Abstract
Type 1 diabetes develops when most insulin-producing β cells of the pancreas are killed by an autoimmune attack. The in vivo conditions modulating the sensitivity and resistance of β cells to this attack remain largely obscure. Here, we show that connexin 36 (Cx36), a trans-membrane protein that forms gap junctions between β cells in the pancreatic islets, protects mouse β cells against both cytotoxic drugs and cytokines that prevail in the islet environment at the onset of type 1 diabetes. We documented that this protection was at least partially dependent on intercellular communication, which Cx36 and other types of connexin channels establish within pancreatic islets. We further found that proinflammatory cytokines decreased expression of Cx36 and that experimental reduction or augmentation of Cx36 levels increased or decreased β cell apoptosis, respectively. Thus, we conclude that Cx36 is central to β cell protection from toxic insults.
Introduction
Pancreatic β cells are functionally heterogeneous (1) and interconnected by channels made of connexin 36 (Cx36) (2–4), a member of the connexin (Cx) protein family (5). Previous studies have implicated this protein in the synchronization of stimulus-induced Ca2+ waves between β cells and the electrotonic spread of depolarizing and hyperpolarizing currents within the islets (6–8), as well as in the regulation of basal and stimulated insulin secretion (6–10) and insulin expression (3). Here, we have investigated whether this protein also controls β cell survival. We tested the effects of streptozotocin (STZ) and alloxan (AX), 2 drugs that mimic in laboratory rodents the selective and massive death of β cells observed in type 1 diabetes (11), on a series of null and transgenic mice whose β cells feature various Cx and coupling patterns (7, 8, 12–14). We further tested the in vitro resistance of islets isolated from these mice to 3 Th1 cytokines that are implicated at the onset of type 1 diabetes (15, 16). We then investigated the mechanisms linking these cytokines, β cell apoptosis, and Cx36 in primary pancreatic islets and several lines of insulin-producing cells in which Cx36 was experimentally down- or upregulated.
Results
Comparison of mice featuring β cells with different Cx patterns.
To test the influence of Cx on the in vivo resistance of β cells, we compared mice whose β cells (a) differed in Cx36 gene dosage (Cx36+/+, Cx36+/–, Cx36–/–; refs. 7, 8, 13); (b) were specifically targeted, through the control of the rat insulin promoter (RIP), for the expression of transgenic Cx36 (RIP-Cx36+/–, RIP-Cx36+/+; ref. 14); or (c) coexpressed Cx36 with either Cx32 (RIP-Cx32+/–, RIP-Cx32+/+; ref. 11) or Cx43 (RIP-Cx43+/–, RIP-Cx43+/+; ref. 14), whose de novo β cell–specific expression was also directed by RIP. The control mice of all lines expressed Cx36, even though the native levels of this Cx were higher in Cx36+/+ mice than in RIP-Cx36–/–, RIP-Cx32–/–, and RIP-Cx43–/– mice (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI40509DS1). Cx36–/– mice did not express Cx36 (Supplemental Figure 1). RIP-Cx36+/– and RIP-Cx36+/+ mice expressed higher levels of Cx36 than did Cx36+/+ and RIP-Cx36–/– mice (Supplemental Figure 1). RIP-Cx32 and RIP-Cx43 mice expressed Cx32 and Cx43, respectively, in addition to native Cx36 (Supplemental Figures 1 and 2). The international nomenclature of the mouse lines, and the abbreviated names used herein, are shown in Supplemental Figure 3. Experiments were performed with null and transgenic mice that had been backcrossed with C57BL/6J mice for 5–10 (Cx36) or 3–8 (RIP-Cx36, RIP-Cx32, and RIP-Cx43) generations.
Loss of Cx36 sensitizes mice to the effects of cytotoxic drugs.
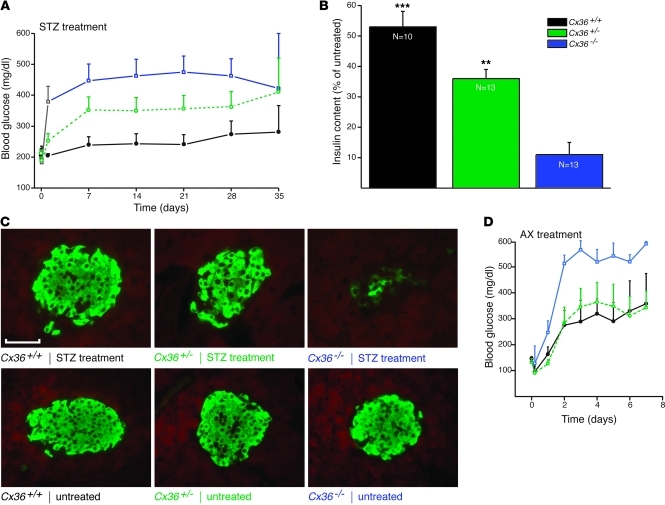
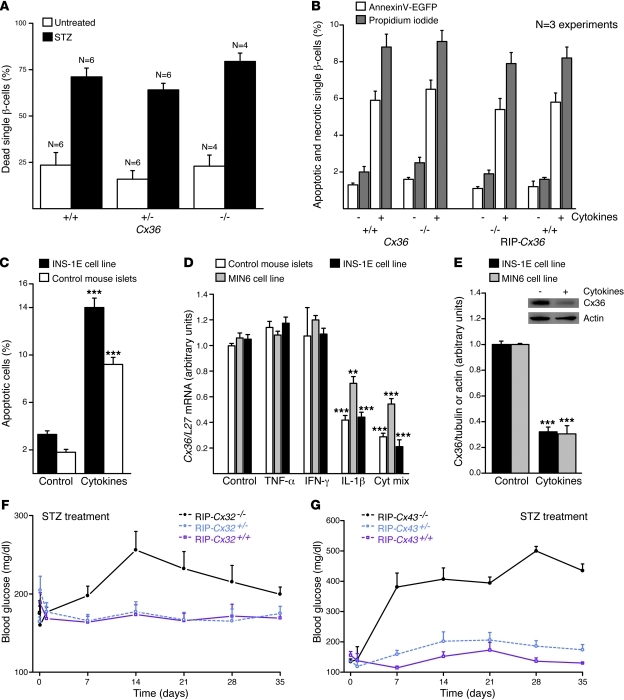
To test whether reduced levels of Cx36 modified the in vivo resistance of β cells, mice of the Cx36 line were given a single i.p. injection of 200 mg/kg BW STZ. The drug caused Cx36+/+ mice to develop a mild hyperglycemia (Figure 1A). This alteration was more pronounced in Cx36+/– littermates, and even further enhanced in Cx36–/– littermates (Figure 1A), which lack Cx36 (refs. 7, 8, 13, and Supplemental Figure 1). These differences were the result of a larger drop in insulin content and in the mass of residual β cells (Figure 1, B and C). After injection of 60 mg/kg BW AX, a drug that kills β cells by a different mechanism than STZ (11), Cx36–/– mice also became more hyperglycemic than Cx36+/+ and Cx36+/– littermates (Figure 1D). These experiments showed that loss of Cx36 sensitized β cells to the in vivo effects of 2 cytotoxic drugs.
