Abstract
Tuberculosis remains a leading cause of death resulting from an infectious agent, and the spread of multi- and extensively drug-resistant strains of Mycobacterium tuberculosis poses a threat to management of global health. New drugs that effectively shorten the duration of treatment and are active against drug-resistant strains of this pathogen are urgently required to develop effective chemotherapies to combat this disease. Two nitroimidazoles, PA-824 and OPC-67683, are currently in Phase II clinical trials for the treatment of TB and the outcome of these may determine the future directions of drug development for anti-tubercular nitroimidazoles. In this review we summarize the development of these nitroimidazoles and alternative analogs in these series that may offer attractive alternatives to PA-824 and OPC-67683 for further development in the drug-discovery pipeline. Lastly, the potential pitfalls in the development of nitroimidazoles as drugs for TB are discussed.
Tuberculosis is the second leading cause of death resulting from an infectious agent worldwide, with nine million cases. A total of 1.8 million deaths are attributed annually to this debilitating disease [1]. Calculations based on purified protein derivative skin test results, which measure infection of individuals by Mycobacterium tuberculosis (Mtb), have estimated that approximately a third of the world’s population is latently infected with Mtb. Such individuals have a 10% lifetime risk of developing active TB and this risk increases to a 10% annual risk in HIV infected individuals [1,2]. TB is the leading cause of death in HIV-infected individuals with the spread of HIV being a major factor in the inability to combat the global spread of Mtb. The current chemotherapy of drug-sensitive TB requires 6–9 months of multidrug treatment and under ideal situations can achieve a 95% cure rate. However, in the real world, the cure rates are much lower, with poor treatment and non-compliance having given rise to selection and spread of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB. The treatment options of MDR, and especially XDR-TB, are limited, and are generally longer than 24-month treatments, associated with much lower cure rates [3].
The WHO’s ‘Stop TB’ strategy aims to halve the incidence and number of deaths due to TB relative to their respective 1990 levels by 2015, and to reduce the incidence of new cases to one in a million by 2050. Achieving this goal will be extremely challenging and the development of new drugs that will effectively treat both drug-sensitive as well as drug-resistant TB and, ideally, also target latent TB, will be key to this success. This review will discuss the development of a series of nitroimidazoles, which are currently one of the most promising series of new anti-tubercular drugs in the clinical drug development pipeline.
From anti-infectives to anti-tuberculars: history of nitroimidazole drugs
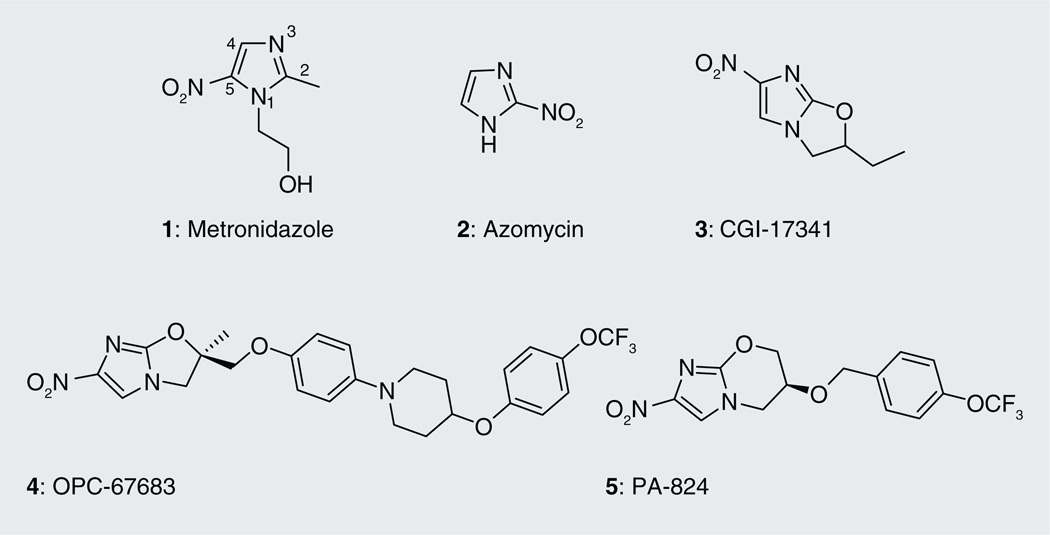
Metronidazole (1; Figure 1), the first class of nitroimidazoles, currently in the 16th edition of the WHO’s model lists of essential medicines [301], was discovered in mid 1950s at Rhône-Poulenc during the search for a cure for the sexually transmitted disease tricho-miniasis [4], caused by Trichomonas vaginalis. Extracts from streptomycete 6670 were found to have potent activity against T. vaginalis and the purified active component was identified as azomycin (2) (chemically known as 2-nitro-imidazole) [4,5]. Consequently, Rhône-Poulenc developed an array of azomycin derivatives to explore this trichomonacidal activity and came up with metronidazole (l-(β-hydroxyethyl)-2-methyl-5-nitroimidazole), which is still used today for the treatment of trichominiasis [6]. The antiprotozoan activity of the metronidazole was not restricted to T. vaginalis, since it was found to be effective against Giardia lamblia, the causative agent of giardiasis, [7], as well as Trypanosoma cruzi, which causes Chagas disease (trypanosomiasis) [8]. 5 years after its discovery, metronidazole was clinically demonstrated to cure amoebic dysentery caused by Entamoeba histolytica [5]. Since its discovery, metronidazole has been used successfully for the treatment of diseases caused by anaerobic bacteria, such as the Gram-negative Bacteroides fragilis, which causes peritoneal infections, the Gram-positive bacteria Clostridium difficile, which causes pseudomembranous colitis [6] and Helicobacter pylori, which causes stomach ulcers [9]. It is used extensively for the treatment of abscesses (e.g., brain, pelvis, pulmonary and tubo-ovarian), septicemias, pneumonia, endodermitis and bacterial vaginosis, as well as anaerobic growth in the periodontal cavity [10]. In the mid-1990s, metronidazole was shown to have bactericidal activity against dormant Mtb. No activity was seen against aerobic actively replicating Mtb, underscoring its utility against anaerobically adapted bacteria [11]. Granulomas in lungs infected with Mtb have been shown to become very hypoxic [12]. Restriction of oxygen is thought to be an important factor that maintains Mtb under a state of low metabolic activity in latently infected lungs [13]. Consequently, the ability of drugs to kill Mtb under hypoxia-induced nonreplicating conditions is assumed to be critical in the development of drugs that will lead to shortening of chemotherapy [13,14]. Thus, the discovery that metronidazole has activity against anaerobic nonreplicating Mtb was significant as it raised the prospect of the use of combination drugs (metronidazole with rifampicin [RIF] and isoniazid [INH]) for treatment of both actively replicating and nonreplicating persistent Mtb. There is no standardized method for testing the activity of drugs under anaerobic conditions. Assays that report the minimum anaerobicidal concentration measure the concentration of drug that results in a tenfold drop in bacterial numbers in hypoxically adapted Mtb treated for a week under anaerobic conditions with the compound under investigation. INH is used as a negative control under these conditions since it has no activity against Mtb persisting under anaerobic conditions whereas metronidazole is used as positive control because it has exclusive activity under anaerobic conditions against this organism. In the low oxygen recovery assay, hypoxically adapted Mtb is treated for 1 week under anaerobic conditions with the compound under investigation, but in contrast to the previous assay, activity of the compounds is measured after an aerobic outgrowth period as a surrogate for anaerobic activity.
Figure 1.
Anti-tubercular nitroimidazoles. The numbering of the atoms in the imidazole ring is shown in metronidazole (1).
Prior to the establishment of the anti-tubercular activity of metronidazole, which belongs to the class of 5-nitroimidazoles, 2-nitroimidazoles were the first in this class of compounds reported to have antimycobacterial activity in early 1970s [15]. Derivatives of 2-nitroimidazoles substituted at the 1 and 5 positions were found not only to be moderately active against Mtb, but also showed activity against many other organisms [16]. 2-nitroimidazoles have a reduction potential approximately 150 mV higher than the 5-nitroimidazoles and are, therefore, readily reduced relative to 5-nitroimidazoles. It is to be noted here that, in general, the nitroimidazoles require bioreductive activation for their cidal activity (see section on ‘Mechanism of action’). Nitroimidazole derivatives with lower reduction potential can selectively tap into the redox system of the microbe (as opposed to mammals) and produce cidal activity specific to the microbe [17]. Thus, it became increasingly difficult to exploit the structure–activity relationships (SARs) of the 2-nitro series, due to their reduction by mammalian enzymes, and interest in anti-infective research gradually shifted towards other 4- and 5-nitroimidazole derivatives.
In cancer drug development, the search for good radiosensitizers for improving the sensitivity of tumor cells towards radiation therapy led to efforts directed at incorporating an additional nitro group onto the 2-nitroimidazole scaffold to increase its electron affinity in order to further increase reduction potential and subsequently alkylate the 2, 4(5)-dinitroimidazole with a series of oxirane derivatives. In addition to obtaining the desired product (1-(2-hydroxyalkyl)-2,4-dinitroimidazole), nitroimidazo [2,1-b]oxazoles were unexpectedly also produced, by intermolecular cyclization of the alcohol with the elimination of the 2-nitro group [18]. In 1989, Hindustan Ciba-Geigy demonstrated the anti-tubercular activity of these bicyclic nitroimidazoles [19] with further optimization of various structural analogs generating the lead compound CGI-17341 (3; Figure 1), which was found to be active against drug-susceptible as well as MDR Mtb [20]. However, further development was abandoned due to its mutagenicity.
More than a decade later, Otsuka Pharmaceutical Co. Ltd, overcame the mutagenicity problem of the nitroimidazooxazole series of compounds by substituting the 2-position of the side chain with a heteroatom and developed a series of nitroimidazooxazoles, which led to the compound OPC-67683 (4; Figure 1) [21], which is currently in Phase II clinical trials for the treatment of TB [302]. A few years prior to the discovery of OPC-67683, PathoGenesis (now Novartis) came out with their lead compound PA-824 (5; Figure 1), from a series of over 300 nitroimidazooxazines, which showed increased activity against Mtb with potential to decrease the duration of therapy [22,23]. This compound is also currently in Phase II clinical trials [303]. The reason for choosing oxazines over oxazoles for anti-tubercular drug development by PathoGenesis was probably driven by the need to patent compounds distinct from those made by Hindustan Ciba-Geigy [10].
Structure–activity relationships of anti-tubercular nitromidazoles
5-nitroimidazoles
An important consideration in nitroimidazole drug development has been generating compounds that are selectively reduced by microbes as opposed to their mammalian hosts. The 5-nitroimidazoles have a lower reduction potential than the 2-nitroimidazoles and this lower reduction potential is beyond the reach of the aerobic, and specifically the mammalian, redox systems, thereby making them harder to reduce. This lower reduction potential therefore makes the 5-nitroimidazoles selective for anaerobic microorganisms, including anaerobically persisting Mtb, where favorable, low reduction systems prevail. Thus, the 5-nitroimidazole, metronidazole, has better activity against anaerobes than the 2-nitroimidazole, benznidazole (6), whereas the latter, in turn, has better activity against aerobes (Table 1) [24]. Thus, more elaborate SAR has been established for the 5-nitroimidazoles relative to the 2-nitroimidazoles.
Table 1.
Minimum inhibitory concentration of 2- and 5-nitroimidazoles against aerobes and anaerobes†.
 |
||
|---|---|---|
| Organism | MIC (µM) | MIC (µM) |
| Aerobes | ||
| Pseudomonas aeruginosa ATTC 27853 | >748.54 | 490.61 |
| Staphylococcus aureus ATTC 25293 | >748.54 | 245.30 |
| Escherichia coli ATTC 25922 | >748.54 | >490.06 |
| E. coli W3110 rec A‡ | >748.54 | >490.06 |
| E. coli W3110 rec A | 748.54 | 30.66 |
| E. coli WP2 polA‡uvrA‡ | >748.54 | 245.30 |
| E. coli WP67 polA uvrA | 748.54 | 61.33 |
| Salmonella typhimurium LT2 uvrB | >748.54 | >490.06 |
| S. typhimurium LT2 uvrB NR | >748.54 | >490.06 |
| S. typhimurium TA98 uvrB | >748.54 | 490.61 |
| S. typhimurium TA98 uvrB, NR | >748.54 | 490.61 |
| S. typhimurium TA1538 uvrB | 748.54 | 490.61 |
| Anaerobes | ||
| Bacteroides fragilis ATTC 25285 | 2.92 | 7.67 |
| Bacteroides variabilis | 5.85 | 15.33 |
| Bacteroides distasonis | 5.85 | 7.67 |
| Clostridium perfringens | 2.92 | 7.67 |
| Clostridium bifermentans | 2.92 | 15.33 |
| Clostridium septicum | 2.92 | 3.83 |
| Clostridium sordelii | 5.85 | 7.67 |
| Clostridium tetani | 2.92 | 7.67 |
| Clostridium sporogenes | 2.92 | 15.33 |
| Peptostreptococcus sp. | 5.85 | 7.67 |
| Actinomyces sp. | 46.78 | 30.66 |
Data from [24].
uvr, recA and polA are the genes of the SOS DNA repair system.
MIC: Minimum inhibitory concentration; NR: Nitroreductase deficient.
N1-substituted 5-nitroimidazoles were evaluated for activity against Bacteroides spp. and ranked according to their activity: tinidazole (7) > panidazole (8) > ornidazole (9) > metronidazole ≥ secnidazole (10) > carnidazole (11) > dimetridazole (12; Table 2) [25]. Another 5-nitroimidazole GO-10213 (13) was found to be more active than metronidazole against aerobes, microaerophilic organisms and anaerobes but development halted due to the mutagenicity of the imidazolidinone ring [26].
Table 2.
