Abstract
Objective
To investigate the molecular-genetic heterogeneity associated with the t(6:9) in adenoid cystic carcinoma (ACC) and correlate the findings with patient clinical outcome.
Experimental Design
Multi-molecular and genetic techniques complemented with massive pair-ended sequencing and SNP array analyses were used on tumor specimens from 30 new and 52 previously RT-PCR analyzed fusion transcript negative ACCs. MYB mRNA expression level was determined by quantitative RT-PCR. The results of 102 tumors (30 new and 72 previously reported cases) were correlated with the clinicopathologic factors and patients’ survival.
Results
The FISH analysis showed 34/82 (41.5%) fusion positive tumors and molecular techniques identified fusion transcripts in 21 of the 82 (25.6%) tumors. Detailed FISH analysis of 11 out the 15 tumors with gene fusion without transcript formation showed translocation of NFIB sequences to proximal or distal sites of the MYB gene. Massive pair-end sequencing of a subset of tumors confirmed the proximal translocation to an NFIB sequence and led to the identification of a new fusion gene (NFIB-AIG1) in one of the tumors. Overall, MYB-NFIB gene fusion rate by FISH was in 52.9% while fusion transcript forming incidence was 38.2%. Significant statistical association between the 5′ MYB transcript expression and patient survival was found.
Conclusions
We conclude that: 1) t(6;9) results in a complex genetic and molecular alterations in ACC, 2) MYB-NFIB gene fusion may not always be associated with chimeric transcript formation, 3) non-canonical MYB, NFIB gene fusions occur in a subset of tumors, 4) high MYB expression correlates with worse patient survival.
Keywords: Gene fusion, Gene fusion, chromosomal translocations, salivary gland carcinomas, molecular alterations
INTRODUCTION
Salivary adenoid cystic carcinoma, a relatively uncommon malignancy, is known for its progressive and heterogeneous clinical behavior (1-3). The primary treatment for patients with ACC is surgical resection with and without post-operative radiotherapy dependent upon the presence or the lack of adverse pathologic findings (4). More than 60% of these patients succumb to recurrent and/or metastatic disease with limited therapeutic options (4-6). Several recent genomic studies have attempted to unravel the events associated with ACC development and to identify molecular and biological markers for better management of patients with advanced disease (9, 12). Although no definitive marker(s) has been identified, recurrent loss of the terminal region of the long arm of chromosome 6 and translocation involving chromosomes 6q and 9p regions on different partners were the most consistently reported findings (7-13).
Recently, a fusion between the MYB and NFIB genes resulting from t(6;9)(q22-23;p24) regions have been identified in all 11 ACCs and were found to be associated with high 5′-MYB gene expression (14). Our group subsequently reported a lower incidence of MYB-NFIB fusion transcript, numerous fusion variants (15) and high level of the 5′-segment of the MYB transcript in the majority of fusion positive tumors. We also noted that a subset of fusion negative tumors express MYB level similar to those of fusion positive tumors. These observations, together with the complex fusion variants and the high incidence of MYB-NFIB gene fusion by in-situ hybridization (22), support the involvement of different molecular events associated with the t(6;9) in the MYB gene regulation (16-23) and other yet to be identified aberrations. We contend that accounting for these alterations is critical to understanding the role of the MYB-NFIB gene fusion in ACC development and progression.
To thoroughly account for the molecular genetic alterations associated with the t(6;9) in ACC and to understand their biological implications, we performed detailed cytogenetic and molecular analyses on 30 new ACCs and the 52 previously screened MYB-NFIB fusion transcript negative tumors and correlated the findings with the clinicopathologic parameters and the patient outcome.
MATERALS and METHODS
Tissue specimens and cohort analysis
We used fresh frozen tissue specimens from eighty-two primary ACCs accessioned at the head and neck section from 1989 to 2010 which comprised of 30 previously unanalyzed specimens and the 52 fusion transcript negative tumors from our earlier report (15). Tumors were classified into tubular and cribriform if they lacked any solid component and manifested at 75% of either form. (6) Tumors were categorized as solid if this feature is identified in any area. For clinical correlation of fusion positive and negative tumors, we also included all fusion positive tumors previously reported (15) for a combined total of 102 patients.
RNA extraction and fusion transcript sequencing
Total RNA was extracted with the TRIzol reagent (Invitrogen) and treated with recombinant DNase I, RNase-free (Roche) prior to RT-PCR and converted subsequently to cDNA using the SuperScript™ III First-Strand Synthesis System for RT-PCR (Invitrogen) with oligo(dt) primers according to the manufacturer’s instructions. The amplification of the MYB-NFIB fusion transcripts and the primers used were described previously (Supplementary Table 1) (15). The MYB-NFIB fusion transcripts were detected by PCR analysis with Platinum Taq DNA polymerase (Invitrogen). We designed a new set of primers (Supplementary Table 1) in addition to the previously published primer sets (Supplementary Table 1). ACTB primer (Supplementary Table 1) was used as internal control. RT-PCR products were purified and sequenced directly or cloned into the pCR2.1 vector (Invitrogen). The PCR fragments were sequenced with an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems) at the DNA sequencing core facility. The MYB-NFIB variants, the nucleotide sequences and genomic organization of MYB (accession number NM_001130173) and NFIB (ENSG00000147862) were determined using the NCBI (http://www.ncbi.nlm.nih.gov) and Ensemble (http://ensembl.org), respectively.
Quantitative RT-PCR
Quantitative RT-PCR was performed using the Applied Biosystems 7900HT Real-time PCR systems (Applied Biosystems) with Power SYBR® Green PCR Master Mix (Applied Biosystems). The primer sequences are shown in Supplementary Table 1. The ACTB gene was used as internal control. Duplicate samples for each tumor or tissue were analyzed. The expression of MYB transcripts were determined by the ΔCT method (Average CT-MYB-Average CT-ACTB), and relative MYB expression in each tumor was determined based on MYB expression in a pooled normal salivary gland standard (Clontech Laboratories). The average of each individual tumor and this value was used to determine the average for fusion positive and negative tumor groups. In this study, we considered the upper quartile of MYB values to represent the high expression.