Figure 1. Mice lacking Cx36 are sensitized to the toxic effects of STZ and AX.
(A) A single injection of 200 mg/kg STZ induced mild hyperglycemia in control Cx36+/+ mice (black; n = 9). This hyperglycemia was more pronounced in Cx36+/– (green; n = 14) and Cx36–/– (blue; n = 14) littermates, which expressed reduced and nil doses, respectively, of the Cx36 gene. (B) 5 weeks after STZ injection, pancreatic insulin content was highest in control Cx36+/+ mice, lowest in Cx36–/– mice, and intermediate in Cx36+/– mice. Values are expressed as percent of untreated; n as indicated. **P < 0.01, ***P < 0.001 versus Cx36–/–. (C) At the same time point, the proportion of surviving β cells, identified by insulin immunostaining, was highest in Cx36+/+, lowest in Cx36–/–, and intermediate in Cx36+/– mice. Compared with insulin staining of corresponding untreated mice, STZ-treated Cx36–/– mice revealed major loss of β cells. Scale bar: 120 μm. (D) A single injection of 60 mg/kg AX induced sustained hyperglycemia in Cx36+/+ (black; n = 4) and Cx36+/– (green; n = 4) mice. This hyperglycemia was significantly more pronounced in Cx36–/– littermates (blue; n = 5). All data are mean ± SEM.
Overexpression of Cx36 protects transgenic mice against cytotoxic drugs.
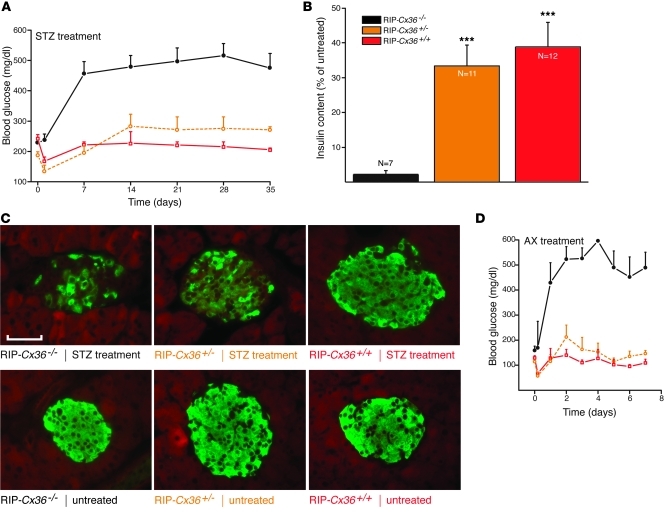
To test whether increased levels of Cx36 could protect β cells, we repeated the experiments in transgenic mice of the RIP-Cx36 line, which we developed to overexpress Cx36 in β cells (Supplemental Figures 3 and 4). RIP-Cx36–/– mice, which had the same Cx36 gene dosage as Cx36+/+ mice, expressed lower levels of Cx36 protein (Supplemental Figures 1, 3, and 4) and became more hyperglycemic after injection of STZ (Figure 2A), caused by a marked loss of pancreatic β cells and insulin content (Figure 2, B and C). These alterations were significantly decreased in RIP-Cx36+/– and RIP-Cx36+/+ littermates, which featured increased insulin content and levels of Cx36 (Figure 2, A–C, and Supplemental Figures 1 and 4). Comparable observations were made after injection of AX (Figure 2D). These experiments showed that increased expression of Cx36 protected β cells against the in vivo effects of 2 cytotoxic drugs.
Figure 2. Mice overexpressing islet Cx36 are protected against STZ and AX.
(A) STZ-induced hyperglycemia was observed in RIP-Cx36–/– mice (black; n = 15); in contrast, RIP-Cx36+/– (orange; n = 13) and RIP-Cx36+/+ (red; n = 11) mice did not become hyperglycemic for the duration of the experiment. (B) 5 weeks after STZ injection, residual insulin content was significantly higher in the pancreas of RIP-Cx36+/+ and RIP-Cx36+/– mice than in that of RIP-Cx36–/– littermates. Values are expressed as percent of the insulin content of untreated mice; n as indicated. ***P < 0.001 versus RIP-Cx36–/–. (C) The proportion of surviving insulin-containing β cells of RIP-Cx36+/+ and RIP-Cx36+/– mice was substantially higher than that of RIP-Cx36–/– littermates. As compared with insulin staining of the corresponding untreated islets, most β cells in RIP-Cx36+/– and RIP-Cx36+/+ islets were found to survive STZ treatment. Scale bar: 120 μm. (D) AX-induced hyperglycemia was also observed in RIP-Cx36–/– mice (black; n = 6), but not in RIP-Cx36+/– (orange; n = 4) and RIP-Cx36+/+ (red; n = 3) littermates. All data are mean ± SEM.
Pancreatic islets are protected by Cx36 in vitro.