Minimum inhibitory concentration of 5-nitroimidazoles against Bacteroides.
 | ||||
|---|---|---|---|---|
| Compound number |
Compound name | R1 | R2 | MIC (µM) |
| Against Bacteroides fragilis ATCC 23745† | ||||
| 7 | Tinidazole | CH2CH2SO2CH2CH3 | CH3 | 0.8 |
| 8 | Panidazole | CH2CH2-(4)-pyridyl | CH3 | 2.2 |
| 9 | Ornidazole | CH2CH(OH)CH2Cl | CH3 | 3.7 |
| 1 | Metronidazole | CH2CH2OH | CH3 | 3.7 |
| 10 | Secnidazole | CH2CH(OH)CH3 | CH3 | 3.7 |
| 11 | Carnidazole | CH2CH2NHC(S)OCH3 | CH3 | 6.3 |
| 12 | Dimetridazole | CH3 | CH3 | 10.0 |
| Against Bacteroides spp.‡ | ||||
| 1 | Metronidazole | CH2CH2OH | CH3 | 2.92 |
| 13 | GO-10213 | CH3 |  |
0.43 |
2-nitroimidazoles
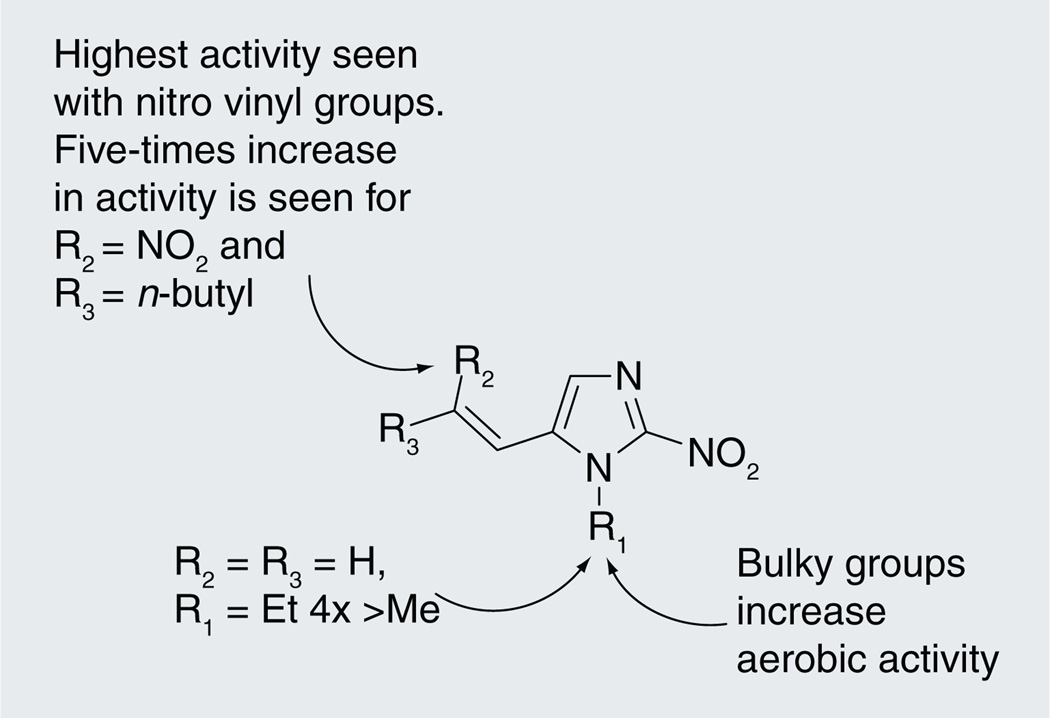
2-nitroimidazoles were the first class of nitroimidazoles with reported anti-tubercular activity. A large array of compounds belonging to this class substituted at 1- and 5-positions was screened against Gram-positive and Gram-negative bacteria, as well as fungi. The anti-tubercular activity of a selected set of compounds defining the SAR of this series is represented in Table 3 [15]. Alkyl (14–17), halide (18–20) and amide (21 & 22) substitution at the 1- as well as 5-position showed poor activity, whereas vinyl (25 & 26) substituents at the 5-position showed increased potency. The most active compound in the initial series ((26), minimum inhibitory concentration [MIC] = 29.93 µM) had an ethyl at N1 and an unsubstituted vinyl at the 5-position [15]. Subsequently, further vinyl-substituted 2-nitroimidazoles were made with only marginal improvement in antimycobacterial activity (e.g., 1-methyl-2-nitro-5-(2-nitro-hex-1-enyl)-1H-imidazole (31)) [27]. Further probing of the substitution at the 5-position with larger substituents yielded a marginal improvement in anti-tubercular activity with the most active compound being n-decyl-substituted oxime at the vinylic position (33) [28,29]. It is notable that 2-amino imidazoles [16], which are thought to be the end-product of intracellular nitroimidazole bioreduction, were also investigated for antimicrobial activity with similar substitutions at the 5-position yielding compounds with moderate anti-tubercular as well as generalized antimicrobial activity. In general, increase in the lipophilicity at the 5-position of the 2-nitroimidazoles increased the antimicrobial activity of Gram-positive bacteria, including Mtb (Figure 2) [27].
Table 3.
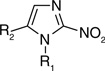
Minimum inhibitory concentrations of 2-nitroimidazoles against Mycobacterium tuberculosis H37Rv†.
 | |||
|---|---|---|---|
| Compound number | R1 | R2 | MIC (µM) |
| 14 | H | n-C3H7 | >128.97 |
| 15 | CH3 | CH3 | >1417.94 |
| 16 | CH3 | C2H5 | >1289.74 |
| 17 | CH3 | CH2CH2OH | >1169.18 |
| 18 | CH3 | CH2CH2Cl | >1058.03 |
| 19 | C2H5 | CH2CH2Cl | 98.50 |
| 20 | CH2CH2Cl | CH3 | >1058.03 |
| 21 | CH2CONH2 | CH3 | >543.30 |
| 22 | CH2CONHCH3 | CH3 | >1009.69 |
| 23 | CH3 | CHO | 64.50 |
| 24 | CH3 | COCH3 | 295.77 |
| 25 | CH3 | CH2 =CH | 130.68 |
| 26 | C2H5 | CH2 =CH | 29.93 |
| 27 | CH3 | C6H5CH = CH | >87.30 |
| 28 | C2H5 | C6H5CH = CH | 41.14 |
| 29 | CH3 | HC(NO2) = CH | 25.25 |
| 30 | CH3 | CH3C(NO2) = CH | 23.58 |
| 31 | CH3 | n-C4H9C(NO2)=CH | 19.68 |
| 32 | CH3 | n-C5H11N(O)=CH | 208.23 |
| 33 | CH3 | n-C10H21N(O)=CH | 16.12 |
| 34 | CH3 | 210.86 | |
Figure 2.
Structure–activity relationships of 2-nitroimidazoles.
Imidazo[2,1-b]oxazoles
Structure–activity relationships of imidazo[2,1-b]oxazoles were explored on discovering that compound 35 (Table 4) exhibited anti-tubercular activity. Substitution of the 2-position of the oxazole ring with various alkyl and alkyl halides resulted in compounds with mostly improved in vitro anti-tubercular activity as represented in Table 4 [19]. Substitution of the methyl of 35 with ethyl resulted in the lead compound in this study, CGI-17341 (3) with 35-fold increased activity above 35, while substitution with a phenyl group (37) only marginally improved activity. Alkyl mono halide substitutions 36 and 38 had greatly improved activity, whereas the trichloromethyl group (39) resulted in a tenfold decrease in activity. It is not clear if the compounds that were tested were enantiomerically pure or not, since the R-enantiomer was later shown to be the active enantiomer for the 4-nitro imidazo[2,1-b]oxazole series, whereas the S-enantiomer was the active enantiomer in the 4-nitro imidazo[2,1-b]oxazine series [10]. Thus, testing of racemic mixtures would have underestimated the true potency of these compounds. Dialkyl substitution at the 2-position resulted in the most active compounds (43 & 44) with activity proportional to the chain length. A spiro cyclopentyl (45) substitution at the 2-position resulted in an inactive compound, but the spiro cyclohexyl (46) and cycloheptyl (47) substituent resulted in improved activity.
Table 4.
Anti-tubercular activity of 4-nitroimidazo[2,1-b]oxazoles†.
| Compound number |
R1 | R2 | Anti-tubercular activity | |
|---|---|---|---|---|
| In vitro MIC (µM) |
In vivo ED50‡ (mg/kg) p.o. |
|||
| 35 | CH3 | H | 11.54 | 25 |
| 3 | C2H5 | H | 0.33 | 10 |
| 36 | CH2 Cl | H | 0.59 | 30–100 |
| 37 | C6H5 | H | 4.11 | >100 |
| 38 | CH2Br | H | 0.97 | 30–100 |
| 39 | CCl3 | H | 115.16 | ND |
| 40 | CH2OiPr | H | 17.17 | >200 |
| 41 | CH2Oallyl | H | 17.33 | >200 |
| 42 | CH2OC6H5 | H | 0.92 | >200 |
| 43 | n-C4H9 | CH3 | 0.07 | ND |
| 44 | n-C7 H15 | CH3 | 0.01 | ND |
| 45 | −(CH2)4- | 149.23 | ND | |
| 46 | −(CH2)5- | 0.03 | 0.13 | |
| 47 | −(CH2)6- | 0.015 | 0.06 | |
Data from [19].
Drug dose that allowed the survival of 50% of mice, when all the untreated control groups died on day 23.
MIC: Minimum inhibitory concentration; ND: Not determined; p.o.: per os.
5-nitro analogs with a 2-methyl or 2-chloromethyl substituent were 100- and 2000-fold less active than their 4-nitro counterparts, respectively, showing a clear preference for the position of the nitro group for anti-tubercular activity [18,19].
Nitroimidazo[2,1-b]oxazines
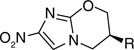
Initial SAR studies leading to the identification of PA-824 ((6S)-2-nitro-6-{[4-(trifuoromethoxy) benzyl]oxy}-6,7-dihydro-5H–imidazo[2,1-b] [1,3]oxazine) (5), the lead compound in the series of bicyclic compounds containing nitroimidazo [2,1-b]oxazines, have been patented [201,202]. Most of the compounds were assayed for activity against Mycobacterium bovis strains while only a selected few were assayed against drug susceptible and drug resistant Mtb. Most of the compounds had a benzyloxy group attached to the oxazine ring (Table 5). Whereas the unsubstituted, the 2,4-disubstituted and 3-substituted benzyloxy analogs were inactive, 4-substituted benzyloxy groups were active with the 4-trifuoromethyl substituent (48) being less active than the 4-trifluoromethoxy group (PA-824) [10]. The benzyloxybenzyloxy substituent (49) was the most active compound in vitro. For the nitroimidazo[2,1-b]oxazines, the S-isomers were 100-fold more active than the corresponding R form [10,23]. Introduction of carbonate, carbamate (52 & 53) and urea (51) linkers in between the oxazine ring and the substituted benzyl ring led to compounds with equal or slightly better MICs against M. bovis strains with p-chlorophenyl urea (51) being the most potent compound in the series. Selected activity results are summarized in Table 5.
Table 5.
Activity of compounds in the nitroimidazo [2,1-b]oxazine series that eventually led to PA-824 (5)†.
 | |||
|---|---|---|---|
| Compound number |
R | MIC (µM) against Mycobacterium bovis BCG |
MIC (µM) against Mycobacterium tuberculosis H37Rv |
| 5 | 0.17 | 0.36 | |
| 48 | 1.46 | ND | |
| 49 | 0.08 | 0.08 | |
| 50 | 0.18 | 0.09 | |
| 51 | 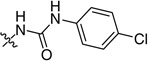 |
0.006 | ND |
| 52 | 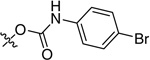 |
0.04 | ND |
| 53 |  |
0.67 | ND |
| 54 | 0.08 | ND | |
| 55 | 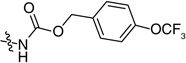 |
0.04 | ND |
The SAR for the anti-tubercular nitroimidazoles have been established based on whole cell activity, yet the basis of this activity is not fully understood. Where metronidazole only kills anaerobically persisting Mtb, PA-824 kills both aerobically replicating as well as anaerobic nonreplicating Mtb. In order to understand the aerobic versus anaerobic activity of various nitroimidazoles, efforts were directed towards the elucidation of the fundamental structure–function relationship of these compounds and how this is related to the aerobic as opposed to anaerobic activity of the analogs (Table 6). Des-nitro PA-824 (56) had neither aerobic nor anaerobic activity, thereby asserting the requirement of the nitro group for activity [30]. The trifluoro-methoxybenzyl ether side chain was critical for both aerobic and anaerobic activity where complete replacement of the side chain to alcohol (57) or methyl ether (58) rendered the molecules inactive. The rigidity conferred by the oxazine ring is crucial since the ring opened form (59) had reduced aerobic activity and a dramatic decrease in anaerobic activity. Loss of oxygen from the 2-position (60) from compound 59 further decreased the aerobic as well as anaerobic activity emphasizing the importance of oxygen at this position for both aerobic as well as anaerobic activity. Not surprisingly, removing the side chain from 60 resulting in 61 also resulted in an inactive compound with this compound being notable since it is the 4-nitro isomer of metronidazole underscoring the importance of the position of the nitro group for the anaerobic activity of metronidazole (Table 6) [30].
Table 6.
Structure–activity relationship of PA-824 (5) analogs leading to identification of key structural features required for aerobic and anaerobic activity against Mycobacterium tuberculosis H37Rv†.
| Compound number |
Structure | MIC (µM) | MAC‡ (µM) |
|---|---|---|---|
| 5 | 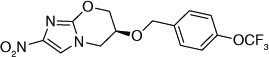 |
0.40 | 8–16 |
| 56 | 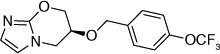 |
>160 | >500 |
| 57 |  |
>100 | 250 |
| 58 |  |
>125 | 250 |
| 59 |  |
6.25 | 250–500 |
| 60 | 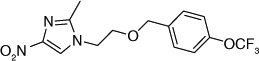 |
>145 | >500 |
| 61 | 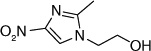 |
>300 | >500 |
| 62 | 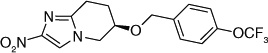 |
25 | 250 |
| 63 | 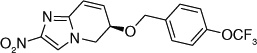 |
25 | 62.5 |
| 64 | 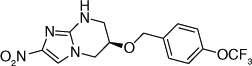 |
0.8 | 125 |
| 65 | 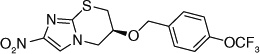 |
0.8 | 25 |
| 66 | 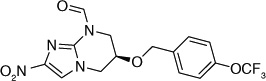 |
3.2 | 25 |
| 67 | 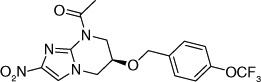 |
6.25 | 31.25 |
| 68 | 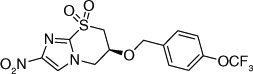 |
>100 | 25 |
| 69 |  |
>100 | 50–100 |
| 70 | 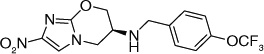 |
0.31 | 12.5 |
| 71 | 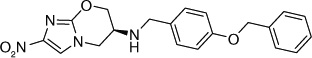 |
0.15 | 6.25 |
| 72 | 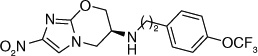 |
0.16 | 6.25 |
| 73 | 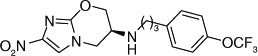 |
0.08 | 3.125 |
| 74 |  |
0.039 | 31–12.5 |
| 75 | 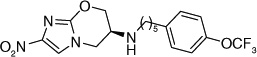 |
0.078 | 6.25 |
The electron-donating potential at the 2-position of the oxazine ring was found to be crucial for activity since the replacement of the oxygen with carbon in 62 dramatically affected both aerobic as well as anaerobic activity with some restoration of anaerobic activity seen with the unsaturated species (63) suggesting that SAR for aerobic and anaerobic activity are different and are determined by the electronics at this position [30,31]. This notion was further supported by the observation that replacement of the 2-position oxygen with electron-donating groups, such as nitrogen (64) or sulfur (65), had no effect on the aerobic activity but diminished anaerobic potency, whereas replacement with electron-withdrawing groups (66–69) dramatically reduced or abrogated aerobic activity without much effect on anaerobic activity (Table 6) [31,32].
The fact that the benzyloxybenzyloxy substituent on the oxazine ring generates a compound (49; Table 5) more active than PA-824 suggested the presence of a larger hydrophobic pocket near the active site of the enzyme that interacts with the drug [301,302]. To explore the depth of this hydrophobic pocket, SAR of the tail of PA-824 was investigated. The problem of solubility of these compounds with the additional hydrophobe was eliminated by substituting the ether analog with the corresponding amine analog where the amino-derivatives of PA-824 and 49 yielded compounds 70 and 71 with slightly improved activity (Table 6) [31]. On increasing the linker size connecting the 6-position amine with the trifuoromethoxybenzene aromatic moiety from two to four carbons (72–75), the aerobic activity was found to sequentially improve with aerobic activity reaching a maximum with the aminobutyl-824 (74), whereas the 5-carbon linker in aminopentyl-824 (75) had decreased activity. There was no significant improvement of the anaerobic activity on changing the linker size, suggesting a different SAR for aerobic and anaerobic activity with respect to the hydrophobic tail region of the drug (Table 6) [31].
