ABSTRACT
The stress-responsive alternative sigma factor σB is conserved across diverse Gram-positive bacterial genera. In Listeria monocytogenes, σB regulates transcription of >150 genes, including genes contributing to virulence and to bacterial survival under host-associated stress conditions, such as those encountered in the human gastrointestinal lumen. An inhibitor of L. monocytogenes σB activity was identified by screening ~57,000 natural and synthesized small molecules using a high-throughput cell-based assay. The compound fluoro-phenyl-styrene-sulfonamide (FPSS) (IC50 = 3.5 µM) downregulated the majority of genes previously identified as members of the σB regulon in L. monocytogenes 10403S, thus generating a transcriptional profile comparable to that of a 10403S ΔsigB strain. Specifically, of the 208 genes downregulated by FPSS, 75% had been identified previously as positively regulated by σB. Downregulated genes included key virulence and stress response genes, such as inlA, inlB, bsh, hfq, opuC, and bilE. From a functional perspective, FPSS also inhibited L. monocytogenes invasion of human intestinal epithelial cells and bile salt hydrolase activity. The ability of FPSS to inhibit σB activity in both L. monocytogenes and Bacillus subtilis indicates its utility as a specific inhibitor of σB across multiple Gram-positive genera.
IMPORTANCE
The σB transcription factor regulates expression of genes responsible for bacterial survival under changing environmental conditions and for virulence; therefore, this alternative sigma factor is important for transmission of L. monocytogenes and other Gram-positive bacteria. Regulation of σB activity is complex and tightly controlled, reflecting the key role of this factor in bacterial metabolism. We present multiple lines of evidence indicating that fluoro-phenyl-styrene-sulfonamide (FPSS) specifically inhibits activity of σB across Gram-positive bacterial genera, i.e., in both Listeria monocytogenes and Bacillus subtilis. Therefore, FPSS is an important new tool that will enable novel approaches for exploring complex regulatory networks in L. monocytogenes and other Gram-positive pathogens and for investigating small-molecule applications for controlling pathogen transmission.
Introduction
Listeria monocytogenes causes a rare but potentially fatal food-borne disease called listeriosis. With its high fatality rate, listeriosis accounts for ~10% of all deaths from food-borne diseases in the United States (1). L. monocytogenes can transition from a saprotrophic existence under a wide range of environmental conditions (2) to intracellular infection in a diverse array of hosts (3). The ability of L. monocytogenes to transform from saprotroph to intracellular pathogen is influenced by regulatory networks that enable bacterial survival and control virulence factor expression in response to environmental signals (4).
Sigma B is one important component of a network that links environmental stress survival and virulence in L. monocytogenes (5, 6). Sigma factors are dissociable subunits of prokaryotic RNA polymerase. The association of a specific alternative sigma factor, e.g., σB, with core RNA polymerase under appropriate environmental conditions enables the rapid redirection of regulon transcription in response to environmental signals. More than 150 genes comprise the L. monocytogenes σB regulon (7, 8).
σB networks, including its interactions with PrfA, influence transmission of L. monocytogenes during both the gastrointestinal (9) and systemic stages of infection (5, 10). Complex interactions occur between σB and PrfA-dependent gene regulation (5, 10); PrfA is the master regulator of L. monocytogenes virulence gene expression. σB directly regulates prfA transcription via the P2prfA promoter (11–13) and also indirectly regulates PrfA activity. Specifically, σB downregulates PrfA activity in intracellular L. monocytogenes, thus moderating expression of PrfA-dependent virulence genes and thereby reducing host cell damage incurred by these virulence gene products (5).
A general strategy for exploring complex biological networks is to disrupt a targeted element of that network and then examine the consequences. High-throughput screening of small-molecule libraries has been used effectively to identify agents that disrupt specific bacterial targets, including an inhibitor of the virulence regulator ToxT in Vibrio cholerae (14). We screened multiple small-molecule libraries to identify an inhibitor of the stress response and virulence-associated regulator σB. The most promising small molecule was further assessed using an L. monocytogenes whole-genome microarray, quantitative reverse transcription-PCR (qRT-PCR) of σB-dependent genes, and phenotypic profiling, including Caco-2 cell invasion assays and qualitative assessment of bile salt hydrolase activity. The compound also was evaluated for its ability to inhibit σB activity in B. subtilis.
RESULTS
A high-throughput cell-based screen identifies promising small molecules that interfere with σB activity.
A high-throughput cell-based screening assay (HTS) was used to identify compounds that inhibit expression of the σB-dependent opuCA promoter (15) without affecting L. monocytogenes growth (Chembank Screening Project: SigBInhibition). Based on the primary screen, 41 putative inhibitors of σB activity were selected for secondary cell-based screening (Fig. S1). Compounds that induced σB activity were not analyzed further.
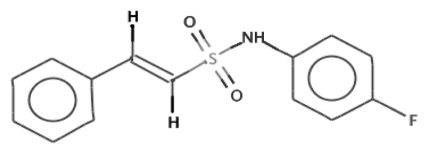
IC50 values, i.e., compound concentrations needed to inhibit 50% of σB activity, were determined from secondary screening results for each of the 41 compounds. For 14 compounds, σB activity was inhibited at a concentration lower than that used in the primary screen; however, 11 compounds were eliminated from further consideration based on mammalian cell cytotoxicity data in ChemBank (http://chembank.broad.harvard.edu). The three remaining L. monocytogenes σB inhibitors were 4-hydrazino[1]benzofuro[3,2-d]pyrimidine; 3-(cyclohexylacetyl)-4-hydroxy-2H-chromen-2-one; and (E)-N,2-diphenylethenesulfonamide. Among these, the most effective σB activity inhibitor, (E)-N,2-diphenylethenesulfonamide (IC50 = ~15 µM), which was a member of the ChemDiv3 library (Table S1), was not commercially available. Therefore, fluoro-phenyl-styrene-sulfonamide (FPSS), an analog of the original compound, was obtained for further study. Relative to (E)-N,2-diphenylethenesulfonamide, FPSS has fluorine substituted for a hydrogen (Fig. 1). Based on quantitative reverse transcriptase PCR (qRT-PCR) results, FPSS was the most effective σB inhibitor among the three compounds. Data available in ChemBank indicated minimal evidence and no evidence of mammalian cell cytotoxicity for (E)-N,2-diphenylethenesulfonamide and FPSS, respectively.
FIG 1 .
Chemical structure of the σB activity inhibitor FPSS (fluoro-phenyl-styrene-sulfonamide). FPSS is a derivative of (E)-N,2-diphenylethenesulfonamide, the compound originally identified by HTS as the most effective inhibitor of σB activity. Relative to the structure of (E)-N,2-diphenylethenesulfonamide, FPSS has a fluorine substituted for a hydrogen.
We hypothesized that a small molecule that directly binds σB might also prevent σB from associating with core polymerase, thereby inhibiting σB activity. Therefore, the ability of various small molecules to bind σB was assessed using a small-molecule (SMM) screen with His-tagged σB (Fig. S2; Table S1). Of three putative ligands—i.e., 3-amino-4-oxo-N-(pyridin-3-ylmethyl)-3,4-dihydroquinazoline-2-carboxamide [Maybridge], ethyl 1-benzyl-5-[3-(tert-butylamino)-2-hydroxypropoxy]-2-methyl-1H-indole-3-carboxylate [Chemical Diversity], and 5-phenyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine [Maybridge])—none inhibited σB activity in the bile salt hydrolase activity assay, and therefore, none were evaluated further.
Multiple lines of evidence support σB activity inhibition by FPSS.