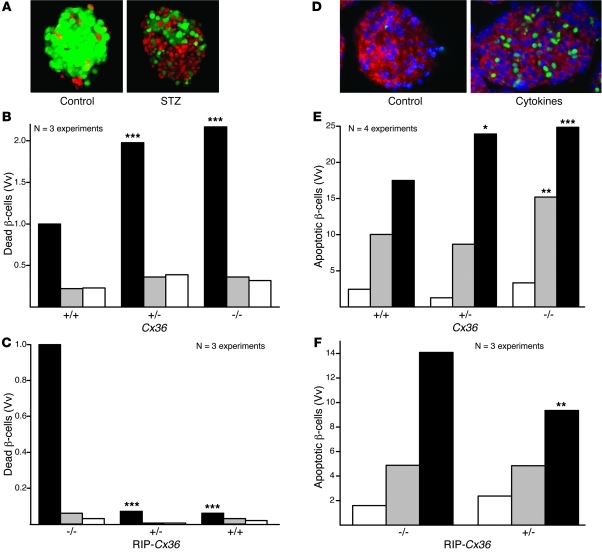
To assess whether the Cx36-dependent protection was an intrinsic property of pancreatic islets, we exposed islets isolated from Cx36 and RIP-Cx36 mice to STZ. After 15 hours of exposure to 2.2 mM (Cx36 line) or 4.4 mM (RIP-Cx36 line) STZ, the islets of Cx36+/+ and RIP-Cx36–/– mice, which had the same Cx36 dosage, featured a comparable proportion of ethidium bromide–labeled (EB-labeled) dead cells (Figure 3, A–C). This proportion was increased in the islets of Cx36–/– mice (Figure 3B). Islets from RIP-Cx36+/– and RIP-Cx36+/+ mice contained mostly living, calcein-labeled cells (Figure 3C). These observations showed that in 2 independent mouse lines, islets differing in Cx36 expression also differed in sensitivity to STZ.
Figure 3. Cx36 protects β cells from Th1 cytokines.
(A) Islets isolated from C57BL/6 mice showed much more healthy cells (calcein stain; green) than dead cells (EB stain; red). The proportion of dead cells substantially increased after 24 hours exposure to STZ. (B) In islets of Cx36–/– mice, STZ (black) increased the volume density (Vv) of dead cells over that in untreated islets (white) and in islets exposed to citrate buffer (gray). The volume density of dead β cells was lower in the islets of STZ-treated Cx36+/+ mice than in those of Cx36+/– and Cx36–/– littermates. (C) Dead cell volume density was significantly higher in STZ-treated RIP-Cx36–/– islets than in islets of RIP-Cx36+/– and RIP-Cx36+/+ littermates. (D) TUNEL labeling showed that Cx36+/+ islets contained rare apoptotic cells (green). The number of apoptotic cells increased after 24 hours exposure to IL-1β, IFN-γ, and TNF-α. (E) Exposure of Cx36–/– islets to either IL-1β and IFN-γ (gray) or IL-1β, IFN-γ, and TNF-α (black) increased the volume density of apoptotic cells over that in untreated mice (white). Apoptosis was lower in the islets of cytokine-treated Cx36+/+ mice than in those of Cx36+/– and Cx36–/– littermates. (F) The volume density of apoptotic cells was higher in RIP-Cx36–/– islets than in islets of RIP-Cx36+/– littermates exposed to the 3 cytokines. Values are medians of the indicated number of experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus respective cognate control (Cx36+/+ or RIP-Cx36–/–).
To investigate such a difference, we assessed whether increased levels of Cx36 abolished the expression and function of the GLUT-2 transporter, preventing the uptake of both STZ and AX (11, 17, 18), in islets of RIP-Cx36 mice. Immunolabeling of pancreatic sections showed expression of GLUT-2 in most β cells of all mouse genotypes and proper localization of the protein at the cell membrane (Supplemental Figure 5C). Most β cells also incorporated 6-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino)-6-deoxyglucose (NDBG), a nonhydrolyzable analog of glucose that enters β cells only via GLUT-2 (ref. 17 and Supplemental Figure 5D). These experiments showed that the resistance of RIP-Cx36 islets against STZ and AX could not be attributed to loss of the transporter whereby these drugs enter β cells.
To test whether Cx36 also protects β cells against endogenous signals implicated in the pathogenesis of type 1 diabetes, we exposed islets to a cytokine mix of 50 U/ml IL-1β, 1,000 U/ml IFN-γ, and 1,000 U/ml TNF-α for a 24-hour period (15, 16, 19, 20) and monitored β cell apoptosis. In islets of RIP-Cx36–/– and Cx36+/+ mice, which had a similar Cx36 gene dosage, TUNEL labeling showed that 2 cytokines in combination — and all 3 even more so — increased the proportion of apoptotic β cells (Figure 3, D–F). Higher numbers of apoptotic β cells were found in islets of Cx36+/– and Cx36–/– mice (Figure 3E). Conversely, lower numbers of apoptotic β cells were found in islets of RIP-Cx36+/– mice (Figure 3F), which showed increased levels of Cx36 compared with RIP-Cx36–/– mice (Supplemental Figures 1, 2, and 4). Our findings indicated that Cx36 protected β cells against the cytokines found in the islet environment at the early stages of an autoimmune attack.
Th1 cytokines similarly activate apoptotic pathways in β cells featuring different Cx36 expression.