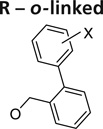
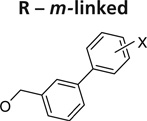
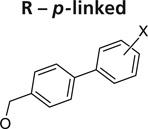
Further exploration of the hydrophobic binding pocket was undertaken with o-, m- and p-linked biphenyl analogs attached to the nitroimidazooxazine via ether linkage. The o-linked biphenyls (76–86) showed poorest activity, followed by the m-linked analogs (87–97), while the p-linked biphenyl analogs (98–108) were the most active. The activity trend did not alter significantly with substitutions in the second aryl ring (Table 7). This suggested that the hydrophobic pocket is more or less linear with moderate tolerability around the terminus of the second aryl ring (86, 97 & 108). The difference in the MIC values between the aerobic (microplate Alamar blue assay) and the low oxygen recovery assays for the o-linked compounds is smaller compared with the m- and p-linked compounds attesting to the fact that the mechanism of aerobic and anaerobic activities are significantly distinct [33]. These compounds had an ether linkage instead of the amino linkage and hence the addition of a second aryl moiety made them less soluble. The solubility problem was overcome by the attachment of amino (113) or alcohol (110) groups to the second aryl group, but this did not have any marked improvement on the aerobic activity. The p-linked biphenyl analogs were more active than PA-824 and SARs of these classes of analogs were further explored (Table 8), which showed that substitution at the 4-position (104 & 105) of the distal aryl ring had marginal improvement in activity compared with substitutions at the 2- and 3-positions (100 & 101) with bisubstituted aryl rings showing similar or better potency (107 & 108). The SAR studies of the lipophilic tail in summary have shown a positive correlation between the aerobic activity and the lipophilicity of PA-824 analog as well as the electron-withdrawing potential of the substituent on the distal aryl group [33] (see Tables 8, 9 & 10 and the section regarding PA-824 in preclinical testing of anti-TB nitroimidazoles for discussion of microsomal stability).
Table 7.
Structure–activity relationships of o-, m- and p-linked biphenyl analogs of PA-824 (5)†.
 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
 |
 |
 |
|||||||
| Substituent X | Compound number |
MABA | LORA | Compound number |
MABA | LORA | Compound number |
MABA | LORA |
| H | 76 | 0.64 | 2.5 | 87 | 0.095 | 2.1 | 98 | 0.045 | 3.9 |
| 2-OCF3 | 77 | 1.2 | 6.2 | 88 | 0.46 | 3.3 | 99 | 0.035 | 0.97 |
| 3-CN | 78 | 0.8 | 8.2 | 89 | 0.17 | 3.7 | 100 | 0.12 | 1.3 |
| 3-F | 79 | 0.62 | 3 | 90 | 0.18 | 2.7 | 101 | 0.045 | 2.2 |
| 3-OCF3 | 80 | 1.9 | 6.3 | 91 | 0.4 | 3.5 | 102 | 0.077 | 1.4 |
| 4-COMe | 81 | 17 | 36 | 92 | 0.3 | 1.9 | 103 | 0.04 | 0.73 |
| 4-CN | 82 | 8.4 | 19 | 93 | 0.23 | 4.8 | 104 | 0.025 | 0.58 |
| 4-F | 83 | 1.2 | 3.4 | 94 | 0.077 | 3.8 | 105 | 0.015 | 1.4 |
| 4-OCF3 | 84 | 2.3 | 4.3 | 95 | 0.11 | 2.2 | 106 | 0.035 | 1.3 |
| 3-F, 4-OMe | 85 | 1.8 | 3.8 | 96 | 0.19 | 2.2 | 107 | 0.04 | 1.9 |
| 3,4-benz | 86 | 1.6 | 3.1 | 97 | 0.12 | 3.2 | 108 | 0.045 | 0.87 |
Data from [33].
LORA: Low-oxygen recovery assay where hypoxically adapted cells are incubated with compound under anaerobic conditions followed by an aerobic recovery to measure luminescence as a surrogate readout of the number of cells prior to recovery; MABA: Microplate Alamar blue assay. Aerobic minimum inhibitory concentration as determined by Alamar Blue-based readout of redox activity in cells; MIC: Minimum inhibitory concentration.
Table 8.
Structure–activity relationships of p-linked biphenyl analogs of PA-824 (5)†.
 | ||||||
|---|---|---|---|---|---|---|
| Compound number |
Substituent X | MIC (µM) | Microsomal stability after 1 h (ratio remaining) |
In vivo efficacy (% relative to PA-824, (5)) |
||
| MABA | LORA | Human | Mouse | |||
| 5 | 0.5 | 2.6 | 0.82 | 0.94 | 100 | |
| 109 | 2-OEt | 0.03 | 0.82 | 0.5 | 0.002 | 15 |
| 110 | 2-O(CH2)3OH | 0.34 | 2.7 | 0.59 | 0.21 | <1 |
| 111 | 3-OCH2Ph | 0.12 | 0.88 | 0.64 | 0.5 | 66 |
| 112 | 4-CF3 | 0.03 | 1.4 | 0.6 | 0.85 | 7200 |
| 113 | 4-CH2NHPh | 0.06 | 0.63 | 0.12 | 0.52 | 3 |
| 104 | 4-CN | 0.025 | 0.58 | 0.95 | 0.58 | 360 |
| 103 | 4-COMe | 0.04 | 0.73 | 0.36 | 0.7 | <1 |
| 105 | 4-F | 0.015 | 1.4 | 0.75 | 0.55 | 300 |
| 114 | 4-OPh | 0.04 | 2.7 | 0.22 | 0.48 | <1 |
| 106 | 4-OCF3 | 0.035 | 1.3 | 0.97 | 0.96 | >25000 |
| 115 | 2-Cl, 4-CF3 | 0.03 | 1.4 | 0.92 | 0.93 | 720 |
| 116 | 2-Cl, 4-OCF3 | 0.04 | 0.78 | 0.91 | 0.86 | 2300 |
| 117 | 2-F, 4-OCF3 | 0.045 | 0.72 | 0.88 | 0.89 | 4600 |
| 118 | 3-Cl, 4-OCF3 | 0.03 | 0.90 | 0.9 | 0.84 | 28100 |
| 119 | 3-OCF3, 4-Cl | 0.04 | 0.95 | 0.95 | 0.92 | 970 |
| 120 | 3-CF3, 4-Cl | 0.035 | 1.9 | 0.92 | 0.96 | 770 |
| 107 | 3-F, 4-OMe | 0.04 | 1.9 | 0.8 | 0.5 | 2 |
| 121 | 3-F, 4-OCF3 | 0.03 | 0.34 | 0.93 | 0.86 | 41900 |
| 122 | 3-OCF2H, 4-Cl | 0.03 | 1.1 | 0.83 | 0.86 | 1000 |
Data from [33].
LORA: Low-oxygen recovery assay where hypoxically adapted cells are incubated with compound under anaerobic conditions followed by an aerobic recovery to measure luminescence as a surrogate readout of the number of cells prior to recovery; MABA: Microplate Alamar blue assay, aerobic minimum inhibitory concentration as determined by Alamar Blue-based readout of redox activity in cells; MIC: Minimum inhibitory concentration.
Table 9.
Structure–activity relationships of selected five-membered heterobiaryl analogs of PA-824 (5)†.
 | ||||||
|---|---|---|---|---|---|---|
| Compound number |
Substituent X | MIC (µM) | Microsomal stability after 1 h (ratio remaining) |
In vivo efficacy (% relative to PA-824, (5)) |
||
| MABA | LORA | Human | Mouse | |||
| 5 | 0.5 | 2.6 | 0.82 | 0.94 | 100 | |
| 106 | 0.035 | 1.3 | 0.97 | 0.96 | >25000 | |
| 123 | 0.06 | 0.58 | 0.86 | 0.81 | 1200 | |
| 124 | 0.05 | 0.61 | 0.87 | 0.67 | 4100 | |
| 125 | 0.075 | 3.3 | 0.99 | 0.74 | 34 | |
| 126 | 0.035 | 1.3 | 0.97 | 0.81 | 4300 | |
Data from [34].
LORA: Low-oxygen recovery assay where hypoxically adapted cells are incubated with compound under anaerobic conditions followed by an aerobic recovery to measure luminescence as a surrogate readout of the number of cells prior to recovery; MABA: Microplate Alamar blue assay, aerobic MIC as determined by Alamar Blue-based readout of redox activity in cells; MIC: Minimum inhibitory concentration.
Table 10.
Structure–activity relationships of selected six-membered heterobiaryl analogs of PA-824 (5)†.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound number |
Link | Position of the N |
Substituent X | Solubility (µg/ml) |
MIC (µM) | Microsomal stability after 1 h (ratio remaining) |
In vivo efficacy (% relative to PA-824, (5)) |
||
| MABA | LORA | Human | Mouse | ||||||
| 5 | 19 | 0.50 | 2.6 | 0.82 | 0.94 | 100 | |||
| 106 | 1.2 | 0.035 | 1.30 | 0.97 | 0.96 | >20500 | |||
| 126 | para | 2 | 4-CF3 | 0.2 | 0.06 | 1.0 | 0.93 | 0.9 | 3300 |
| 127 | para | 2 | 4-CF3, 6-Cl | 3.3 | 0.025 | 1.0 | 0.86 | 0.8 | 510 |
| 128 | para | 2 | 4-F | 67 | 0.035 | 1.3 | 0.83 | 0.61 | 65 |
| 129 | para | 2 | 4-OCF2H | 2.9 | 0.08 | 1.5 | 0.98 | 0.8 | 330 |
| 130 | para | 3 | 4-CF3 | 1.0 | 0.03 | 2.1 | 0.86 | 0.91 | 1500 |
| 131 | para | 3 | 4-F | 8.0 | 0.095 | 2.1 | 0.88 | 0.79 | 66 |
| 132 | para | 3 | 4-OCF2H | 3.2 | 0.045 | 0.66 | 0.95 | 0.75 | 190 |
| 133 | para | 3 | 4-OCH3 | 4.4 | 0.045 | 0.61 | 0.4 | 0.61 | 26 |
| 134 | para | 4 | 3-F | 14 | 0.23 | 1.4 | 0.93 | 0.86 | 95 |
| 135 | meta | 4′ | 4-F | 31 | 0.17 | 1.2 | ND | ND | ND |
| 136 | meta | 4′ | 4-OCF3 | 3.9 | 0.035 | 2.4 | 0.54 | 0.31 | 12 |
| 137 | meta | 5′ | 4-F | 45 | 0.31 | 7.7 | 0.71 | 0.39 | 2 |
| 138 | para | 2′ | 4-OCF3 | 2.5 | 0.065 | 3.7 | 0.97 | 0.97 | 2700 |
| 139 | para | 2′ | 3-F, 4-OCF3 | 30 | 0.05 | 1.3 | 0.97 | 0.86 | 23300 |
| 140 | para | 3′ | 4-F | 3.8 | 0.06 | 2.9 | 0.88 | 0.8 | 780 |
| 141 | para | 3′ | 4-OCF3 | 2.3 | 0.05 | 0.54 | 0.83 | 0.87 | >8900 |
| 142 | para | 3′ | 3-Cl, 4-OCF3 | 0.18 | 0.017 | 1.0 | 0.87 | 0.77 | >93300 |
| 143 | para | 3′ | 3-F, 4-OCF3 | 3.0 | 0.025 | 0.93 | 1 | 0.9 | >84000 |
| 144 | para | 2′,3′ | 4-OCF3 | 6.1 | 0.075 | 1.7 | 0.94 | 0.92 | 1100 |
| 145 | para | 2′, 5′ | 4-OCF3 | 2.6 | 0.023 | 1.0 | 0.98 | 0.91 | 16700 |
| 146 | para | 2′,6′ | 4-OCF3 | 8.1 | 0.11 | 1.9 | 0.92 | 0.87 | 370 |
| 147 | para | 3′,5′ | 4-OCF3 | 2.1 | 0.027 | 1.8 | 0.96 | 0.87 | >11200 |
Data from [35].
LORA: Low-oxygen recovery assay where hypoxically adapted cells are incubated with compound under anaerobic conditions followed by an aerobic recovery to measure luminescence as a surrogate readout of the number of cells prior to recovery; MABA: Microplate Alamar blue assay, aerobic MIC as determined by Alamar Blue-based readout of redox activity in cells; MIC: Minimum inhibitory concentration; ND: Not determined.
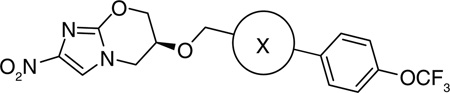
In an attempt to increase the solubility of the biphenyl analogs, the proximal phenyl ring was replaced with hydrophilic five-membered heterocycles (containing mono, mixed, di, tri and tetra heteroatoms) [34] all of which, except the thiophene and thiazole heterocycles, had improved solubility. Of the various heterocycles tested, four series (3-aryl-1-methylpyrazole (123), 1-aryl-3-linked-pyrazole (124), 2-aryl-4-linked-triazole (125) and 2-aryl-5-linked-tetrazole (126) analogues) showed good aerobic as well as anaerobic anti-tubercular activity (Table 9).
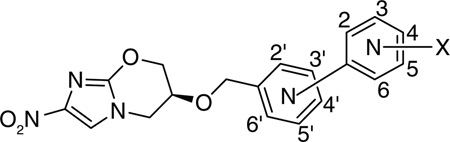
Further structure–function relationship studies were carried out with biaryl analogs of PA-824 with the replacement of proximal, distal as well as both aryl groups with six-membered nitrogen-containing heterocycles [35]. This allowed the overall structure to be nearly linear and, hence, a better ft in the putative hydrophobic pocket of the enzyme [33]. Solubility improved when one of the phenyl rings (distal or proximal) was replaced with pyridine and was further improved when both the phenyl rings changed to pyridine. Solubility at neutral pH was highest for mono pyridine analogs lacking a substituent at the o- to the nitrogen and the bipyridine analogs and improved for pyridylpyrazine and pyridylpyrimidine analogs. The p-linked biaryls were less soluble than the o- and m-linked counterparts [35].
Replacement of the distal phenyl ring with substituted pyridine ring showed that the position of the nitrogen in the terminal ring did not affect the activity significantly for these analogs. In accordance with the previous studies, potencies ranked p- > m- > o-linked compounds. Despite the improved solubility of many of the p-linked substituted pyridine series (126–134), only the trifluoromethyl analogs (126, 127 & 130) had better aerobic and anaerobic activities than PA-824 but these had considerably lower solubility than PA-824. In contrast, replacement of the proximal aryl with a pyridine did show that activity was dependent on the position of the pyridyl nitrogen (138 & 141) and in this series, only two compounds (135 & 139) had a somewhat improved solubility as well as improved aerobic and anaerobic activities, with the most potent compounds having much poorer solubility than PA-824. As before, optimization of aerobic activity did not correlate with optimal anaerobic activity. Of the m-linked compounds, the most aerobically active compounds were those in which the 4′-position was a nitrogen atom (136), yet compounds with a 2′-aza showed better anaerobic activity. Of the p-linked compounds, anaerobic activity was best with 3′-aza groups (141) relative to the 2′-aza groups (138). Disubstituted 3′-aza compounds (142 & 143) were in general the most potent of the heterobiaryl compounds but were up to 100-fold less soluble than PA-824 (Table 10) [35]. The poor solubility did not translate to poor in vivo efficacy as seen by their substantially improved activity relative to PA-824 in the mouse model.
p-linked bipyridine compounds with substituents were more soluble than the mono-pyridine counterparts, but showed decreased aerobic as well as anaerobic activity. Further SAR studies were performed with compounds in which the proximal pyridine ring was replaced with diaza substituent (pyridazine, pyrazine or pyrimidine). In this category the compounds belonging to the pyridazine class (144) were very hydrophilic (based on CLogP values) with moderate potency; the pyrazine class (145) was more lipophilic with somewhat improved anaerobic activity while the pyrimidine class (146) had more solubility with activities less potent than some of the other heterobiaryl compounds yet better than that of PA-824.
The crystal structure of PA-824 revealed that the pseudoaxial orientation of the trifluoromethoxybenzyl ether contributed towards tight packing of this compound [36]. In an effort to disrupt the pseudoaxial conformation of PA-824 with the aim of improving solubility, 7-(S)-and 7-(R)-methyl-nitroimidazole-oxazines (148 & 149) were synthesized and the latter found to have pseudoequatorial geometry. However, although both isomers had similar activity, there was no improvement in the solubility, especially for the (R)-isomer, suggesting that the crystal packing of the compound did not contribute to solubility. It also indicated that the active site of the enzyme that recognizes PA-824 had a big enough pocket to fit both the 7-(S)- (148) and 7-(R)-methyl (149) groups, such that their activities were comparable (Table 11) [36].
Table 11.