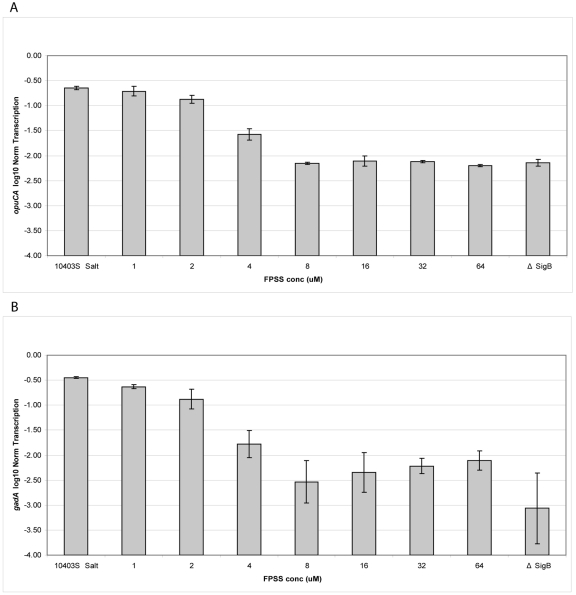
Quantitative qRT-PCR assessment of the effects of FPSS concentrations from 1 µM to 64 µM on σB-dependent transcription showed that exposure to 64 µM FPSS resulted in a ~40-fold reduction in transcript levels for both σB-dependent genes opuCA and gadA relative to their transcript levels in cells not treated with FPSS (Fig. 2) (P < 0.05, GLM [general linear model] with post-hoc Tukey's honestly significant difference [HSD] test). opuCA and gadA transcript levels in cells treated with FPSS (ranging from 8 µM to 64 µM) were not significantly different from those in the ∆sigB strain (P > 0.05). At 4 µM, FPSS significantly reduced opuCA and gadA transcript levels compared to those in 10403S without FPSS (P < 0.05) but not to levels equivalent to those in the ∆sigB strain (Fig. 2). The FPSS concentration yielding half the maximal inhibition (IC50) was calculated as 3.5 µM for opuCA and 3.0 µM for gadA. Importantly, absolute transcript levels for the housekeeping genes rpoB and gap were not different in L. monocytogenes with and without exposure to FPSS, indicating that FPSS specifically inhibits transcription of σB-dependent genes without affecting transcription of housekeeping genes.
FIG 2 .
FPSS treatment reduces transcript levels of σB-dependent opuCA and gadA. Normalized log-transformed opuCA (A) and gadA (B) transcript levels in L. monocytogenes 10403S exposed to 0.3 M NaCl to induce σB activity in the presence of FPSS at concentrations ranging from 1 to 64 µM; controls included strains 10403S and its isogenic ∆sigB mutant exposed to 0.3 M NaCl. Transcript levels were quantified by qRT-PCR, log10 transformed, and normalized to the geometric mean of the transcript levels for the housekeeping genes rpoB and gap. The data are means from three replicates; error bars show the standard deviations.
The phenotypic effects of various concentrations of FPSS on the activity of bile salt hydrolase, the product of the σB-dependent gene bsh, which is required for L. monocytogenes survival in vivo (6), were qualitatively assessed. L. monocytogenes treated with 96 µM and 193 µM FPSS showed no bile salt hydrolase (BSH) activity, with no apparent effect on the ability of L. monocytogenes to grow on brain heart infusion (BHI) agar. When treated with 290 µM FPSS, L. monocytogenes produced no BSH activity but also grew poorly on BHI (data not shown).
L. monocytogenes whole genome microarray identified 208 genes downregulated by treatment with FPSS.
Transcriptional consequences of FPSS treatment were profiled using an L. monocytogenes whole-genome microarray. FPSS treatment downregulated transcript levels for 208 genes and upregulated transcript levels for 32 genes (adjusted P value of <0.05 and an absolute fold change [FC] value of ≥2). In previous studies with L. monocytogenes 10403S and EGD-e, 281 genes were identified as positively regulated by σB under at least one assay condition, and 137 genes as positively regulated by σB under two or more of the seven assay conditions examined (5–8, 16, 17) (Table S2). Overall, FPSS significantly reduced transcript levels of 56% (156/281) of genes previously identified as being upregulated by σB in at least one study and of >91% (125/137) of genes identified as being upregulated by σB in two or more studies (Table 1; Table S3). Of the 208 FPSS-downregulated genes, 115 were reported to be positively regulated by σB in both 10403S and EGD-e (5–8, 16, 17), with an additional 21 genes reported to be positively regulated by σB in 10403S (5, 7, 8, 17) and 20 reported to be positively regulated by σB in EGD-e (6, 16). FPSS downregulated transcript levels for >90% of genes with previously reported hidden Markov model-identified σB-dependent promoters (17). A number of operons previously identified as being positively regulated by σB (8) were also significantly downregulated after treatment with FPSS, including inlAB, which mediates entry into nonprofessional phagocytes (18), and opuCABCD, which is involved in compatible solute transport. The autoregulated sigB operon (7, 8, 19), consisting of lmo0893 to lmo0896 (rsbV, rsbW, sigB, and rsbX), was also downregulated by FPSS.
TABLE 1 .
Relationships between genes identified as differentially expressed after treatment with FPSS and genes identified previously as σB dependent
| Gene type | No. of genes (no. with an upstream σB-dependent promotera) |
|
|---|---|---|
| Downregulated by FPSS |
Upregulated by FPSS |
|
| Identified previously as positively regulated by σBb |
152 (86) | 2 (0) |
| Identified previously as negatively regulated by σBc |
2 (0) | 7 (0) |
| Reported previously to be positively or negatively regulated under different conditions or in different studiesd |
4 (0) | 0 |
| Having no previous evidence of σB-dependent transcript levels |
50 (0) | 23 (0) |
| Total | 208 (86) | 32 (0) |
Genes were classified with upstream σB-dependent promoters by in silico analysis using a hidden Markov model as described by Oliver et al. (17).
Genes were classified as positively regulated by σB based on evidence from at least one of six previous microarray or RNA-Seq studies (5–8, 16, 17) (see Tables S2 and S3 for details).
Genes were classified as negatively regulated by σB based on evidence from at least one of six previous microarray or RNA-Seq studies (5–8, 16, 17) (see Tables S2 and S4 for details).
Genes reported as differentially regulated in previous microarray or RNA-Seq studies (5–8, 16, 17), including genes reported as negatively regulated by σB in one study and positively regulated by σB in another study and genes that were found to be negatively and positively regulated by σB under different conditions in the same study (see Table S2).
To evaluate FPSS effects on the function of other alternative sigma factors, transcript levels for genes in the σH and σL regulons were assessed. Among the 30 genes previously identified as σH dependent (with an FC ≥ 2.0), 14 were significantly downregulated by FPSS (adjusted P < 0.05, fold change ≤ −2); however, 12 of those 14 genes are also σB dependent. Gene set enrichment analysis (GSEA) showed that the σH-only regulon (i.e., genes that are regulated only by σH and not coregulated by σB) was not significantly enriched among the genes differentially transcribed as a result of FPSS treatment (false discovery rate [FDR] q = 0.472). GSEA also showed that the σL regulon was not significantly enriched as a result of treatment with FPSS (FDR q = 0.836).
GSEA was used to determine if genes from specific biological role categories were overrepresented among those differentially affected by FPSS. Consistent with σB’s role in bacterial stress response, gene sets enriched among FPSS-downregulated genes included those classified as (i) “Cellular Processes: Adaptations to Atypical Conditions” and (ii) “Energy Metabolism (other)” (FDR q = 0.060 and q = 0.201, respectively). Previously identified σB-regulated genes also were significantly enriched among FPSS-downregulated genes (FDR q < 0.0001). Gene sets enriched among FPSS-upregulated genes included those classified as (i) “Cellular Processes: Chemotaxis and Motility,” (ii) “Protein Fate: Protein Folding and Stabilization,” and (iii) “Amino Acid Biosynthesis: Histidine Family” (FDR q < 0.0001, q = 0.008, and q = 0.031, respectively).
Of the 208 FPSS-downregulated genes, 126 were positively regulated by σB during infection in the murine intestine (6), including 17 genes that had been identified as σB dependent only in the intestinal environment (Table S2). Among these 17 genes, 9 genes were of unknown or hypothetical function. Several FPSS-downregulated genes are recognized as contributing to virulence and host infection (i.e., inlA, inlB inlD, bilEAB, bsh, hfq, clpC, opuC, and gadA). Further, the PrfA regulon (i.e., genes regulated by the pleiotropic virulence gene regulator PrfA) was significantly enriched among the genes downregulated by FPSS (FDR q = 0.095). Interestingly, 19 genes that were previously classified as coregulated by PrfA and σB (10, 20) were both upregulated in the mouse spleen (10) and downregulated by FPSS, further supporting their σB-dependent transcription. Among these 19 genes, three were identified as potential virulence factors in murine and tissue culture models (10, 22), including lmo1601 (encoding a general stress protein), lmo1602 (encoding an unknown protein), and lmo2157 (encoding SepA, a metalloprotease in Staphylococcus epidermidis [21]), which is upregulated in L. monocytogenes during intracellular infection (22). FPSS also downregulated lmo0937, a PrfA-regulated gene that is upregulated in the mouse spleen at 48 h postinfection (10), and lmo0915, which encodes a component of a phosphotransferase system identified as a potential virulence factor by Camejo et al. (10); neither gene had been identified previously as σB dependent. Other σB-dependent genes downregulated by FPSS and upregulated during intracellular infection (22) include lmo0232 (clpC); lmo0445, which encodes a transcriptional regulator; lmo2672, which encodes a protein similar to a transcriptional regulator; and lmo0783, which is a member of an operon encoding mannose phosphotransferase system components. FPSS-treated cells had lower transcript levels for a number of genes that encode cell wall-associated proteins previously shown to be upregulated under intracellular conditions (22) and in the murine intestine (6); these genes include inlA, inlD, lmo0610, lmo0880, and lmo2085, which all encode proteins with an LPXTG sorting motif for cell wall anchoring, and inlB, which encodes a protein with a GW domain that is important for binding host ligands (23).