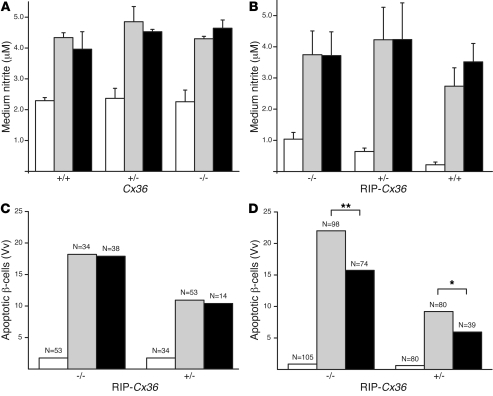
To investigate the molecular mechanism of the β cell protection, we first assessed the production of NO, which is released by β cells exposed to cytokines (19, 20). We found that similar levels of nitrite, a stable product of NO oxidation, accumulated in the media of all islets exposed for 24 hours to IL-1β, IFN-γ, and TNF-α, regardless of the Cx36 levels of their β cells (Figure 4, A and B). Immunolabeling for cytochrome C resulted in the staining of bright spots, compatible with a mitochondrial localization of the enzyme, in both MIN6 cells and primary β cells of untreated Cx36+/+ and RIP-Cx36–/– mice (Supplemental Figure 6, A–C). After exposure to the cytokines, which caused β cell apoptosis (Figure 4, C and D), many MIN6 cells and β cells of Cx36–/– and RIP-Cx36–/– islets showed less punctate and more homogeneous labeling (Supplemental Figure 6, A–C), consistent with the release of cytochrome C into the cytosol. The cytokine-induced apoptosis was unaffected by cyclosporin A in both RIP-Cx36–/– and RIP-Cx36+/– islets (Figure 4C), which indicated that under these conditions, cytochrome C was not released through the canonical permeation transition pore (19–22). Furthermore, apoptosis was similarly inhibited in RIP-Cx36–/– (P < 0.01) and RIP-Cx36+/– (P < 0.05) islets by exposure to 50 μM of the pan-caspase inhibitor Z-Val-Ala-Asp(OMe)-FMK (Z-VAD), added 1 hour before exposing the islets to cytokines and maintained throughout the 24-hour duration of the experiments (Figure 4D). These findings indicate that islets featuring different Cx36 levels were similar with regard to the receptors and signaling cascades that lead to caspase-dependent β cell apoptosis.
Figure 4. Cytokines activate apoptotic pathways in β cells of mice from both Cx36 and RIP-Cx36 lines.
(A and B) A 24-hour exposure to either IL-1β plus IFN-γ (gray) or IL-1β, IFN-γ, and TNF-α (black) increased NO production to the same extent in islets isolated from mice of Cx36 (A) and RIP-Cx36 (B) lines. Values are mean ± SEM of the indicated number of independent experiments. (C) Exposure to 10 μM cyclosporin A (black) did not decrease the cytokine-induced (gray) apoptosis of RIP-Cx36 β cells. (D) β cell apoptosis increased over basal levels (white) in RIP-Cx36 islets isolated and exposed for 24 hours to a mix of IL-1β, IFN-γ, and TNF-α (gray). This increase was larger in islets isolated from RIP-Cx36–/– than in those of RIP-Cx36+/– littermates. In both groups, incubation in the presence of Z-VAD (black; added 1 hour prior to the cytokine and maintained throughout the incubation period) reduced the cytokine-induced apoptosis. Values are medians of the indicated number of islets. White bars throughout denote untreated (basal) levels. *P < 0.05, **P < 0.01.
Junctional signaling is increased in the protected islets.
To test whether the β cell protection provided by Cx36 depends on the establishment of intercellular channels, we dissociated the cells of isolated islets. Exposure to 1.1 mM STZ for 15 hours resulted in a comparable increase of EB-stained necrotic cells from Cx36+/+, Cx36+/–, and Cx36–/– mice (Figure 5A). Because cytokines induce both necrosis and apoptosis of rodent β cells (23–26), we further isolated islet cells from the Cx36 and RIP-Cx36 lines and exposed them for 36 hours to the 3 cytokines described above. These conditions markedly increased the proportion of annexin V–EGFP– and propidium iodide–stained β cells in Cx36+/+ and RIP-Cx36–/– mice (Figure 5B and Supplemental Figure 7). Similar proportions of apoptotic and necrotic single β cells were found in samples dispersed from islets of Cx36–/– and RIP-Cx36+/+ mice (Figure 5B). Our observations indicated that all β cells featured a similar sensitivity to a cytotoxic environment when junctional coupling was abrogated.
Figure 5. β cell protection requires cell contact and Cx expression.
(A) The proportion of dead cells was similar in all control islet cell suspensions. STZ similarly increased this proportion in all groups. n as indicated. (B) Islet cells of control and homozygous mice of both Cx36 and RIP-Cx36 lines showed increased apoptosis (white) and necrosis (gray) after exposure to the cytokine mix of IL-1β, IFN-γ, and TNF-α. (C) The cytokine mix increased apoptosis of INS1E cells and control C57BL/6 islets. (D) The cytokine mix decreased Cx36 mRNA in mouse islets and in MIN6 and INS-1E cells. Values are expressed relative to L27 gene level. (E) The cytokine mix also decreased Cx36 in extracts of INS1E and MIN6 cells. Values are shown relative to the tubulin signal, normalized to control. Cx36 and actin Western blot immunolabeling, from which the quantitative data were generated, is also shown (inset). **P < 0.01, ***P < 0.001 versus corresponding control. (F) STZ injection induced hyperglycemia in RIP-Cx32–/– mice, but not in RIP-Cx32+/– and RIP-Cx32+/+ littermates. (G) RIP-Cx43–/– mice also became hyperglycemic after STZ injection, whereas RIP-Cx43+/– and RIP-Cx43+/+ littermates did not. Data are mean ± SEM of 4–6 experiments (A), of 3–5 experiments (B–E), or of 3–12 mice (F and G).
To test whether the protection dependent on Cx36 could be provided by other types of Cx channels, we tested the RIP-Cx32 and RIP-Cx43 lines. After injection of STZ, control RIP-Cx32–/– and RIP-Cx43–/– mice became hyperglycemic; in contrast, the corresponding heterozygous and homozygous littermates remained normoglycemic throughout the experiment (Figure 5, F and G). These observations suggest that other Cx isoforms can protect β cells in vivo in the presence of native levels of Cx36.
