Abstract
Two new metabolites, including a lindenane-type sesquiterpenoid, menelloide C (1), and a germacrane-type sesquiterpenoid, menelloide D (2), were isolated from a Formosan gorgonian coral identified as Menella sp. The structures of 1 and 2 were established by spectroscopic methods and 2 displayed a weak inhibitory effect on the release of elastase by human neutrophils.
Keywords: menelloide, lindenane, germacrane, sesquiterpenoid, Menella, elastase
1. Introduction
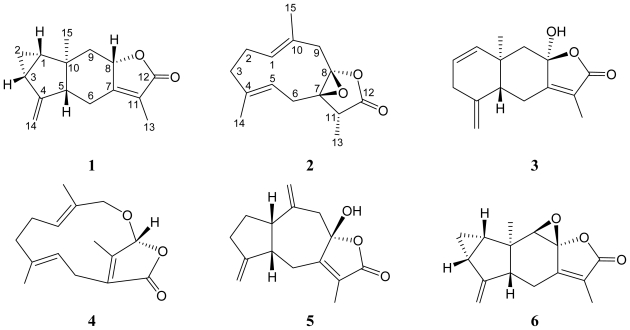
Previous chemical investigations on gorgonian corals belonging to genus Menella (family Plexauridae) [1] have yielded a series of interesting natural products [2–8]. In continuation of our search for new substances from the invertebrates collected off the waters of Taiwan, four new sesquiterpenoid derivatives, (−)-hydroxylindestrenolide (3) [9], menelloide A (4), menelloide B (5), and (+)-chloranthalactone B (6) [10] have been isolated from the gorgonian Menella sp. We have further isolated two new sesquiterpenoids, including a lindenane-type sesquiterpenoid, menelloide C (1), and a germacrane-type sesquiterpenoid, menelloide D (2) (Figure 1) from Menella sp. In this paper, we describe the isolation, structure characterization, and bioactivity of sesquiterpenoids 1 and 2.
Figure 1.
The structures of menelloides C (1), D (2), (−)-hydroxylindestrenolide (3), menelloide A (4), menelloide B (5), and (+)-chloranthalactone B (6).
2. Results and Discussion
Menelloide C (1) was isolated as a needle solid and the molecular formula for this compound was determined to be C15H18O2 (7° of unsaturation) using HRESIMS (C15H18O2Na, m/z 253.1206, calculated 253.1204). The IR spectrum of 1 showed a strong band at 1744 cm−1, consistent with the presence of ester group. From the 13C NMR data (Table 1), a suite of resonances at δC 174.8 (C-12), 162.4 (C-7), 122.6 (C-11), 78.4 (CH-8), and 8.6 (CH3-13), could be assigned to the α-methyl-α,β-unsaturated-γ-lactone moiety in 1. An additional unsaturated functionality was indicated by 13C NMR resonances at δC 151.4 (C-4) and 106.6 (CH2-14), suggesting the presence of an exocyclic carbon-carbon double bond. On the basis of overall unsaturation data, compound 1 was concluded to be a molecule possessing four rings.
Table 1.
NMR Spectroscopic Data (500 MHz, CDCl3) for Menelloide C (1).
| Menelloide C (1)
| ||||
|---|---|---|---|---|
| Position | δC, Mult. | δH (J in Hz) | 1H–1H COSY | HMBC |
| 1 | 28.9, CH | 1.38, ddd (7.5, 7.5, 3.5) | 2, 3 | n.o. a |
| 2α | 16.6, CH2 | 0.70, m | 1, 2β, 3 | n.o. |
| β | 0.84, m | 1, 2α, 3 | 4, 10 | |
| 3 | 23.8, CH | 2.02, m | 1, 2 | n.o. |
| 4 | 151.4, C | |||
| 5 | 56.5, CH | 3.02, m | 6 | n.o. |
| 6α | 22.9, CH2 | 2.35, dd (18.0, 12.5) | 5, 6β | 5, 7, 8 |
| β | 2.54, m | 5, 6α | n.o. | |
| 7 | 162.4, C | |||
| 8 | 78.4, CH | 5.19, m | 9 | n.o. |
| 9α | 43.3, CH2 | 1.82, dd (13.0, 9.0) | 8, 9β | 5, 7, 8, 10, 15 |
| β | 2.62, dd (13.0, 11.5) | 8, 9α | 7, 8, 15 | |
| 10 | 38.9, C | |||
| 11 | 122.6, C | |||
| 12 | 174.8, C | |||
| 13 | 8.6, CH3 | 1.82, s | 7, 11, 12 | |
| 14a | 106.6, CH2 | 5.03, s | 14b | 5 |
| b | 4.75, s | 14a | 5 | |
| 15 | 21.2, CH3 | 0.51, s | 1, 5, 9, 10 | |
n.o. = not observed.
