Abstract
Five new nardosinane-type sesquiterpenoids, paralemnolins Q–U (1–5), along with three known compounds (6–8), were isolated from the Formosan soft coral Paralemnalia thyrsoides. The structures of new metabolites were elucidated on the basis of extensive spectroscopic methods, and the absolute configuration of 1 was determined by the application of Mosher’s method on 1. Among these metabolites, 1 and 3 are rarely found nardosinane-type sesquiterpenoids, possessing novel polycyclic structures. Compounds 1, 3, 6 and 7 were found to possess neuroprotective activity.
Keywords: soft coral, Paralemnalia thyrsoides, nardosinane, neuroprotective activity
1. Introduction
Soft corals of the genus Paralemnalia [1–7] and Lemnalia [8–12] have been found to be rich sources of sesquiterpenoids of nardosinane [1–3,9,12,13], neolemnane [3,4,10,11] and africanane-type [3,8] compounds and norsesquiterpenoids of nornardosinane-type [3,5,6,9,10] compounds. Our previous study on the secondary metabolites of a Taiwanese soft coral Paralemnalia thyrsoides, collected from the coast of Green Island, has resulted in the isolation of two norsesquiterpenoids [6], ten sesquiterpenoids [4,6,7] and three novel sesquiterpenoids derived from a nardosinane precursor [7]. In our continuing search for new and bioactive metabolites from Formosan soft corals, Paralemnalia thyrsoides collected from Orchid Island were chemically investigated for the first time, and the investigation has resulted in the isolation of five new nardosinane-type sesquiterpeneoids, paralemnolins Q–U (1–5), along with three known compounds 2-deoxylemnacarnol (6) [2], 2-deoxy-7-O-methyllemnacarnol (7) [14] and 2-oxolemnacarnol (8) [15] (Chart 1). The structures of sesquiterpenoids 1–5 were elucidated by spectroscopic analysis and the absolute configurations were established by application of modified Mosher’s method on 1 [16]. The inhibitory activity of compounds 1–8 against three human cancer cell lines was investigated, however, none of these metabolites was found to possess useful cytotoxicity. Furthermore, a study of the neuroprotective effect of these metabolites revealed that 1, 3, 6 and 7, in particular 6, could reduce 6-OHDA (6-hydroxydopamine)-induced neurotoxicity in neuroblastoma SH-SY5Y cells.
Chart 1.
Structures of metabolites 1–8.
2. Results and Discussion
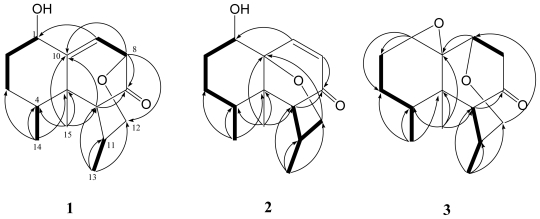
Paralemnolin Q (1) was obtained as a white powder. The HRESIMS (m/z 273.1468 [M + Na]+) of 1 established the molecular formula C15H22O3, appropriate for five degrees of unsaturation, and its IR spectrum revealed the presence of carbonyl (1735 cm−1) and hydroxy (3429 cm−1) groups. The 13C NMR and DEPT (Table 1) spectroscopic data showed signals of three methyls, three sp3 methylenes (including one oxymethylene appearing at δC 69.5), five sp3 methines, one sp2 methine, one sp3 and two sp2 quaternary carbons (including one carbonyl carbon appearing at δC 211.6). The above data accounted for two of the five degrees of unsaturation, indicating a tricyclic structure for 1. From the COSY spectrum measured in CDCl3, it was possible to establish four proton sequences from H-1 to H2-3, H-4 to H3-14, H-8 to H-9 and H-11 to H3-13 (Figure 1). Key HMBC correlations of H-6 to C-7; H-8 to C-7 and C-10; H-9 to C-1; H3-13 to C-6, C-11 and C-12; H3-14 to C-3, C-4 and C-5; and H3-15 to C-4, C-5, C-6 and C-10, permitted the connection of the carbon skeleton. Furthermore, the HMBC cross-peak from H-8 to C-12 suggested that C-8 and C-12 were linked through an oxygen to form a tetrahydropyran ring. On the basis of the above analysis, the gross planar structure of 1 was established.
Table 1.