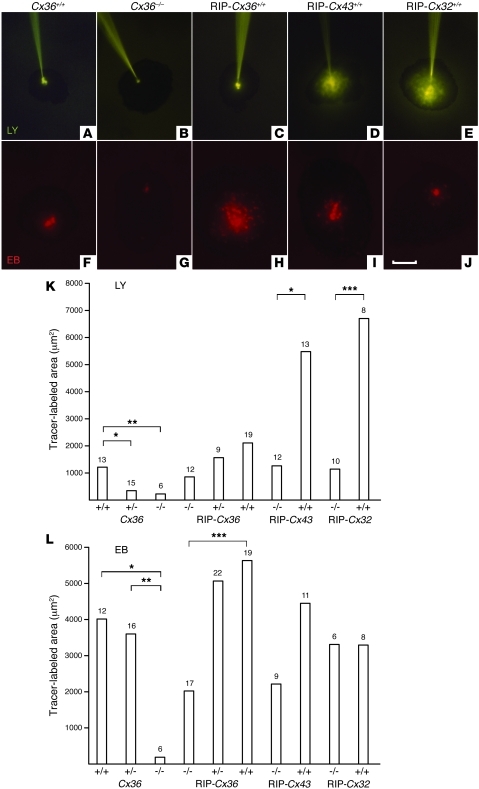
To test whether Cx-mediated coupling is a critical event in this protection, we microinjected isolated islets with either EB or Lucifer Yellow (LY), which differently permeate Cx36, Cx32, and Cx43 channels (27, 28). In islets of Cx36+/+ and RIP-Cx36–/– mice, coupling of β cells was larger when evaluated with EB than LY (Figure 6). This coupling was absent in islets of Cx36–/– mice. When tested with EB, the extent of coupling was greater in the islets of RIP-Cx36+/+ mice. β cells of RIP-Cx43–/– and RIP-Cx32–/– mice also showed a control coupling pattern. In contrast, homozygous littermates of each line featured much larger territories of coupled β cells when tested with LY (Figure 6). The incidence of coupling varied little in the different islet groups, except in the Cx36–/– mice, in which it was nil (Supplemental Figure 8).
Figure 6. Increased junctional coupling in islets resistant to β cell–toxic conditions.
(A–E) Microinjection of islets with LY showed limited β cell coupling in islets of Cx36+/+ and RIP-Cx36+/+ mice (A and C), no coupling in islets of Cx36–/– mice (B), and greater coupling in islets of RIP-Cx43+/+ and RIP-Cx32+/+ mice (D and E). (F–J) Microinjection with EB showed sizable coupling in islets of Cx36+/+, RIP-Cx43+/+, and RIP-Cx32+/+ mice (F, I, and J), no coupling in islets of Cx36–/– mice (G), and greater coupling in islets of RIP-Cx36+/+ animals (H). Scale bar: 100 μm. (K and L) Coupling extent in islets from Cx36+/+, Cx36+/–, RIP-Cx43–/–, and RIP-Cx32–/– mice, as well as all mice from the RIP-Cx36 line, was larger after EB (L) than LY (K) injection. No coupling was observed with either tracer in Cx36–/– mice. Values show medians of the indicated number of islet microinjections. *P < 0.05, **P < 0.01, ***P < 0.001, median test.
Th1 cytokines decrease Cx36 expression, and experimental alterations in Cx36 expression modulate the cytokine-induced death of insulin-producing cell lines.
We further tested whether the conditions eliciting β cell apoptosis affected the expression of Cx36. Using the insulin-producing MIN6 and INS1E cell lines, which share with β cells the native expression of Cx36 (6, 10), we found that the mix of cytokines mentioned above also elicited apoptosis and release of cytochrome C (Figure 5C and Supplemental Figure 6). Under these conditions, the levels of Cx36 mRNA and of the cognate protein were reduced in both cells (Figure 5, D and E, and Supplemental Figure 9). At least in cell lines, this decrease was mostly caused by IL-1β (Figure 5D), the cytokine that predominantly causes β cell apoptosis (24–26). Thus, the cytokines that are cytotoxic to β cells downregulated the levels of Cx36.
To test whether experimental down- and upregulation of Cx36 affected cytokine-induced apoptosis, we first transfected INS1E cells with 2 different siRNAs specifically targeting Cx36 mRNA or with an irrelevant control siRNA that did not alter the cytokine-induced downregulation of Cx36 (Supplemental Figure 9). Compared with this control, the 2 Cx36-specific siRNAs reduced by about 50% the levels of Cx36 (Supplemental Figure 9). Under these conditions, different combinations of IL-1β, TNF-α, and IFN-γ increased the incidence of INS1E cell apoptosis compared with both the control levels observed in the absence of the cytokines and the levels observed in cells exposed to these cytokines after transfection with the control siRNA (Supplemental Figure 9). In a second set of experiments, WT MIN6 cells were compared with a clone of companion cells that we selected for their spontaneously higher expression of Cx36, which was shown to be stable for years, and to a clone of MIN6 cells transfected with an antisense cDNA for mCx36 (1). Immunostaining of both intact cells and protein extracts revealed that the 3 clones featured markedly different levels of Cx36 protein (in decreasing order of expression: overexpressing MIN6 cells, WT MIN6 cells, and antisense MIN6 cells), which were reduced after overnight exposure of each clone to the Th1 cytokines mentioned above (Supplemental Figure 9). Under such conditions, the incidence of total cell death resulting from both apoptosis and necrosis significantly increased after exposure to the cytokines, and this increase was inversely related to the levels of Cx36 (in decreasing order of expression: antisense MIN6 cells, WT MIN6 cells, overexpressing MIN6 cells; Supplemental Figure 9). When only apoptosis was scored, the clone of MIN6 cells overexpressing Cx36 was significantly less affected by cytokines than either the control or the antisense clone (Supplemental Figure 9). These observations indicate that the experimental down- and upregulation of Cx36 increased and decreased, respectively, the cytokine-induced death of different lines of insulin-producing cells.
Discussion
In the present study, we found that Cx36, the protein that forms gap junctions of native pancreatic islets (1–9), protected the insulin-producing β cells against both cytotoxic drugs and the Th1 cytokines that predominate in the islet environment at the onset of type 1 diabetes (15, 16, 19–22). Thus, β cells lacking Cx36 were more sensitive to the lethal effects of these molecules, whereas β cells overexpressing Cx36 were protected against proapoptotic conditions both in vivo and in vitro.