Three genes important for glycerol utilization (i.e., lmo1538, lmo1539, and lmo1293) were also downregulated by FPSS; utilization of glycerol as a carbon source in intracellular environments (22) is required for intracellular survival (24). While lmo1538 (encoding a glycerol kinase) and lmo1539 (encoding a glycerol uptake facilitator) were downregulated by FPSS, they were previously reported to be negatively regulated by σB in stationary phase and under salt stress conditions (8). Interestingly, however, both genes were upregulated by σB in the intestine (6) and during intracellular replication (22). lmo1293 (glpD), which encodes a glycerol-3-phosphate dehydrogenase, was previously reported to be positively regulated by σB in L. monocytogenes exposed to salt stress (8) or grown intracellularly (24) and in the gastrointestinal tract (6) but was downregulated by σB in stationary-phase cells (8). Taken together, our data provide additional evidence supporting the hypothesis that the composition of the σB regulon is dynamically dependent on environmental conditions (7). Importantly, our data also demonstrate that a number of genes downregulated by FPSS are specifically regulated by σB in the gastrointestinal environment. For example, three additional genes downregulated by FPSS (i.e., lmo0642, lmo1251, and lmo1930) had higher transcript levels in the L. monocytogenes parent strain than in the ∆sigB strain when both were grown in the murine intestine (6), but these genes did not appear to be σB dependent under other in vitro conditions (5, 7, 8, 16, 17)
Only a small number of genes upregulated by FPSS have been identified previously as negatively regulated by σB.
Overall, 32 genes were identified with significantly higher transcript levels in FPSS-treated L. monocytogenes than in untreated cells (Table 1), suggesting negative regulation of these genes by σB. While 264 genes were previously identified as negatively regulated by σB under at least one condition (5–8, 16, 17), only 7 of the 32 FPSS-upregulated genes were represented among these 264 genes. Further, only 14 genes were previously identified as negatively regulated by σB under at least two environmental conditions (5–8, 16, 17) and none of the 32 FPSS-upregulated genes were represented among these 14 genes. Very few genes appear to be consistently repressed by σB under various conditions, likely because these genes are indirectly rather than directly regulated by σB. Six of the 7 FPSS-upregulated genes previously identified as negatively regulated by σB encode proteins with known functions, including an ABC transporter (lmo2114), a posttranslocation chaperone (prsA), a methyl-accepting chemotaxis protein (lmo1699 and lmo1700), NADP glutamate dehydrogenase (lmo0560), and a d-alanine-activating enzyme (dltA). While dltA (the first gene in an operon encoding proteins that modify lipoteichoic and wall teichoic acids) was previously shown to be negatively regulated by σB, other genes in this operon (i.e., lmo0973 [dltB] and lmo0971 [dltD]) not previously identified as σB dependent were also significantly upregulated following FPSS treatment, suggesting that this entire operon is negatively regulated by σB, at least under some conditions. Two genes, lmo2568 (unknown function) and lmo1637 (encoding a protein similar to a membrane protein), were upregulated by FPSS but had previously been reported to be positively regulated by σB in the intestine (6) and during various growth phases (16), respectively. Among the genes not previously reported as σB dependent that were upregulated by FPSS, some were involved in ABC transport, motility, and cell division, but most had unknown functions (Table S4).
Further supporting the idea that FPSS treatment of L. monocytogenes generates the equivalent of a ΔsigB phenotype, GSEA identified genes in the “motility and chemotaxis” role category as enriched among genes upregulated by FPSS (FDR q < 0.0001), consistent with previous reports that σB negatively regulates motility-related functions (8). In addition, six FPSS-upregulated genes (lmo0678 to lmo0681, lmo0685, and lmo0686) not described previously as negatively regulated by σB were located in a large flagellar biosynthesis and motility operon (lmo0673 to lmo0718) containing 13 genes recognized as negatively regulated by σB(8). As transcription of a number of motility related genes was affected by FPSS, GSEA was also performed on the regulons of the known chemotaxis- and motility-related regulators DegU, MogR, and CodY. The DegU operon, as defined by Williams et al. (25), was enriched among FPSS-upregulated genes (FDR q < 0.0001); DegU is an activator of flagellum biosynthesis (26). Specific DegU-regulated genes identified among the FPSS-upregulated genes include six genes in the flagellum biosynthesis operon (lmo0673 to lmo0718) as well as the σB-repressed (8) methyl-accepting chemotaxis operon (lmo1699 and lmo1700) (25). The CodY regulon was also significantly enriched among FPSS-upregulated genes (FDR q < 0.0001); CodY is a negative regulator of genes encoding flagellar components in L. monocytogenes (27). Although MogR, the transcriptional repressor of flagellum genes (28, 29), was shown previously to be σB dependent (6), its regulon (29) was not significantly enriched among the genes differentially regulated by FPSS (FDR q = 0.257).
FPSS reduces L. monocytogenes invasion of human enterocytes.
FPSS treatment (at either 8 µM or 64 µM) reduced L. monocytogenes’ ability to invade Caco-2 human enterocytes by 1.50 log (± 0.49) and 1.42 log (± 0.39), respectively, compared to the untreated control bacteria (Fig. S3) (P < 0.05). Invasion of L. monocytogenes treated with 8 µM FPSS was not significantly different from invasion by the ∆sigB strain, which also showed significantly reduced invasion compared to the untreated parent strain (P < 0.05), providing phenotypic evidence of FPSS inhibition of σB-regulated virulence functions that contribute to orally acquired listeriosis (9).
FPSS inhibits σB activity in B. subtilis, indicating effectiveness across genera.
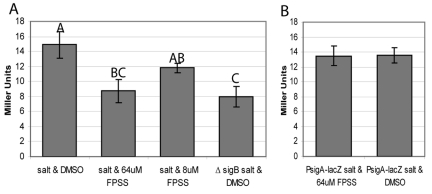
To determine if FPSS affects σB activity in bacteria other than L. monocytogenes, the compound was tested for its ability to specifically inhibit σB activity in B. subtilis. FPSS (64 µM) significantly inhibited σB-dependent ctc-lacZ activity (P < 0.05; GLM with post-hoc Tukey’s HSD test) to levels equivalent to those in a ∆sigB strain (Fig. 3) (P > 0.05) without reducing β-galactosidase activity from a σA-dependent lacZ fusion (30) (Fig. 3), further supporting FPSS specificity for inhibiting σB activity.
FIG 3 .
B. subtilis β-galactosidase assay. (A) β-Galactosidase activity of a B. subtilis strain with a σB-dependent Pctc-lacZ reporter fusion treated with (i) 0.3 M NaCl and DMSO, (ii) 0.3 M NaCl and 64 µM FPSS, and (iii) 0.3 M NaCl and 8 µM FPSS; this panel also shows the activity for an isogenic ΔsigB strain with the Pctc-lacZ reporter fusion treated with 0.3 M NaCl and DMSO. (B) β-Galactosidase activity of a B. subtilis strain with a σA-dependent PrsbRSTU-lacZ fusion treated with either (i) 0.3 M NaCl and 64 µM FPSS or (ii) 0.3 M NaCl and DMSO. The data are means from at least three biological replicates; error bars show the standard deviations. Different letters above the bars indicate strains or treatments that differed significantly (P < 0.05; GLM Tukey).