From the 1H–1H COSY spectrum of 1 (Table 1), it was possible to differentiate between the separate spin systems of H-1/H2-2/H-3, H-5/H2-6, and H-8/H2-9. These data, together with the key HMBC correlations between protons and quaternary carbons of 1, such as H-2β/C-4; H-6α, H2-9, H3-13/C-7; H-2β, H-9α, H3-15/C-10; H3-13/C-11; and H3-13/C-12 permitted the elucidation of the carbon skeleton of 1 (Table 1). The exo-cyclic carbon-carbon double bond at C-4 was confirmed by the HMBC correlations between H2-14/C-5. The vinyl methyl group at C-11 was established by the HMBC correlations between H3-13/C-7, C-11, C-12. The ring junction CH3-15 was positioned at C-10 from the HMBC correlations between H3-15/C-1, C-5, C-9, C-10 and H2-9/C-15. Therefore, the proposed skeleton of 1 was established and suggested to be a lindenane-type sesquiterpenoid.
The relative configuration of 1 was elucidated by a NOESY spectrum which was compatible with those of 1 offered by computer modeling (Table 2), in which the close contacts of atoms calculated in space were consistent with the NOESY correlations. In the NOESY experiment of 1, H-8 showed correlations with H-5 and H-9β, indicating that these protons were situated on the same face and assigned as β-protons. Furthermore, H3-15 showed correlations with H-9α, but not with H-5, suggesting that CH3-15 was α-oriented. H-1 exhibited correlations with H-3 and H-9β, indicating that the cyclopropane ring was positioned on the α face in 1.
Table 2.
The Stereoview of 1 (Generated from Computer Modeling) and the Calculated Distances (Å) between Selected Protons Having Key NOESY Correlations.
| Menelloide C (1) | H/H | (Å) |
|---|---|---|
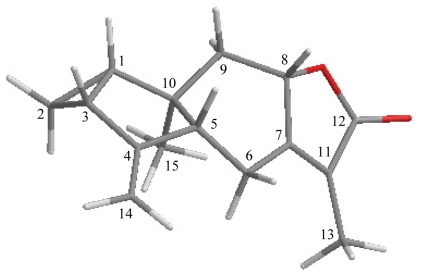 |
H-1/H-3 | 2.43 |
| H-1/H-9β | 2.42 | |
| H-5/H-8 | 2.68 | |
| H-8/H-9β | 2.30 | |
| H-9α/H3-15 | 2.58 |
In our previous study, a lindenane-type sesquiterpenoid, (+)-chloranthalactone B (6) ([α]25D +136 (c 0.05, CHCl3)), was isolated from this study material Menella sp. [10] and this compound was proven to be an enantiomer of a known compound, chloranthalactone B (7) ([α] −130.3 (c 0.1, MeOH)) (Figure 2), which was isolated from the roots of Chloranthus glaber and Chloranthsu japonicus, respectively [11–13]. It was found that the structure of 1 was similar to those of lindenanes 6 and 7 except for the 8,9-epoxy group [10–12]. It is interesting to note that the lindenane-type sesquiterpenoids possessing a cyclopropane moiety, presented as structures 1 (menelloide C, ([α]25D +57 (c 0.04, CHCl3)) and 6 ((+)-chloranthalactone B) [10], isolated from Menella sp. were suggested to possess the same configurations for the chiral carbons C-5 and C-10 because these two compounds were isolated from the same organisms.
Figure 2.
The structures of chloranthalactone B (7) and shizukanolide (8).