Although different mouse stains are known to have different sensitivities to β cell–toxic insults (29, 30), within each mouse line studied herein, we found no obvious intrinsic difference in the behavior of individual β cells obtained from WT, heterozygous, and homozygous null animals. Thus, in all the animal models investigated, β cells expressed similar key molecules and pathways, including the GLUT-2 transporter, which is required for the uptake of STZ and AX (11, 18, 31); produced similar levels of NO when exposed to cytokines; and activated the caspases, which are the main mediators of cytokine-induced β cell apoptosis (15, 16, 19). Accordingly, disssociated β cells of all mice were equally sensitive to the cytokine-activated apoptotic mechanisms. In contrast, intact islets isolated from the native vascular, neural, and exocrine pancreas environment revealed that the levels of Cx36 modulated the sensitivity of β cells to identical cytotoxic conditions, implying an islet-autonomous difference.
This difference can first be accounted for by a negative effect of Th1 cytokines on Cx36 expression. Thus, the mix of IL-1β, IFN-γ and TNF-α, which promoted β cell apoptosis, also markedly decreased the levels of Cx36. Specifically, the cytokine IL-1β, the predominant trigger of β cell apoptosis (16, 19–26), also had the largest effect on decreasing the levels of Cx36 transcript and protein. Conversely, the in vitro down- and upregulation of Cx36 expression inversely related to the proapoptotic effect of the Th1 cytokines. The mechanism underlying this relationship remains to be unraveled and may involve any intermediate of the complex cascade of signal molecules that ultimately elicits a (pro)apoptotic effect. Whatever this intermediate, our data are consistent with the view that β cells expressing high levels of Cx36 and exposed to cytokines can retain sufficient levels of the Cx to somewhat resist immunological aggression, whereas β cells featuring low levels of Cx36 can become essentially deprived of this protein and thus be sensitized to the aggressive conditions.
While our study has not identified the mechanism whereby Cx36 protects β cells, it indicates that the β cell–to–β cell transfer of gap junction–permeant moieties may be key to this protection. First, all the pancreatic islets that were protected against both pharmacological and immunological attacks featured significantly greater β cell coupling than did normally sensitive native islets, in which this coupling is of modest amplitude (1–4, 7–9). Conversely, the islets of Cx36–/– mice, in which this coupling is absent (7–9), were more sensitized to the same conditions. Second, in the presence of native Cx36 levels, the protection of β cells was increased by the de novo expression of either Cx32 or Cx43, 2 isoforms that are not natively expressed by β cells (1, 2, 4) and form channels with biophysical and regulatory characteristics different from those made of Cx36 (5, 27, 28). It would be important to validate this tentative conclusion using drugs that specifically and reversibly block Cx36 channels. At this time, however, the available drugs all have pleiotropic effects, and cannot sustain cell uncoupling for a time sufficient to observe the eventual effects on cell function and death without inducing multiple confounding effects (32, 33).
It is worth stressing that the Cx36-dependent protection was effective against molecules that activate a variety of signaling pathways within β cells, including DNA alkylation, depletion in NAD+ and ATP, liberation of NO, production of reactive oxygen species, and [Ca2+] alterations (11, 18, 19–26, 31). Several of the second messengers, which are central to these pathways and converge to modulate β cell apoptosis, permeate Cx channels (5, 27, 28). Therefore, it is conceivable that toxic molecules generated by a proapoptotic trigger, or their metabolites, may be more efficiently diluted within the increased volume of cytoplasm that results from enlarged Cx-dependent cell coupling. This dilution would be impeded within both single cells, which cannot establish functional Cx channels, and β cells that are uncoupled from their neighbors, as a result of downregulation or loss of Cx36 (7–9). This mechanism does not exclude the parallel possibility that increased β cell communication could also allow a healthy β cell to rescue an adjacent dying cell with an as-yet putative survival factor that could be transferred through Cx36 channels. Indeed, a unique feature of Cx channels is the permeability to multiple moieties and the bidirectional diffusion of these molecules (5, 27, 28), specifically between functionally heterogeneous cells, such as β cells (1). In this context, it is worth noting that in a quite different cell system, Cx43 has been shown to promote cell survival, either by forming hemichannels that allow for the uptake by cells of extracellular survival factors (34) or by interacting with cytosolic proteins that regulate cAMP-dependent survival pathways (35). These observations open the further possibility that Cx proteins may mediate the islet prosurvival signaling by mechanisms independent of the formation and function of cell-to-cell channels, for example, by affecting gene expression, interacting with other proteins, and/or forming hemichannels (32). Even though Cx36 hemichannels have not yet been identified in control pancreatic islets (36), a coupling-independent mechanism would be consistent with our observations that different Cx isoforms protected β cells in spite of largely different effects on the cell-to-cell exchange of cationic and anionic tracers, that Cx36+/– mice were protected against different cytotoxic insults in spite of modest cell coupling changes, and that RIP-Cx36+/– and RIP-Cx36+/+ mice accumulated Cx36 in the cytoplasm, conceivably altering its possible interaction with a variety of proteins. Of note, the levels of circulating glucose reached by RIP-Cx36–/– mice treated with either STZ or AX were similar to those reached by Cx36–/– mice under the same treatments, in spite of the fact that the former animals had control levels of Cx36, whereas the latter ones lacked this Cx. These data, which are consistent with the differential sensitivity of different mouse lines to the effects of both STZ and AX (29, 30), indicate that several factors in addition to Cx36 contribute to control β cell resistance, and thus the levels of circulating glucose.