13C NMR data for compounds 1–5.
| Position | 1, a δC, mult. | 2, b δC, mult. | 3, a δC, mult. | 4, a δC, mult. | 5, a δC, mult. |
|---|---|---|---|---|---|
| 1 | 69.5, CH c | 73.1, CH | 59.0, CH | 118.5, CH | 121.7, CH |
| 2 | 36.4, CH2 | 29.4, CH2 | 25.5, CH2 | 77.8, CH | 80.8, CH |
| 3 | 28.8, CH2 | 24.1, CH2 | 23.3, CH2 | 30.5, CH2 | 31.1, CH2 |
| 4 | 36.3, CH | 30.0, CH | 32.5, CH | 29.2, CH | 33.7, CH |
| 5 | 48.7, C | 41.0, C | 37.6, C | 41.0, C | 40.9, C |
| 6 | 60.6, CH | 56.9, CH | 61.6, CH | 58.9, CH | 59.1, CH |
| 7 | 211.6, C | 201.8, C | 210.6, C | 107.3, C | 107.5, C |
| 8 | 73.7, CH | 133.9, CH | 40.3, CH2 | 33.2, CH2 | 33.4, CH2 |
| 9 | 111.3, CH | 143.6, CH | 78.2, CH | 27.6, CH2 | 27.4, CH2 |
| 10 | 156.6, C | 74.9, C | 62.8, C | 149.7, C | 145.2, C |
| 11 | 35.3, CH | 26.4, CH | 32.0, CH | 37.1, CH | 37.2, CH |
| 12 | 61.9, CH2 | 64.6, CH2 | 67.6, CH2 | 72.2, CH2 | 72.2, CH2 |
| 13 | 18.8, CH3 | 14.9, CH3 | 17.2, CH3 | 18.7, CH3 | 18.6, CH3 |
| 14 | 14.2, CH3 | 13.8, CH3 | 14.5, CH3 | 15.9, CH3 | 16.3, CH3 |
| 15 | 19.5, CH3 | 17.6, CH3 | 17.7, CH3 | 19.7, CH3 | 21.1, CH3 |
Spectrum recorded at 100 MHz in CDCl3;
125 MHz in CDCl3;
Attached protons deduced by DEPT experiment.
Figure 1.
Selected 1H–1H COSY (—) and HMBC (→) correlations of 1–3.
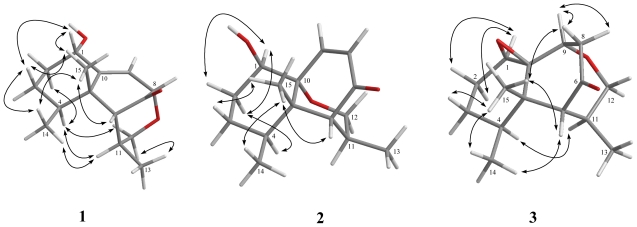
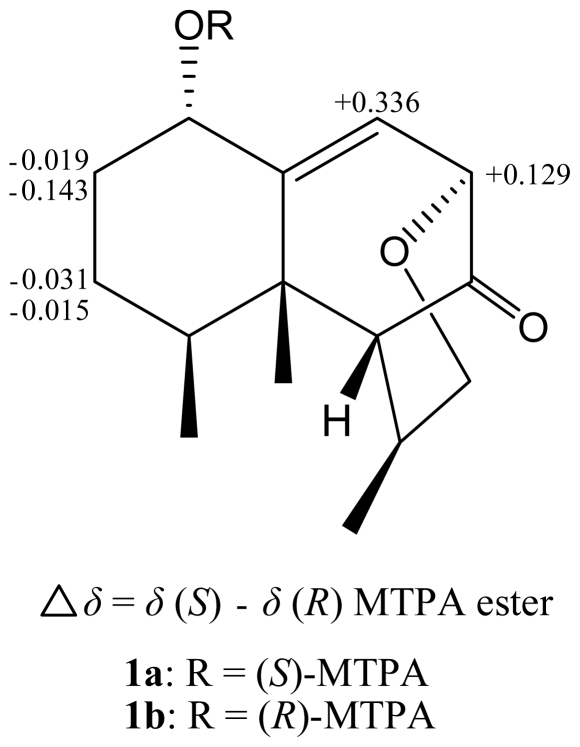
The relative configuration of 1 was elucidated by the analysis of NOE correlations, as shown in Figure 2. It was found that H3-15 (δH 0.98, s) showed NOE interactions with H-1 (δH 4.22, dd, J = 12.0, 4.8 Hz), H-6 (δH 2.10, brs), and H3-14 (δH 0.79, d, J = 6.4 Hz); therefore, assuming the β-orientation of H3-15, all of H-1, H-6, and H3-14 should also be positioned on the β face. Furthermore, H-4 (δH 2.02, ddq, J = 12.0, 4.0, 6.4 Hz) exhibited NOE correlations with H-11 (δH 2.39, ddq, J =3.6, 3.2, 7.2 Hz) and one proton of H2-12 (δH 4.18, dd, J = 12.0, 3.2 Hz), revealing the α-orientation of H-11, and the β-orientations of H-8 and H3-13. On the basis of the above findings and other detailed NOE correlations (Figure 2), the relative structure of 1 was determined. In order to resolve the absolute structure of 1, we determined the absolute configuration at C-1 using a modified Mosher’s method [15]. The (S)- and (R)-MTPA esters of 1 (1a and 1b, respectively) were prepared using the corresponding R-(−)- and S-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl chlorides, respectively. The determination of the chemical shift differences (δS – δR) for the protons neighboring C-1 led to the assignment of the S configuration at C-1 of 1 (Figure 3). Thus, the absolute configuration of 1 has been determined.
Figure 2.
Key NOESY correlations for 1–3.
Figure 3.
1H NMR chemical shift differences of MTPA esters of 1.