Fluorescent in situ hybridization (FISH)
FISH was performed on touch preparations of ACCs to identify MYB/NFIB rearrangements. We initially used BAC clones containing MYB gene (RP11-104D9) and NFIB gene (RP11-54D21 and RP11-79B9) for screening of the gene fusion (Figure. 1A). The probes were labeled with Spectrum Green and Spectrum Red, respectively (Abbott Laboratories, Abbott Park, IL). Hybridization and detection were performed according to the manufacturer’s protocols. 200 individual nuclei were analyzed for each case and the interphase nuclei was captured and processed using the Quantitative Image Processing System (Applied Imaging, Santa Clara, CA). In nuclei containing the MYB-NFIB fusion, green and red signals from the MYB and NFIB genes overlap in a red/green (yellow) signal.
Figure 1.
A) Schematic representation of the location of the MYB chromosome 6q23 and the NFIB on 9p22-23 and the BAC clones used for the FISH analysis. RP11-104D9 was used as a probe for MYB and probes RP11-79B and RP11-54D21 were used for the NFIB gene in the FISH analysis. B) Schematic structure of the MYB and the NFIB genes and the primers used for the RT-PCR and the 3′RACE analysis. The exon numbers of MYB are based on NCBI database (accession number NM_001130173). NFIB exon numbers were obtained from accession number ENSG0000147862 for NFIB in the Ensembl database; note MYB exon 10 is not included any MYB-NFIB chimeric transcripts and intact MYB. ‡MYB exon 10 is also known well as exon 9B (accession number HSU22376).
C) RT-PCR analysis of MYB-NFIB fusion transcripts using new primer sets. Asterisk points to case #161B4 where gene fusion was detected by FISH without transcript formation. D) FISH analysis using BAC clones of MYB (green) and NFIB (red) genes in transcript negative gene fusion positive ACC (394D7 case). White arrows point to the yellow signal representing the MYB and NFIB gene fusion. E) Sequence illustration shows fusion of MYB exon 15 with NFIB 3′UTR, as detected by 3′RACE in 394D7 case. F) Represents the MYB transcript expression of the 30 new cases. The red bars denote the MYB-NFIB transcript positive samples, whereas the blue bars represent the expression level in fusion negative tumors. The asterisks point to tumors with MYB/NFIB gene fusion by FISH only. Results are represented as fold increase relative to MYB expression in pooled normal salivary gland tissue.
To localize the alternate breakpoints in fusion positive transcript negative tumors, we selected two BAC clones; one at 5′ of MYB (RP11-378M4) and the other at 3′ of MYB gene (RP11-55H4). Both of these clones overlap with the MYB clone used in the first FISH (RP11-104D9) screening probe (Figure 3A).
Figure 3.
Alternative breakpoint in the proximal or the distal sites of the MYB gene. A) The schematic representation of FISH probe for MYB gene displays the overlapping of RP11-378M and RP11-55H4 probes with the initial screening probe (RP11-104D9). The arrows showed the break point locations, and the asterisk means a breakage at 99kb upstream of MYB gene in 485F7 samples. B) The white arrows point to a yellow signal representing the t(6;9) translocation. Case 78 is positive for both.
3′ Rapid Amplification of cDNA Ends (3′RACE)
To determine the 3′ end of MYB transcript sequence or detect the unknown gene fusion of MYB, first-strand cDNA was synthesized 2μg of total RNA by M-MLV reverse transcriptase (Ambion) using 3′RACE adapter primer (AP; Invitrogen, Supplementary Table 1). 3′RACE nested PCR was done using 2 sets of MYB gene specific primers (Supplementary Table 1) and 3′RACE universal primer (AUAP; Invitrogen, Supplementary Table 1) to generate a specific amplification product. PCR products were purified and sequenced directly or cloned into the pCR2.1 vector (Invitrogen), and then were sequenced.
DNA copy number analysis
Genomic DNA was extracted with Gentra Puregene Tissue Kit (QIAGEN) according to the manufacturer’s instructions. DNA copy number (CN) from nineteen tumors and corresponding normal specimens was analyzed by affymetrix GeneChip Human Mapping 250k NSP array. The mapping information of the SNP sites was provided by the Human genome sequence version NCB136/hg18. Array data analyses were performed using Partek software and R packages.
Massive parallel sequencing
The procedures for genome wide paralleled paired-end sequencing to identify somatic genomic alterations and rearrangements in tumor specimens were performed as previously described (24, 25). Briefly, 5ug of genomic DNA from tumor and normal specimens were sheared to 400-500 bp fragments. Sequencing of 37 bp from either end was performed on the Illumina Genome Analyzer II platform. Reads were aligned to reference human genome (NCB1 build 36) using MAQ (26), with a coverage up to 1-2X sequence (50-60 million reads, 37 bp paired and N400 6p inserts), giving a physical coverage of 6-8X (25). Putative genomic rearrangements were screened by PCR across the breakpoint in tumor DNA samples and germline DNA (27).
Statistical analysis
Descriptive statistics for scaled values and frequencies of study patients within the categories for each of the parameters of interest were enumerated with the assistance of commercial statistical software. Correlations between categorical parameters and endpoints were assessed by Pearson’s Chi-squared or, where there are fewer than ten subjects in any cell of a 2 × 2 grid, by the two-tailed Fisher exact test. Since the values for expression of MYB at exons 2-3 and at exons 15-16 did not meet tests for normality, possible differences between groups were assessed by the non-parametric Mann-Whitney U test. Curves describing overall survival were generated by the Kaplan-Meier product limit method. The statistical significance of differences between the actuarial curves was tested by the log rank test. Follow-up time was the time from first appointment at the University of Texas M. D. Anderson Cancer Center for the primary tumor of concern until the date of last contact or death. Proportional hazard ratios and multivariate models were assessed by Cox regression analysis. These statistical tests were performed with the assistance of the Statistical (StatSoft, Inc., Tulsa, OK) and SPSS (IBM SPSS, IBM Corporation, Somers, NY) statistical software applications.