Structure–activity relationship of 7-methyl analogs of PA-824 (5) against Mycobacterium tuberculosis H37Rv†
| Compound number |
Structure | MIC (µM) | MAC (µM) | Solubility (µg/ml) |
|---|---|---|---|---|
| 5 | 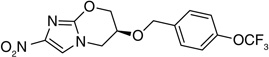 |
0.4 | 8–16 | 10.2 |
| 148 | 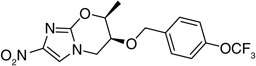 |
0.2–0.4 | 16 | 9.89 |
| 149 | 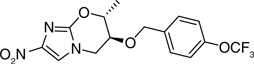 |
0.2 | 8 | 10.3 |
Data from [36].
MAC: Minimum anaerobicidal concentration; MIC: Minimum inhibitory concentration
In another study the SAR of substitution at the 5-position of the nitroimidazooxazine ring of PA-824 was explored [37]. It is the center where the enzymatic reductive activation of PA-824 initiates with the transfer of hydride ion from F420 to PA-824 (see the subsection on PA-824 in ‘Mechanism of action’) [38]. Substitution of hydrogen at the 5-position of the nitroimidazooxazine ring with an electron-withdrawing nitrile group (150) and electron-donating amino group (151) generated inactive compounds suggesting that gross changes in the electron distribution of the nitroimidazole ring is not tolerated. However, substitution with a halogen resulted in compounds (152 & 153) with some, albeit poor, in vitro aerobic as well as anaerobic activity, suggesting toleration of small substituents at this position (Table 12). The halogen substituted compounds (152 & 153) not only showed in vitro activity against wild-type Mtb, but also against mutants that were resistant to PA-824 due to Rv3547 inactivation or inability to synthesize F420 where both these components are required for PA-824 activation(see the subsection on PA-824 in ‘Mechanism of action’) [38]. This suggested the possibility of an alternate bioreductive activation pathway of certain other nitroimidazoles [37]. A summary of the SAR of PA-824 is represented in Figure 3.
Table 12.
Structure–activity relationships of 5-substituted analogs of PA-824 (5) against Mycobacterium tuberculosis H37Rv†.
 | |||
|---|---|---|---|
| Compound number |
Substituent X | MIC (µM) | MAC (µM) |
| 5 | H | 0.4 | 8–16 |
| 150 | CN | >100 | ND |
| 151 | NH2 | >100 | >500 |
| 152 | Br | 25 | 32.5 |
| 153 | Cl | 25 | ND |
Data from [37].
MAC: Minimum anaerobicidal concentration; MIC: Minimum inhibitory concentration; ND: Not determined.
Figure 3.
Summary of structure–activity relationships of bicyclic nitroimidazoles PA-824 (5).
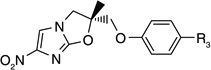
Nitroimidazo[2,1-b]oxazoles
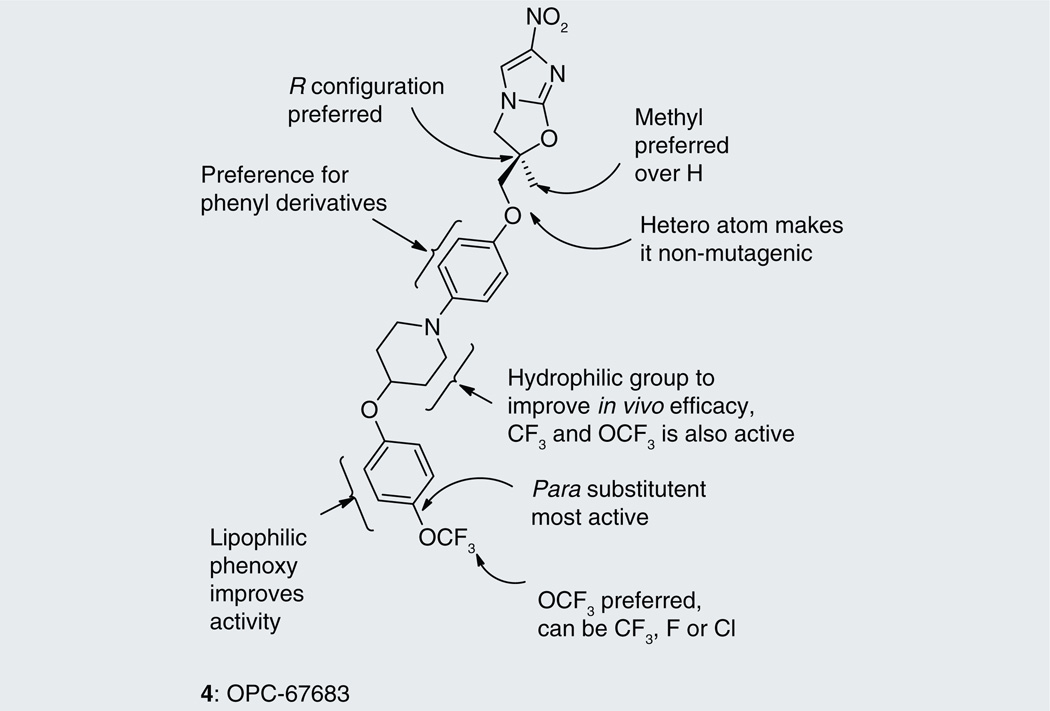
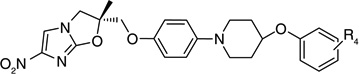
Otsuka Pharmaceuticals Co. Ltd., developed 6-nitro-2,3-dihydroimidazo[2,1-b]oxazoles as potential anti-tubercular agents because these inhibited mycolic acid biosynthesis in Mtb [39]. These share a core structure with CGI-17341 (3), the lead compound from the series of bicyclic nitroimidazoles with promising anti-tubercular activity that could not be pursued because of its mutagenicity [19,20]. The mutagenicity of 6-nitro-2,3-dihydroimidazo[2,1-b]oxazole was circumvented by incorporation of a heteroatom at the 2-position of the oxazole ring [40]. Subsequently, various phenoxymethyl substituents were made and tested for aerobic growth inhibition, which showed that, in contrast to the nitroimidazooxazines, the R-isomer (158) was the more active (Table 13) than the S-isomer (159) prompting further exploration of the R-isomer. Various analogs with substituents at the p-position of the phenyl ring of (158) were synthesized and tested for in vitro as well as in vivo efficacy (see the subsection on OPC-67683 in ‘Pre-clinical testing of anti-TB nitroimidazoles’). The results showed that in vitro efficacy did not always match in vivo efficacy (160–167), which may be related to pharmacokinetic parameters that are not reported. Even for compounds designed to improve bioavailability by incorporating a hydrophilic group at the 4-position of the benzene ring (165–167), the in vivo efficacy did not match those of less soluble compounds such as 161, 163 and 164. These compounds (165–167) had comparable in vitro activity, but the piperidino substituent 165 was selected for further development because of its significantly improved relative in vivo efficacy. An array of compounds with lipophilic phenoxy groups at the 4-position of the piperidine ring were assayed for anti-tubercular activity (168–173) with OPC-67683 (4; Table 13) being selected above the rest due to its superior in vitro combined with in vivo efficacy [40]. The nitroimidazooxazoles that led to the lead compound OPC-67683 had equipotent activity against INH- as well as RIF-resistant Mtb [40]. The SAR for this series is summarized in Figure 4 [41].
Table 13.
Structure–activity relationships showing the development of the lead compound OPC-67683 (4)†.
 | ||||
|---|---|---|---|---|
| Compound number | R1 | R2 | Confguration | MIC (µM) |
| 154 | H | OPh | Racemic | 2.98 |
| 155 | H | OCH2Ph | Racemic | 10.82 |
| 156 | H | O(CH2)2Ph | Racemic | 5.15 |
| 157 | CH3 | OPh | Racemic | 0.36 |
| 158 | CH3 | OPh | (R) | 0.18 |
| 159 | CH3 | OPh | (S) | 11.37 |
 | ||||
| Compound number | R3 | MIC (µM) | In vivo efficacy | |
| 160 | H | 0.18 | 2.0 | |
| 161 | Cl | 0.08 | >3.1 | |
| 162 | CH3O | 0.16 | 0.72 | |
| 163 | CF3 | 0.58 | >4.4 | |
| 164 | OCF3 | 0.56 | >3.6 | |
| 165 | 2.18 | 1.9 | ||
| 166 | 2.16 | 1.3 | ||
| 167 | 2.07 | 0.0 | ||
 | ||||
| Compound number | R4 | MIC (µM) | In vivo efficacy | |
| 168 | H | 0.87 | 2.8 | |
| 169 | p-Cl | 0.10 | 2.2 | |
| 170 | p-F | 0.83 | 2.2 | |
| 171 | p-CF3 | 0.02 | 2.2 | |
| 4 | p-OCF3 | 0.01 | >3.8 | |
| 172 | o-OCF3 | 0.73 | 3.0 | |
| 173 | m-OCF3 | 0.04 | >4.4 | |
MIC reported in this table was determined against drug-sensitive Mycobacterium tuberculosis strain (H37Rv). In vivo efficacy is measured as the reduction in viable bacterial counts (log CFU) in lungs of infected mice with respect to untreated controls with the compounds at a daily dose of 50 mg/kg for 10 days. Treatment was initiated a day afterinfection.
MIC: Minimum inhibitory concentration.
Figure 4.
Summary of structure–activity relationships of bicyclic nitroimidazoles OPC-67683 (4).
Mechanism of action
Metronidazole
Nitroimidazoles are activated by bioreduction for which a low redox potential electron-transfer system is a prerequisite, and this activation is essential for their cidal activity. The single-electron redox potential for 2-nitroimidazoles and 5-nitroimidazoles are −0.27 to −0.44 V and −0.4 to −0.5 V, respectively, and the latter is beyond the reduction capacity of mammalian redox systems. The redox potentials of the electron-transport system in microbes, especially those under limiting-oxygen conditions, are in the range of −0.42 V or below and are thus capable of reducing nitroimidazoles. Single-electron reduction of 5-nitroimidazoles produces a nitro radical anion, which is unstable and can decompose to form nitrite anion and imidazole radical [42]. This pathway is particularly favored under anaerobic conditions. Alternatively, the nitro radical anion can be further reduced by single-electron reduction to the nitroso and hydroxylamine species and all these nitroimidazole species are capable of causing DNA damage and resultant cell death [43]. In a futile cycling reaction, under aerobic conditions the nitro radical anion can reduce oxygen in microaerophilic organisms to form superoxide [44,45], which can be inactivated by superoxide dismutase and catalase enzymes. However, in the presence of transition elements, such as iron or copper, which are present in the cell bound to a variety of proteins, superoxide reacts with hydrogen peroxide produced during oxidative metabolism to form hydroxyl radical by the Haber–Weiss reaction, which in turn is a potent agent of DNA damage causing DNA fragmentation thereby inhibiting DNA synthesis. This process is thought to be responsible for the cidal activity of metronidazole [10,42,46] and, as a result, mutants in DNA repair pathways are hypersensitive to metronidazole [47,48].
Metronidazole sensitivity in eukaryotic parasites [10,42,49–53] and anaerobic and microaerophilic bacteria [10,54–62] has been studied extensively and has been reviewed comprehensively in the literature. Mtb is moderately sensitive to metronidazole under anaerobic conditions with exposure to oxygen abrogating activity arguing that futile cycling unlikely plays a role in the cidal consequences of this compound. Mtb lacks the typical pyruvate:ferrodoxin oxidoreductase [63] as well as pyruvate:flavodoxin oxidoreductase system, which is required for nitroimidazole activation in some eukaryotes [64,65] and microaerophilic organisms, respectively [66]. Rv2454c and Rv2455c, encoding an anaerobic-type a-ketoglutarate ferredoxin oxidoreductase [67], might substitute for the pyruvate:ferrodoxin oxidoreductase system for activation of metronidazole [10]. The low activity of this drug in Mtb is often attributed to low percentage of total adenine and thymine in mycobacterial DNA since organisms containing DNA with high percentage of total adenine and thymine are more susceptible to nitroimidazoles [10,49].
PA-824
PA-824 shows activity against both actively replicating, as well as hypoxic nonreplicating Mtb. Under aerobic conditions, PA-824 was shown to inhibit biosynthesis of proteins and lipids in a dose-dependent manner without disrupting nucleic acid biosynthesis [23]. Disruption of lipid biosynthesis (the step involving the formation of ketomycolate from hydroxymycolic acid) was shown to be independent of the effect on protein synthesis. Transcriptional profiling studies of Mtb treated with PA-824 under aerobic conditions suggested that inhibition of both respiratory processes, as well as cell wall biosynthesis, attributed towards aerobic activity as seen by the upregulation of respiratory genes, fatty acid biosynthetic genes and signature genes that characterize inhibition of cell wall biosynthesis [68]. The disruption of the cell wall biosynthetic machinery is thought to be the main mechanism of aerobic activity [23]. This mechanism is, however, unlikely to play a role in the activity against hypoxically adapted nonreplicating cells since these bacilli do not undergo extensive remodeling of mycolic acids under anaerobic conditions [69].
Three different components have been described to be essential for the intracellular activation of PA-824 in Mtb with mutations in any of these resulting in resistance to this compound: Rv0407 encoding a nonessential F420-dependent glucose-6-phosphate dehydrogenase, genes in the F420 biosynthetic pathway, as well as Rv3547 [23,70]. Rv3547, encoding a 151 amino acid protein with no similarity to any proteins with known function, was characterized as a F420-dependent nitroreductase [38,70]. F420-dependent glucose-6-phosphate dehydrogenase, which catalyzes the oxidation of glucose-6-phosphate to 6-phosphogluconolactone, is required for the intracellular reduction of the deazaflavin cofactor F420, which serves as the hydride donor to PA-824 in the Rv3547-catalyzed reduction of this compound. In contrast to the reduction of metronidazole, PA-824 reduction occurs by a hydride (2-electron) addition to the 5-position of the nitroimidazooxazine ring with subsequent protonation at the 6-position, resulting in three major metabolites of which the predominant one corresponds to des-nitro-PA-824 (56), which is also the predominant intracellular metabolite [64]. The formation of these metabolites is associated with the formation of reactive nitrogen intermediates and it is specifically the formation of nitrous acid associated with des-nitro (56) formation that was correlated with the anaerobic cidal effect of this compound [64]. Thus, the anaerobic activity of PA-824 is attributed to the internal release of NO in Mtb, which could react with cytochromes/cytochrome oxidase to meddle with ATP homeostasis under hypoxic nonreplicating conditions [68,71,72]. In addition, NO could target 29 mycobacterial enzymes [73], DNA [74] as well as displace copper from metallothioneins [75]. There exists a poor correlation between NO release from PA-824 analogs and its aerobic activity [38], suggesting that the aerobic mechanism of action is distinct. This notion is also supported by the observation that the SAR for aerobic activity is distinct from the anaerobic whole cell activity of nitroimidazooxazines. It is hypothesized that under aerobic conditions, the inhibition of cytochrome c oxidase by NO is reversed by molecular oxygen [76,77].
OPC-67683
Like PA-824, OPC-67683 is also a prodrug that requires in vivo activation by Rv3547 in Mtb [21] with mutations conferring resistance to OPC-67683, mapping to Rv3547. In contrast to PA-824, the only metabolite that was detected when M. bovis Bacille Calmette-Guerin was incubated with OPC-67683 was the des-nitro derivative of OPC-67683.
Macromolecular incorporation assays using 14C-acetate to label fatty acids, showed that OPC-67683 inhibits mycolic acid biosynthesis in M. bovis. Unlike INH, which inhibits total mycolic acid biosynthesis, OPC-67683 only inhibited the biosynthesis of methoxy- and ketomycolates, while the biosynthesis of a-mycolic acid was unaffected. The concentration of OPC-67683 and INH resulting in 50% inhibition of mycolic acid synthesis in M. bovis clearly correlated with their anti-tubercular activity reinforcing the notion that mycolate biosynthesis was the primary target. The enzymatic target in fatty acid biosynthesis leading to the observed effects on mycolate profiles, has not been identified for either PA-824 or OPC-67683.