The HRESIMS spectrum of paralemnolin R (2) showed a molecular formula of C15H22O3, the same as that of 1. The NMR data revealed the presence of an α,β-unsaturated ketone (δC 201.8 C), and one trisubstituted double bond (δC 143.6 CH, 133.9 CH). The above functionalities account for two of the five degrees of unsaturation, suggesting a tricyclic structure in 2. 1H–1H COSY and HMBC spectra (Figure 1) further revealed that 2 possesses an α,β-unsaturated ketone at C-7(C=O), C-8 and C-9. Furthermore, the HMBC cross-peak from H-12 to C-10 suggested that C-10 and C-12 are linked through an oxygen. On the basis of the above observations, and by the assistance of additional 2D NMR (1H–1H COSY and HMBC) correlations, it was possible to establish the planar structure of 2 as illustrated in Figure 1. The relative configurations of the six chiral centers at C-1, C-4, C-5, C-6, C-10 and C-11 in 2 were further determined on the basis of NOE correlations (Figure 2). It was found that H3-15 showed NOE interactions with both H3-14 and H-6, while H-4 was NOE correlated with H-11. Therefore, H-6, H3-14 and H3-15 were positioned on the same β-face, and in contrast, H-4 and H-11 should be placed on the α-face. Moreover, one of the methylene protons at C-2 (δH 2.18, dddd, J = 15.0, 15.0, 5.0, 3.5 Hz) exhibited NOE correlations with H-4 and was assigned as H-2α. The NOE correlation observed between H-2α and H-1 reflected the β-orientation of hydroxy group. Further NOE analysis revealed that 2 possesses the same configurations at C-4, C-5, C-10, and C-11, as in known compounds flavalins B-D [12]. Thus, the structure of 2 was fully established.
The HRESIMS of paralemnolin S (3) showed that it possesses the molecular formula C15H22O3 (m/z 251.1634 [M + H]+). The IR spectrum of 3 showed the absorption of a carbonyl group (1719 cm−1). The NMR data showed the presence of one trisubstituted epoxide (δH 3.31, 1H, brs; δC 62.8, C and 59.0, CH), and one ketone (δC 210.6, C). The above functionalities and 1H and 13C NMR spectroscopic data (Tables 1 and 2) showed a polycyclic structure in 3. On the basis of the above results and by the assistance of 1H–1H COSY and HMBC spectroscopic analyses (Figure 1), the molecular framework of 3 could be established. This metabolite was found to be a rare nardosinane containing an oxacycloheptane. The 4S*, 5S*, 11R* configurations of 3 were revealed from the similar NOE interactions (Figure 2) as in 1 and 2. Moreover, by NOESY spectrum (Figure 2), it was found that the α-oriented H-1 showed NOE interactions with H2-2, but not with H3-15, indicating the α-orientation of H-1. Furthermore, the NOE correlation observed between one proton (δH 2.80, dd, J =19.2, 2.8 Hz) of H2-8 with H3-15 and H-9 reflected the β-orientation of H-9. From the above evidences and the other NOE correlations (Figure 3), the structure of 3 was determined.