RESULTS
In the initial phase of the study, we analyzed 30 new ACCs representing equal number of patients by multiple complementary techniques to account for heterogeneity of the molecular genetic alterations associated with the t(6;9). The patients comprised of 17 males and 13 females who ranged in age from 39 to 94 with a mean of 62 years. The tumor size ranged from 0.2 – 12.0 cm, with mean of 3.1 cm and twenty tumors (66.7%) had perineural invasion. All patients underwent surgical resection with curative intent and post-operative radiotherapy. Table 1 presents the genetic and molecular findings of 30 new ACCs by different techniques.
Table 1. Molecular and Genetic results of the 30 new Adenoid Cystic Carcinomas.
| Sample Number |
MYB-NFIB (new primer) |
3’RACEa | FISH | Q-PCR_MYB | |
|---|---|---|---|---|---|
| (exon 2-3) | (exon 15-16) | ||||
| 197-E8 |
MYB exon 9b- NFIB exon 11 MYB exon 9b- NFIB exon 12 |
N.P. | + | 10 | 1 |
| 327-B4 | MYB exon15- NFIB exon 12 | MYB exon 15- NFIB exon 12 | + | 62 | 0 |
| 236-A1 | MYB exon 15- NFIB exon 12 |
MYB exon 15- NFIB exon 12 MYB exon 15- NFIB exon 11 |
+ | 90 | 20 |
| 235-D4 |
MYB exon 9b- NFIB exon 11 MYB exon 9b- NFIB exon 12 |
N.P. | + | 161 | 5 |
| 290-F8 | - | MYB exon 15- NFIB 3’UTR | + | 162 | 1 |
| 381-C7 | MYB exon 8b- NFIB exon 11 | N.P. | + | 194 | 0 |
| 73-C3 |
MYB exon 15- NFIB exon 12 MYB exon 15- NFIB exon 11 |
N.P. | + | 266 | 18 |
| 394-D7 | - | MYB exon 15- NFIB 3’UTR | + | 358 | 0 |
| 404-D3 | MYB exon 8b- NFIB exon 12 | N.P. | + | 392 | 0 |
| 369-B5 | MYB exon 8b- NFIB exon 12 | N.P. | + | 760 | 4 |
| 133-C4 | MYB exon 8b- NFIB exon 12 | N.P. | + | 1516 | 33 |
| 163-D8 | - | N.P. | − | 0 | 0 |
| 537-G8 | - | N.P. | − | 0 | 1 |
| 124-C1 | - | N/A | + | 0 | 0 |
| 626-H6 | - | N.P. | − | 3 | 1 |
| 598-D6 | - | N.P. | − | 22 | 0 |
| 626-D1 | - | N.P. | − | 25 | 0 |
| 319-H5 | - | N.P. | − | 30 | 185 |
| 454-A8 | - | N.P. | − | 62 | 1 |
| 321-C6 | - | N.P. | − | 86 | 0 |
| 139-D5 | - | N.P. | − | 93 | 69 |
| 391-F7 | - | N/A | − | 133 | 388 |
| 383-H8 | - | N/A | − | 158 | 80 |
| 161-B4 | - | N/A | − | 226 | 150 |
| 610-H4 | - | N/A | + | 271 | 160 |
| 603-D6 | - | N.P. | − | 326 | 171 |
| 627-C2 | - | N/A | + | 342 | 180 |
| 392-B2 | - | N/A | + | 388 | 113 |
| 288-F7 | - |
MYB exon 13- MYB intron 13- EFR3A intron 22 (Chr8q24.22) |
+ | 416 | 6 |
| 78-C8 | - | N/A | + | 691 | 237 |
| Molecular genetic results of the subset of ACC with alterations from previous study. | |||||
|---|---|---|---|---|---|
| Sample Number |
MYB-NFIB (new primer) |
3’RACEa | FISH | Q-PCR_MYB | |
| (exon 2-3) | (exon 15-16) | ||||
| 405-B2 | - |
MYB exon 15- PDCDILG2 (chr 9; intron 3, inversion) |
+ | 106 | 0 |
| b485-F7 | - | N/A | + | 231 | 421 |
| 233-C2 | - | N/A | + | 177 | 188 |
| 526-B5 | - | N/A | + | 368 | 213 |
| 471-F2 | - | N/A | + | 105 | 288 |
| 484-H3 | - | N/A | + | 105 | 213 |
| 594-D3 | - | N/A | + | 128 | 34 |
| 542-A1 | - | MYB exon 14- NFIB 3’UTR | + | 881 | 29 |
| 502-A5 | - | N/A | + | 606 | 384 |
| 185-G8 | MYB exon 11- NFIB exon 12 | MYB exon 11- NFIB exon 12 | + | 1264 | 635 |
| 335-C6 | MYB exon 8b- NFIB exon 12 | MYB exon 8b- NFIB exon 12 | + | 1229 | 5 |
| 191-D6 | MYB exon11- NFIB exon 12 | MYB exon 11- NFIB exon 12 | + | 488 | 62 |
| 318-H3 | - |
MYB exon/intron 11- NFIB 3’UTR |
+ | 0 | 0 |
| 391-D6 | MYB exon 12- NFIB exon 11 | MYB exon 15- NFIB exon 11 | + | 23 | 0 |
| 436-E2 |
MYB exon 8a- NFIB exon 12 MYB exon 8a- NFIB exon 11 |
MYB exon 8a- NFIB exon 12 | + | 1217 | 5 |
| 436-H3 | - | N/A | + | 229 | 476 |
| 570-H7 | MYB exon 8a- NFIB exon 12 | MYB exon 8a- NFIB exon 12 | + | 446 | 0 |
Note:
N.P., not performed; N/A, No-Abnormality (MYB gene is intact at 3’region). (−), negative; (+), positive.
Note:
N/A, No-Abnormality (MYB gene is intact at 3’region). (−), negative; (+), positive.