Preclinical testing of anti-TB nitroimidazoles
Metronidazole & other 5-nitroimidazoles
Metronidazole is only active against anaerobic Mtb cells [11] and its activity can be further enhanced in the presence of the transcriptional inhibitor RIF, which has moderate potency against anaerobic Mtb, whereas addition of INH, which has no effect against anaerobic persisting Mtb, does not potentiate the cidal effect of this nitroimidazole [10,78]. This raised the possibility that TB chemotherapy could be significantly shortened by a combination of INH, RIF and metronidazole based on the hypothesis that INH would target actively replicating populations; RIF would target both replicating as well as nonreplicating organisms, whereas metronidazole would kill those populations persisting in hypoxic granulomas.
Despite in vivo studies that have reported some additive effect of metronidazole in infected mice treated with INH or RIF [79], it is perhaps not surprising that at least two studies of metronidazole efficacy in infected mice have reported no or poor efficacy of this drug [80,81] since TB lesions in mice are not hypoxic enough to allow reductive activation of metronidazole in Mtb cells [82,83]. Metronidazole has no activity in vitro against Mtb under microaerophilic conditions, which may explain why even in animals containing granulomas that are sufficiently hypoxic to be labeled with the hypoxic inducible marker pimonidazole, metronidazole did not show any anti-tubercular activity while activity with RIF was seen [84]. The inactivity of metronidazole in this model can also be attributed, amongst others, to poor penetration in granulomatous lesions [84].
Pharmacokinetic studies in humans showed that various 5-nitroimidazoles had similar pharmacokinetic parameters and were readily bioavailable when administered orally with moderate to low protein binding [85]. A 500-mg oral dose of metronidazole and 750-mg oral dose of ornidazole resulted in a highest concentration of a drug in serum (Cmax, time of residence of a therapeutic drug at its maximum concentration in the serum) of 8–13 mg/l (0.25–4 h) [86] and 9.1–14.8 mg/l (2–4 h) [85], respectively. The Cmax for tinidazole and secnidazole at a 2-g oral dose was found to be 58.0 mg/l [87] and 35.7–46.3 mg/l [88], respectively. Tissue penetration of 5-nitroimidazole class of compounds is good but is not specific. Thus, metronidazole was distributed in pelvic tissues, teeth, peritoneal fluid, pancreas, colorectal tissues as well as in the central nervous system [89]. In acute studies in rats, metronidazole was well tolerated (5 g/kg) with no reported chronic toxicity problems up to 80 weeks at a dose of 150 mg/kg [5,90]. Metronidazole is fairly well tolerated in humans as it is also one of the drugs that can be used during pregnancy, with very minimal reversible clinical side effects [91]. These considerations are critical for anti-tubercular drug development where chemotherapy is of extended duration and where noncompliance to treatment regimens due to adverse effects is a major problem in disease management.
Metronidazole has been tested in a clinical study of its efficacy in the treatment of pulmonary TB in patients. In this study, patients were treated with INH, RIF and streptomycin with or without metronidazole. It was found that patients receiving 400 mg of metronidazole three times daily showed clinical improvements as measured by radiographic improvement as well as overall well-being over patients receiving placebo. Both metronidazole- and placebo-treated patients showed similar sputum clearance rates, which measures reduction in the number of acid fast bacilli in the sputum during chemotherapy. This is not surprising since metronidazole is postulated to be ineffective against the bacterial populations in cavities that have eroded into the airways since these are assumed to be aerobic or microaerophilic [92], although transcriptional profiling of sputum-derived mycobacteria has indicated that these may originate from hypoxic environments as evidenced by the upregulation of the dormancy response regulon [93]. Unfortunately, the relapse rates of the patients from this study are unknown so it will never be known whether the apparent clinical efficacy of metronidazole correlated with an effect on the recalcitrant populations of cells that persist in the face of INH and RIF treatment [10].
CGI-17341 (3)
In vivo studies with nitroimidazo[2,1-b]oxazoles series of compounds that were synthesized by Hindustan Ciba-Geigy Ltd were carried out in murine M. bovis infection. For many of the compounds, the in vitro activity was not reflected in their in vivo potency, as seen, for example, with the spiro-cyclohexyl derivative 47, which showed promising in vitro activity (0.06 µM) but was inactive in vivo (even at 100 mg/kg). CGI-17341 (3), which had an in vitro MIC value of 0.32 µM and an in vivo ED50 of 7.7 mg/kg (effective dose of compound at which 50% of mice infected with Mtb survived) was found to be active against ten clinical isolates and several drug resistant Mtb with MICs of 0.43– 1.6 µM [20]. Treatment of mice infected with Mtb after 11 and 12 days post infection with CGI-17341 showed activity of this compound at a dose of 80 mg/kg for 2 months [20]. The mutation frequency in Mtb to CGI-17341 resistance was low enough to allow the compound to be effective in vivo without major toxicity issues or causing rapid development of resistance of the pathogen even though it, along with many others in this series of compounds, showed positive Ames test results.
PA-824
Nitroimidazo[2,1-b]oxazines were found to be superior to the CGI-17341 compounds due to their non-mutagenicity. Like CGI-17341, they were found to be highly specific for the Mtb complex (M. bovis, Mycobacterium afri-canum, Mycobacterium canetti and Mycobacterium microti) and demonstrated little or no activity against other mycobacteria (Mycobacterium avium, Mycobacterium smeg-matis, Mycobacterium cheolonae and Mycobacterium fortuitum) [23] highlighting its potential utility for the treatment of disease caused by members of the Mtb complex but not nontuberculous mycobacterial disease. In addition, the activity of PA-824 against clinical isolates as well as MDR strains, with no cross resistance to current anti-tubercular drugs [94], as well as its efficacy against both replicating as well as nonreplicating Mtb further emphasized the importance of exploring the utility of this drug for TB chemotherapy (5).
The very first studies on the in vivo efficacy of nitroimidazooxazines indicated that although PA-824 was not the most active compound against in vitro-grown Mtb from the first series under investigation, in vivo studies showed that it is the most active compound in infected mice [23]. PA-824 only demonstrated toxicity in mice at very high doses (a single dose >1000 mg/kg or daily dosing at >500 mg/kg for 28 days). It was found that PA-824 demonstrated dose-dependent activity against Mtb in infected mice and at a dose of >50 mg/kg was equipotent to INH at 25 mg/kg [23]. The drug even appeared to have a postantibiotic effect in infected mice as seen by apparent decreases in bacterial numbers for several weeks after cessation of treatment, but these studies probably overestimated the true killing of the pathogen since the readout was based on an unstable luciferase reporter that was likely lost during host pathogenesis in the absence of selection [10,23]. Importantly, PA-824 was also found to be effective in guinea pigs, an animal model that recapitulates aspects of granuloma development typical of human disease [23]. In a patent published in the same year by PathoGenesis [302], other nitroimidazooxazines (52, 53 & 55) were found to be substantially more effective than PA-824 (Table 14) in vivo but were dropped from further development, presumably due to the poor chemical stability of carbonates and carbamates [95].
Table 14.
Efficacy of the first nitroimidazooxazines in infected mice.
| Compound number |
Dose (mg/kg) |
Fold reduction in | |
|---|---|---|---|
| Lung | Spleen | ||
|
Mice infected with Mycobacterium tuberculosis† | |||
| 5 | 25 | 47 | 8 |
| 48 | 25 | 20 | 5 |
| 52 | 25 | 73 | 5 |
| 53 | 25 | 75 | 6 |
| 54 | 25 | 39 | 2 |
| 55 | 25 | 194 | 8 |
|
Mice inoculated with Mycobacterium bovis BCG‡ | |||
| 49 | 50 | 55 | ND |
| 50 | 50 | 5 | ND |
All readouts were based on reduction in luminescence in organ homogenates.
Mice were intravenously infected with Mycobacterium tuberculosis expressing luciferase and treatment for 10 days initiated 7 days after infection.
Mice were intravenously inoculated with Mycobacterium bovis BCG expressing luciferase and 10 days of treatment initiated 1 day after infection.
BCG: Bacille Calmette-Guerin; ND: Not determined.
Further studies on the in vivo efficacy of PA-824 have shown that a dose of 12.5 mg/kg was the minimal dose required for bacteriostasis in the lungs but that 100 mg/kg was required to reduce bacterial burdens 100-fold after 4 weeks of treatment [96]. The caveat of these studies is that treatment was initiated 1 day after infection, which bears no reflection on the established infections with which TB patients would present. Subsequent studies in mice with established infection (3 weeks postinfection) have, however, confirmed that PA-824 at 100 mg/kg is equipotent to INH, gatifloxacin and moxifloxacin at 25, 100 and 100 mg/kg, respectively, during 12 weeks of therapy [94].
The suitability of PA-824 in replacing standard anti-tubercular drugs in the initial or continuation phases of TB chemotherapy has been investigated in several studies in mice [96–98] where standard therapy consists of an initial 2 months of RIF/pyrazinamide (PZA)/INH followed by a continuation phase with RIF/INH. It has been established that PA-824 is not additive or synergistic to INH in the initial intensive 2-month treatment phase, although, as expected, its combination with INH did prevent the emergence of INH resistance. The use of PA-824 alone in the continuation phase was not as effective as RIF/INH although better than monotherapy with moxifloxacin or INH [96]. Follow-up studies to investigate the utility of PA-824 in replacing drugs in standard drug-combination regimens, confirmed that PA-824 could replace, and was somewhat better than, INH in the intensive as well as continuation phases of therapy. However, it was found that it could not replace PZA in the 2-month intensive phase and that RIF was essential in all drug combinations with PA-824 in both the intensive as well as continuation phases of treatment [97]. There was no statistically significant difference, however, in the proportion of mice relapsing after 6 months of treatment in drug combinations containing PA-824 preventing any conclusions to be made as to the utility of PA-824 in shortening standard therapy, although, as acknowledged in this study, the difference between murine and human TB makes direct extrapolation of results from mouse studies to human treatment impossible [97]. More extensive studies showed that PA-824, in combination with PZA, demonstrated synergistic bactericidal activity in the murine model of TB with comparable potency to the standard anti TB regimen of INH, RIF and PZA [98]. More importantly, this study demonstrated that replacement of INH in standard regimens with 100 mg/kg of PA-824 led to apparent sterilization of organs after only 2 months of treatment and with no evidence of relapse observed 4 months after cessation of therapy [98]. Nuermberger et al. also investigated novel drug combinations in the search of therapies that would significantly reduce the duration of chemotherapy. They found that PA-824 in combination with moxifloxacin and PZA was able to cure mice faster than INH, RIF and PZA and that 2 months of PA-824/moxifloxacin/PZA followed by 2 months of PA-824/moxifloxacin led to apparent cure as seen by the absence of relapse 3 months after cessation of therapy [99].
In addition, in an effort to increase the efficacy of PA-824, methods to allow pulmonary delivery were developed in order to release compound at the site of infection [100]. A formulation of PA-824, l-leucine and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine in 70% ethanol was spray-dried to make porous particles suitable for aerosolization. The drug load in these dry powdered porous particles was 75% by weight and had long-term stability at room temperature. These particles were aerosolized into guinea pigs and compared with intravenous and pulmonary administration, with the guinea pig being the animal model of choice due to the higher similarity in TB disease pathology to humans than mice [101,102]. It was established that even though the pulmonary aerosol administration of PA-824 in guinea pigs gave comparable or lower systemic exposure of the drug relative to the oral route, this delivery route gave higher lung concentrations of drug [100] with dose-dependent decreases in tissue damage and bacterial burdens in the lungs of infected animals [103]. Direct delivery of drugs by aerosolization to the lungs, the predominant site of infection, may avert some of the toxicity issues that can accompany systemic administration such as elevated serum creati-nine levels (see later discussion) although would substantially raise the costs of treatment [100,103].
Pharmacokinetic and efficacy studies have also been performed in mice on other nitroimidazo-oxazine derivatives in attempts to improve on the in vivo potency of PA-824 (Tables 8–10). In several cases the interpretation of these studies is limited by the fact that pharmacokinetic parameters in the mice were unknown or not reported. Microsome stability assays of biphenyl analogs of the nitroimidazooxazines with improved in vitro activity suggested that alkoxy (109 & 110), ketone (103), phenoxy (114) and the free amine (113) substituents had poor microsomal stability, whereas substituent with halogens (105) and/or trifuoro methyl (112) or trifluormethoxy groups (106) showed greater microsomal stability (Table 8). Mouse efficacy studies with these biphenyl analogs were performed (at a daily oral dose of 100 mg/kg for 5 days/week for 3 weeks) and the best leads, 106, 121 and 118, were found to be greater than 200-fold more efficacious than PA-824 despite their poor solubility although the blood serum and lung accumulation levels were unknown (Table 8). The five-membered heterobiaryl nitroimidazooxazine compounds were more soluble than PA-824 and had better microsomal stability than PA-824. Of these, 1-aryl-3-linked-pyrazole (124) and the 2-aryl-5-linked-tetrazole (126) were much more effective in vivo (Table 9) [34]. Of the 6-membered heterobiaryl analogs of PA-824, microsomal stability and in vivo acute efficacy studies identified five compounds (139, 142, 143, 145 & 147), which were more than two logs-fold efficacious in infected mice compared with PA-824, with two of these (142 & 143) being more than threefold efficacious than OPC-67683 in the chronic infection mouse model (Table 10) [35].
The success of animal studies paved the way to testing in humans. Pharmacokinetic studies of PA-824 in healthy individuals in single as well as multiple-dose studies have shown that the drug is readily absorbed, orally bioavailable, safe and well tolerated, with no serious adverse effects [104]. The pharmacokinetic parameters for single and multiple dose studies are represented in Supplementary Tables 1 & 2, respectively. Independent of the dose of PA-824, its maximal plasma concentration was reached in 4 to 5 h. The average elimination half life was 16 to 20 h with steady state reached in 5 to 6 days for multiple dosing. PA-824 was well tolerated at 1000 mg once a day for 5 days and 600 mg once a day for a week [104]. The pharmacokinetics parameters were consistent with once a day regimen. The adverse effects on administration of PA-824 to healthy volunteers were insignificant and the only one of note was the dose-dependent reversible elevation of serum creatinine level [104,105]. Pharmacodynamic studies of renal function indicated that the increase in the serum creatinine levels could thus not be ascribed to pathological effects of the drug on renal functions [105], but may be attributed to the inhibition of tubular secretion of creatinine, which is a clinically benign phenomenon also observed in marketed drugs such as pyrimethamine, cimetidine and trimethoprim [105–110].
In order to identify the lowest efficacious dose of PA-824 for the treatment of pulmonary TB, studies were carried out in drug-sensitive, smear-positive patients at a dose of 200, 600, 1000 and 1200 mg/day of PA-824 for two weeks [111], which showed that PA-824 had similar pharmacokinetics to healthy volunteers and demonstrated substantial and linear early bactericidal activity (EBA) comparable to existing frontline drugs. The EBA was similar at all PA-824 doses probably because the plasma concentration of PA-824 was above the MIC even at the lowest dose, predicating the need for extended EBA studies at lower doses [111]. Adverse effects were generally mild and dose-dependent and arose at a frequency similar to the standard treatment regimen of INH, RIF, PZA and ethambutol (EMB) [111].
OPC-67683
OPC-67683 is non-mutagenic, more potent in vitro than clinically approved anti-tubercular drugs, bactericidal and has equipotent activities against drug-sensitive strains, as well as strains resistant to current anti-tubercular drugs [21]. OPC-67683 was also found to superior to RIF, INH and PA-824 against Mtb growing in human macrophages even when the exposure was limited to 4 h [21]. In mice, OPC-67683 was found to have the longest half life (t1/2 = 7.6 h) and lowest plasma concentration (Cmax = 0.56 µM), among all the front-line anti-tubercular drugs and found to exhibit the most potent anti-tubercular activity amongst all the front line drugs as well as PA-824 [21]. Co-administration of OPC-67683 with RIF and PZA in infected mice led to a rapid reduction in bacterial burdens in the first three months of therapy and after four months the organs were sterilized in contrast to the standard regimen of RIF, INH, EMB and PZA, which does not cause complete sterilization even after 6 months of treatment. Thus the inclusion of OPC-67683 reduced the duration of treatment [21].