DISCUSSION
By using a high-throughput screen of approximately 57,000 small molecules, 41 candidate compounds were identified as potential inhibitors of L. monocytogenes σB activity. Through subsequent screens, we identified a compound designated FPSS that specifically inhibits σB-mediated transcription, as shown by qRT-PCR of σB-dependent genes and whole-genome microarray analysis of cells treated with the compound. This compound also significantly reduces L. monocytogenes invasion into human intestinal epithelial cells and inhibits σB-directed activity in the Gram-positive bacterium B. subtilis, indicating that this compound inhibits σB-mediated transcription across genera. Overall, our data show that FPSS (i) inhibits expression of the σB regulon with high specificity, yielding transcriptional profiles similar to those generated by a genetic null mutation of the sigB gene, and (ii) specifically inhibits expression of σB-dependent genes important for virulence, stress response, and other functions associated with L. monocytogenes survival and growth in the gastrointestinal tract. In combination with previous reports that identified small molecules that interfere with virulence factors and virulence activation and that show therapeutic promise (14, 31), our results suggest that, in addition to its role as a promising tool for studying regulatory networks involving σB, FPSS also may represent a compound that can be developed into a therapeutic agent.
FPSS specifically inhibits expression of σB-dependent virulence, stress response, and other functions that are associated with L. monocytogenes growth and survival in the gastrointestinal tract.
σB is well recognized as an important transcriptional regulator in multiple Gram-positive genera. For example, σB regulates transcription of genes contributing to stress response, virulence, or both in low-GC Gram-positive microbes, including human pathogens such as Bacillus cereus (32, 33), Bacillus anthracis (34), Staphylococcus aureus (35, 36), and the opportunistic pathogen S. epidermidis (37). σB activates transcription of a large number of target genes across the genera and species reported to date (e.g., L. monocytogenes, Listeria innocua, S. aureus, and B. subtilis) (8, 38, 39). FPSS treatment of L. monocytogenes affects expression of σB-dependent genes that are upregulated in the host intestine but that had not been identified previously as σB dependent under other in vitro conditions. Specifically, among 172 genes in L. monocytogenes EGD-e that were upregulated by σB in the murine intestinal lumen (6), FPSS treatment significantly downregulated 126 genes; 17 genes downregulated by FPSS had been identified as σB dependent only in the intestinal lumen (6) but not in other in vitro test systems. Thus, identification of σB-dependent genes in L. monocytogenes treated with FPSS may provide new insight into σB-dependent gene regulation that may be critical during the gastrointestinal stage of infection. For example, the PrfA regulon was significantly enriched among genes downregulated by FPSS treatment, including two PrfA-dependent genes (i.e., lmo0937 and plcA) that had not been identified previously as σB regulated. These findings are consistent with σB’s role, via the P2prfA promoter, in directly upregulating prfA transcription (11–13) and also support the idea that σB-dependent upregulation of prfA transcription plays a critical role during intestinal stages of infection. Activation of σB in the intestinal lumen thus appears to increase expression of σB-dependent inlA, which is required for intestinal epithelial cell invasion (40), and also primes expression of PrfA, which is critical for regulating virulence gene expression during the subsequent intracellular stages of infection.
FPSS-treated L. monocytogenes also had higher transcript levels than nontreated cells for a number of genes involved in chemotaxis and motility. Several genes in a large operon encoding flagellar structural components were previously reported to be negatively regulated by σB (8), and sigB-null mutants also exhibited increased swarming (6, 8). σB-dependent downregulation of transcripts encoding flagellar components and overall motility appear to be at least partially due to σB-dependent transcription of a long untranslated region (UTR) upstream of mogR, which encodes a negative regulator of L. monocytogenes motility. Reduced transcription of this UTR not only reduces mogR transcript levels (thereby increasing flagellar motility) but also appears to increase transcript levels for some flagellar genes, as the σB-dependent UTR also decreases flagellin gene transcripts through an antisense-RNA-type mechanism (6, 8). While L. monocytogenes flagellar motility appears to contribute to intestinal invasion (41), σB-dependent downregulation of flagellar expression in the intestinal lumen may be critical for subsequent stages of infection, as Listeria downregulates flagellar gene expression during infection (10) to evade the immune system; increased expression of flagellar components can induce potent proinflammatory effects via TLR5-mediated immunogenicity (42).
Inhibitors of alternative σ factor activation represent potential avenues for development into therapeutics.
In addition to its value as a compound that can be used to study regulatory pathways involving σB, FPSS also may provide a starting point for development of new therapeutic compounds that interfere with regulatory pathways critical for infection and virulence. Several small molecules that target transcription regulators inhibit virulence and virulence-associated characteristics in vitro and in vivo, suggesting that these targets are suitable for development of novel therapeutics against bacterial infections (43, 44). Importantly, prokaryotic transcriptional machinery, as represented by interactions between σ70 and the β′ subunit of core RNA polymerase in Escherichia coli, can be disrupted by small molecules without affecting eukaryotic transcription (45). Consequently, the therapeutic potential of novel compounds that interfere with transcriptional regulation of bacterial virulence functions is of emerging interest.
Virstatin is an example of a small molecule with therapeutic potential that has been shown to inhibit transcriptional regulation in V. cholerae. Virstatin interferes with the virulence gene regulator ToxT, a member of the AraC family of transcriptional regulators, thus showing potential for treatment of Vibrio infections (14). Small-molecule inhibitors also have been identified for other members of the AraC transcription factor family, e.g., MarA, SoxS and Rob in E. coli (43) and LcrF in Yersinia spp. (44). As with σB, AraC-type regulators typically contribute to transcription of multiple stress response (46) and virulence factors (47, 48); therefore, inhibition of these and similar transcriptional regulators can result in broad physiological consequences for the affected microbes (43).
The small molecule identified here, FPSS, inhibits σB activity at an IC50 of 3 to 3.5 µM. By comparison, the ToxT inhibitor virstatin (14) has an MIC between 3 and 40 µM, depending on the target strain. Minimal bactericidal concentrations of gentamicin, ampicillin, and streptomycin against L. monocytogenes range from 2 to 46 µM (49). In addition to its promising IC50 prior to structural optimization, FPSS produces highly specific, genome-wide reduction of σB-directed activity, including inhibited expression of σB-dependent virulence genes such as inlAB, bsh, bilE, clpC, and hfq (5–8, 16, 17). Furthermore, opuC (50) and gadA (51), which are important for gastrointestinal survival in the host, are also significantly downregulated by FPSS. FPSS clearly inhibits transcription of a number of genes with functions in virulence and infection, thus increasing its therapeutic potential over compounds that target only one virulence factor (43). The contributions of σB to L. monocytogenes virulence are also supported by phenotypic evidence, including reduced virulence of a ΔsigB strain in a guinea pig model of infection (9) and reduced invasion of human Caco-2 cells by a ΔsigB strain (9, 40), consistent with the reduced invasiveness for FPSS-treated L. monocytogenes observed here. Importantly, σB also contributes to establishment of infection and virulence in other Gram-positive pathogens, including B. anthracis and S. aureus. A B. anthracis sigB mutant is less virulent than the parent strain, producing a 1-log reduction in 50% lethal dose, perhaps because σB enhances the ability of B. anthracis to persist in the bloodstream of a mammalian host (34). In S. aureus, σB directly and indirectly modulates global regulatory elements involved in virulence functions (52). Functional loss of σB results in decreased S. aureus virulence in central venous catheter-related diseases manifested by significantly reduced multiorgan infection (53). Similar to B. anthracis, σB is suggested to promote S. aureus survival in the bloodstream, preventing clearance and allowing establishment of infection (54). Further development and optimization of FPSS thus may provide an opportunity to develop novel therapeutics for some important Gram-positive pathogens.
MATERIALS AND METHODS
Strain and media selection.
Strains used in this study included the L. monocytogenes parent strain 10403S (serotype 1/2a) (55), its otherwise isogenic ∆sigB derivative (FSL A1-254) (56), a reporter strain for σB activity (FSL S1-063 [10403S opuCA-gus]) (7, 57, 58), and a negative-control reporter strain for σB activity (FSL S1-059 [∆sigB opuCA-gus]) (Table S5). To evaluate the effectiveness of a selected small molecule to inhibit σB activity in a Gram-positive genus other than Listeria, B. subtilis strains bearing reporter fusions for either σB or σA activity and a ΔsigB negative-control reporter strain (Table S5) were also tested. To achieve low background fluorescence, a chemically defined minimal medium (59) with 25 mM glucose (DMG) (60) was used for the high-throughput screen. Cells were grown in brain heart infusion broth (BHI; Difco, Sparks, MD) for phenotypic and transcriptional profiling assays.