485-F7 sample had a NFIB-AIG1 gene fusion
MYB-NFIB genomic translocation and fusion transcript of the 30 new ACCs
To screen for the MYB-NFIB gene fusion and the transcript formation, we performed FISH using BAC clones for the MYB gene (green, RP-11-104D9, Figure 1A) and the NFIB gene (red, RP11-54D21 and RP11-79B9, Figure 1A) and RT-PCR using the original set of and newly designed primers on all 30 tumors (Figure 1B) and all fusion PCR products were sequenced. None of the FISH negative tumors had fusion transcripts formation by RT-PCR. FISH analysis showed 17 (56.7%) tumors to be positive for the MYB and NFIB genes translocation (Table 1). Nine (52.9%) of the FISH positive samples had detectable fusion transcript and eight (26.7%) lacked transcript product (Figure 1C and Table 1); these data suggest that additional rearrangements or breakpoints other than MYB-NFIB fusions are present. Considering the complex and evolving information on the structural formation of the MYB gene, (27) we observed that exon 10 of the gene is lost in all sequenced MYB-NFIB chimeric transcript and the intact form of MYB transcript in ACC. This exon is also known as exon 9B, using NCBI accession #HSU22376.
To assess alteration at the 3′end of the MYB transcript, 3′RACE amplification was performed and led to the identification of three fusion transcripts; these were not detected by RT-PCR analysis (Table 1). Interestingly, two FISH positive tumors (#290F8 and 394D7, Figure 1D) had fusion transcripts comprised of MYB exon 15 and the 3′UTR of the NFIB gene and manifested the expected high level of MYB truncated transcript (Figure 1E and Supplementary Figure 1); one tumor (# 288F7) had fusion between MYB exon 13 and intron 22 of the EFR3A gene on chromosome 8q24 (Table 1 and Supplementary Figure 2); in addition to a stop codon at intron 13 and MYB truncated transcript.
MYB transcript expression
We previously reported loss or marked reduction of the full-length MYB (exon 15-16) transcript expression in all MYB-NFIB fusion positive tumors. To confirm this finding, we analyzed the expression levels of MYB transcripts in 30 salivary tumors by quantitative RT-PCR using primers for MYB exons 2-3 and the last MYB exon. Overall, the expression of MYB exon 2-3 in fusion transcript positive tumors (average 361) was more than two-fold higher than the majority of their fusion transcript negative ACCs (average 172) (Table 1). The analysis also shows that the expression levels of MYB exon 2-3 and the last exon were comparable in the majority of tumors with genomic MYB-NFIB fusion without transcript formation. In one tumor (#288F7, Table 1) with MYB and EFR3A gene fusion, the expression of the last MYB exon was markedly reduced while the 5′-segment was moderately elevated. We also observed that the three tumors with the highest 5′-segment MYB transcript (cases # 404D3, 369B5, and 133C4) had fusion between MYB exon 8b and exon 12 of NFIB gene (Figure 1F and Table 1).
Analysis of 52 fusion transcript negative of 72 ACCs previously studied
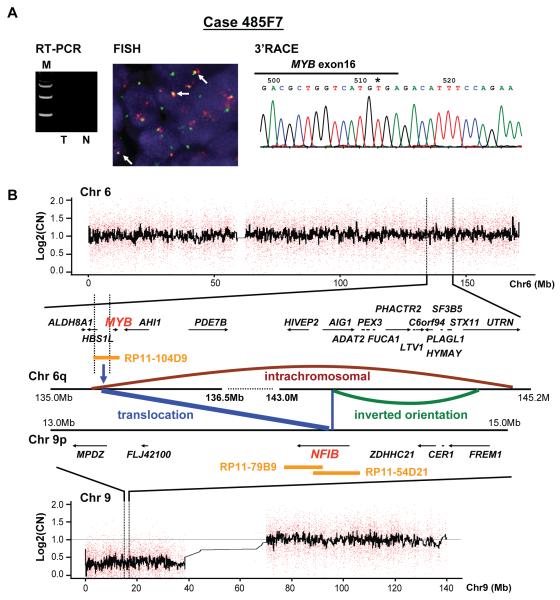
Based on these findings, we extended our analysis to include the 52 MYB-NFIB fusion transcript negative ACCs from our previous study (15). The analysis showed that seventeen (32.7%) of the 52 tumors to be positive for gene fusion by FISH, and only eight of these (6 by PCR and 2 by the 3′RACE) were MYB/NFIB transcript forming tumors (Table 1). Two of these tumors (542A1 and 318H3) had fusion between MYB exon 14 (Supplementary Figure 3) and intron 11 (Supplementary Figure 4) and the NFIB 3′UTR, respectively. In addition, we identified a novel t(6;9) rearrangement involving MYB exon15 and an inverted sequence of the PDCD1LG2 intron 3 (Table 1 and Supplementary Figure 5) in another tumor (# 405B2). Another tumor (485F7) showed MYB/NFIB gene fusion by FISH but no transcript formation or MYB alterations was found (Figure 2A).
Figure 2.
The chromosomal rearrangements in case 485F7, as an example of genomic MYB-NFIB fusion without transcript formation. A) RT-PCR analysis shows as MYB-NFIB transcript negative (T) and corresponding normal (N). The 3′RACE analysis reveals that the last exon 16 of MYB gene is intact. B) Genomic rearrangement and copy number changes of chromosomes 6 and 9 of the same tumor. The center schematic representation depicts the intra- and inter-chromosomal structures generated from the massively pared-end tag sequencing data. Blue bars, chromosomal rearrangement (MYB-NFIB and NFIB-AIG1); brown bar, intra-chromosomal rearrangement between HBS1L and UTRN gene; green bar, inverted orientation between AIG1 and UTRN gene. Blue vertical arrow indicates NFIB gene breaks at intron 7 that translocates to chromosome 6q22 just proximal to the MYB upstream. MYB probe (RP11-104D9) and NFIB probe (RP11-79B and RP11-54D21) are shown as FISH probes.
Detailed FISH analysis
To account for the translocation sites in gene fusion positive but transcript negative ACCs, we selected additional BAC clones at the 3′ and 5′ of the MYB gene that overlaps with the original MYB probe (Figure 3A). The results revealed, 5′ fusion signal proximal to the MYB gene in one tumor (Supplementary Table 2), a positive 3′ probe signals in eight, and fusion signals were found for both the 3′ and the 5′ flanking probes of the MYB in two tumors. Surprisingly, the two cases (288F7 and 405B2) where MYB fused with EFR3A or PDCD1LG2 showed as a positive by 3′ probe signal suggesting that each allele fused different genes. These findings localize the breakpoints distal to the 3′ end of the MYB gene in 8 tumors and to the proximal 5′ end of the gene in one tumor. In the two tumors positive for both probes, either reciprocal translocation and/or translocation in one allele and insertion involving the other allele may have occurred. Detailed molecular analyses of these two tumors are underway.
