Abstract
Objective
To assess the function of the new system of review by multicentre research ethics committees and to highlight areas where improvement is still needed.
Design
Prospectively collected data from a multicentre study was examined with respect to the ethics review process. Administrative, financial, and time elements of the review process were audited.
Setting
A single multicentre research ethics committee and 125 local ethics committees from six regions of England.
Main outcome measures
Time to reply, time to approval, and number of non-local changes to the application requested.
Results
Only 40% of local ethics committees considered our study in the manner specified in the 1998 directive. Less than a third of committees replied within the 21 day period stipulated, although committees acting by executive subcommittee replied more quickly than those not acting by executive subcommittee. There was a tendency for executive subcommittees to approve studies in a shorter time. Local ethics committees asked for a large number of non-local changes to the application. The financial cost of applying to multiple ethics committees remains high, mainly because multiple copies of research applications are being requested.
Conclusions
The new system of approval by multicentre research ethics committee for multicentre studies was introduced to reduce administrative costs, speed up the process of reviews by multiple research ethics committees, and standardise the conclusions of the local research ethics committees. Since its introduction an improvement has been seen, but the system is not yet universally functioning as intended. Ethics review still remains a hindrance to the financial resources and commencement of national studies. We strongly support the structure of review by multicentre research ethics committees but suggest that the system has yet to achieve its aims.
Introduction
The new UK multicentre research ethics committees were set up in autumn 1997 following concerns about the process of ethical review for multicentre studies.1,2 Both administrative and ethical problems had been encountered when applying to large numbers of local research ethics committees. The diversity of ethical requirements between local research ethics committees has been criticised as possibly inhibiting useful research2 or allowing studies of doubtful quality to take place.3 It had been suggested that the development of a central ethics committee at regional or national level would solve the problem of multilocation research.1 The new multicentre research ethics committees in each region were given responsibility for reviewing proposals taking place within the boundaries of five or more local research ethics committees. Approval given by a multicentre research ethics committee would have national acceptance. Local research ethics committees were then to consider the study only with respect to issues that may affect acceptability locally.
Feedback after the first six months of the system indicated that local research ethics committees were finding the new system difficult. In response, the Department of Health issued a directive reinforcing the purpose of the new system and requesting local research ethics committees to abide by guidelines. In September 1998 the NHS Executive distributed interim guidance to local research ethics committees outlining the manner in which studies approved by multicentre research ethics committees should be handled (box below).4
NHS Executive guidance points to local research ethics committees, September 1998
1 A standing executive subcommittee should be established to consider applications approved by multicentre research ethics committees (quorum shall be two members)
2 A meeting of this executive subcommittee should be called within 2 weeks of receipt of an application approved by a multicentre research ethics committee
3 The decision of the executive subcommittee should be communicated to the researcher within 5 working days. This does not require ratification by the full committee, and if approval is granted the research work may commence
4 Rejection of the application can only be for local reasons (see below) and must be accompanied by a full explanation for this decision
5 Local issues the executive subcommittee is asked to consider:
Suitability of the local researcher
Suitability of the site
Suitability of the subjects
Patient information sheets and consent forms to carry local information as required or to be produced in a locally appropriate language. No other changes to the information sheets or consent forms can be made
We describe the experience of applying to a multicentre research ethics committee and multiple local research ethics committees under this new system for the approval of a large multicentre study.
Methods
Data were prospectively recorded. Our research proposal was submitted to the North Thames multicentre research ethics committee in September 1998. Five changes were requested to the protocol, consent procedure, and information sheets, and final approval was received two months later. The proposal was then submitted to 125 local research ethics committees in early December 1998. All were unaware that responses were audited.
Each committee was contacted to ascertain the number of copies of the application required. Each application consisted of 15 documents totalling 96 pages. Requirements ranged from 1 to 21 applications per committee. All were posted by 3 December 1998. The responses from the local research ethics committees were recorded and reviewed in the light of the guidelines shown in the box on the previous page. Responses were categorised according to criteria shown in the box above.