The finding that this protein is expressed and functions in the islets of the human pancreas (3), in a pattern like that seen in rodents (2, 4, 7, 8), strengthens the interest to search for pharmacological tools promoting Cx36 expression and/or Cx36 channel function (1, 32). From the results of this study and previous reports (7–10), the prediction would be that these agents could foster both the survival of β cells and their insulin secretion. In this context, it is interesting to note that glibenclamide, one of the rare molecules that increases gap junctions (32, 33), promotes the Cx36-dependent coupling of rodent β cells in vivo (27, 32, 37, 38). This sulphonylurea is largely used to stimulate insulin release from the glucose-insensitive islets of type 2 diabetic patients (39) and appears to have some antiapoptotic effect on human islets (40). The search for novel drugs specifically targeting Cx36 and inducing larger and more selective effects now requires the development of devoted methods and innovative algorithms (41). Hopefully, some of these drugs could block the apoptosis that decreases β cell mass in both type 1 and type 2 diabetes (1, 32). Given that Cx36 is mostly restricted to pancreatic β cells, neurons, and few related neuron-endocrine cells (5, 13, 32, 42), such pharmacological tools may also be of value in a variety of neurodegenerative diseases.
Methods
Animals.
Mice of the Cx36 line were generated by homologous recombination (13). The RIP-directed lines were generated by microinjecting a Cx32 (12), Cx36, or Cx43 (14) transgene into zygotic pronuclei (Supplemental Figures 2 and 5 and Supplemental Methods). Genotype of littermates was determined by PCR amplification of tail DNA (7, 9, 12–14). 3- to 6-month-old mice backcrossed with C57BL/6 controls were used in all experiments. Experiments in RIP-Cx32, RIP-Cx36, and RIP-Cx43 lines were initiated with mice of the third generation and repeated with mice of the eighth generation; experiments in the Cx36 line were initiated with mice of the seventh generation and repeated with mice of the tenth generation. No significant difference was observed between generations for the initial and repeat experiments (data not shown).
In vivo treatments.
STZ (Sigma-Aldrich) was freshly dissolved in 10 mM citrate buffer, pH 4.5, and 200 mg/kg BW was immediately injected i.p. (11, 18, 31). AX (Sigma-Aldrich) was freshly dissolved in 0.9 % NaCl containing 1 mM HCl, and 60 mg/kg BW (Cx36 mice) or 70 mg/kg BW (RIP-Cx36 mice) was immediately injected i.v. (11, 18, 31). Levels of circulating glucose were measured in tail blood (7, 9, 12, 14).
In vitro experiments.
RPMI 1640 medium was supplemented with one of the following: 10 mM citrate buffer (all controls); 1.1 mM (single islet cells), 2.2 mM (islets of the Cx36 line), or 4.4 mM (islets of the RIP-Cx36, RIP-Cx32, and RIP-Cx43 lines) STZ for 15 and 24 hours (single islet cells and isolated islets, respectively); 50 U/ml IL-1β (R&D Systems) plus 1,000 U/ml IFN-γ (Biosciences) and 1,000 U/ml TNF-α (R&D Systems) for 24 and 36 hours (islets and islet cells, respectively); 10 μM cyclosporin A (Calbiochem); 50 μM Z-VAD (Enzyme System Products). The latter 2 drugs were added 1 hour before addition of the cytokines and maintained thereafter throughout the experiment.
Other methods.
Procedures for islet and cell isolation, culture, transfection, immunofluorescence, biochemistry, apoptosis, and necrosis tests and dye coupling experiments were as previously reported (2–4, 6–10, 12, 14, 27). See Supplemental Methods for details.
Statistics.
Values are expressed as either mean ± SEM or median (depending on whether they had normal or non-Gaussian distribution, respectively) of the indicated number of experiments. Comparison of means was made by 1-way ANOVA, whereas comparison of medians was made using a median test. The additional nonparametric Kolmogorov-Smirnov, Mann-Whitney, and χ2 tests were further used to assess differences between non-Gaussian distributions. All tests were run using SPSS (Windows version; SPSS Inc.). P values less than 0.05 were considered significant.
Study approval.
All animal experiments were conducted as per the regulations of the Geneva veterinary office (Geneva, Switzerland; authorization no. 1034/3552/1).
Supplementary Material
Acknowledgments
The authors’ work was supported by grants from the Swiss National Science Foundation (310000-122423, 310000-109402, CR32I3_129987), the Juvenile Diabetes Research Foundation (40-2011-11), and the European Union (BETAIMAGE 222980; IMIDIA, C2008-T7).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(12):4870–4879. doi:10.1172/JCI40509.