Massively pair-ended sequencing analysis
To survey the genomic findings in tumors representing the MYB-NFIB gene fusion status, four tumors [two fusion negative, one FISH positive/transcript negative (485F7, Supplementary Figure 6) and one fusion positive by both FISH and RT-PCR (325E5, Supplementary Figure 6)] were analyzed by massively pair-ended sequencing to confirm the molecular results and to screen for new alterations. The analysis confirmed the lack of any abnormalities in the two fusion negative tumors and validated the presence of MYB-NFIB gene fusion in the fusion positive tumor. In the FISH positive/transcript negative tumor (#485F7, Figure 2A), complex alterations were observed; Figure 2B represents schematic illustration of the inter- and intra-chromosomal changes in this tumor; these included a breakage and translocation of intron-7 sequence of the NFIB to a 99kb upstream location of the MYB coding region (Supplementary Table 3) and a novel fusion between NFIB gene and two alternative variants of the AIG1 gene on chromosome 6q24. The MYB-NFIB fusion transcript resulting from this translocation was confirmed by RT-PCR and sequencing analyses (Supplementary Figure 7).
The SNPs copy number analysis of chromosomes 6 and 9 are shown above and below the schematic illustration in Figure 2B. Comparison of the massively parallel paired-end sequencing (Supplementary Figure 6) and Affymetrix 250k SNP genomic array data confirmed that the genomic alterations occurred at copy number neutral region of chromosome 6 (Figure 2B and Supplemental Figure 8).
Combined Fusion analysis and Clinicopathologic parameters
The combined analysis of the 102 (30 new and 72 previously reported) tumors showed that 54 (52.9%) had genomic MYB-NFIB gene fusion, 39 (38.2%) of these formed fusion transcript and 48 (47.1%) were negative for any fusion related alterations (Supplementary Table 4). The expression of both MYB exon 2-3 and exon 15-16 segments in transcript forming tumors was significantly (Mann-Whitney U-test) higher than those with transcript negative tumors (p < 0.001 and p = 0.003, respectively). The expression of MYB exon 2-3 in tumors with only genomic fusion (by FISH) was significantly higher than in fusion transcript negative tumors (p < 0.001). The expression level of MYB transcript (exon 15-16) in fusion transcript negative tumor was not significantly different from the expression of gene fusion negative tumors (Supplementary Table 4).
MYB expression and patient survival in ACCs
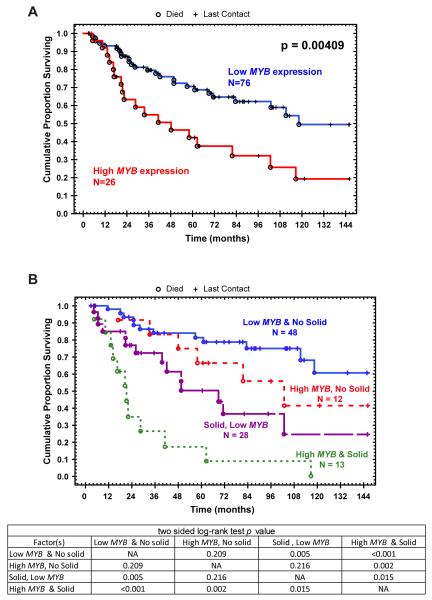
We further examined the association of tumors with MYB-NFIB fusion with and without transcript formation and level of MYB expression (exon 2-3) and the clinicopathologic factors and patients outcome. An arbitrary cut-off level of 470 for MYB expression was based on the upper quartile of MYB (exon 2-3) expression among all the samples tested. This value was used in the statistical correlative analysis. Kaplan-Meier analysis showed significant correlation between high MYB expression and poor survival (p=0.004 log-rank test). Univariate Cox proportional hazard regression analyses showed that high MYB expression, age of 60 or more years, and tumor with solid component were significant prognostic factors (Wald p=0.005 p=0.008 and p<0.001, respectively, Table 2). Interestingly, significantly different survival plot for patients with low MYB and solid tumors with those who had high MYB and solid phenotype tumors was found suggesting that high MYB expression correlates with poor outcome independent of the solid phenotype.
Table 2.
Multifactorial (Cox) regression analysis of factors affecting 12-year overall survival of ACC patients
| Variable | No. | Univariate analysis HR (95% Cl) |
pa | Multivariate analysisc HR (95% Cl) |
pa |
|---|---|---|---|---|---|
| MYB-NFIB transcript | |||||
| negative | 63 | 1.00 | 0.12 | N/A | N/A |
| positive | 39 | 1.602 (0.892-2.844) |
|||
| FISH | |||||
| negative | 48 | 1.00 | 0.07 | N/A | N/A |
| positive | 54 | 1.734 (0.947-3.173) |
|||
| MYB expression (exon2-3) b | |||||
| Low | 76 | 1.00 | 0.005 | 1.00 | 0.014 |
| High | 26 | 1.003 (1.001-1.005) |
1.003 (1.001-1.005) |
||
| Age | |||||
| <60 | 67 | 1.00 | 0.008 | 1.00 | 0.008 |
| ≥60 | 35 | 1.057 (1.016-1.100) |
1.055 (1.014-1.097) |
||
| Gender | |||||
| Female | 41 | 1.00 | 0.64 | N/A | N/A |
| Male | 61 | 1.154 (0.639-2.087) |
|||
| Size | |||||
| <4cm | 63 | 1.00 | 0.94 | N/A | N/A |
| ≥4cm | 35 | 0.987 (0.723-1.349) |
|||
| Pattern | |||||
| Not Solid | 60 | 1.00 | <0.001 | 1.00 | <0.001 |
| Solid | 41 | 3.706 (2.027-6.777) |
3.596 (1.963-6.589) |
||
| PNI | |||||
| No | 7 | 1.00 | 0.58 | N/A | N/A |
| Yes | 78 | 1.499 (0.361-6.229) |
|||
| Stage c | |||||
| I-II | 20 | 1.00 | 0.14 | N/A | N/A |
| III-IV | 35 | 1.919 (2.027-6.777) |
|||
| Metastasis | |||||
| No | 50 | 1.00 | 0.97 | N/A | N/A |
| Yes | 52 | 1.012 (0.564-1.817) |
Wald p-value.
