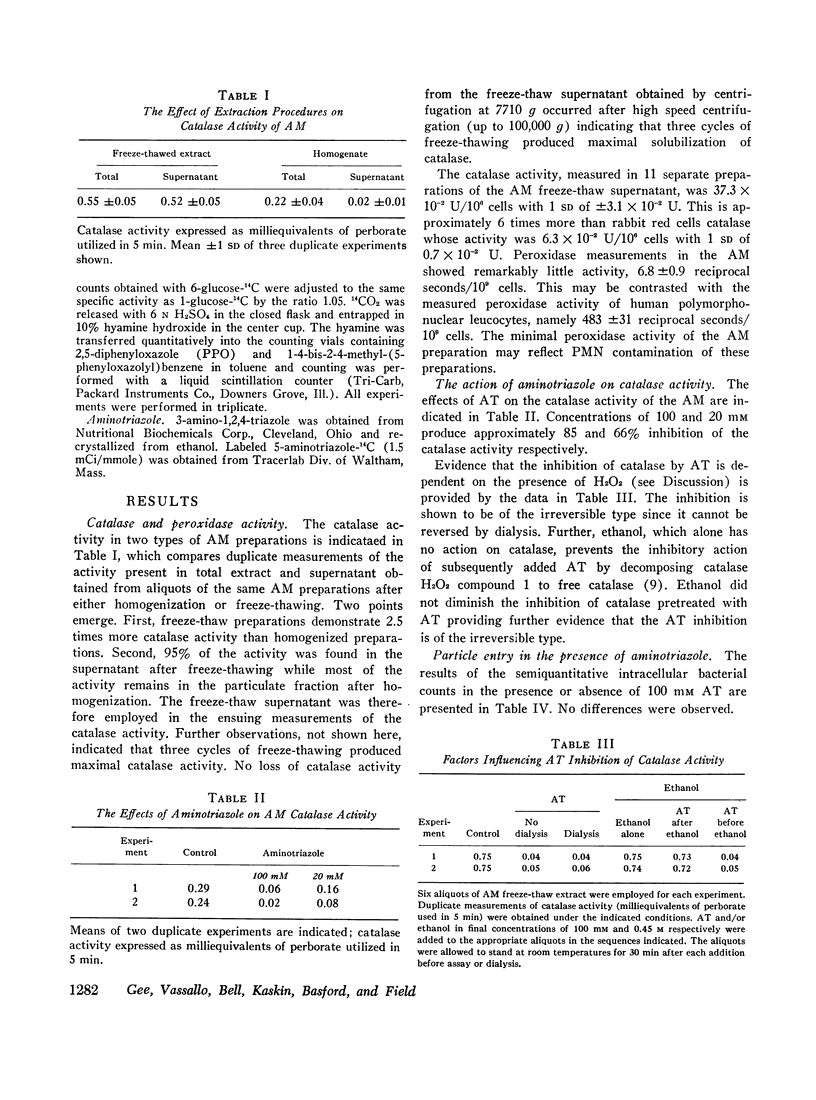
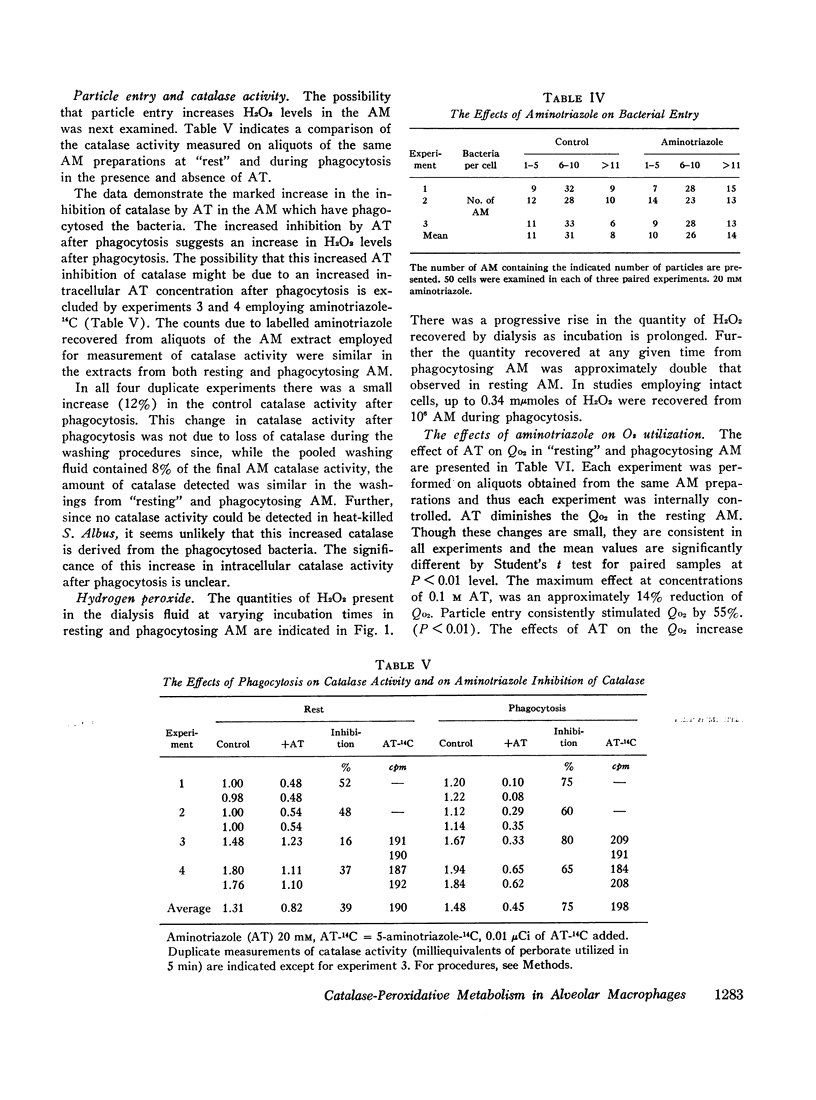
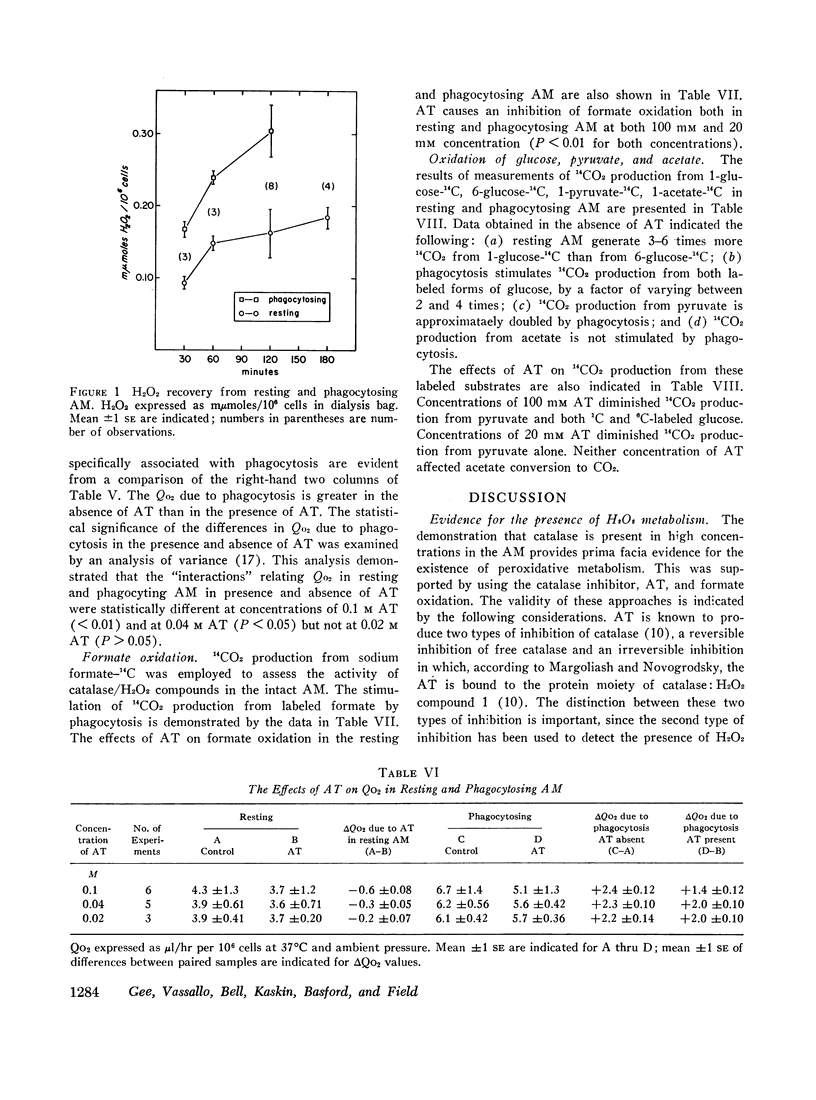
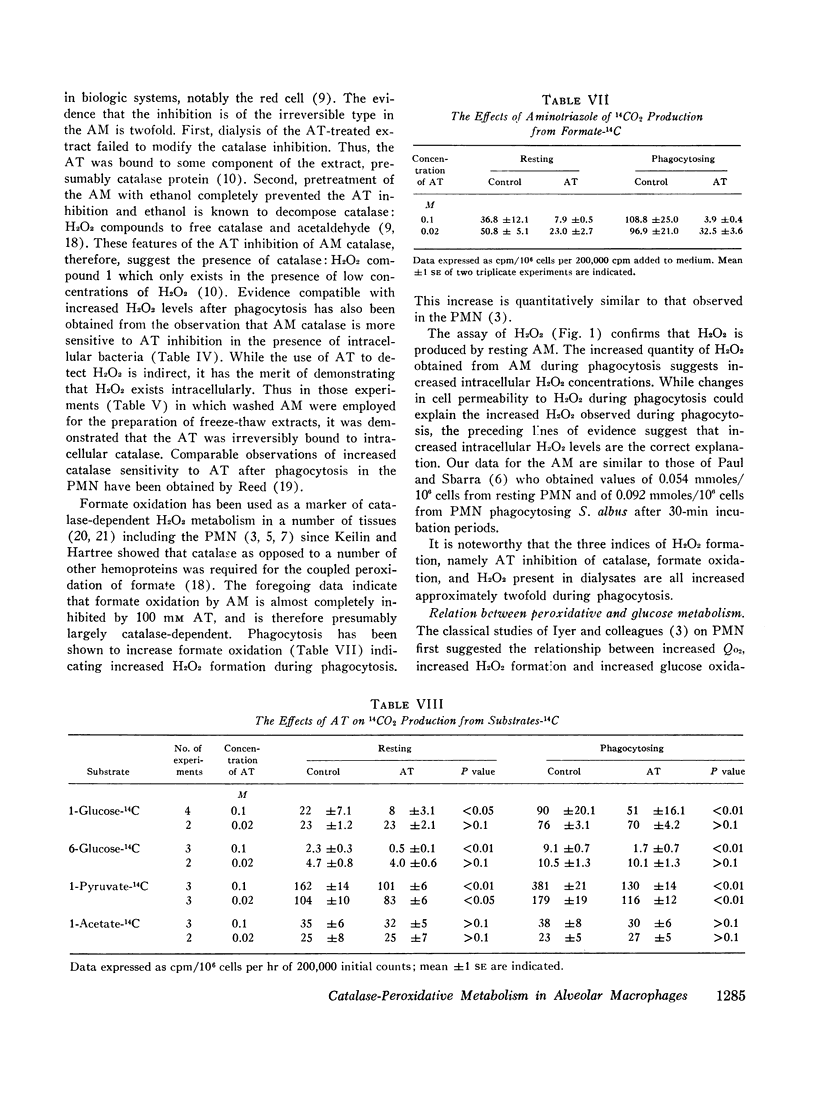
Evidence for the presence of peroxidative metabolism in rabbit alveolar macrophages (AM) has been obtained from the following observations: (a) catalase is present in high concentrations; (b) peroxidase activity could not be detected employing guaiacol as substrate; (c) the irreversible inhibition of AM catalase by aminotriazole served as a detection system for H2O2 and demonstrated increased intracellular H2O2 after phagocytosis; (d) formate oxidation, a marker of catalase-dependent peroxidations, occurs in resting AM and is increased by phagocytosis; (c) measurements of H2O2 accumulation in a dialysate of AM demonstrated twofold increase during phagocytosis; and (f) aminotriazole diminishes O2 utilization and 14CO2 production from labelled glucose and pyruvate. It is concluded that, while catalase-dependent H2O2 metabolism is not essential for particle entry, this pathway represents one of the metabolic pathways stimulated by particle entry in the AM.