Moreover, the structure of 1 was compared with that of a known sesquiterpenoid metabolite, shizukanolide (8) (Figure 2), which was first isolated from a Japanese plant Chloranthus japonicus (Chloranthaceae) [14,15]. It was found that these two compounds possessed the same planar structures and 1 was found to be a diastereomer of shizukanolide (8) by comparison of the NMR data of 1 with those of 8.
Compound 2 (menelloide D), obtained as a colorless oil, showed an [M + Na]+ signal at m/z 271.1312 in the HRESIMS, suggesting the molecular formula C15H20O3 (calcd C15H20O3Na, 271.1310), with 6° of unsaturation. The IR spectrum of 2 showed a band at 1798 cm−1, consistent with the presence of γ-lactone group. The 13C NMR and DEPT spectra of 2 showed that this compound has 15 carbons (Table 3), including three methyls, four sp3 methylenes, an sp3 methine, two sp2 methines, two sp3 quaternary carbons, and three sp2 quaternary carbons. From the 1H and 13C NMR spectra (Table 3), 2 was found to possess a γ-lactone moiety (δC 175.6, C-12) and two trisubstituted olefins (δH 4.93, 1H, dd, J = 11.0, 5.0 Hz, H-1; δC 131.3, C-10; 129.6, CH-1; δH 4.41, 1H, d, J = 11.0 Hz, H-5; δC 130.5, C-4; 121.3, CH-5). The presence of a tetrasubstituted epoxy group was confirmed from the signals of two oxygenated quaternary carbons at δC 92.8 (C-8) and 71.0 (C-7) and this epoxy group could be a part of a hemiketal constellation in the γ-lactone moiety on the basis of a characteristic carbon signal at δC 92.8 (C). Thus, from the above data, compound 2 was identified as a tricyclic compound.
Table 3.
NMR Spectroscopic Data (500 MHz, CDCl3) for Menelloide D (2).
| Menelloide D (2)
| ||||
|---|---|---|---|---|
| Position | δC, Mult. | δH (J in Hz) | 1H–1H COSY | HMBC (H→C) |
| 1 | 129.6, CH | 4.93, dd (11.0, 5.0) | 2 | 2, 9, 15 |
| 2α | 26.7, CH2 | 2.03, m | 1, 2β, 3 | 1, 3, 4, 10 |
| β | 2.12, m | 1, 2α, 3 | n.o. a | |
| 3α | 38.9, CH2 | 2.20, ddd (12.0, 3.0, 3.0) | 2, 3β | 1 |
| β | 1.74, ddd (12.0, 12.0, 4.0) | 2, 3α | 1, 2, 4, 5, 14 | |
| 4 | 130.5, C | |||
| 5 | 121.3, CH | 4.41, d (11.0) | 6 | 3 |
| 6α | 25.9, CH2 | 2.91, d (17.0) | 5, 6β | 4, 5, 7, 8 |
| β | 2.62, dd (17.0, 11.0) | 5, 6α | 4, 5, 7, 8 | |
| 7 | 71.0, C | |||
| 8 | 92.8, C | |||
| 9α | 40.6, CH2 | 3.01, d (14.5) | 9β | 1, 8, 10, 15 |
| β | 3.14, d (14.5) | 9α | 1, 7, 8, 10, 15 | |
| 10 | 131.3, C | |||
| 11 | 43.4, CH | 2.72, q (7.0) | 13 | 6, 7, 12, 13 |
| 12 | 175.6, C | |||
| 13 | 10.1, CH3 | 1.36, d (7.0) | 11 | 7, 11, 12 |
| 14 | 17.0, CH3 | 1.59, s | 3, 4, 5 | |
| 15 | 17.0, CH3 | 1.34, s | 1, 9, 10 | |
n.o. = not observed.
From the 1H–1H COSY spectrum of 2, three different structural units, C-1/C-2/C-3, C-5/C-6, and C-11/C-13, were identified (Table 3), which were assembled with the assistance of an HMBC experiment (Table 3). The HMBC correlations between protons and quaternary carbons such as H-2α, H-3β, H2-6, H3-14/C-4; H2-6, H-9β, H-11, H3-13/C-7; H2-6, H2-9/C-8; H-2α, H2-9, H3-15/C-10; and H-11, H3-13/C-12 were employed successfully to establish the planar structure of 2.