470 was defined as a cut-off value for MYB expression (exon2-3).
55 patients are available for the staging analysis.
Overall Model, p<0.00001. N/A = not applicable
PNI: Perineural invasion
DISCUSSION
Our study identified novel and a spectrum of complex cytogenetic and molecular alterations associated with the t(6;9) event in ACC. The results show that approximately 53% of ACCs showed genomic MYB-NFIB fusion with and without fusion transcript formation. These findings are in agreement with those recently reported in a retrospective study of this entity (22). The majority of tumors with genomic fusion represented in-frame translocation of the MYB and the NFIB genes with the formation of variable chimeric fusion transcripts in a cell and tissue specific context (15). Interestingly, in the subset of non-transcript forming MYB-NFIB gene fusion tumors, we identified translocation breakpoints at the flanking sites of the MYB gene on chromosome 6q24 region with no evidence of MYB transcript alteration. Similar breakpoints at the flanking sequences of fusion genes in several neoplastic entities have also been reported (16, 17). The predicted biological consequences of these alterations are most likely the disregulation of critical oncogenes neighboring these sites (29-32).
In this study, multiple breakpoints exclusive of those reported between the MYB and the NFIB genes were identified. These included translocations involving, exon 15 of MYB and intron 3 of the PDCD1LG2 gene on chromosome 9p24 and MYB exon 13, intron 22 of the EFR3A gene on chromosome 8q24 (16, 33, 34) and the NFIB with the AIG1 on chromosome 6q24. In addition, a separate intragenic translocation of an NFIB sequence to a proximal site of the MYB coding region was also identified in the latter tumor. The translocation involving intron sites in these instances have previously been reported in a benign (HMGA2 and COG5) (34) (NFIB and to the HMGA2 gene) (36-40) and malignant tumors (EML4-ALK fusion gene) (40, 41). The mechanistic association of these uncommon events in the oncogenesis of ACC, however, remains to be elucidated.
Our findings strongly link the MYB-NFIB gene fusion to the upregulation of the MYB gene in ACCs. This may likely be due either to the lack of the 3′UTR, which includes the regulatory microRNA target sites, or to the deletion of the terminal negative regulatory domain (NRD) of the MYB fusion transcript positive ACCs. The translocation of other genetic sequences to the non-coding flanking sites of the MYB gene can also lead to the MYB transcriptional activation perhaps through epigenetic modification including histone acetylation especially in MYB-NFIB fusion transcript negative tumors. (19, 21, 23, 34, 43). Other upstream events affecting the transcription of the MYB gene in fusion transcript negative tumors including (43) the NFIB sequence translocation upstream of the coding region leading to high MYB expression can be involved. Interestingly, our findings are distinctly different from those associated with the activation of this gene in other solid tumors including colon (19, 36, 44) and breast carcinomas and suggest that MYB regulation varies in a tissue and tumor specific context. (33, 34, 37, 38, 42, 45-48)
In this study, clinicopathologic analysis identified three factors including patients older than 60 years, solid phenotype and high MYB expression to be significantly associated with poor survival. In both, multi-factorial cox hazard ratio, log-rank testing and the survival plot for patients with low MYB and solid type was significant different from patients with high MYB and solid type. We contend, however, that further studies are required to assess the functional threshold of MYB expression, to identify chimeric fusion protein and to determine the significance of the selective MYB expression to myoepithelial cells in the pathobiology of ACC. These studies will be further advanced by the availability of reagents that distinguish between the MYB protein variants as well as results from second generation deep sequencing of these tumors. Overall, the data indicates that the juxtaposition of the terminal sequences of the NFIB within and around the coding MYB gene sequence is the main genetic event in the t(6;9) positive ACCs and this leads to an elevated 5′ segment of the MYB gene in ACC. (31, 32, 49)
In conclusion, we comprehensively accounted for the alternative MYB-NFIB gene fusions and the other molecular-genetic alterations associated with the t(6;9) in ACCs and showed that marked genetic heterogeneity are associated with this event. The study characterized two main events resulting from the t(6;9) one with gene fusion alone and another with gene fusion and chimeric transcript formation. The former is due to breakpoints at the flanking sites of the MYB gene. These alterations require multiple methodological approaches for their detection. The results also show that transcript forming ACCs express high 5′-truncated MYB segment and pursue aggressive behavior. We therefore contend that the translocation of the NFIB terminal sequences as a result of the t(6;9) and different molecular events, may underlie the transcriptional regulation of the MYB gene in ACC.
Supplementary Material
Statement of Translational Relevance.
This study identifies previously unreported chromosomal breakpoints and complex genetic and molecular alterations associated with the t(6;9) in salivary adenoid cystic carcinoma (ACC). These events result in MYB-NFIB gene fusion with and without chimeric transcript formation. Tumors with MYB-NFIB gene fusion without transcript had the translocation of terminal sequences of the NFIB to the flanking sites of the MYB gene. Gene fusion resulting in transcript formation was associated with high level expression of the MYB 5′ segment and significantly correlated with patients’ survival. The study provides detailed characterization of the t(6;9) alterations and their link to MYB expression in an effort to define targets for therapeutic stratification of patients with ACC.
Figure 4.
Kaplan-Meier survival curves of ACCs patients: A) correlation between high MYB exon 2-3 expression and poor patient survival (p=0.004, log-rank test). B) survival curves of MYB transcript expression, Adenoid Cystic Carcinoma with solid component and patients survival.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Scott A. Ness for update information on the MYB gene and to Deborah A. Rodriguez and Stella U. Njoku for technical and Wendy Garcia for secretarial assistance.
GRANT SUPPORT:
The study is supported in part by the NIH National Institute of Dental and Craniofacial Research (NIDCR) and the NIH Office of Rare Diseases Research (ORDR) Grant Number U01DE019765, the Head and Neck SPORE program Grant Number P50 CA097007, The Kenneth D. Muller professorship and the NCI-CA-16672 grant. AF and PS acknowledge the support of the Wellcome Trust under grant reference number 077012/Z/05/Z. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institute of Health.