OPC-67683 is not metabolized by the cytochrome P450 (CYP) enzymes of liver microsomes of both human and animals and no induction of these enzymes has been observed at concentrations up to 100 µM, which makes it suitable to be co-administered with CYP-metabolized drugs such as RIF as well as antiretrovirals that are inactivated by CYPs [21].
Clinical testing of nitroimidazoles
PA-824
PA-824, the lead compound in the nitroimidazooxazine series, is currently in Phase II clinical trials in Cape Town (South Africa) and is sponsored by the Global Alliance for TB Drug Development. Two of the three clinical studies have already been completed. In the study ‘PA-824-CL-007: Phase IIa Evaluation of Early Bactericidal Activity in Pulmonary Tuberculosis’, 68 patients with newly diagnosed uncomplicated, smear-positive TB received PA-824 orally at a dose of 200, 600, 1000 and 2000 mg once daily for 2 weeks [303]. EBA, which measures the daily reduction in mycobacterial counts in sputum, was measured in these patients and compared with a control group receiving a combination of RIF (150 mg), INH (75 mg), PZA (400 mg) and EMB (275 mg), although the results of this study have not yet been reported (ClinicalTrials. gov Identifier: NCT00567840) [303]. The utility of using EBA as a predictor of whether a drug will affect outcome of chemotherapy has not yet been demonstrated as evidenced by the poor EBA of highly effective drugs such as RIF and PZA in this test [112], thus poor performance of drugs in EBA studies needs to be interpreted with care. A similar Phase II study ‘Evaluation of Early Bactericidal Activity in Pulmonary Tuberculosis’ with lower dose of PA-824 (50, 100, 150 and 200 mg once daily for 14 days) was also performed and completed in 2010 (ClinicalTrials.gov Identifier: NCT00944021) with official results pending [304]. Currently a third study ‘Evaluation of Early Bactericidal Activity in Pulmonary Tuberculosis With (J-M-Pa-Z)’ is ongoing with current recruitment of patients with smear positive pulmonary TB [305]. In this study (ClinicalTrials. gov Identifier: NCT01215851) the EBA will be evaluated for two weeks in 68 patients divided into four groups based on drug combination: PA-824 plus PZA; PA-824 plus PZA plus moxifloxacin; TMC207 plus PZA; and TMC207 only. A control group would be treated with standard RIF/INH/PZA/EMB combination therapy [305].
OPC-67683
OPC-67683 the lead compound from the nitroimidazooxazole series is currently in Phase II clinical trials sponsored by Otsuka Pharmaceutical Development & Commercialization, Inc. and Otsuka Frankfurt Research Institute GmbH. In this study (‘Safety, Efficacy and Pharmacokinetics of OPC-67683 in Patients With Pulmonary Tuberculosis’), patients with uncomplicated, smear-positive pulmonary TB were administered different doses (100, 200, 300 or 400 mg once daily) OPC-67683 for 14 consecutive days with the control group receiving standard combination therapy [306]. This study has been completed although the results have to date not yet been disclosed (ClinicalTrials. gov Identifier: NCT00401271) [306]. In a parallel study (‘A Placebo-controlled, Phase 2 Trial to Evaluate OPC-67683 in Patients With Pulmonary Sputum Culture-positive, Multidrug-resistant Tuberculosis [TB]’), 430 patients with culture-positive sputum resistant to INH and RIF or only to RIF (conformed by positive rapid test on direct sputum) and sputum smears positive for acid fast bacilli within 60 days before enrollment were treated with 100 mg or 200 mg of OPC-67683 (twice daily), in addition to the optimized background regimen whereas the placebo group received only the optimized background regimen for the same period of time [307]. Pharmakokinetics, safety and efficacy of the drug OPC-67683 are to be evaluated during the study and post treatment with the study still in progress in nine different geographical locations (ClinicalTrials.gov Identifier: NCT00685360) [307]. In another study (‘Safety and Pharmacokinetics [PK] in Multidrug-Resistant [MDR] Refractive Tuberculosis’), which is currently recruiting patients diagnosed as sputum positive for MDR-TB 2 months prior to enrollment and at least three times in the prior 9 months despite treatment with standard anti-tubercular regimens will evaluate pharmacokinetics, metabolite formation and the safety and tolerability of OPC-67683 administered twice a day at a dose of 100 mg in addition to the optimized background regimen (ClinicalTrials.gov Identifier: NCT01131351) [302].
What defines an optimal nitroimidazole for drug development?
New drugs are urgently required to combat TB, and to improve TB chemotherapy it is critical that: the current duration of chemotherapy is shortened, the regimen of drugs is simplified, new regimens are effective against MDR- and XDR-TB, therapies are compatible with antiretrovirals administered to HIV patients, and the regimens include drugs that eradicate the persistent bacteria thought to characterize latent disease.
To shorten therapy and, ideally, to eradicate persistent bacteria, it is essential to understand the metabolism of the pathogen in the human host, since the vulnerable drug targets or processes in the microbe are ultimately a function of its metabolism. TB in humans presents with a variety of clinical manifestations ranging from various degrees of severity of lung disease to extra-pulmonary dissemination [13]. Even within the same patient, there is considerable heterogeneity in the granulomas, the hallmark of this disease in humans, which by implication would be expected to harbor different micro-environments [13]. The metabolism of the pathogen is expected to be a function of its microenvironment as determined by factors such as oxygen availability, carbon-source availability, pH and the presence of reactive nitrogen intermediates. The extensive duration of chemotherapy required to significantly reduce relapse rates has been attributed to different populations of mycobacteria as defined by their metabolic status where rapidly growing bacilli are efficiently eliminated by drugs such as INH that target cell wall biosynthesis, the slowly or sporadically dividing cells being most effectively eradicated by RIF and the bacilli residing in acidified compartments being particularly susceptible to PZA [13,113]. In this respect, the in vitro demonstration that nitroimidazooxazines, such as PA-824, kill both aerobically replicating as well as hypoxic nonreplicating bacteria has indicated that these compounds may target a variety of bacterial populations in the human host, which may lead to shortening of treatment duration and allow the elimination of drugs such as INH from combination treatments, which will, at the very least not add additional drugs to current regimens.
In addition, the demonstration that the nitroimidazooxazines and nitro-imidazooxazoles affect cellular processes distinct from current anti-tubercular drugs has meant that these compounds remain effective against MDR and XDR strains of Mtb. However, these compounds are prodrugs that are activated by an enzyme and co-factors that are seemingly nonessential, and as a result, a variety of mutations can give rise to resistance. Thus, not surprisingly, the mutation frequency to PA-824 resistance is comparable to that of INH [70] and has similarly be found to be high in infected mice during PA-824 treatment (Manjunatha et al., Unpublished Data). This is a significant drawback for the introduction of drugs that require bioactivation, such as nitroimidazoles, into anti-tubercular regimens since the emergence of resistance at a rate comparable to INH resistance would not reduce the global emergence of drug resistance. In addition, it may suggest that PA-824 and related compounds, are less ideal for the treatment of MDR- and XDR-TB.
Another factor that has received little attention in terms of nitroimidazole drug development for TB is bioavailability. Comparative mouse studies of highly insoluble nitroimidazooxazines and nitroimidazooxazoles (Tables 8–13) have demonstrated that oral bioavailability and accumulation in tissues were not addressed by the way in which these were tested at doses of 100 mg/kg in a formulation that may never have applicability beyond clinical trial settings and that these studies may give inaccurate impressions about which nitroimidazoles should enter the drug-development pipeline. The limited solubility of the nitroimidazoles PA-824 and OPC-67683, which are currently in clinical development, would imply that bioavailability after oral intake would be a function of intake of fatty foods. This would add complications to the administration of such drugs. Since TB patients in large parts of the developing world are often under-nourished, with HIV infection often further adding to malabsorption of drugs [2,114], nitroimidazole drug development may require significant more input to find oral formulations that increase their bioavailability. Separate formulations of nitroimidazoles may thus not simplify current regimens. A more soluble nitromidazole may address these issues. On a positive note, the Global Alliance for TB drug development has demonstrated in healthy volunteers that at anticipated clinical doses there is no clinically significant effect of a high-fat, high-calorie meal on plasma levels of PA-824 relative to those seen in the fasted state [GINSBERG AM PERS COMM] . Currently, the pharmacokinetics of many nitroimidazoles have been established [21,35,100,104], but all of these studies have evaluated concentrations of drug in the blood. However, the site of infection in the human is the granuloma, thus the ability of the drug to penetrate into granulomas and the half-life of the drug in gramulomas may ultimately determine the true efficacy of these drugs in humans. It has, for example, been established that moxifloxacin accumulates in granulomas with drug concentrations being dependent on granuloma type [115], which may be an important reason underlying the efficacy of moxifloxacin against TB. Such studies can of course only be done on animal models that generate granulomas comparable to human TB granulomas, which limits the large-scale applicability of lesion-penetration determination to new compounds, but could ideally be applied to compounds that have been prioritized based on in vitro efficacy, absence of adverse metabolic profiles as well as pharmacokinetic properties. Similar studies on nitroimidazoles will prove invaluable in selecting an optimal compound for clinical development.
Although not discussed in this review, two other compound series (nitrofurans [116] and 1,4-di-N-oxide derivatives [117]) that are activated by bioreduction are in preclinical development. It has been proposed that co-administration of nitroimidazoles in combination with nitrofurans or quinoxaline-di-N-oxides may generate a chemotherapeutic cocktail with optimal killing of cells since these prodrugs exploit different bioreductive pathways [118]. The assumption that cocktails of prodrugs that are activated by bioreduction and thus, due to the often nonessential nature of the activation mechanisms, are associated with high mutation frequencies, will lead to killing on a scale that warrants their development as drug candidates, currently has no basis.
Future perspective
Two nitroimidazole compounds are currently in clinical evaluation as anti-tubercular drugs. Current Phase II clinical trials may provide some information about the clinical utility of PA-824 and OPC-67683. With limited in vivo information available on the efficacy of these compounds in an animal model that recapitulates the important characteristics of human disease, as well as better knowledge about drug concentrations at the site of infection, we will have to wait for the results of these trials before we can assess whether these nitroimidazoles will address the critical issues in anti-tubercular drug development.
Based on published data, there is much more known about the SAR and microbiological effects of the nitroimidazooxazines compared with the nitroimidazooxazoles. Thus, PA-824 and related compounds, have submicromolar MIC values against Mtb and, in addition, have been shown to be effective against anaerobically persisting Mtb (Table 6). In contrast, little is known about the anaerobic activity of OPC-67683 although it can be predicted, based on the similar activation pathways of PA-824 and OPC-67683, that reactive nitrogen intermediates are also formed during formation of the desnitro product of the nitroimidazooxazole. It is the formation of the des-nitro end metabolite of PA-824 activation that is correlated with the anaerobic cidal activity of this compound [38]. Mouse efficacy studies are often performed a day after infection of the animal with Mtb. However, the relevance of this could be a concern given that human TB patients generally present with established mycobacterial infections. In this respect, numerous independent studies have established that PA-824 is efficacious in mice with established mycobacterial infection [94,96–98], although OPC-67683 at 50 mg/kg was reported to be more efficacious than PA-824 at a similar dose in chronically infected mice [21]. Both PA-824 and OPC-67683 have good microsomal stabilities and the reported serum concentrations and half-lives are favorable relative to their in vitro MIC values. If insolubility is a concern in drug development, then the challenges facing an OPC-67683 formulation that would meet the economic and stability criteria for an antitubercular drug would be more considerable than that of PA-824.
The cost of clinical trials to fully evaluate the efficacy of these compounds for anti-tubercular chemotherapy will likely require a choice to be made between these compounds unless highly compelling data are provided for the progression of one of the nitroimidazooxazines currently under investigation (Table 8). The evaluation of current clinical trial results in combination with the accumulated data reported for each of these will eventually determine which compound will most likely proceed into the next phase of clinical testing.
Regardless of which compound proceeds further, the scientific benefit of having a new anti-tubercular agent progress through clinical trials cannot be understated. We will learn valuable information about both in vivo treatment and disease biology of the TB patient. Furthermore, a modern TB clinical trial will provide valuable data that will allow comparison with previous and current clinical trials of anti-tubercular agents, which will inform future trials using next-generation nitroimidazoles and/or anti-tubercular agents.
Executive summary.
-
▪
The nitroimidazole class of compounds shows promise as new anti-tubercular agents. Two members of this class, PA-824 and OPC-67683, are undergoing Phase II clinical trials.
-
▪
2-nitroimidazole derivatives modified at the 1- and 5-positions were among the first series of this class reported to display antimycobacterial activity. Metronidazole, which belongs to a class of 5-nitroimidazole is effective against hypoxically adapted nonreplicating Mycobacterium tuberculosis (Mtb).
-
▪
CGI-17341, the lead compound from a series of bicyclic 4- and 5-nitroimidazole[2,1-b]oxazoles, showed dose dependent activity against drug susceptible and MDR Mtb. The bicyclic 5-nitroimidazole[2,1-b]oxazole series showed much lower activity than its 4-nitro counterpart. CGI-17341 was discontinued because of its mutagenic properties.
-
▪
PA-824, the lead compound from over 300 nitroimidazopyrans, and OPC-67683, the lead compound from the series of 6-nitro-2,3-dihydroimidazo [2,1-b] oxazoles, were found to be non-mutagenic and showed activity against slow, rapidly replicating, drug-susceptible as well as resistant strains of Mtb, and showed no cross-resistance with current drugs and was compatible with antiretrovirals.
-
▪
Structure–activity relationship (SAR) studies on PA-824 showed that the S-enantiomer was the active form and the presence of the nitro group at 4-position of the imidazole ring, lipophilic tail at 6-position of the oxazine ring and a rigid bicyclic system were crucial for its activity. Activity studies with the biaryl linker (activity: para>meta>ortho; most compounds have poor solubility) and heterobiaryl (with improved solubility) identified some candidates with >200 fold in-vivo efficacy compared with PA-824 as well as identified the presence of a large hydrophobic binding pocket in the active site of Rv3547.
-
▪
Rv3547 is a deazaflavin (F420 cofactor)-dependent nitroreductase that participates in bioreductive activation of the prodrug PA-824, releasing NO, among other reactive nitrogen species, the production of which correlates with anaerobic anti-tubercular activity.
-
▪
Dose-dependent inhibition of cell wall and protein biosynthesis has been demonstrated for PA-824. There is poor correlation between aerobic and anaerobic activity, and transcriptional profile analysis indicates inhibition of both cell wall and respiratory genes.
-
▪
Pharmacokinetic studies of PA-824 in healthy volunteers showed that it was orally bioavailable, readily absorbed, had a substantial half life, and was safely tolerated without any serious adverse effects. Studies with drug-sensitive smear-positive patients demonstrated substantial and linear early bactericidal activity compared with current frontline drugs.
-
▪
The mutagenicity problem of CGI-17341 was overcome by incorporation of a heteroatom at the 2-position of the oxazole ring. SAR studies with derivatives of 6-nitro-2,3-dihydroimidazo [2,1-b] oxazole with various phenoxymethyl groups showed that the R- form as the active isomer. Further SAR studies in conjunction with in vivo efficacy studies identified the lead compound OPC-67683.
-
▪
OPC-67683 has been shown to inhibit methoxy-mycolic and keto-mycolic acid synthesis, does not affect nor is affected by liver microsome enzyme. It is absorbed better in high-fat diets, without significant adverse side effects. However, it has a low early bactericidal activity in patients with pulmonary TB.
-
▪
Both PA-824 and OPC-67683 are in Phase II clinical trials, the results of which are not yet available.
Supplementary Material
Acknowledgements
We are grateful to Clifton Barry 3rd, Cynthia Dowd and Ujjini Manjunatha for valuable discussions.
This work was funded by a grant from the Bill and Melinda Gates Foundation and the Wellcome Trust through the Grand Challenges in Global Health Initiative and by the intramural research program of the NIH National Institute of Allergy and Infectious Diseases.