The relative stereochemistry of 2 was established on the basis of a NOESY experiment and by vicinal 1H–1H coupling constant analysis. In the NOESY experiment of 2 (Table 4), correlations observed between H3-14 and δH 2.62 as H-6β; and H3-15 and δH 2.03 as H-2α, as well as the lack of correlation observed between H-1 and H3-15 and H-5 and H3-14, reflected the E geometry of double bonds at C-1/10 and C-4/5. H-5 showed a NOESY correlation with δH 2.91 as H-6α and no coupling constant (J = 0.0 Hz) was found between these two protons indicating the dihedral angle between these two protons is approximately 90° by modeling analysis. H3-13 showed a correlation with H-6α, which suggests that H-11 was β-oriented in the γ-lactone moiety. Moreover, there is no correlation between H-11 and any proton in 2 except with H3-13. Based on this finding, the epoxy group between C-7/8 should be β-oriented and led to the stereohindrance between H-11 and C-6 methylene protons by modeling analysis.
Table 4.
The Stereoview of 2 (Generated from Computer Modeling) and the Calculated Distances (Å) between Selected Protons Having Key NOESY Correlations.
| Menelloide D (2) | H/H | (Å) |
|---|---|---|
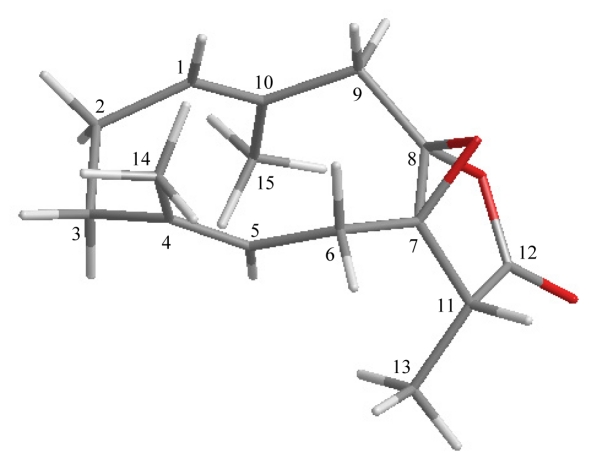 |
H-2α/H3-15 | 2.50 |
| H-5/H-6α | 2.89 | |
| H-6α/H3-13 | 2.44 | |
| H-6β/H3-14 | 2.45 |
The in vitro anti-inflammatory effects of 2 were tested. Sesquiterpenoid 2 displayed a weak inhibitory effect on the release of elastase by human neutrophils (inhibition rate 10.5%) at a concentration of 10 μg/mL.
3. Experimental Section
3.1. General Experimental Procedures
Melting points were determined using a Fargo apparatus and were uncorrected. Optical rotations were measured on a Jasco P-1010 digital polarimeter. Infrared spectra were recorded on a Varian Diglab FTS 1000 FT-IR infrared spectrophotometer; peaks are reported in cm−1. The NMR spectra were recorded on a Varian Inova 500 NMR spectrometer using the residual CHCl3 signal (δH 7.26 ppm) as an internal standard for 1H NMR and CDCl3 (δC 77.1 ppm) for 13C NMR. Coupling constants (J) are given in Hz. ESIMS and HRESIMS were recorded on a Bruker APEX II mass spectrometer. Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck) and spots were visualized by spraying with 10% H2SO4 solution followed by heating. HPLC was performed using a system comprised of a Hitachi L-7100 pump, a Hitahci L-7455 photodiode array detector, and a Rheodyne injection port. A normal phase column (Hibar 250 × 10 mm, Merck, silica gel 60, 5 μm) was used for HPLC.
3.2. Animal Material
Specimens of the gorgonian corals Menella sp. were collected by trawling off the coast of southern Taiwan at a depth of 100 m in December 2004 and stored in a freezer until extraction. A voucher specimen (NMMBA-TW-GC-005) was deposited in the National Museum of Marine Biology and Aquarium, Taiwan. This organism was identified by comparison with previous descriptions [1].