REFERENCES
- 1.Batsakis JG, Regezi JA, Luna MA, El-Naggar AK. Histogenesis of salivary gland neoplasms: a postulate with prognostic implications. J Laryngol Otol. 1989;103:939–44. doi: 10.1017/s0022215100110552. [DOI] [PubMed] [Google Scholar]
- 2.Batsakis JG, Luna MA, El-Naggar AK. Histopathologic grading of salivary gland neoplasms: III. Adenoid cystic carcinomas. Ann Otol Rhinol Laryngol. 1990;99:1007–9. doi: 10.1177/000348949009901215. [DOI] [PubMed] [Google Scholar]
- 3.Chomette G, Auriol M, Tranbaloc P, Vaillant JM. Adenoid cystic carcinoma of minor salivary glands. Analysis of 86 cases. Clinico-pathological, histoenzymological and ultrastructural studies. Virchows Arch A Pathol Anat Histol. 1982;395:289–301. doi: 10.1007/BF00429355. [DOI] [PubMed] [Google Scholar]
- 4.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177–84. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 5.Fordice J, Kershaw C, El-Naggar AK, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125:149–52. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 6.El-Naggar AK, Huvos AG. Adenoid cystic carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumors Pathology and Genetics of Head and Neck Tumors. IARC Press; Lyon, France: 2005. pp. 221–2. [Google Scholar]
- 7.Bell D, Zhao YJ, Rao PH, Weber RS, El-Naggar AK. Translocation t(6;14) as the sole chromosomal abnormality in adenoid cystic carcinoma of the base of tongue. Head Neck Pathol. 2007;1:165–8. doi: 10.1007/s12105-007-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higashi K, Jin Y, Johansson M, Heim S, Mandahl N, Biorklund A, et al. Rearrangement of 9p13 as the primary chromosomal aberration in adenoid cystic carcinoma of the respiratory tract. Genes Chromosomes Cancer. 1991;3:21–3. doi: 10.1002/gcc.2870030105. [DOI] [PubMed] [Google Scholar]
- 9.Frierson HF, Jr., El-Naggar AK, Welsh JB, Sapinoso LM, Su AI, Cheng J, et al. Large scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Pathol. 2002;161:1315–23. doi: 10.1016/S0002-9440(10)64408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutherford S, Hampton GM, Frierson HF, Moskaluk CA. Mapping of candidate tumor suppressor genes on chromosome 12 in adenoid cystic carcinoma. Lab Invest. 2005;85:1076–85. doi: 10.1038/labinvest.3700314. [DOI] [PubMed] [Google Scholar]
- 11.Queimado L, Reis A, Fonseca I, Martins C, Lovett M, Soares J, et al. A refined localization of two deleted regions in chromosome 6q associated with salivary gland carcinomas. Oncogene. 1998;16:83–8. doi: 10.1038/sj.onc.1201480. [DOI] [PubMed] [Google Scholar]
- 12.Stallmach I, Zenklusen P, Komminoth P, Schmid S, Perren A, Roos M, et al. Loss of heterozygosity at chromosome 6q23-25 correlates with clinical and histologic parameters in salivary gland adenoid cystic carcinoma. Virchows Arch. 2002;440:77–84. doi: 10.1007/s004280100523. [DOI] [PubMed] [Google Scholar]
- 13.Rao PH, Roberts D, Zhao YJ, Bell D, Harris CP, Weber RS, et al. Deletion of 1p32-p36 is the most frequent genetic change and poor prognostic marker in adenoid cystic carcinoma of the salivary glands. Clin Cancer Res. 2008;14:5181–7. doi: 10.1158/1078-0432.CCR-08-0158. [DOI] [PubMed] [Google Scholar]
- 14.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106:18740–4. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitani Y, Li J, Rao PH, Zhao YJ, Bell D, Lippman SM, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res. 2010;16:4722–31. doi: 10.1158/1078-0432.CCR-10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clappier E, Cuccuini W, Kalota A, Crinquette A, Cayuela JM, Dik WA, et al. The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood. 2007;110:1251–61. doi: 10.1182/blood-2006-12-064683. [DOI] [PubMed] [Google Scholar]
- 17.Tomita A, Watanabe T, Kosugi H, Ohashi H, Uchida T, Kinoshita T, et al. Truncated c-MYB expression in the human leukemia cell line TK-6. Leukemia. 1998;12:1422–9. doi: 10.1038/sj.leu.2401113. [DOI] [PubMed] [Google Scholar]
- 18.Harper ME, Franchini G, Love J, Simon MI, Gallo RC, Wong-Staal F. Chromosomal sublocalization of human c-MYB and c-fes cellular onc genes. Nature. 1983;304:169–71. doi: 10.1038/304169a0. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–34. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 20.Nomura N, Takahashi M, Matsui M, Ishii S, Date T, Sasamoto S, et al. Isolation of human cDNA clones of MYB-related genes, A-MYB and B-MYB. Nucleic Acids Res. 1988;16:11075–89. doi: 10.1093/nar/16.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellon T, Perrotti D, Calabretta B. Granulocytic differentiation of normal hematopoietic precursor cells induced by transcription factor PU.1 correlates with negative regulation of the c-MYB promoter. Blood. 1997;90:1828–39. [PubMed] [Google Scholar]
- 22.West RB, Kong C, Clarke N, Gilks T, Lipsick JS, Cao H, et al. MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Pathol. 2011;35:92–9. doi: 10.1097/PAS.0b013e3182002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubendorff JW, Lipsick JS. Transcriptional regulation by the carboxyl terminus of c-MYB depends upon both the MYB DNA-binding domain and the DNA recognition site. Oncogene. 1999;18:3452–60. doi: 10.1038/sj.onc.1202679. [DOI] [PubMed] [Google Scholar]