Key Terms
- Structure–activity relationship
The study of the relationship between the structural feature of a molecule and its biological activity. Structure–activity relationship studies in whole cells are difficult to rationalize due to multifactorial events that affect permeability, efflux, inactivation as well as binding of compounds to their target. Structure–activity relationship studies between compounds and their ‘cognate’ proteins provide a more direct readout of binding to the target. Such studies provide indispensable knowledge for rational drug design.
- Radiosensitizers
Chemical compounds that selectively make tumor cells susceptible to radiation therapy. Cells within tumors are often hypoxic and the lack of oxygen minimizes the production of reactive oxygen intermediates that cause nucleic acid damage during radiation. In the presence of radio-sensitizers these hypoxic radiation-resistant tumor cells become susceptible to radiation.
- Bicyclic nitroimidazole
Nitrogroup-containing imidazole ring fused to another ring system such as an oxazole ring (five-membered aromatic cyclic compound containing an oxygen and a nitrogen separated by one carbon atom) or an oxazine ring (six-membered cyclic compound containing an oxygen and a nitrogen). OPC-67683 and PA-824 are examples of bicyclic nitroimidazooxazole, and bicyclic nitroimidazooxazine, respectively.
- Mutagenicity
The ability of an external agent (radiation or chemical compounds) to increase the mutation frequency of DNA above the background level of naturally occurring spontaneous mutations. This is generally tested through the Ames test where Salmonella typhimurium auxotrophic mutants in histidine biosynthesis are screened for reversion to histidine prototrophy in the absence or presence of the test agent.
- Bioavailability
The fraction of the administered dose of a drug that reaches the blood.
- Early bactericidal activity
Early bactericidal activity studies are often used in TB drug clinical trials and measure the rate at which the mycobacterial load in sputum samples of TB patients decreases upon administration of drug for the first 7–14 days. The drug under investigation is often administered as monotherapy in early bactericidal activity studies since the risk of emergence of drug resistance is extremely low in this short treatment period.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/suppl/10.4155/FMC.11.90.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Dye C, Lonnroth K, Jaramillo E, Williams BG, Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull. World Health Organ. 2009;87(9):683–691. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young D, Dye C. The development and impact of tuberculosis vaccines. Cell. 2006;124(4):683–687. doi: 10.1016/j.cell.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Jordan TS, Davies PD. Clinical tuberculosis and treatment outcomes. Int. J. Tuberc. Lung Dis. 2010;14(6):683–688. [PubMed] [Google Scholar]
- 4.Voogd CE. On the mutagenicity of nitroimidazoles. Mutat. Res. 1981;86(3):243–277. doi: 10.1016/0165-1110(81)90006-3. [DOI] [PubMed] [Google Scholar]
- 5.Roe FJ. Metronidazole: review of uses and toxicity. J. Antimicrob. Chemother. 1977;3(3):205–212. doi: 10.1093/jac/3.3.205. [DOI] [PubMed] [Google Scholar]
- 6.Roe VA. Antimicrobial agents: pharmacology and clinical application in obstetric, gynecologic, and perinatal infections. J. Obstet. Gynecol. Neonatal Nurs. 1999;28(6):639–648. doi: 10.1111/j.1552-6909.1999.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 7.Schneider J. Treatment of giardiasis (lambliasis) by metronidazole. Bull. Soc. Pathol. Exot. Filiales. 1961;54:84–95. [PubMed] [Google Scholar]
- 8.Rodriques Coura J, De Castro SL. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz. 2002;97(1):3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 9.Veldhuyzen Van Zanten SJ, Sherman PM, Hunt RH. Helicobacter pylori: new developments and treatments. CMAJ. 1997;156(11):1565–1574. [PMC free article] [PubMed] [Google Scholar]
- 10.Barry CE, 3rd, Boshoff HI, Dowd CS. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr. Pharm. Des. 2004;10(26):3239–3262. doi: 10.2174/1381612043383214. [DOI] [PubMed] [Google Scholar]
- 11.Wayne LG, Sramek HA. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1994;38(9):2054–2058. doi: 10.1128/aac.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Via LE, Lin PL, Ray SM, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 2008;76(6):2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barry CE, 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009;7(12):845–855. doi: 10.1038/nrmicro2236.. ▪ Discusses latent TB and drug-development strategies
- 14.Young DB, Perkins MD, Duncan K, Barry CE., 3rd Confronting the scientific obstacles to global control of tuberculosis. J. Clin. Invest. 2008;118(4):1255–1265. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalleri B, Ballotta R, Arioli V, Lancini G. New 5-substituted 1-alkyl-2-nitroimidazoles. J. Med. Chem. 1973;16(5):557–560. doi: 10.1021/jm00263a035. [DOI] [PubMed] [Google Scholar]
- 16.Cavalleri B, Volpe G, Arioli V, Parenti F. Synthesis and biological activity of some 2-aminoimidazoles. Arzneimittelforschung. 1977;27(10):1889–1895. [PubMed] [Google Scholar]
- 17.Schmid A, Schmid H. Pharmaco-toxicological mode of action of antimicrobial 5-nitroimidazole derivatives. Zentralbl Veterinarmed A. 1999;46(9):517–522. doi: 10.1046/j.1439-0442.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- 18.Sehgal RK, Agrawal KC. Novel Nitroimidazo[2,1-b] oxazole formation from reaction of 2,4(5)-dinitroimidazole with Oxiranes (1) J. Heterocyclic Chem. 1979;16:1499–1500. [Google Scholar]
- 19.Nagarajan K, Shankar RG, Rajappa S, Shenoy SJ, Costa-Pereira R. Nitroimidazole XXI 2,3-dihtdro-6-nitroimidazo [2,1-b] oxazoles with antitubercular activity. Eur. J. Med. Chern. 1989;24:631–633. [Google Scholar]
- 20. Ashtekar DR, Costa-Perira R, Nagrajan K, Vishvanathan N, Bhatt AD, Rittel W. In vitro and in vivo activities of the nitroimidazole CGI 17341 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1993;37(2):183–186. doi: 10.1128/aac.37.2.183.. ▪ Activity (both in vivo and in vitro) of CGI-17341 against H37Rv, as well as resistant strains, is reported showing the importance of bicyclic nitroimidazoles as possible anti-tubercular drugs
- 21. Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3(11):e466. doi: 10.1371/journal.pmed.0030466.. ▪▪ Reports the discovery, activity studies (both in vivo and in vitro) and pharmacokinetic parameters of OPC-67683 and that this nitroimidazo oxazole inhibits the mycolate biosynthetic pathway
- 22.Denny WA, Palmer BD. The nitroimidazooxazines (PA-824 and analogs): structure-activity relationship and mechanistic studies. Future Med Chem. 2010;2(8):1295–1304. doi: 10.4155/fmc.10.207. [DOI] [PubMed] [Google Scholar]
- 23. Stover CK, Warrener P, Vandevanter DR, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405(6789):962–966. doi: 10.1038/35016103.. ▪▪ Reports the initial activity studies (both in vivo and in vitro) of PA-824 against drug resistant clinical strains. It is the first report of a nitroimidazole that inhibits an aspect of fatty acid biosynthesis
- 24.Hof H. Antibacterial activities of the antiparasitic drugs nifurtimox and benznidazole. Antimicrob. Agents Chemother. 1989;33(3):404–405. doi: 10.1128/aac.33.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jokipii L, Jokipii AMM. Comparative-evaluation of the 2-methyl-5-nitroimidazole compounds dimetridazole, metronidazole, secnidazole, ornidazole, tinidazole, carnidazole, and panidazole against Bacteroides-fragilis and other bacteria of the Bacteroides-fragilis group. Antimicrob. Agents Chemother. 1985;28(4):561–564. doi: 10.1128/aac.28.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hof H, Stroder J. Antibacterial Activity of Go 10213, a Nitroimidazole Derivative. Antimicrob Agents Ch. 1986;29(5):953–954. doi: 10.1128/aac.29.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalleri B, Volpe G, Arioli V. Synthesis and biological activity of some vinyl-substituted 2-nitroimidazoles. J. Med. Chem. 1977;20(5):656–660. doi: 10.1021/jm00215a007. [DOI] [PubMed] [Google Scholar]
- 28.Cavalleri B, Volpe G, Arioli V, Lancini G. Synthesis and biological activity of two metabolites of 1-methyl-5-(1-methylethyl)-2-nitro-1 H-imidazole, an antiprotozoal agent. J. Med. Chem. 1977;20(11):1522–1525. doi: 10.1021/jm00221a035. [DOI] [PubMed] [Google Scholar]
- 29.Cavalleri B, Volpe G, Ripamonti A, Arioli V. 1-methyl-2-nitroimidazol-5-yl derivatives. IIIrd communication. Arzneimittelforschung. 1977;27(6):1131–1134. [PubMed] [Google Scholar]
- 30. Kim P, Zhang L, Manjunatha UH, et al. Structure-activity relationships of antitubercular nitroimidazoles. 1. Structural features associated with aerobic and anaerobic activities of 4- and 5-nitroimidazoles. J. Med. Chem. 2009;52(5):1317–1328. doi: 10.1021/jm801246z.. ▪ Reports key structural features that are essential for bicyclic nitroimidazoles
- 31.Kim P, Kang S, Boshoff HI, et al. Structure-activity relationships of antitubercular nitroimidazoles. 2. Determinants of aerobic activity and quantitative structure-activity relationships. J. Med. Chem. 2009;52(5):1329–1344. doi: 10.1021/jm801374t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson AM, Blaser A, Anderson RF, et al. Synthesis, reduction potentials, and antitubercular activity of ring A/B analogues of the bioreductive drug (6S)-2-nitro-6-{[4-(trifluoromethoxy) benzyl] oxy}-6,7-dihydro-5H–imidazo[2, 1-b][1,3]oxazine (PA-824) J. Med. Chem. 2009;52(3):637–645. doi: 10.1021/jm801087e. [DOI] [PubMed] [Google Scholar]
- 33. Palmer BD, Thompson AM, Sutherland HS, et al. Synthesis and structure-activity studies of biphenyl analogues of the tuberculosis drug (6S)-2-nitro-6-{[4-(trifluoromethoxy) benzyl] oxy}-6,7-dihydro-5H–imidazo[2, 1-b][1,3]oxazine (PA-824) J. Med. Chem. 2010;53(1):282–294. doi: 10.1021/jm901207n.. ▪ Reports three compounds with more than 200-fold in vivo efficacy compared with PA-824 in structure-activity relationship studies
- 34.Sutherland HS, Blaser A, Kmentova I, et al. Synthesis and structure-activity relationships of antitubercular 2-nitroimidazooxazines bearing heterocyclic side chains. J. Med. Chem. 2010;53(2):855–866. doi: 10.1021/jm901378u. [DOI] [PubMed] [Google Scholar]
- 35.Kmentova I, Sutherland HS, Palmer BD, et al. Synthesis and structure–activity relationships of aza- and diazabiphenyl analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy) benzyl] oxy}-6,7-dihydro-5H–imidazo [2, 1-b] [1,3]oxazine (PA-824) J. Med. Chem. 2010 doi: 10.1021/jm101288t. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Manjunatha UH, Goodwin MB, et al. Synthesis and antitubercular activity of 7-(R)- and 7-(S)-methyl-2-nitro-6-(S)-(4-(trifluoromethoxy) benzyloxy)-6,7-dihydro-5H- imidazo [2,1-b] [1,3] oxazines, analogues of PA-824. Bioorg. Med. Chem. Lett. 2008;18(7):2256–2262. doi: 10.1016/j.bmcl.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bollo S, Nunez-Vergara LJ, Kang S, et al. The effect of 5-substitution on the electrochemical behavior and antitubercular activity of PA-824. Bioorg. Med. Chem. Lett. 2011;21(2):812–817. doi: 10.1016/j.bmcl.2010.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh R, Manjunatha U, Boshoff HI, et al. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science. 2008;322(5906):1392–1395. doi: 10.1126/science.1164571.. ▪▪ A novel bactericidal mechanism is described that explains the anaerobic efficacy of PA824. The metabolites generated in PA824 activation are identified and correlated with their biological activities against Mycobacterium tuberculosis
- 39.Matsumoto M, Hashizume H, Tsubouchi H, et al. Screening for novel antituberculosis agents that are effective against multidrug resistant tuberculosis. Curr. Top. Med. Chem. 2007;7(5):499–507. doi: 10.2174/156802607780059727. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki H, Haraguchi Y, Itotani M, et al. Synthesis and antituberculosis activity of a novel series of optically active 6-nitro-2,3-dihydroimidazo[2,1-b] oxazoles. J. Med. Chem. 2006;49(26):7854–7860. doi: 10.1021/jm060957y. [DOI] [PubMed] [Google Scholar]
- 41. Rivers EC, Mancera RL. New antituberculosis drugs with novel mechanisms of action. Curr. Med. Chem. 2008;15(19):1956–1967. doi: 10.2174/092986708785132906.. ▪ Discusses the discovery, characterization, activity and preclinical studies of potential first line anti-tubercular drugs that are currently in clinical trials
- 42.Edwards DI. Nitroimidazole drugs - action and resistance mechanisms. 1. Mechanisms of action. J. Antimicrob. Chemother. 1993;31(1):9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 43.Edwards DI. Reduction of nitroimidazoles in vitro and DNA damage. Biochem. Pharmacol. 1986;35(1):53–58. doi: 10.1016/0006-2952(86)90554-x. [DOI] [PubMed] [Google Scholar]
- 44.Perezreyes E, Kalyanaraman B, Mason RP. The reductive metabolism of metronidazole and ronidazole by aerobic liver-microsomes. Mol. Pharmacol. 1980;17(2):239–244. [PubMed] [Google Scholar]
- 45.Sealy RC, Swartz HM, Olive PL. Electron-spin resonance spin trapping - detection of superoxide formation during aerobic microsomal reduction of nitro-compounds. Biochem. Bioph. Res. Co. 1978;82(2):680–684. doi: 10.1016/0006-291x(78)90928-2. [DOI] [PubMed] [Google Scholar]
- 46.Sisson G, Jeong JY, Goodwin A, et al. Metronidazole activation is mutagenic, causes DNA fragmentation in Helicobacter pylori, in Escherichia coli containing a cloned H. pylori rdxA plus (nitroreductase) gene. J. Bacteriol. 2000;182(18):5091–5096. doi: 10.1128/jb.182.18.5091-5096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson SA, Blaser MJ. Isolation of the Helicobacter-pylori reca gene and involvement of the reca region in resistance to low ph. Inf. Immun. 1995;63(6):2185–2193. doi: 10.1128/iai.63.6.2185-2193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeung TC, Beaulieu BB, Mclafferty MA, Goldman P. Interaction of metronidazole with DNA-repair mutants of Escherichiacoli. Antimicrob. Agents Chemother. 1984;25(1):65–70. doi: 10.1128/aac.25.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards DI. Nitroimidazole drugs - action and resistance mechanisms. 2. Mechanisms of resistance. J. Antimicrob. Chemother. 1993;31(2):201–210. doi: 10.1093/jac/31.2.201. [DOI] [PubMed] [Google Scholar]
- 50.Ragsdale SW. Pyruvate ferredoxin oxidoreductase and its radical intermediate. Chem. Rev. 2003;103(6):2333–2346. doi: 10.1021/cr020423e. [DOI] [PubMed] [Google Scholar]
- 51.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 2001;14(1):150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borst P, Ouellette M. New mechanisms of drug-resistance in parasitic protozoa. Ann. Rev. Microbiol. 1995;49:427–460. doi: 10.1146/annurev.mi.49.100195.002235. [DOI] [PubMed] [Google Scholar]
- 53.Dunne RL, Dunn LA, Upcroft P, O’Donoghue PJ, Upcroft JA. Drug resistance in the sexually transmitted protozoan Trichomonas vaginalis. Cell Res. 2003;13(4):239–249. doi: 10.1038/sj.cr.7290169. [DOI] [PubMed] [Google Scholar]
- 54.Carlier JP, Sellier N, Rager MN, Reysset G. Metabolism of a 5-nitroimidazole in susceptible and resistant isogenic strains of Bacteroides fragilis. Antimicrob. Agents Chemother. 1997;41(7):1495–1499. doi: 10.1128/aac.41.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendz GL, Trend MA. Intracellular redox status and antibiotic resistance in enterogastric micro-aerophilic bacteria: evidence for the ‘scavenging of oxygen’ hypothesis. Redox Rep. 2001;6(3):179–181. doi: 10.1179/135100001101536292. [DOI] [PubMed] [Google Scholar]
- 56.Rafii F, Wynne R, Heinze TM, Paine DD. Mechanism of metronidazole-resistance by isolates of nitroreductase-producing Enterococcus gallinarum and Enterococcus casseliflavus from the human intestinal tract. Fems Microbiol. Lett. 2003;225(2):195–200. doi: 10.1016/S0378-1097(03)00513-5. [DOI] [PubMed] [Google Scholar]
- 57.Mendz GL, Megraud F. Is the molecular basis of metronidazole resistance in microaerophilic organisms understood? Trends Microbiol. 2002;10(8):370–375. doi: 10.1016/s0966-842x(02)02405-8. [DOI] [PubMed] [Google Scholar]
- 58.Goodwin A, Kersulyte D, Sisson G, Van Zanten SJOV, Berg DE, Hoffman PS. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 1998;28(2):383–393. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- 59.Kwon DH, Osato MS, Graham DY, El-Zaatari FaK. Quantitative RT-PCR analysis of multiple genes encoding putative metronidazole nitroreductases from Helicobacter pylori. Int. J. Antimicrob. Agents. 2000;15(1):31–36. doi: 10.1016/s0924-8579(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 60.Sisson G, Goodwin A, Raudonikiene A, et al. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob. Agents Chemother. 2002;46(7):2116–2123. doi: 10.1128/AAC.46.7.2116-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffman PS, Goodwin A, Johnsen J, Magee K, Veldhuyzen SJO, Vanzanten SJOV. Metabolic activities of metronidazole-sensitive and -resistant strains of Helicobacter pylori: Repression of pyruvate oxidoreductase and expression of isocitrate lyase activity correlate with resistance. J. Bacteriol. 1996;178(16):4822–4829. doi: 10.1128/jb.178.16.4822-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith MA, Edwards DI. Redox potential and oxygen concentration as factors in the susceptibility of Helicobacter-pylori to nitroheterocyclic drugs. J. Antimicrob. Chemother. 1995;35(6):751–764. doi: 10.1093/jac/35.6.751. [DOI] [PubMed] [Google Scholar]
- 63.Lim A, Eleuterio M, Hutter B, Murugasu-Oei B, Dick T. Oxygen depletion-induced dormancy in Mycobacterium bovis BCG. J. Bacteriol. 1999;181(7):2252–2256. doi: 10.1128/jb.181.7.2252-2256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulda J. Trichomonads, hydrogenosomes and drug resistance. Int. J. Parasitol. 1999;29(2):199–212. doi: 10.1016/s0020-7519(98)00155-6. [DOI] [PubMed] [Google Scholar]
- 65.Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 1999;43(7):1533–1541. doi: 10.1128/aac.43.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes NJ, Chalk PA, Clayton CL, Kelly DJ. Identification of carboxylation enzymes and characterization of a novel 4-subunit pyruvate-flavodoxin oxidoreductase from Helicobacter-pylori. J. Bacteriol. 1995;177(14):3953–3959. doi: 10.1128/jb.177.14.3953-3959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baughn AD, Garforth SJ, Vilcheze C, Jacobs WR., Jr An anaerobic-type a-ketoglutarate ferredoxin oxidoreductase completes the oxidative tricarboxylic acid cycle of Mycobacterium tuberculosis. PLoS Pathog. 2009;5(11):e1000662. doi: 10.1371/journal.ppat.1000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manjunatha U, Boshoff HI, Barry CE. The mechanism of action of PA-824: novel insights from transcriptional profiling. Commun. Integr. Biol. 2009;2(3):215–218. doi: 10.4161/cib.2.3.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boshoff HI, Barry BC. Is the mycobacterial cell wall a hopeless drug target for latent tuberculosis? Drug Discov. Today Dis. Mech. 2006;3:237–245. [Google Scholar]
- 70. Manjunatha UH, Boshoff H, Dowd CS, et al. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA. 2006;103(2):431–436. doi: 10.1073/pnas.0508392103.. ▪▪ Identifies a novel family of nitroreductases that likely utilize F420 as a hydride donor that is responsible for activation of the prodrug PA-824.