Table 2.
1H-NMR spectral data for compounds 1–5.
| Position | 1, a δH (J in Hz) c | 2, b δH (J in Hz) | 3, a δH (J in Hz) | 4, a δH (J in Hz) | 5, a δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 4.22, dd (12.0, 4.8) c | 3.81, brs | 3.31, brs | 5.57, dd (4.0, 1.6) | 5.60, brs |
| 2 | 2.19, dddd (14.0, 4.8, 2.4, 2.4) | 2.18, dddd (15.0, 15.0, 5.0, 3.5) | 2.19, dddd (15.6, 4.8, 2.4, 2.4) | 4.37, t (4.0) | 4.59, t (7.2) |
| 1.48, m | 1.65, m | 1.92, dddd (15.6, 12.0, 6.0, 1.6) | |||
| 3 | 1.74, ddd (14.0, 4.0, 4.0, 4.0) | 1.70, m | 1.37, m | 1.93, m | 1.84, m |
| 1.57, m | 1.37, m | 1.24, m | 1.57, m | 1.56, m | |
| 4 | 2.02, ddq (12.0, 4.0, 6.4) | 2.55, ddq (15.5, 3.5, 7.0) | 1.73, ddq (12.4, 2.8, 6.8) | 2.02, ddq (12.8, 3.2, 6.8) | 1.95, m |
| 6 | 2.10, brs | 2.25, d (5.0) | 2.40, m | 1.82, m | 1.80, m |
| 8 | 4.10, d (6.4) | 6.39, s | 2.80, dd (19.2, 2.8) | 1.94, m | 1.95, m |
| 2.64, dd (19.2, 2.8) | 1.82, m | 1.79, m | |||
| 9 | 5.77, dd (6.4, 2.4) | 6.39, s | 3.53, t (2.8) | 2.44, m; 2.30, m | 2.46, m; 2.26, m |
| 11 | 2.39, ddq (3.6, 3.2, 7.2) | 2.36, m | 2.38, m | 1.92, m | 1.91, m |
| 12 | 4.18, dd (12.0, 3.2) | 3.75, dd (12.5, 6.5) | 3.81, dd (13.2, 4.8) | 3.87, t (8.8) | 3.88, t (8.8) |
| 3.38, dd (12.0, 1.2) | 3.19, dd (12.5, 12.5) | 3.31, dd (13.2, 9.6) | 3.48, t (8.8) | 3.49, t (8.8) | |
| 13 | 1.10, d (7.2) | 0.70, d (7.0) | 0.93, d (7.2) | 1.10, d (5.2) | 1.09, d (6.0) |
| 14 | 0.79, d (6.4) | 0.75, d (7.0) | 0.76, d (6.8) | 0.90, d (6.8) | 0.92, d (6.8) |
| 15 | 0.98, s | 1.06, s | 0.87, s | 1.11, s | 1.18, s |
Spectrum recorded at 400 MHz in CDCl3;
500 MHz in CDCl3;
J values in Hz in parentheses.
The HRESIMS of paralemnolin T (4) showed that it possesses the molecular formula C15H24O4 (m/z 269.1742 [M + H]+). The IR spectrum of 4 showed the absorption of a hydroxy group (3361 cm−1). Comparison of the 1H and 13C NMR spectroscopic data (Tables 1 and 2) of compounds 4 and 8 [15] suggested that the structure of 4 should be very similar to that of 8, with the exception of signals assigned to C-2, where a ketone (δC 198.0, C) in 8 was replaced by one hydroperoxy-bearing methine (δH 4.37, 1H, t, J = 4.0 Hz, δC 77.8, CH) in 4. Furthermore, H3-15 was found to show NOE correlations with H-6, H3-14 which further correlates with H3-13, and one proton of H2-3 (δH 1.57, m), and the other proton of H2-3 (δH 1.93, m) showed an NOE correlation with hydroperoxy proton (δH 7.83, brs), suggesting that H-2, H-6, H3-13, H3-14 and H3-15 should be placed on the same β-face and in contrast, hydroperoxy group should be positioned on the α-face. Further analysis of other NOE correlations revealed that 4 possesses the same relative configurations at C-4, C-5, C-6, C-7 and C-11 as those of 8 (Figure 4). Based on the above results, the structure of 4 was established.