Primary high-throughput cell-based small-molecule screen.
The L. monocytogenes opuCA-gus fusion strain FSL S1-063 was used in a cell-based high-throughput screen (HTS) against ~57,000 compounds. As reported at http://ChemBank.Broad.Harvard.edu, the libraries included the following: (i) known bioactive compounds, including FDA-approved drugs (i.e., the SPBio and SMP libraries); (ii) synthetic compounds from diversity-oriented synthesis (e.g., the CMLD, ICCB, PK04, Ald1.1-H, and Sulf1.1-A libraries); (iii) natural products (i.e., the PhilEx and ICBGEx libraries); and (iv) commercially available compounds (e. g., the ChemDiv3, Maybridge4, and TimTec1 libraries). Table S1 contains a complete listing of libraries screened for this study.
Multidrop liquid-handling robots (Matrix, Thermo Fisher) were used to dispense 27 µl of DMG into black-walled clear-bottom 384-well plates (Nunc, Rochester, NY), and then 100 nl of each small-molecule stock was transferred from the library stock or source plate to the assay plates with a CyBi-Well Vario pipettor (CyBio AG, Jena, Germany). Final experimental concentrations of the small molecules used in the assays were dependent on each stock concentration [e.g., (E)-N,2-diphenylethenesulfonamide had a stock concentration of 19.3 mM, producing a 64.3 µM final concentration in each well]. Each source plate contained approximately 15 wells to which only dimethyl sulfoxide (DMSO) was added, as the small molecules were dissolved in DMSO; these wells are referred to as DMSO-only negative-internal-control wells. All source plates were prepared in duplicate to provide experimental replicates (i.e., plates A and B). Two plates in which all wells contained medium with DMSO and L. monocytogenes (inoculated as detailed below) were included as external plate controls. L. monocytogenes strains were grown to an optical density at 600 nm (OD600) of approximately 0.4 (3 hours) in BHI, cultures were diluted 1:50 with DMG, and then 3 µl of the appropriate diluted culture was added to each well. As a control, a custom assay plate containing 192 wells of the 10403S opuCA-gus strain FSL S1-063 and 192 wells of the otherwise isogenic ΔsigB opuCA-gus strain FSL S1-059 was treated with only DMSO.
All plates were sealed and incubated for 18 h at 37°C. To determine bacterial growth or inhibition in the presence of the compounds, absorbance (OD600) was measured using a Synergy HT multimode microplate reader (Biotek Instruments, Winooski, VT) after ~18 h of incubation. To measure fluorescence for β-glucuronidase (GUS) activity determination, black seals (PerkinElmer, Waltham, MA) were affixed to the bottoms of the plates after the absorbance readings were completed. Cells were then lysed using 5 µl of 2× CelLyticB (Sigma, St. Louis, MO) and a protease inhibitor cocktail mixture (1 ml 2× CelLyticB and 0.05 ml protease inhibitor cocktail; Sigma), immediately prior to the addition of 4 µl of 1.6 mg/ml 4-methylumbelliferyl β-d-glucuronide hydrate (4-MUG; Sigma) in DMSO. Reaction mixtures were incubated in the dark for 1 h at room temperature (~23°C), and reactions were stopped by the addition of 0.2 M Na2CO3. Fluorescence was read using a Wallac 2102 EnVision Multilabel Reader (PerkinElmer) with an excitation wavelength of 355 nm and an emission of 460 nm.
Statistical analysis of primary screen data.
To identify compounds that inhibited σB activity without affecting L. monocytogenes growth, opuCA-directed GUS activity in the presence of each compound was calculated by dividing relative fluorescence units (RFU) by cell density in OD600 units (RFU/OD) (61). Statistical analyses were conducted in collaboration with the Broad Institute and performed as previously described (62, 63). Raw and analyzed data were deposited in ChemBank (64, 65). The software package Spotfire DecisionSite Analytics (TIBCO Spotfire, Somerville, MA) was used for two-dimensional data visualization.
Secondary screen and dose response curve.
Forty-one compounds that appeared to inhibit σB activity (Z score of ≤-3 in both replicates) were selected for secondary cell-based screening using the assay and reporter fusion described above to calculate initial IC50s. Each compound was diluted in DMSO in a series of six 1/5 dilutions of the initial stock concentration [e.g., starting from 19.3 mM stock, (E)-N,2-diphenylethenesulfonamide was diluted in a series of six 1 to 5 dilutions, yielding concentrations of 3.86 mM to 1.24 µM]. The small molecules at these concentrations were then dispensedinto the assay plates.
Small-molecule microarray screens.
Two different arrays, each printed with 8,500 small-molecule (SM) spots and 1,500 DMSO control spots, were used to screen for binding of σB to the small molecules. Small-molecule microarrays (SMMs) were printed on glass slides at the Broad Institute as described previously (66–68). The immobilized SMs included 8,500 compounds created by diversity-oriented synthesis and 8,500 compounds representing natural products, FDA-approved drug-like compounds, commercial compounds, and known bioactive compounds (Table S1) (68; http://Chembank.broadinstitute.org). SMM screening (three replicates) was performed as described by Bradner et al. (66). His-tagged σB was purified from E. coli M15, kindly provided by W. Goebel (69). Data analyses included (i) assessment of signal-to-noise ratio (SNR) of the spot feature; (ii) Z score calculations based on comparison of signals from compound spots to signals from DMSO control spots within a slide; and (iii) composite Z score calculations for data from the three replicates. Spotfire Analytics software was used for three-dimensional data visualization.
FPSS.
(E)-N,2-diphenylethenesulfonamide, the compound identified by HTS as being responsible for the greatest inhibition of σB activity, was not commercially available. Therefore, the analog fluoro-phenyl-styrene-sulfonamide [IUPAC name (E)-N-(4-fluorophenyl)-2-phenylethenesulfonamide; ChemBank ID, 2063822; MW 277.3] was obtained from Enamine Ltd. (Kiev, Ukraine). FPSS was dissolved in DMSO to a concentration of 10 mM. The solution was filter sterilized using with a 0.1-µm filter (Omnipore membrane filter; Millipore Corporation, Billerica, MA) and a Swinney stainless 13-mm holder for syringe filtration (Millipore Corporation).
Bile salt hydrolase (BSH) activity assay.
As L. monocytogenes bsh, which encodes bile salt hydrolase, is σB dependent (5, 7, 15), a qualitative BSH activity assay was used to determine the FPSS concentration needed for σB inhibition. Four-well multidish plates (26 mm by 33 mm; Nunc) containing 6 ml of either BHI agar or de Man, Rogosa and Sharp (MRS) agar medium (BD Biosciences, San Jose, CA) containing 0.5% (wt/vol) glycodeoxycholic acid sodium (GDCA) salt (Calbiochem, San Diego, CA) (70) with either no FPSS or 96, 193, or 290 µM of FPSS [1.5, 3, or 4.5 times the 64.3 µM concentration used for (E)-N,2-diphenylethenesulfonamide in the HTS] were prepared and allowed to dry overnight. L. monocytogenes 10403S and ∆sigB were grown in BHI broth to exponential phase, defined as an OD600 of 0.4, and then 4 µl of culture was spotted in parallel on MRS and BHI agars. The MRS agar plates were incubated anaerobically using the BD-BBL GasPak anaerobic system (Becton Dickinson, Franklin Lakes, NJ), while BHI plates were incubated aerobically. Both sets of plates were incubated for 48 h at 37°C and then were visually assessed for growth (BHI plates) or the presence of a white precipitate comprised of deconjugated bile salts indicating BSH activity (MRS plates). The assay was performed three times.
RNA isolation.