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRANDT R., KESTON A. S. SYNTHESIS OF DIACETYLDICHLOROFLUORESCIN: A STABLE REAGENT FOR FLUOROMETRIC ANALYSIS. Anal Biochem. 1965 Apr;11:6–9. doi: 10.1016/0003-2697(65)90035-7. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. D-amino acid oxidase in leukocytes: a possible D-amino-acid-linked antimicrobial system. Proc Natl Acad Sci U S A. 1969 Mar;62(3):756–763. doi: 10.1073/pnas.62.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Hochstein P., Utley H. Hydrogen peroxide detoxication by glutathione peroxidase and catalase in rat liver homogenates. Mol Pharmacol. 1968 Nov;4(6):574–579. [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Jandl J. H. Effects of sulfhydryl inhibition on red blood cells. 3. Glutathione in the regulation of the hexose monophosphate pathway. J Biol Chem. 1966 Sep 25;241(18):4243–4250. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Catalase, peroxidase and metmyoglobin as catalysts of coupled peroxidatic reactions. Biochem J. 1955 Jun;60(2):310–325. doi: 10.1042/bj0600310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., White L. R. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N Engl J Med. 1969 Feb 27;280(9):460–466. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A. A study of the inhibition of catalase by 3-amino-1:2:4:-triazole. Biochem J. 1958 Mar;68(3):468–475. doi: 10.1042/bj0680468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi E., Selvaraj R. J., Sbarra A. J. The biochemical activities of rabbit alveolar macrophages during phagocytosis. Exp Cell Res. 1965 Dec;40(3):456–468. doi: 10.1016/0014-4827(65)90226-0. [DOI] [PubMed] [Google Scholar]
- PORTWICH F., AEBI H. [Determination of the peroxide formation of animal tissue by means of peroxidative oxidation]. Helv Physiol Pharmacol Acta. 1960;18:312–327. [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- Roberts J., Camacho Z. Oxidation of NADPH by polymorphonuclear leucocytes during phagocytosis. Nature. 1967 Nov 11;216(5115):606–607. doi: 10.1038/216606a0. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Cline M. J., Schultz J., Lehrer R. I. Myeloperoxidase deficiency. Immunologic study of a genetic leukocyte defect. N Engl J Med. 1970 Jan 29;282(5):250–253. doi: 10.1056/NEJM197001292820505. [DOI] [PubMed] [Google Scholar]