Figure 4.
Key NOE correlations of 4 and 5.
Paralemnolin U (5) was isolated as a white solid. Its HRESIMS exhibited a [M + H]+ ion peak at 269.1746 m/z, establishing a molecular formula of C15H24O4. By 2D NMR spectroscope data, including COSY, HMQC, and HMBC, compound 5 was shown to possess the same molecular framework as that of 4. Furthermore, the NMR data of 5 were very similar to those of 4, suggesting that 5 is an isomer of 4. By NOESY spectrum (Figure 2), it was found that the β-oriented H3-15 showed NOE correlations with one proton of H2-3 (δH 1.56, m) and the other proton of H2-3 (δH 1.84, m) showed NOE correlation with H-2, indicating the β-orientation of the hydroperoxy group. On the basis of the above findings and other NOE correlations (Figure 4), 5 was revealed to be the C-2 epimer of 4.
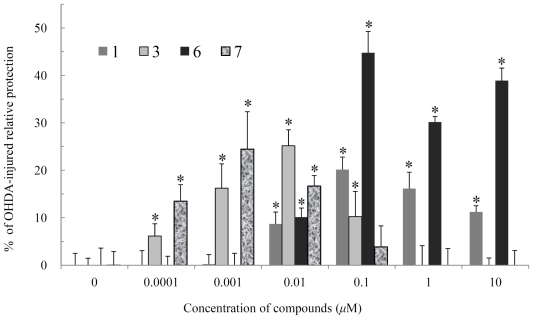
In order to explore the biological activities of the isolated compounds, cytotoxicity of these compounds against the proliferation of a limited panel of cancer cell lines, including mouse melanoma (B16), human epithelial carcinoma (HeLa), human hepatoma carcinoma (HepG2) cell lines, was evaluated. The results showed that all of the compounds were not cytotoxic toward the above cancer cells (IC50’s > 20 μg/mL). Furthermore, the neuroprotective assay of 1–8 using 6-OHDA-induced neurotoxicity in neuroblastoma SHSY5Y, a human dopaminergic neuron often used for study of Parkinson’s disease [17], was performed by a method reported previously [18]. It was observed that the cytotoxicity of 6-OHDA on SH-SY5Y cells could be reduced by pretreatment with 1, 3, 6 and 7 at various concentrations. The relative neuroprotective activities of 1 at 10−2, 10−1, 1 and 10 μM were 8.7 ± 2.5, 20.2 ± 14.0, 16.2 ± 3.5 and 11.2 ± 1.3%, 3 at 10−4, 10−3, 10−2 and 10−1 μM were 6.1 ± 2.6, 16.2 ± 5.1, 25.2 ± 3.4 and 10.2 ± 5.3%, 6 at 10−2, 10−1, 1 and 10 μM were 10.1 ± 1.9, 44.8 ± 4.5, 30.2 ± 1.2 and 38.9 ± 2.7%, and 7 at 10−4, 10−3 and 10−2 μM were 13.5 ± 3.5, 24.5 ± 7.9 and 16.7 ± 2.2%, respectively (Figure 5). From the neurological activity results, we suggest that further investigation of 1, 3, 6 and 7 for their therapeutic potential against neurodegenerative diseases is worthwhile.
Figure 5.
The neuroprotective effects of 1, 3, 6 and 7 on 6-OHDA-induced neurotoxicity in human neuroblastoma SH-SY5Y cells. SH-SY5Y cells were pre-incubated for 1 h with the indicated concentration of test compound and then stimulated with 6-OHDA (20 μM) or vehicle. Relative neuroprotection of control (without the treatment of 6-OHDA and compound) and 6-OHDA-treated alone group were taken to be 100% and 0%, respectively. The experiment was repeated three times. * Significantly different from the 6-OHDA-treated alone group (P < 0.05).