Criteria for categorisation of local research ethic committee responses
Executive subcommittee
A committee was defined as an executive subcommittee either if they stated that this was the case or if they requested three or fewer copies of the application
Uncontested approval
Uncontested approval was that granted without comments or changes requested. Other forms of approval such as approval accompanied by request for comments were not deemed to be uncontested
Local changes
Local changes were defined according to the NHS Executive interim guidance report. These included changes or comments relating to the local investigator, individual hospital, or local patient group, or local changes to the study documents
Data are presented as median values (25th-75th centile) as they were not normally distributed. For continuous data, medians were compared with the Kruskal-Wallis test. Proportions were compared with the χ2 test (with Yates correction).
Results
Organisation of committees
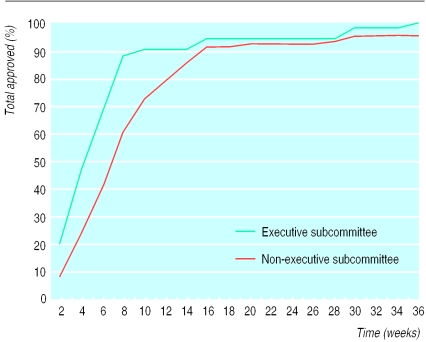
A total of 50 committees (40%) were organised as executive subcommittees. The proportion of committees acting by executive subcommittee ranged from 13% to 54% depending on region. Overall, 39 committees (31%) replied within the 21 day period stipulated in the guidelines (figure). A higher proportion of committees acting by executive subcommittee replied within this 21 day period (40% executive subcommitte v 25% non-executive subcommittee) although this difference was not statistically significant (relative risk 1.2, P=0.12). The median time taken to receive an approval from all committees was 41 days. Those committees acting by executive subcommittee were significantly quicker to give approval than those not acting by executive subcommittee (median 28.5 days for executive subcommitte v 46 days for non-executive subcommittee, P=0.0002). At six months after application nine committees had not approved the study, five acting by executive subcommittee.
Processing of application
Requests for amendments were classified as local or non-local (box above). Fifty two committees (42%) granted uncontested approval. More committees acting by executive subcommittee gave an uncontested approval (64% executive subcommittee v 53% non-executive subcommittee; relative risk 1.4 (95% confidence interval 0.9 to 2.1), P=0.23). Of those committees that asked for amendments 67% asked for non-local ones. The table provides examples of such changes. The vast majority of these committees later approved the resubmission despite our non-compliance with their requests.
Effects on research study
Delay receiving ethics approval had a significant effect on study commencement and recruitment. An up to date list held centrally of all local research ethics committees with contact details was not available. Delays occurred while correct addresses and administrative details were identified. Seventeen per cent of patients referred to date were not recruited because ethical approval had not been granted by the relevant local research ethics committee.
Financial aspects of application
The high cost of applying to multiple local research ethics committees was not anticipated. Extra funding was sought to cover this cost. Photocopying was contracted out. The total number of pages consumed by applications for local research ethics committees was 105 888 (1103 applications of 96 pages each). The total cost of application was £6132.90 (£2950 photocopying, £1200 postage, and £1982.90 paper).
Discussion
High hopes had been expressed that a new system of ethics review would solve the problems of multicentre research.3,5 Our prospectively collected data illustrate that the system led by new multicentre research ethics committees has the potential to function well. Unfortunately many of the problems inherent in the old system have not yet been solved.
Review of our proposal by the multicentre research ethics committee was straightforward. Administrative procedures were uncomplicated, and assistance was readily forthcoming when dealing with individual inquiries on local research ethics committees.
Administrative issues
Identifying local research ethics committees and obtaining contact details was difficult. The available list was out of date and incomplete. It would be helpful if multicentre research ethics committees could provide up to date information on disk including contact details of each local research ethics committee in their region as well as number of applications required. It should be the responsibility of the local research ethics committee to inform multicentre research ethics committees if details change. This would immediately alleviate many of the early difficulties experienced.
Cost
The high cost of obtaining national ethics approval3persists, mainly due to the requirement by local research ethics committees for multiple copies of the application. The new system of review by executive subcommittee should have alleviated the need to supply large committees with multiple copies. The failure of local research ethics committees to operate according to guidelines places an unnecessarily heavy burden on funding bodies at a time when research funds are scarce.