3.3. Extraction and Isolation
The freeze-dried and minced material of Menella sp. (wet weight 451 g, dry weight 411 g) was extracted with ethyl acetate (EtOAc) at room temperature. The EtOAc layer (5.07 g) was separated on silica gel and eluted using n-hexane/EtOAc (stepwise from 100:1 to 0:100 n-hexane/EtOAc) to yield fractions 1–16. Fraction 3 was separated by normal-phase HPLC (NP-HPLC), using the mixtures of n-hexane and EtOAc (15:1–pure EtOAc) to yield the fractions 3A–3Z. Fraction 3H was purified by NP-HPLC using the mixtures of n-hexane and acetone (20:1) to afford 2 (1.0 mg). Compound 1 (0.8 mg) was obtained from fraction 3S by NP-HPLC (n-hexane/EtOAc, 10:1).
Menelloide C (1): needle solid; mp 97–99 °C; ([α]25D +57 (c 0.04, CHCl3); IR (neat) νmax 1744 cm−1; 1H (CDCl3, 500 MHz) and 13C (CDCl3, 125 MHz) NMR data, see Table 1; ESIMS: m/z 253 [M + Na]+; HRESIMS: m/z 253.1206 (calcd for C15H18O2 + Na, 253.1204).
Menelloide D (2): colorless oil; ([α]25D −36 (c 0.05, CHCl3); IR (neat) νmax 1798 cm−1; 1H (CDCl3, 500 MHz) and 13C (CDCl3, 125 MHz) NMR data, see Table 3; ESIMS: m/z 271 [M + Na]+; HRESIMS: m/z 271.1312 (calcd for C15H20O3 + Na, 271.1310).
3.4. Molecular Mechanics Calculations
Implementation of the MM2 force field [16] in CHEM3D PRO software from CambridgeSoft Corporation (Cambridge, MA, USA; ver 9.0, 2005) was used to calculate molecular models.
3.5. Elastase Release by Human Neutrophils
Human neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Measurements of elastase release were carried out according to previously described procedures [17]. Elastase release experiments were performed using MeO-Suc-Ala-Ala-Pro-Valp-nitroanilide as the elastase substrate.
4. Conclusions
In previous studies, a series of interesting natural products, including steroids [4,6,8], guaiane lactones [5,7], briarane diterpenoids [8], menellin A (a highly oxygenated racemate with C8 skeleton) [8], picolinic acid N-methyl betaine [3,4], n-hexadecanol [4], 9H-purin-6-amino-N-9-dimethyl [4], thymidine [4], and batyl alcohol [2,4], were isolated from gorgonian corals belonging to genus Menella, collected off the South China Sea. In our studies on the chemical constituents of a gorgonian coral identified as Menella sp., collected off the waters of Taiwan, various sesquiterpenoids featuring the guaiane, lindenane, and germacrane-type carbon skeletons, containing a γ-lactone in their structures, were isolated. As described in previous studies, the organic extract of Menella sp. displayed significant inhibitory effects on the generation of superoxide anion and the release of elastase [9,10]. However, at this stage, the results showed that the compounds that we isolated only showed weak activity. We suggested that the active components are still existed in the other fractions and these fractions will be studied in the future.
Acknowledgments
This research was supported by grants from the National Museum of Marine Biology and Aquarium (Grant No. 100100101 and 100200311); National Dong Hwa University; Division of Marine Biotechnology, Asia-Pacific Ocean Research Center, National Sun Yat-sen University (Grant No. 00C-0302-05); and the National Science Council (Grant No. NSC 100-2325-B-291-001, 99-2323-B-291-001, and 98-2320-B-291-001-MY3), Taiwan, awarded to P.-J.S.
Footnotes
Samples Availability: Not available.