3. Experimental Section
3.1. General Experimental Procedures
Melting points were determined using a Fisher-Johns melting point apparatus. Optical rotations were measured on a JASCO P-1020 polarimeter. Ultraviolet spectrum was recorded on a JASCO V-650 spectrophotometer. IR spectra were recorded on a JASCO FT/IR-4100 infrared spectrophotometer. The NMR spectra were recorded on a Varian 400MR FT-NMR (or Varian Unity INOVA500 FT-NMR) instrument at 400 MHz (or 500 MHz) for 1H and 100 MHz (or 125 MHz) for 13C in CDCl3. ESIMS data were obtained with a Finnigan LCQ ion-trap mass spectrometer. HRESIMS data were recorded on a LTQ Orbitrap XL mass spectrometer. Silica gel (Merck, 230–400 mesh) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F-254, 0.2 mm) were used for analytical TLC. High-performance liquid chromatography was performed on a Hitachi L-2455 HPLC apparatus with a Supelco C18 column (250 × 21.2 mm, 5 μm).
3.2. Animal Material
Soft coral P. thyrsoide was collected by hand using SCUBA off the coast of Orchid Island, located off Taiwan’s southeastern coast, in August 2008, at a depth of 10–15 m, and stored in a freezer until extraction. A voucher sample was deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Separation
The frozen bodies of P. thyrsoide (3.1 kg, wet wt) were sliced and exhaustively extracted with dichloromethane (1 × 10 L). The EtOAc extract (30.4 g) was chromatographed over silica gel by column chromatography and eluting with EtOAc in n-hexane (0–100%, stepwise) then with acetone in EtOAc (50–100%, stepwise) to yield 30 fractions. Fraction 21, eluting with n-hexane–EtOAc (2:1), was further purified over silica gel using n-hexane–acetone (7:1) to afford six subfractions (A1–A6). Subfraction A2 was separated by reversed-phase HPLC using MeOH–H2O (4:1) to afford 4 (1.8 mg), 5 (0.6 mg), 6 (18.3 mg) and 7 (8.8 mg), and subfraction A4 was separated by reversed-phase HPLC using MeOH–H2O (2:1) to afford 1 (10.2 mg), 2 (14.6 mg), 3 (0.8 mg) and 8 (5.1 mg).
Paralemnolin Q (1): white solid; mp 118 °C; [α]26 D +26 (c 0.24, CHCl3); IR (neat) νmax 3429, 2963, 2931, 2877 and 1735 cm−1; 13C and 1H NMR data, see Tables 1 and 2; ESIMS m/z 273 [M + Na]+; HRESIMS m/z 273.1468 [M + Na]+ (calcd for C15H22O3Na, 273.1467).
Paralemnolin R (2): white solid; mp 120 °C; [α]26 D −42 (c 1.46, CHCl3); UV (MeOH) λmax 211 (log ɛ = 3.5); IR (neat) νmax 3401, 2960, 2937 and 1654 cm−1; 13C and 1H NMR data, see Tables 1 and 2; ESIMS m/z 273 [M + Na]+; HRESIMS m/z 273.1465 [M + Na]+ (calcd for C15H22O3Na, 273.1467).
Paralemnolin S (3): colorless oil; [α]24 D +83 (c 0.08, CHCl3); IR (neat) νmax 2958, 2933, 2881, 1719 and 1676 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 251 [M + H]+; HRESIMS m/z 251.1634 [M + H]+ (calcd for C15H23O3, 251.1642).
Paralemnolin T (4): colorless oil; [α]24 D −28 (c 0.18, CHCl3); IR (neat) νmax 3361, 2933, 2881 and 1652 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 269 [M + H]+; HRESIMS m/z 269.1742 [M + H]+ (calcd for C15H25O4, 269.1747).
Paralemnolin U (5): colorless oil; [α]24 D −140 (c 0.06, CHCl3); IR (neat) νmax 3365, 2937, 2877 and 1652 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 269 [M + H]+; HRESIMS m/z 269.1746 [M + H]+ (calcd for C15H25O4, 269.1747).