For RNA isolation, L. monocytogenes 10403S and ΔsigB strains were initially grown overnight in 5 ml of BHI broth at 37°C with shaking (230 rpm), followed by subculturing twice, each time at an OD600 of 0.4, using a 1% (vol/vol) transfer into 5 ml of prewarmed BHI. When the second subculture reached an OD600 of 0.4, cells were treated with a total volume of 76 µl comprising FPSS (to yield final concentrations ranging from 1 µM to 128 µM) and/or DMSO, followed immediately by addition of 324 µl of either 5 M NaCl (to yield a final concentration of 0.3 M NaCl, an osmotic stress that induces σB activity [8]) or (ii) sterile distilled water. Treated cultures were then incubated at 37°C with shaking (230 rpm) for 10 min, followed by addition of 2 volumes of RNAprotect (Qiagen Inc., Valencia, CA) and subsequent incubation at room temperature for 10 min. The cells were harvested following centrifugation for 10 min at 5,000 × g, and cell pellets were stored at −80°C until RNA was extracted and DNase treated using an Ambion RiboPure kit (Ambion, Austin, TX) according to the manufacturer’s instructions. RNA concentrations and purity were assessed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE). RNA quality was analyzed using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), and only RNA with an integrity number of ≥8 was used. Each treatment was performed 3 times.
TaqMan qRT-PCR.
Transcript levels for the σB-dependent genes opuCA and gadA and the housekeeping genes rpoB and gap were quantified with TaqMan primers and probes (13, 58, 71) using RNA prepared as described above and an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) as previously described (71). qRT-PCR was also used to determine the FPSS IC50. Inhibition was measured using opuCA transcript levels determined with RNA isolated from cells treated with a series of 1:2 dilutions of FPSS, with concentrations ranging from 128 to 1 µM. These data were assessed using SigmaPlot 10.0 (SYSTAT Software Inc., Evanston, IL) standard curve analysis under the pharmacology function.
Whole-genome microarray.
cDNA labeling for microarray analyses and microarray hybridization were performed as previously described (5) using L. monocytogenes whole-genome microarrays (8, 72). Raw intensity values for all probes on each array were normalized using pin-tip LOWESS (8) in R version 2.2.1 with the LIMMA package. Signals from two replicate probes on each array were then averaged and log2 transformed. Differences in transcript levels between strains were determined using a linear model, and P values were determined using eBayes. Differences in transcript levels were considered meaningful if they met the following three criteria: (i) adjusted P values of <0.05 (ii) absolute fold changes of ≥2 and (iii) a probe cross-hybridization index (CHI) of >90%. One gene (i.e., lmo0263) fulfilled criteria (i) and (ii) but not (iii) (probe CHI was 80%) and therefore was not included in our analyses.
Gene set enrichment analysis (GSEA; Broad Institute, Cambridge, MA) (73) was used to identify gene sets that were significantly enriched among up- or downregulated genes. GSEA was run on the ranked list of log fold change values obtained from the fitted normalized data in LIMMA with 1,000 permutations and exclusion of gene sets with <5 or >2,000 members. Genes were classified into sets based on the TIGR Comprehensive Microbial Resource (http://cmr.tigr.org) subrole categories for L. monocytogenes EGD-e. False discovery rate q values of <0.25 were considered significant (73).
Caco-2 invasion assay.
L. monocytogenes invasion assays using the human colorectal adenocarcinoma epithelial cell line Caco-2 (ATCC HTB-37) were performed as described by Garner et al. (9). L. monocytogenes 10403S and ΔsigB strains were initially grown overnight in 5 ml of BHI broth at 37°C with shaking (230 rpm), then were subcultured twice, at an OD600 of 0.4, using a 1% (vol/vol) transfer into 5 ml of prewarmed BHI. When the second subculture reached an OD600 of 0.4, cells were treated with (i) FPSS to yield final concentrations of 64 µM or 8 µM (lowest concentration with full efficacy according to qRT-PCR) or (ii) DMSO as well as NaCl (0.3 M final concentration) as described above, except that treated cultures were incubated at 37°C for 30 min. For infection, the Caco-2 cells were inoculated with approximately 2 × 107 L. monocytogenes organisms; bacterial numbers were confirmed by plating on BHI agar. Four biological replicates were each performed in triplicate wells. Statistical analysis was performed using one-way analysis of variance (ANOVA) and Tukey’s studentized range (honestly significant difference [HSD]) test, performed in SAS 9.0 (SAS Institute).
β-Galactosidase enzyme assays in B. subtilis.
B. subtilis strain PB198 (amyE::pDH32-ctc trpC2 [74]) and the otherwise isogenic ΔsigB strain PB345 (amyE::pDH32-ctc sigB∆3::spc trpC2 [75]) were used as reporter strains for measuring σB activity (Table S5). Effects of FPSS on the σA housekeeping sigma factor in B. subtilis were tested using B. subtilis strain PB252 (amyE::PA-lacZ trpC2 [30]). Strains were grown overnight in 5 ml of buffered Luria-Bertani (LB) broth at 37°C with shaking (230 rpm) and then were subcultured twice, at an OD600 of 0.4, using a 1% (vol/vol) transfer into 5 ml of prewarmed LB. When the second subculture reached OD600 of 0.4, cells were treated with (i) FPSS (8 or 64 µM) or DMSO only (as a control) and (ii) DMSO as well as NaCl (0.3 M final concentration) as described above, with treated cultures being incubated at 37°C for 30 min. After this incubation, the OD600 was recorded, and 0.2 ml of the culture was added to a tube containing 2.8 ml Z buffer, followed by the addition of 20 µl toluene to permeabilize the cells. A prewarmed 0.4-ml volume of 4-mg/ml ortho-nitrophenyl-β-galactoside (ONPG) was added, and the time of addition was noted. After 85 min, 1 ml of 1 M sodium carbonate was added to stop the reaction, and the OD420 was read. Miller units were calculated as previously described (76). β-Galactosidase activity results were analyzed using one-way ANOVA and Tukey’s studentized range (HSD) test.
Microarray data accession number.
Data from microarray experiments were submitted to the Gene Expression Omnibus (GEO) database, assigned accession number GSE16887, and approved.
SUPPLEMENTAL MATERIAL
Scatterplot generated from high-throughput screens of multiple small-molecule libraries with L. monocytogenes strain bearing a σB-dependent opuCA-gus fusion. Z scores shown in the plot were calculated from GUS activities in relative fluorescent units (RFU) normalized to cell density (in OD600 units) for 9,985 of the approximately 57,000 small molecules that tested in duplicate in the initial screen. Red dots represent DMSO controls; blue dots represent the small molecules tested. Dots in the upper right corner box labeled “induction” represent compounds that induced σB activity, indicated by a Z score of ≥3; dots in the lower left corner box labeled “inhibition” represent compounds that inhibited σB activity, indicated by a Z score of ≤−3. Download Figure S1, TIF file, 0.5 MB.
Scatterplot of Z scores from small-molecule microarray screen. Three-dimensional scatterplot of Z scores calculated from normalized fluorescence intensity resulting from interaction between σB and printed small-molecule ligands. Arrays were tested in triplicate, and bound His-tagged σB was detected using Alexa Fluor 647-labeled anti-His antibody. Red dots represent DMSO controls; blue dots are the small-molecule ligands tested. This plot represents all data from all three replicate experiments performed with the array that includes 8,500 compounds created by diversity-oriented synthesis. Download Figure S2, TIF file, 1.8 MB.
Caco-2 cell invasion efficiencies of L. monocytogenes treated with FPSS. Strains and corresponding treatments are indicated on the x axis, including L. monocytogenes 10403S (wt) treated with 0.3 M NaCl, 10403S treated with 0.3 M NaCl and 64 µM FPSS, 10403S treated with 0.3 M NaCl and 8 µM FPSS, and the ΔsigB strain treated with 0.3 M NaCl. Invasion efficiencies were calculated as the number of bacteria recovered relative to the number of bacteria used for inoculation (i.e., log[CFU/ml recovered/CFU/ml inoculated]). Data represent the means of four biological replicates, each performed in triplicate wells. Different letters on the bars indicate strains or treatments that differed significantly (P < 0.05; GLM Tukey). Download Figure S3, TIF file, 0.7 MB.
Small molecule libraries used for screening in this study.
Summary of genes previously identified as positively regulated by σB in L. monocytogenes 10403S and EGD-e and effects of FPSS on their transcript levels.
Comparison of genes downregulated by FPSS and σB-dependent genes previously identified in L. monocytogenes 10403S and EGD-e.
Comparison of genes upregulated by FPSS and σB-dependent genes previously identified in L. monocytogenes 10403S and EGD-e.
Strains used in this study.