- 71.Boshoff HI, Barry CE., 3rd Tuberculosis - metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 2005;3(1):70–80. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- 72.Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA. 2008;105(33):11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rhee KY, Erdjument-Bromage H, Tempst P, Nathan CF. S-nitroso proteome of Mycobacterium tuberculosis: enzymes of intermediary metabolism and antioxidant defense. Proc. Natl Acad. Sci. USA. 2005;102(2):467–472. doi: 10.1073/pnas.0406133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Darwin KH, Ehrt S, Gutierrez-Ramos JC, Weich N, Nathan CF. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science. 2003;302(5652):1963–1966. doi: 10.1126/science.1091176. [DOI] [PubMed] [Google Scholar]
- 75.Gold B, Deng H, Bryk R, et al. Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat. Chem. Biol. 2008;4(10):609–616. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brunori M, Forte E, Arese M, Mastronicola D, Giuffre A, Sarti P. Nitric oxide and the respiratory enzyme. Biochim. Biophys. Acta. 2006;1757(9–10):1144–1154. doi: 10.1016/j.bbabio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 77.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345(1):50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 78.Sun Z, Zhang Y. Antituberculosis activity of certain antifungal and antihelmintic drugs. Tuber. Lung Dis. 1999;79(5):319–320. doi: 10.1054/tuld.1999.0212. [DOI] [PubMed] [Google Scholar]
- 79.Paramasivan CN, Kubendiran G, Herbert D. Action of metronidazole in combination with isoniazid & rifampicin on persisting organisms in experimental murine tuberculosis. Indian J. Med. Res. 1998;108:115–119. [PubMed] [Google Scholar]
- 80.Brooks JV, Furney SK, Orme IM. Metronidazole therapy in mice infected with tuberculosis. Antimicrob. Agents Chemother. 1999;43(5):1285–1288. doi: 10.1128/aac.43.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dhillon J, Allen BW, Hu YM, Coates ARM, Mitchison DA. Metronidazole has no antibacterial effect in Cornell model murine tuberculosis. Int. J. Tuber. Lung Dis. 1998;2(9):736–742. [PubMed] [Google Scholar]
- 82.Aly S, Wagner K, Keller C, et al. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J. Pathol. 2006;210(3):298–305. doi: 10.1002/path.2055. [DOI] [PubMed] [Google Scholar]
- 83.Tsai MC, Chakravarty S, Zhu GF, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell. Microbiol. 2006;8(2):218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 84.Klinkenberg LG, Sutherland LA, Bishai WR, Karakousis PC. Metronidazole lacks activity against Mycobacterium tuberculosis in an in vivo hypoxic granuloma model of latency. J. Infect. Dis. 2008;198(2):275–283. doi: 10.1086/589515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lau AH, Lam NP, Piscitelli SC, Wilkes L, Danziger LH. Clinical pharmacokinetics of metronidazole and other nitroimidazole antiinfectives. Clin. Pharmacokinet. 1992;23(5):328–364. doi: 10.2165/00003088-199223050-00002. [DOI] [PubMed] [Google Scholar]
- 86.Freeman CD, Klutman NE, Lamp KC. Metronidazole - a therapeutic review and update. Drugs. 1997;54(5):679–708. doi: 10.2165/00003495-199754050-00003. [DOI] [PubMed] [Google Scholar]
- 87.Carmine AA, Brogden RN, Heel RC, Speight TM, Avery GS. Tinidazole in anaerobic infections - a review of its antibacterial activity, pharmacological properties and therapeutic efficacy. Drugs. 1982;24(2):85–117. doi: 10.2165/00003495-198224020-00001. [DOI] [PubMed] [Google Scholar]
- 88.Gillis JC, Wiseman LR. Secnidazole - a review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis. Drugs. 1996;51(4):621–638. doi: 10.2165/00003495-199651040-00007. [DOI] [PubMed] [Google Scholar]
- 89.Lamp KC, Freeman CD, Klutmam NE, Lacy MK. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin. Pharmacokinet. 1999;36(5):353–373. doi: 10.2165/00003088-199936050-00004. [DOI] [PubMed] [Google Scholar]
- 90.Roe FJC. Toxicologic evaluation of metronidazole with particular reference to carcinogenic, mutagenic, and teratogenic potential. Surgery. 1983;93(1):158–164. [PubMed] [Google Scholar]
- 91.Roe FJC. Safety of nitroimidazoles. Scand. J. Infect. Dis. 1985:72–81. [PubMed] [Google Scholar]
- 92.Desai CR, Heera S, Patel A, Babrekar AB, Mahashur AA, Kamat SR. Role of metronidazole in improving response and specific drug sensitivity in advanced pulmonary tuberculosis. J. Assoc. Physicians India. 1989;37(11):694–697. [PubMed] [Google Scholar]
- 93.Garton NJ, Waddell SJ, Sherratt AL, et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008;5(4):e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lenaerts AJ, Gruppo V, Marietta KS, et al. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 2005;49(6):2294–2301. doi: 10.1128/AAC.49.6.2294-2301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carvalho E, Francisco AP, Iley J, Rosa E. Triazene drug metabolites. Part 17: synthesis and plasma hydrolysis of acyloxymethyl carbamate derivatives of antitumour triazenes. Bioorg. Med. Chem. 2000;8(7):1719–1725. doi: 10.1016/s0968-0896(00)00100-0. [DOI] [PubMed] [Google Scholar]
- 96.Tyagi S, Nuermberger E, Yoshimatsu T, et al. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2005;49(6):2289–2293. doi: 10.1128/AAC.49.6.2289-2293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nuermberger E, Rosenthal I, Tyagi S, et al. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2006;50(8):2621–2625. doi: 10.1128/AAC.00451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tasneen R, Tyagi S, Williams K, Grosset J, Nuermberger E. Enhanced bactericidal activity of rifampin and/or pyrazinamide when combined with PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2008;52(10):3664–3668. doi: 10.1128/AAC.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nuermberger E, Tyagi S, Tasneen R, et al. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2008;52(4):1522–1524. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sung JC, Garcia-Contreras L, Verberkmoes JL, et al. Dry powder nitroimidazopyran antibiotic PA-824 aerosol for inhalation. Antimicrob. Agents Chemother. 2009;53(4):1338–1343. doi: 10.1128/AAC.01389-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ordway D, Palanisamy G, Henao-Tamayo M, et al. The cellular immune response to Mycobacterium tuberculosis infection in the guinea pig. J. Immunol. 2007;179(4):2532–2541. doi: 10.4049/jimmunol.179.4.2532. [DOI] [PubMed] [Google Scholar]
- 102.Turner OC, Basaraba RJ, Orme IM. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect. Immun. 2003;71(2):864–871. doi: 10.1128/IAI.71.2.864-871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garcia-Contreras L, Sung JC, Muttil P, et al. Dry Powder PA-824 Aerosols for treatment of tuberculosis in guinea pigs. Antimicrob. Agents Chemother. 2010;54(4):1436–1442. doi: 10.1128/AAC.01471-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob. Agents Chemother. 2009;53(9):3720–3725. doi: 10.1128/AAC.00106-09.. ▪ Study in which pharmacokinetic parameters of PA-824 in healthy subjects are evaluated
- 105.Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. Assessment of the effects of the nitroimidazo-oxazine PA-824 on renal function in healthy subjects. Antimicrob. Agents Chemother. 2009;53(9):3726–3733. doi: 10.1128/AAC.00112-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andreev E, Koopman M, Arisz L. A rise in plasma creatinine that is not a sign of renal failure: which drugs can be responsible? J. Intern. Med. 1999;246(3):247–252. doi: 10.1046/j.1365-2796.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- 107.Gisclon LG, Giacomini KM. Inhibition of cimetidine transport by creatinine in luminal membrane-vesicles prepared from rabbit kidney. Drug Metab. Dispos. 1988;16(2):331–332. [PubMed] [Google Scholar]
- 108.Kastrup J, Petersen P, Bartram R, Hansen JM. The effect of trimethoprim on serum creatinine. Brit. J. Urol. 1985;57(3):265–268. doi: 10.1111/j.1464-410x.1985.tb06340.x. [DOI] [PubMed] [Google Scholar]
- 109.Larsson R, Bodemar G, Kagedal B, Walan A. The effects of cimetidine (tagamet) on renal-function in patients with renal-failure. Acta Med. Scand. 1980;208(1–2):27–31. doi: 10.1111/j.0954-6820.1980.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 110.Opravil M, Keusch G, Luthy R. Pyrimethamine inhibits renal secretion of creatinine. Antimicrob. Agents Chemother. 1993;37(5):1056–1060. doi: 10.1128/aac.37.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob. Agents Chemother. 2010;54(8):3402–3407. doi: 10.1128/AAC.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sirgel FA, Fourie PB, Donald PR, et al. The early bactericidal activities of rifampin and rifapentine in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 2005;172(1):128–135. doi: 10.1164/rccm.200411-1557OC. [DOI] [PubMed] [Google Scholar]
- 113.Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. Int. J. Tuberc. Lung Dis. 2000;4(9):796–806. [PubMed] [Google Scholar]
- 114.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282(7):677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 115.Prideaux B, Dartois V, Staab D, et al. High-Sensitivity MALDI-MRM-MS imaging of moxifloxacin distribution in tuberculosis-infected rabbit lungs and granulomatous lesions. Anal. Chem. 2011 doi: 10.1021/ac1029049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hurdle JG, Lee RB, Budha NR, et al. A microbiological assessment of novel nitrofuranylamides as anti-tuberculosis agents. J. Antimicrob. Chemother. 2008;62(5):1037–1045. doi: 10.1093/jac/dkn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vicente E, Villar R, Burguete A, et al. Efficacy of quinoxaline-2-carboxylate 1,4-di-N-oxide derivatives in experimental tuberculosis. Antimicrob. Agents Chemother. 2008;52(9):3321–3326. doi: 10.1128/AAC.00379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goldman RC. Maximizing bactericidal activity with combinations of bioreduced drugs. Future Med. Chem. 2010;2(8):1253–1271. doi: 10.4155/fmc.10.215. [DOI] [PubMed] [Google Scholar]
Patents
- 201.US5668127. Pathogenesis Corporation. 1997
- 202.US6087358. Pathogenesis Corporation. 2000
Websites
- 301.WHO model list of essential medicines. www.who.int/entity/medicines/publications/essentialmedicines/Updated_sixteenth_adult_list_en.pdf.
- 302.ClinicalTrials.gov. Safety and pharmacokinetics (PK) in multidrug-resistant (MDR) refractive tuberculosis. http://clinicaltrials.gov/ct2/show/NCT01131351.
- 303.ClinicalTrials.gov. PA-824-CL-007: phase IIa evaluation of early bactericidal activity in pulmonary tuberculosis. http://clinicaltrials.gov/ct2/show/NCT00567840.
- 304.ClinicalTrials.gov. Evaluation of early bactericidal activity in pulmonary tuberculosis. http://clinicaltrials.gov/ct2/show/NCT00944021.
- 305.ClinicalTrials.gov. Evaluation of early bactericidal activity in pulmonary tuberculosis with(J-M-Pa-Z) http://clinicaltrials.gov/ct2/show/NCT01215851.
- 306.ClinicalTrials.gov. Safety, efficacy and pharmacokinetics of OPC-67683 in patients with pulmonary tuberculosis. http://clinicaltrials.gov/ct2/show/NCT00401271.
- 307.ClinicalTrials.gov. A placebo-controlled, Phase 2 trial to evaluate OPC 67683 in patients with pulmonary sputum culture-positive, multidrug-resistant tuberculosis (TB) http://clinicaltrials.gov/ct2/show/NCT00685360.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.