Preparation of (S)- and (R)-MTPA esters of 1. To a solution of 1 (1.0 mg) in pyridine (0.1 mL) was added (R)-MTPA chloride (10 μL), and the mixture was allowed to stand for 12 h at room temperature. After the evaporation of the solvent, the residue was subjected to short silica gel column chromatography using n-hexane–acetone (6:1) to yield the (S)-MTPA ester, 1a (0.4 mg). The same procedure was applied to obtain the (R)-MTPA ester 1b (0.9 mg) from the reaction of (S)-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride with 1. Selective 1H-NMR (CDCl3, 400 MHz) data of 1a: δ 2.237 (1H, m, H-2a), 1.513 (1H, m, H-2b), 1.757 (1H, m, H-3a), 1.641 (1H, m, H-3b), 2.123 (1H, brs, H-6), 3.992 (1H, d, J = 6.4 Hz, H-8), 5.488 (1H, dd, J = 6.4, 2.0 Hz, H-9), 1.091 (3H, d, J = 6.8 Hz, H-13), 0.801 (3H, d, J = 6.8 Hz, H-14), 1.046 (3H, s, H-15); selective 1H NMR (CDCl3, 400 MHz) data of 1b: δ 2.256 (1H, m, H-2a), 1.656 (1H, m, H-2b), 1.788 (1H, m, H-3a), 1.656 (1H, m, H-3b), 2.106 (1H, brs, H-6), 3.863 (1H, d, J = 6.8 Hz, H-8), 5.152 (1H, dd, J = 6.4, 2.0 Hz, H-9), 1.078 (3H, d, J = 6.8 Hz, H-13), 0.804 (3H, d, J = 6.8 Hz, H-14), 1.038 (3H, s, H-15).
3.4. Cytotoxicity Testing
Cell lines were purchased from the American Type Culture Collection (ATCC). Cytotoxicity assays of compounds 1–8 were performed using the Alamar Blue assay [19,20].
3.5. Neuroprotective Activity Assay
The method for neuroprotective assay was modified from previous study [18]. The human neuroblastoma SH-SY5Y cell line was cultured on 96-well plates. Compounds 1–8 were added to the cells 1 h before 20 μM 6-hydroxydopamine (6-OHDA) challenge. After 15 h incubation, 10 μL of Alamar Blue (Biosource, CA, USA) was aseptically added. The percentages of neuroprotection in 6-OHDA alone and control (without 6-OHDA and test compounds) groups were defined as 0% and 100%, respectively.
4. Conclusions
Our present investigation demonstrated that the Formosan soft coral Paralemnalia thyrsoides is a good source of bioactive substances. In our investigation of new and bioactive metabolites from the Formosan soft corals, this is the first study of P. thyrsoides collected from Orchid Island. From the neurological activity results, compounds 1, 3, 6 and 7, in particular 6, deserve further study for therapeutic potential against neurodegenerative diseases.
Supplementary Material
Acknowledgements
This work was supported by grants from the Ministry of Education (99C031702) and National Science Council of Taiwan (NSC 98-2113-M-110-002-MY3) awarded to J.-H.S.
Footnotes
Samples Availability: Not available.
References
- 1.Su JY, Zhong YL, Zeng LM. Two new sesquiterpenoids from the soft coral Paralemnalia thyrsoides. J. Nat. Prod. 1993;56:288–291. [Google Scholar]
- 2.Bowden BF, Coll JC, Mitchell SJ. Studies of Australian soft corals. XIX. Two new sesquiterpenes with the nardosinane skeleton from a Paralemnalia species. Aust. J. Chem. 1980;33:885–890. [Google Scholar]
- 3.Izac RR, Schneider P, Swain M, Fenical W. New nor-sesquiterpenoids of apparent nardosinane origin from the pacific soft-coral Paralemnalia thyrsoides. Tetrahedron Lett. 1982;23:817–820. [Google Scholar]
- 4.Huang H-C, Chao C-H, Su J-H, Hsu C-H, Chen S-P, Kuo Y-H, Sheu J-H. Neolemnane-type sesquiterpenoids from a Formosan soft coral Paralemnalia thyrsoides. Chem Pharm Bull. 2007;55:876–880. doi: 10.1248/cpb.55.876. [DOI] [PubMed] [Google Scholar]