ACKNOWLEDGMENTS
We thank C. W. Price and T. A. Gaidenko for the gift of B. subtilis strains used in this study and for helpful discussions. We also thank T. Bergholz for discussions regarding statistical analyses. The project described was supported National Institutes of Health award no. 5R01AI052151-07 (to K. J. B.). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This project has been funded in part with federal funds from the National Cancer Institute’s Initiative for Chemical Genetics, National Institutes of Health, under contract no. N01-CO-12400 and was performed with the assistance of the Chemical Biology Platform of the Broad Institute of Harvard and MIT. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Service, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Footnotes
Citation Palmer ME, Chaturongakul S, Wiedmann M, and Boor KJ. 2011. The Listeria monocytogenes σB regulon and its virulence-associated functions are inhibited by a small molecule. mBio 2(6):e00241-11. doi:10.1128/mBio.00241-11.
REFERENCES
- 1. Scallan E, et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oliver HF, Boor KJ, Wiedmann M. 2007. Environmental reservoir and transmission into the mammalian host, p 111–138 In Goldfine H, Shen H, Pathogenesis and host response of Listeria monocytogenes. Springer-Verlag, New York, NY. [Google Scholar]
- 3. Wesley IV. 1999. Listeriosis in animals, p 39–73 In Ryser ET, Marth EH, Listeria, listeriosis, and food safety, 2nd ed. M Decker Inc, New York, NY. [Google Scholar]
- 4. Chaturongakul S, Raengpradub S, Wiedmann M, Boor KJ. 2008. Modulation of stress and virulence in Listeria monocytogenes. Trends Microbiol. 16:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ollinger J, Bowen B, Wiedmann M, Boor KJ, Bergholz TM. 2009. Listeria monocytogenes σB modulates PrfA-mediated virulence factor expression. Infect. Immun. 77:2113–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toledo-Arana A, et al. 2009. The Listeria transcriptional landscape from saprophytism to virulence. Nature 459:950–956 [DOI] [PubMed] [Google Scholar]
- 7. Kazmierczak MJ, Mithoe SC, Boor KJ, Wiedmann M. 2003. Listeria monocytogenes σB regulates stress response and virulence functions. J. Bacteriol. 185:5722–5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raengpradub S, Wiedmann M, Boor KJ. 2008. Comparative analysis of the σB-dependent stress responses in Listeria monocytogenes and Listeria innocua strains exposed to selected stress conditions. Appl. Environ. Microbiol. 74:158–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garner MR, Njaa BL, Wiedmann M, Boor KJ. 2006. Sigma B contributes to Listeria monocytogenes gastrointestinal infection but not to systemic spread in the guinea pig infection model. Infect. Immun. 74:876–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Camejo A, et al. 2009. In vivo transcriptional profiling of Listeria monocytogenes and mutagenesis identify new virulence factors involved in infection. PLoS Pathog. 5:e1000449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nadon CA, Bowen BM, Wiedmann M, Boor KJ. 2002. Sigma B contributes to PrfA-mediated virulence in Listeria monocytogenes. Infect. Immun. 70:3948–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rauch M, Luo Q, Müller-Altrock S, Goebel W. 2005. SigB-dependent in vitro transcription of prfA and some newly identified genes of Listeria monocytogenes whose expression is affected by PrfA in vivo. J. Bacteriol. 187:800–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwab U, Bowen B, Nadon C, Wiedmann M, Boor KJ. 2005. The Listeria monocytogenes prfAP2 promoter is regulated by sigma B in a growth phase dependent manner. FEMS Microbiol. Lett. 245:329–336 [DOI] [PubMed] [Google Scholar]
- 14. Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. 2005. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 310:670–674 [DOI] [PubMed] [Google Scholar]
- 15. Sue D, Boor KJ, Wiedmann M. 2003. σB-dependent expression patterns of compatible solute transporter genes opuCA and lmo1421 and the conjugated bile salt hydrolase gene bsh in Listeria monocytogenes. Microbiology 149:3247–3256 [DOI] [PubMed] [Google Scholar]
- 16. Hain T, et al. 2008. Temporal transcriptomic analysis of the Listeria monocytogenes EGD-e sigmaB regulon. BMC Microbiol. 8:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oliver HF, et al. 2009. Deep RNA sequencing of L. monocytogenes reveals overlapping and extensive stationary phase and sigma B-dependent transcriptomes, including multiple highly transcribed noncoding RNAs. BMC Genomics 10:641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chatterjee SS, et al. 2006. Invasiveness is a variable and heterogeneous phenotype in Listeria monocytogenes serotype strains. Int. J. Med. Microbiol. 296:277–286 [DOI] [PubMed] [Google Scholar]
- 19. Becker LA, Cetin MS, Hutkins RW, Benson AK. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Milohanic E, et al. 2003. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol. Microbiol. 47:1613–1625 [DOI] [PubMed] [Google Scholar]
- 21. Lai Y, et al. 2007. The human anionic antimicrobial peptide dermcidin induces proteolytic defence mechanisms in staphylococci. Mol. Microbiol. 63:497–506 [DOI] [PubMed] [Google Scholar]
- 22. Chatterjee SS, et al. 2006. Intracellular gene expression profile of Listeria monocytogenes. Infect. Immun. 74:1323–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marino M, Banerjee M, Jonquières R, Cossart P, Ghosh P. 2002. GW domains of the Listeria monocytogenes invasion protein InlB are SH3-like and mediate binding to host ligands. EMBO J. 21:5623–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joseph B, et al. 2006. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J. Bacteriol. 188:556–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams T, Joseph B, Beier D, Goebel W, Kuhn M. 2005. Response regulator DegU of Listeria monocytogenes regulates the expression of flagella-specific genes. FEMS Microbiol. Lett. 252:287–298 [DOI] [PubMed] [Google Scholar]
- 26. Knudsen GM, Olsen JE, Dons L. 2004. Characterization of DegU, a response regulator in Listeria monocytogenes, involved in regulation of motility and contributes to virulence. FEMS Microbiol. Lett. 240:171–179 [DOI] [PubMed] [Google Scholar]
- 27. Bennett HJ, et al. 2007. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol. Microbiol. 63:1453–1467 [DOI] [PubMed] [Google Scholar]
- 28. Gründling A, Burrack LS, Bouwer HG, Higgins DE. 2004. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc. Natl. Acad. Sci. U. S. A. 101:12318–12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen A, Higgins DE. 2006. The MogR transcriptional repressor regulates nonhierarchical expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathog. 2:e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wise AA, Price CW. 1995. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor sigma B in response to environmental signals. J. Bacteriol. 177:123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lieberman LA, Higgins DE. 2009. A small-molecule screen identifies the antipsychotic drug pimozide as an inhibitor of Listeria monocytogenes infection. Antimicrob. Agents Chemother. 53:756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Schaik W, Tempelaars MH, Wouters JA, de Vos WM, Abee T. 2004. The alternative sigma factor σB of Bacillus cereus: response to stress and role in heat adaptation. J. Bacteriol. 186:316–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Schaik W, et al. 2007. Identification of the σB regulon of Bacillus cereus and conservation of σB-regulated genes in low-GC-content Gram-positive bacteria. J. Bacteriol. 189:4384–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fouet A, Namy O, Lambert G. 2000. Characterization of the operon encoding the alternative σB factor from Bacillus anthracis and its role in virulence. J. Bacteriol. 182:5036–5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen HY, et al. 2011. Vancomycin activates σB in vancomycin-resistant Staphylococcus aureus resulting in the enhancement of cytotoxicity. PLoS One 6:e24472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kusch K, et al. 2011. The influence of SaeRS and σB on the expression of superantigens in different Staphylococcus aureus isolates. Int. J. Med. Microbiol. 301:488–499 [DOI] [PubMed] [Google Scholar]
- 37. Knobloch JK, et al. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183:2624–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bischoff M, et al. 2004. Microarray-based analysis of the Staphylococcus aureus σB regulon. J. Bacteriol. 186:4085–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hecker M, Völker U. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 44:35–91 [DOI] [PubMed] [Google Scholar]
- 40. Kim H, Boor KJ, Marquis H. 2004. Listeria monocytogenes σB contributes to invasion of human intestinal epithelial cells. Infect. Immun. 72:7374–7378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O’Neil HS, Marquis H. 2006. Listeria monocytogenes flagella are used for motility, not as adhesins, to increase host cell invasion. Infect. Immun. 74:6675–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Way SS, et al. 2004. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell. Microbiol. 6:235–242 [DOI] [PubMed] [Google Scholar]