References and Notes
- 1.Fabricius K, Alderslade P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. 1st ed. Australian Institute of Marine Science; Queensland, Australia: 2001. pp. 59–60. [Google Scholar]
- 2.Deng S, Peng S, Li F, Tan X, Chen J. A study on chemical constituents of South China Sea gorgonian Menella spinifera Kukenthal (I) Guangzhou Chem. 1993:44–47. [Google Scholar]
- 3.Li F, Deng S, Rao Z, Wu H, Xu J. Studies on chemical constituents of South China Sea gorgonian Menella spinifera Kukenthal (II) Guangzhou Chem. 1996:49–51. [Google Scholar]
- 4.Deng S, Li F, Peng S, Rao Z, Wu H, Xu J. Chemical constituents of the South China Sea gorgonian Menella spinifera Kukenthal. Chin. J. Appl. Chem. 1997;14:80–82. [Google Scholar]
- 5.Zhang W, Guo Y-W, Mollo E, Cimino G. Menverins A–D, new highly oxygenated guaiane lactones from Hainan gorgonian Menella verrucosa (Brundin) Helv. Chim. Acta. 2004;87:2919–2925. [Google Scholar]
- 6.Zhang W, Huang H, Ding Y, Gavagnin M, Mollo E, Cimino G, Guo Y-W. Three polyoxygenated steroids from two species of the South China Sea gorgonian Muricella flexuosa and Menella verrucosa Brundin. Helv. Chim. Acta. 2006;89:813–820. [Google Scholar]
- 7.Li L, Wang C-Y, Huang H, Mollo E, Cimino G, Guo Y-W. Further highly oxygenated guaiane lactones from the South China Sea gorgonian Menella sp. Helv. Chim. Acta. 2008;91:111–117. [Google Scholar]
- 8.Chai X-Y, Sun J-F, Tang L-Y, Yang X-W, Li Y-Q, Huang H, Zhou X-F, Yang B, Liu Y. A novel cyclopentene derivative and a polyhydroxylated steroid from a South China Sea gorgonian Menella sp. Chem. Pharm. Bull. 2010;58:1391–1394. doi: 10.1248/cpb.58.1391. [DOI] [PubMed] [Google Scholar]
- 9.Kao S-Y, Chang Y-C, Su J-H, Lu M-C, Chen Y-H, Sheu J-H, Wen Z-H, Wang W-H, Kuo Y-H, Hwang T-L, Sung P-J. (−)-Hydroxylindestrenolide, a new sesquiterpenoid from a gorgonian coral Menella sp. (Plexauridae) Chem. Pharm. Bull. 2011;59:1048–1050. doi: 10.1248/cpb.59.1048. [DOI] [PubMed] [Google Scholar]
- 10.Kao S-Y, Su J-H, Hwang T-L, Sheu J-H, Su Y-D, Lin C-S, Chang Y-C, Wang W-H, Fang L-S, Sung P-J. Discovery of novel sesquiterpenoids from a gorgonian Menella sp. Tetrahedron. 2011;67:7311–7315. [Google Scholar]
- 11.Uchida M, Kusano G, Kondo Y, Nozoe S, Takemoto T. Two new sesquiterpenoids from Chloranthus glaber Makino. Heterocycles. 1978;9:139–144. [Google Scholar]
- 12.Uchida M, Koike Y, Kusano G, Kondo Y, Nozoe S, Kabuto C, Takemoto T. Studies on the constituents of Chloranthus spp. III. Six sesquiterpenes from Chlorantus japonicus. Chem. Pharm. Bull. 1980;28:92–102. [Google Scholar]
- 13.The optical rotation value for chloranthalactone B (7) was reported as [α] −130.3 in the text of ref. 12. However, in the text of ref. 11 and in the experimental of ref. 12, the optical rotation values for chloranthalactone B (4) were reported as [α] −1303.3. The authors suggested that the optical rotation value [α] −1303.3 are typing errors in the text of ref. 11 and in the experimental of ref. 12.
- 14.Kawabata J, Tahara S, Mizutani J, Furusaki A, Hashiba N, Matsumoto T. Shizukanolides, two sesquiterpenoids from Chloranthus japonicus (Chloranthaceae) Agric. Biol. Chem. 1979;43:885–887. [Google Scholar]
- 15.Kawabata J, Tahara S, Mizutani J. Isolation and structural elucidation of four sesquiterpenes from Chloranthus japonicus (Chloranthaceae) Agric. Biol. Chem. 1981;45:1447–1453. [Google Scholar]
- 16.Allinger NL. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977;99:8127–8134. [Google Scholar]
- 17.Hwang T-L, Su Y-C, Chang H-L, Leu Y-L, Chung P-J, Kuo L-M, Chang Y-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009;50:1395–1408. doi: 10.1194/jlr.M800574-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]