- 5.Wang G-H, Huang H-C, Su J-H, Wu Y-C, Sheu J-H. Paralemnolins J–P, new sesquiterpenoids from the soft coral Paralemnalia thyrsoide. Chem. Pharm. Bull. 2010;58:30–33. doi: 10.1248/cpb.58.30. [DOI] [PubMed] [Google Scholar]
- 6.Huang H-C, Chao C-H, Liao J-H, Chiang MY, Dai C-F, Wu Y-C, Sheu J-H. A novel chlorinated norsesquiterpenoid and two related new metabolites from the soft coral Paralemnalia thyrsoides. Tetrahedron Lett. 2005;46:7711–7714. [Google Scholar]
- 7.Huang H-C, Wen Z-H, Chao C-H, Ahmed AF, Chiang MY, Kuo Y-H, Hsu C-H, Chen S-P, Sheu J-H. Novel sesquiterpenoids from the Formosan soft coral Paralemnalia thyrsoides. Tetrahedron Lett. 2006;47:8751–8755. [Google Scholar]
- 8.Daloze D, Braekman JC, Georget P, Tursch B. Chemical studies of marine invertebrates. XXII. Two novel sesquiterpenes from soft corals of the genera Lemnalia and Paralemnalia. Bull. Soc. Chim. Belg. 1977;86:47–54. [Google Scholar]
- 9.Bowden BF, Coll JC, Mitchell SJ, Skelton BW, White AH. Studies of Australian soft corals. XXII. The structures of two novel sesquiterpenes and a nor sesquiterpene from Lemnalia africana, confirmed by a single-crystal X-ray study. Aust. J. Chem. 1980;33:2737–2747. [Google Scholar]
- 10.El-Gamal AA, Chiu E-P, Li C-H, Cheng SY, Dai C-F, Duh C-Y. Sesquiterpenoids and norsesquiterpenoids from the Formosan soft coral Lemnalia laevis. J. Nat. Prod. 2005;68:1749–1753. doi: 10.1021/np050326r. [DOI] [PubMed] [Google Scholar]
- 11.Izac RR, Fenical W, Tagle B, Clardy J. Neolemnane and eremophilane sesquiterpenoids from the pacific soft coral Lemnalia africana. Tetrahedron. 1981;37:2569–2573. [Google Scholar]
- 12.Lu Y, Li P-J, Hung W-Y, Su J-H, Wen Z-H, Hsu C-H, Dai C-F, Chiang MY, Sheu J-H. Nardosinane Sesquiterpenoids from the Formosan Soft Coral Lemnalia flava. J. Nat. Prod. 2011;74:169–174. doi: 10.1021/np100541a. [DOI] [PubMed] [Google Scholar]
- 13.Bishara A, Yeffet D, Sisso M, Shmul G, Schleyer M, Benayahu Y, Rudi A, Kashman Y. Nardosinanols A–I and lemnafricanol, sesquiterpenes from several soft corals, Lemnalia sp., Paralemnalia clavata, Lemnalia africana, and Rhytisma fulvum fulvum. J. Nat. Prod. 2008;71:375–380. doi: 10.1021/np070550b. [DOI] [PubMed] [Google Scholar]
- 14.Kapojos MM, Mangindaan RE, Nakazawa T, Oda T, Ukai K, Namikoshi M. Three new nardosinane type sesquiterpenes from an Indonesian soft coral Nephthea sp. Chem. Pharm. Bull. 2008;56:332–334. doi: 10.1248/cpb.56.332. [DOI] [PubMed] [Google Scholar]
- 15.Bowden BF, Coll JC, Mitchell SJ. Studies of Australian soft corals. XXIII. The co-occurrence of bicyclogermacrene and lemnacarnol derivatives in Parerythropodium fulvum. Tetrahedron Lett. 1980;21:3105–3108. [Google Scholar]
- 16.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991;113:4092–4096. [Google Scholar]
- 17.Kitamura Y, Kosaka T, Kakimura JI, Matsuoka Y, Kohno I, Nomura Y, Taniguchi T. Protective effects of the antiparkinsonian drugs talipexole and pramipexole against 1-methyl-4-phenylpyridinium-induced apoptotic death in human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1998;54:1046–1054. [PubMed] [Google Scholar]
- 18.Lee KY, Sung SH, Kim YC. Triterpenoids from Schisandra lancifolia with anti-HIV-1 activity. J. Nat. Prod. 2006;69:679–681. doi: 10.1021/np0503303. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama GR, Caton MC, Nova MP, Parondoosh Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods. 1997;204:205–208. doi: 10.1016/s0022-1759(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.