References
- 1.Bavamian S, et al. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes Metab. 2007;9 suppl 2:118–132. doi: 10.1111/j.1463-1326.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 2.Serre-Beinier V, et al. Cx36 preferentially connects beta-cells within pancreatic islets. Diabetes. 2000;49(5):727–734. doi: 10.2337/diabetes.49.5.727. [DOI] [PubMed] [Google Scholar]
- 3.Serre-Beinier V, et al. Cx36 makes channels coupling human pancreatic beta-cells, and correlates with insulin expression. Hum Mol Genet. 2009;18(3):428–439. doi: 10.1093/hmg/ddn370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theis M, et al. Replacement by a lacZ reporter gene assigns mouse connexin36, 45 and 43 to distinct cell types in pancreatic islets. Exp Cell Res. 2004;294(1):18–29. doi: 10.1016/j.yexcr.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 5. Harris AL, Locke D, eds.Connexin: A Guide . New York, New York, USA: Springer; 2009. [Google Scholar]
- 6.Calabrese A, et al. Connexin 36 controls synchronization of Ca2+ oscillations and insulin secretion in MIN6 cells. Diabetes. 2003;52(2):417–424. doi: 10.2337/diabetes.52.2.417. [DOI] [PubMed] [Google Scholar]
- 7.Ravier MA, et al. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54(6):1798–1807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- 8.Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M. Cx36-mediated coupling reduces beta-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes. 2007;56(4):1078–1086. doi: 10.2337/db06-0232. [DOI] [PubMed] [Google Scholar]
- 9.Wellershaus K, et al. A new conditional mouse mutant reveals specific expression and functions of connexin36 in neurons and pancreatic beta-cells. Exp Cell Res. 2008;314(5):997–1012. doi: 10.1016/j.yexcr.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Le Gurun S, et al. Connexin-36 contributes to control function of insulin-producing cells. J Biol Chem. 2003;278(39):37690–37697. doi: 10.1074/jbc.M212382200. [DOI] [PubMed] [Google Scholar]
- 11.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 12.Charollais A, et al. Junctional communication of pancreatic beta cells contributes to the control of insulin secretion and glucose tolerance. J Clin Invest. 2000;106(2):235–243. doi: 10.1172/JCI9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Güldenagel M, et al. Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci. 2001;21(16):6036–6044. doi: 10.1523/JNEUROSCI.21-16-06036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klee P, et al. Connexin implication in the control of the murine beta-cell mass. Pediatr Res. 2011;70(2):142–147. doi: 10.1203/PDR.0b013e318220f106. [DOI] [PubMed] [Google Scholar]
- 15.Cnop M, Welsh N, Jonas J, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 suppl 2:S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 16.Eizirik DL, Mandrup-Poulsen T. A choice of death: the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44(12):2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 17.Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. . J Neurosci. 2003;23(19):7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia. 2000;43(12):1528–1533. doi: 10.1007/s001250051564. [DOI] [PubMed] [Google Scholar]
- 19.Kim KA, Lee MS. Recent progress in research on beta-cell apoptosis by cytokines. Front Biosci. 2009;14:657–664. doi: 10.2741/3271. [DOI] [PubMed] [Google Scholar]
- 20.Eizirik DL, Flodstrom M, Karlsen AE, Welsh N. The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells. Diabetologia. 1996;39(8):875–890. doi: 10.1007/BF00403906. [DOI] [PubMed] [Google Scholar]
- 21.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10(9):369–377. doi: 10.1016/S0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 22.Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- 23.Hoorens A, Stangé G, Pavlovic D, Pipeleers D. Distinction between interleukin-1-induced necrosis and apoptosis of islet cells. Diabetes. 2001;50(3):551–557. doi: 10.2337/diabetes.50.3.551. [DOI] [PubMed] [Google Scholar]
- 24.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5(4):219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Pavlovic D, Chen MC, Flodström M, Sandler S, Eizirik DL. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS–/–). . Diabetes. 2000;49(7):1116–1122. doi: 10.2337/diabetes.49.7.1116. [DOI] [PubMed] [Google Scholar]
- 26.Saldeen J. Cytokines induce both necrosis and apoptosis via a common Bcl-2-inhibitable pathway in rat insulin-producing cells. Endocrinology. 2000;141(6):2003–2010. doi: 10.1210/en.141.6.2003. [DOI] [PubMed] [Google Scholar]
- 27.Charpantier E, Cancela J, Meda P. Beta cells preferentially exchange cationic molecules via connexin 36 gap junction channels. Diabetologia. 2007;50(11):2332–2341. doi: 10.1007/s00125-007-0807-9. [DOI] [PubMed] [Google Scholar]
- 28.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34(3):325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 29.Rossini AA, Appel MC, Williams RM, Like AA. Genetic influence of the streptozotocin-induced insulitis and hyperglycemia. Diabetes. 1977;26(10):916–920. doi: 10.2337/diabetes.26.10.916. [DOI] [PubMed] [Google Scholar]
- 30.Mathews CE, Leiter EH. Constitutive differences in antioxidant defense status distinguish alloxan-resistant and alloxan-susceptible mice. Free Radic Biol Med. 1999;27:449–455. doi: 10.1016/S0891-5849(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 31.Hosokawa M, Dolci W, Thorens B. Differential sensitivity of GLUT1- and GLUT2-expressing beta cells to streptozotocin. Biochem Biophys Res Commun. 2001;289(5):1114–1117. doi: 10.1006/bbrc.2001.6145. [DOI] [PubMed] [Google Scholar]
- 32.Bosco D, Haefliger JA, Meda P. Connexins: key mediators of endocrine function. Physiol Rev. doi: 10.1152/physrev.00027.2010. In press. [DOI] [PubMed] [Google Scholar]
- 33. Srinivas M. Pharmacology of connexin channels. In: Harris AL, Locke D, eds.Connexins. A Guide . New York, New York, USA: Humana Press-Springer; 2009:207–224. [Google Scholar]
- 34.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277(10):8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 35.Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with beta-arrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem. 2011;112(10):2920–2930. doi: 10.1002/jcb.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scemes E, Bavamian S, Charollais A, Spray DC, Meda P. Lack of “hemichannel” activity in insulin-producing cells. Cell Commun Adhes. 2008;15(1):143–154. doi: 10.1080/15419060802014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meda P, Perrelet A, Orci L. Increase of gap junctions between pancreatic B-cells during stimulation of insulin secretion. J Cell Biol. 1979;82(2):441–448. doi: 10.1083/jcb.82.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meda P, Halban P, Perrelet A, Renold AE, Orci L. Gap junction development is correlated with insulin content in the pancreatic B cell. Science. 1980;209(4460):1026–1028. doi: 10.1126/science.6773144. [DOI] [PubMed] [Google Scholar]
- 39.Xu H, Murray M, McLachlan AJ. Influence of genetic polymorphisms on the pharmacokinetics and pharmaco-dynamics of sulfonylurea drugs. Curr Drug Metab. 2009;10(6):643–658. doi: 10.2174/138920009789375388. [DOI] [PubMed] [Google Scholar]
- 40.Del Guerra S, et al. Effects of exposure of human islet beta-cells to normal and high glucose levels with or without gliclazide or glibenclamide. Diabetes Metab. 2009;35(4):293–298. doi: 10.1016/j.diabet.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 41. Bavamian S.Connexin36: A Novel Therapeutic Target To Correct Beta-Cell Dysfunctions? [thesis]. Geneva, Switzerland: University of Geneva; Sc 4151, 2009. [Google Scholar]
- 42.Michon L, et al. Involvement of gap junctional communication in secretion. Biochim Biophys Acta. 2005;1719(1–2):82–101. doi: 10.1016/j.bbamem.2005.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.