- 24.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–13. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens PJ, McBride DJ, Lin ML, Varela I, Pleasance ED, Simpson JT, et al. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature. 2009;462:1005–10. doi: 10.1038/nature08645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Ruan J, Durbin R. Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 2008;18:1851–8. doi: 10.1101/gr.078212.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou YE, O’Rourke JP, Edwards JS, Ness SA. Single molecular analysis of c-MYB alternative splicing reveals novel classifiers for precursor B-ALL. PLoS One. 2011:e22880. doi: 10.1371/journal.pone.0022880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins EC, Rabbitts TH. The promiscuous MLL gene links chromosomal translocations to cellular differentiation and tumour tropism. Trends Mol Med. 2002;8:436–42. doi: 10.1016/s1471-4914(02)02397-3. [DOI] [PubMed] [Google Scholar]
- 30.Lastowska M, Roberts P, Pearson AD, Lewis I, Wolstenholme J, Bown N. Promiscuous translocations of chromosome arm 17q in human neuroblastomas. Genes Chromosomes Cancer. 1997;19:143–9. [PubMed] [Google Scholar]
- 31.Butler MP, Iida S, Capello D, Rossi D, Rao PH, Nallasivam P, et al. Alternative translocation breakpoint cluster region 5′ to BCL-6 in B-cell non-Hodgkin’s lymphoma. Cancer Res. 2002;62:4089–94. [PubMed] [Google Scholar]
- 32.Chen W, Butler M, Rao PH, Chaganti SR, Louie DC, Dalla-Favera R, et al. The t(2;3)(q21;q27) translocation in non-Hodgkin’s lymphoma displays BCL6 mutations in the 5′ regulatory region and chromosomal breakpoints distant from the gene. Oncogene. 1998;17:1717–22. doi: 10.1038/sj.onc.1202098. [DOI] [PubMed] [Google Scholar]
- 33.Catchpole S, Tavner F, Le Cam L, Sardet C, Watson RJ. A B-MYB promoter corepressor site facilitates in vivo occupation of the adjacent E2F site by p107 × E2F and p130 × E2F complexes. J Biol Chem. 2002;277:39015–24. doi: 10.1074/jbc.M202960200. [DOI] [PubMed] [Google Scholar]
- 34.Nicolaides NC, Gualdi R, Casadevall C, Manzella L, Calabretta B. Positive autoregulation of c-MYB expression via MYB binding sites in the 5′ flanking region of the human c-MYB gene. Mol Cell Biol. 1991;11:6166–76. doi: 10.1128/mcb.11.12.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velagaleti GV, Tonk VS, Hakim NM, Wang X, Zhang H, Erickson-Johnson MR, et al. Fusion of HMGA2 to COG5 in uterine leiomyoma. Cancer Genet Cytogenet. 2010;202:11–6. doi: 10.1016/j.cancergencyto.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Drabsch Y, Hugo H, Zhang R, Dowhan DH, Miao YR, Gewirtz AM, et al. Mechanism of and requirement for estrogen-regulated MYB expression in estrogen-receptor-positive breast cancer cells. Proc Natl Acad Sci U S A. 2007;104:13762–7. doi: 10.1073/pnas.0700104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trauth K, Mutschler B, Jenkins NA, Gilbert DJ, Copeland NG, Klempnauer KH. Mouse A-MYB encodes a trans-activator and is expressed in mitotically active cells of the developing central nervous system, adult testis and B lymphocytes. EMBO J. 1994;13:5994–6005. doi: 10.1002/j.1460-2075.1994.tb06945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stenman G. Fusion oncogenes and tumor type specificity--insights from salivary gland tumors. Semin Cancer Biol. 2005;15:224–35. doi: 10.1016/j.semcancer.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Mitelman F, Johansson B, Mertens F. Fusion genes and rearranged genes as a linear function of chromosome aberrations in cancer. Nat Genet. 2004;36:331–4. doi: 10.1038/ng1335. [DOI] [PubMed] [Google Scholar]
- 40.Rabbitts TH. Chromosomal translocations in human cancer. Nature. 1994;372:143–9. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- 41.Nagel S, Kaufmann M, Scherr M, Drexler HG, MacLeod RA. Activation of HLXB9 by juxtaposition with MYB via formation of t(6;7)(q23;q36) in an AML-M4 cell line (GDM-1) Genes Chromosomes Cancer. 2005;42:170–8. doi: 10.1002/gcc.20113. [DOI] [PubMed] [Google Scholar]
- 42.Sinclair P, Harrison CJ, Jarosova M, Foroni L. Analysis of balanced rearrangements of chromosome 6 in acute leukemia: clustered breakpoints in q22-q23 and possible involvement of c-MYB in a new recurrent translocation, t(6;7)(q23;q32 through 36) Haematologica. 2005;90:602–11. [PubMed] [Google Scholar]
- 43.Mukai HY, Motohashi H, Ohneda O, Suzuki N, Nagano M, Yamamoto M. Transgene insertion in proximity to the c-MYB gene disrupts erythroid-megakaryocytic lineage bifurcation. Mol Cell Biol. 2006;26:7953–65. doi: 10.1128/MCB.00718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson MA, Flegg R, Westin EH, Ramsay RG. Microsatellite deletions in the c-MYB transcriptional attenuator region associated with over-expression in colon tumour cell lines. Oncogene. 1997;14:1715–23. doi: 10.1038/sj.onc.1201007. [DOI] [PubMed] [Google Scholar]
- 45.Brenner JC, Chinnaiyan AM. Translocations in epithelial cancers. Biochim Biophys Acta. 2009;1796:201–15. doi: 10.1016/j.bbcan.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aplan PD. Causes of oncogenic chromosomal translocation. Trends Genet. 2006;22:46–55. doi: 10.1016/j.tig.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biroccio A, Benassi B, Agnano I, D’Angelo C, Buglioni S, Mottolese M, et al. c-MYB and Bcl-x overexpression predicts poor prognosis in colorectal cancer: clinical and experimental findings. Am J Pathol. 2001;158:1289–99. doi: 10.1016/S0002-9440(10)64080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabbitts TH, Stocks MR. Chromosomal translocation products engender new intracellular therapeutic technologies. Nat Med. 2003;9:383–6. doi: 10.1038/nm0403-383. [DOI] [PubMed] [Google Scholar]
- 49.Kauraniemi P, Hedenfalk I, Persson K, Duggan DJ, Tanner M, Johannsson O, et al. MYB oncogene amplification in hereditary BRCA1 breast cancer. Cancer Res. 2000;60:5323–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.