- 43. Bowser TE, et al. 2007. Novel anti-infection agents: small-molecule inhibitors of bacterial transcription factors. Bioorg. Med. Chem. Lett. 17:5652–5655 [DOI] [PubMed] [Google Scholar]
- 44. Kim OK, et al. 2009. N-Hydroxybenzimidazole inhibitors of the transcription factor LcrF in Yersinia: novel antivirulence agents. J. Med. Chem. 52:5626–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Glaser BT, Bergendahl V, Thompson NE, Olson B, Burgess RR. 2007. LRET-based HTS of a small-compound library for inhibitors of bacterial RNA polymerase. Assay Drug Dev. Technol. 5:759–768 [DOI] [PubMed] [Google Scholar]
- 46. Li Z, Demple B. 1994. SoxS, an activator of superoxide stress genes in Escherichia coli. Purification and interaction with DNA. J. Biol. Chem. 269:18371–18377 [PubMed] [Google Scholar]
- 47. Bina J, et al. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci. U. S. A. 100:2801–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Finlay BB, Falkow S. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moellering RC, Jr., Medoff G, Leech I, Wennersten C, Kunz LJ. 1972. Antibiotic synergism against Listeria monocytogenes. Antimicrob. Agents Chemother. 1:30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sleator RD, Wouters J, Gahan CGM, Abee T, Hill C. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl. Environ. Microbiol. 67:2692–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cotter PD, Gahan CGM, Hill C. 2001. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 40:465–475 [DOI] [PubMed] [Google Scholar]
- 52. Bischoff M, Entenza JM, Giachino P. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 183:5171–5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lorenz U, et al. 2008. The alternative sigma factor sigma B of Staphylococcus aureus modulates virulence in experimental central venous catheter-related infections. Microbes Infect. 10:217–223 [DOI] [PubMed] [Google Scholar]
- 54. Jonsson I-M, Arvidson S, Foster S, Tarkowski A. 2004. Sigma factor B and RsbU are required for virulence in Staphylococcus aureus-induced arthritis and sepsis. Infect. Immun. 72:6106–6111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bishop DK, Hinrichs DJ. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005–2009 [PubMed] [Google Scholar]
- 56. Wiedmann M, Arvik TJ, Hurley RJ, Boor KJ. 1998. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferreira A, Sue D, O’Byrne CP, Boor KJ. 2003. Role of Listeria monocytogenes σB in survival of lethal acidic conditions and in the acquired acid tolerance response. Appl. Environ. Microbiol. 69:2692–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sue D, Fink D, Wiedmann M, Boor KJ. 2004. σB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology 150:3843–3855 [DOI] [PubMed] [Google Scholar]
- 59. Premaratne RJ, Lin WJ, Johnson EA. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ferreira A, O’Byrne CP, Boor KJ. 2001. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl. Environ. Microbiol. 67:4454–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shen A, Higgins DE. 2005. The 5′ untranslated region-mediated enhancement of intracellular listeriolysin O production is required for Listeria monocytogenes pathogenicity. Mol. Microbiol. 57:1460–1473 [DOI] [PubMed] [Google Scholar]
- 62. Kelly KA, Clemons PA, Yu AM, Weissleder R. 2006. High-throughput identification of phage-derived imaging agents. Mol. Imaging 5:24–30 [PubMed] [Google Scholar]
- 63. Kim YK, et al. 2004. Relationship of stereochemical and skeletal diversity of small molecules to cellular measurement space. J. Am. Chem. Soc. 126:14740–14745 [DOI] [PubMed] [Google Scholar]
- 64. Seiler KP, et al. 2008. ChemBank: a small-molecule screening and cheminformatics resource database. Nucleic Acids Res. 36:D351–D359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Strausberg RL, Schreiber SL. 2003. From knowing to controlling: a path from genomics to drugs using small molecule probes. Science 300:294–295 [DOI] [PubMed] [Google Scholar]
- 66. Bradner JE, McPherson OM, Koehler AN. 2006. A method for the covalent capture and screening of diverse small molecules in a microarray format. Nat. Protoc. 1:2344–2352 [DOI] [PubMed] [Google Scholar]
- 67. Bradner JE, et al. 2006. A robust small-molecule microarray platform for screening cell lysates. Chem. Biol. 13:493–504 [DOI] [PubMed] [Google Scholar]
- 68. Duffner JL, Clemons PA, Koehler AN. 2007. A pipeline for ligand discovery using small-molecule microarrays. Curr. Opin. Chem. Biol. 11:74–82 [DOI] [PubMed] [Google Scholar]
- 69. Böckmann R, Dickneite C, Middendorf B, Goebel W, Sokolovic Z. 1996. Specific binding of the Listeria monocytogenes transcriptional regulator PrfA to target sequences requires additional factor(s) and is influenced by iron. Mol. Microbiol. 22:643–653 [DOI] [PubMed] [Google Scholar]
- 70. Dashkevicz MP, Feighner SD. 1989. Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl. Environ. Microbiol. 55:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kazmierczak MJ, Wiedmann M, Boor KJ. 2006. Contributions of Listeria monocytogenes σB and PrfA to expression of virulence and stress response genes during extra- and intracellular growth. Microbiology 152:1827–1838 [DOI] [PubMed] [Google Scholar]
- 72. Chan YC, Boor KJ, Wiedmann M. 2007. σB-dependent and σB-independent mechanisms contribute to transcription of Listeria monocytogenes cold stress genes during cold shock and cold growth. Appl. Environ. Microbiol. 73:6019–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Subramanian A, et al. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 102:15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boylan SA, Rutherford A, Thomas SM, Price CW. 1992. Activation of Bacillus subtilis transcription factor sigma B by a regulatory pathway responsive to stationary-phase signals. J. Bacteriol. 174:3695–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Boylan SA, Redfield AR, Brody MS, Price CW. 1993. Stress-induced activation of the σB transcription factor of Bacillus subtilis. J. Bacteriol. 175:7931–7937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang X, Bremer H. 1995. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J. Biol. Chem. 270:11181–11189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatterplot generated from high-throughput screens of multiple small-molecule libraries with L. monocytogenes strain bearing a σB-dependent opuCA-gus fusion. Z scores shown in the plot were calculated from GUS activities in relative fluorescent units (RFU) normalized to cell density (in OD600 units) for 9,985 of the approximately 57,000 small molecules that tested in duplicate in the initial screen. Red dots represent DMSO controls; blue dots represent the small molecules tested. Dots in the upper right corner box labeled “induction” represent compounds that induced σB activity, indicated by a Z score of ≥3; dots in the lower left corner box labeled “inhibition” represent compounds that inhibited σB activity, indicated by a Z score of ≤−3. Download Figure S1, TIF file, 0.5 MB.
Scatterplot of Z scores from small-molecule microarray screen. Three-dimensional scatterplot of Z scores calculated from normalized fluorescence intensity resulting from interaction between σB and printed small-molecule ligands. Arrays were tested in triplicate, and bound His-tagged σB was detected using Alexa Fluor 647-labeled anti-His antibody. Red dots represent DMSO controls; blue dots are the small-molecule ligands tested. This plot represents all data from all three replicate experiments performed with the array that includes 8,500 compounds created by diversity-oriented synthesis. Download Figure S2, TIF file, 1.8 MB.
Caco-2 cell invasion efficiencies of L. monocytogenes treated with FPSS. Strains and corresponding treatments are indicated on the x axis, including L. monocytogenes 10403S (wt) treated with 0.3 M NaCl, 10403S treated with 0.3 M NaCl and 64 µM FPSS, 10403S treated with 0.3 M NaCl and 8 µM FPSS, and the ΔsigB strain treated with 0.3 M NaCl. Invasion efficiencies were calculated as the number of bacteria recovered relative to the number of bacteria used for inoculation (i.e., log[CFU/ml recovered/CFU/ml inoculated]). Data represent the means of four biological replicates, each performed in triplicate wells. Different letters on the bars indicate strains or treatments that differed significantly (P < 0.05; GLM Tukey). Download Figure S3, TIF file, 0.7 MB.
Small molecule libraries used for screening in this study.
Summary of genes previously identified as positively regulated by σB in L. monocytogenes 10403S and EGD-e and effects of FPSS on their transcript levels.
Comparison of genes downregulated by FPSS and σB-dependent genes previously identified in L. monocytogenes 10403S and EGD-e.
Comparison of genes upregulated by FPSS and σB-dependent genes previously identified in L. monocytogenes 10403S and EGD-e.
Strains used in this study.