Research delays
Failure to act by executive subcommittee may also have a major impact on timely commencement of research. Executive subcommittees replied more quickly and were able to approve the study in a significantly shorter time than non-executive subcommittees. Despite being organised as executive subcommittees, however, over 50% were still unable to respond in the time specified.
Approval times
The time taken to obtain approval has improved under the new system. In our study the median time for approval from non-executive subcommittees was 46 days, decreasing to 28.5 days for executive subcommittees. This represents an improvement compared with previous work by Foster et al who found that “fast responding committees” had an average approval time of 35 days whereas “slow responders” took 175 days.5 Nevertheless, five executive subcommittees had been unable to approve our study six months after application.
Non-local amendments
Many changes requested by local research ethics committees were of a non-local nature (67%)—that is, requests that are no longer their prerogative under the 1998 directive. Importantly, some of these requests provided constructive criticism that led to improvements in our study protocol. These could perhaps have been identified by the multicentre research ethics committee. Local research ethics committees have considerable experience with the ethical review process and may believe that important issues have been overlooked by a multicentre research ethics committee. It is possible that longer approval times occurred as a result of the multicentre research ethics committee overlooking such issues, which then had to be addressed by local research ethics committees. Many requests were, however, for minor changes to the wording of information sheets and questionnaires, sometimes resulting in considerable delays to recruitment. It is interesting to note that the vast majority of local research ethics committees who had requested such changes later approved the study when the researcher reiterated that documents had been previously approved by the multicentre research ethics committee. It is also possible that slow response times and multiple requests for non-local changes were a reflection of the nature of our study. Although our study dealt with the potentially controversial issue of consent in teenagers, explicit approval to follow the protocol as stated had been granted by the multicentre research ethics committee.
Suggestions for change
Multicentre research ethics committees should provide an up to date list of the administrative details of local committees, supplied to them by each local research ethics committee
A standard number of copies of the application should be requested from all local committees. This should be a realistic number for the members of an executive subcommittee—that is, it should not need to exceed three
Applications should be reviewed promptly by members of the executive subcommittee and a reply sent to the researcher
Requests for minor alterations to the protocol or supporting documentation should be avoided. Other comments or requests for amendments that are not of a local nature should perhaps be addressed to the multicentre research ethics committee. Approval should not be delayed because of non-local issues
What is already known on this topic
Many authors have commented on the difficulties experienced by researchers in obtaining ethics approval for multicentre studies. Much of this work has been anecdotal
Since the introduction of the new system of multicentre research ethics committees a systematic audit has not been undertaken to evaluate its performance
What this study adds
Although review by multicentre research ethics committees could substantially reduce previous difficulties described, changes are still needed to allow the system to function as intended
Conclusions
Past dissatisfaction with the ethical review system for multicentre studies has focused upon procedural difficulties rather than the substance of ethical review itself.6 The multicentre research ethics committee system was set up to deal with such procedural difficulties. Improvements in the system have occurred. However, local research ethics committees have been reluctant to abandon their autonomy sufficiently to allow efficient functioning of this system, and this has been identified as a potential reason for continuing concerns about the new system.7 Whereas administrators of local research ethics committees face significant problems in trying to achieve turnaround targets that may be unrealistic without important new resource input, substantial frustrations remain for researchers working within a system that at times presents an unethical barrier to potentially beneficial research.
Figure.
Survival curve showing time taken for approval of proposal according to type of local research ethics committee
Table.
Non-local and local changes requested by local research ethics committees after approval by multicentre research ethics committees
| Non-local changes or comments requested | No of times requested (% of committees) | Local changes or comments requested | No of times requested (% of committees) |
|---|---|---|---|
| Changes to information sheets | 22 (18) | Local staff to be notified | 11 (9) |
| Changes to consent procedure | 12 (10) | Ethnic mix to be considered | 10 (8) |
| Changes to questionnaire | 9 (7) | Local investigator to be identified | 6 (5) |
| Changes to methods of recruiting subjects | 9 (7) | Information sheets to be on locally headed paper | 4 (3) |
| Changes to protocol | 5 (4) | Approval required from local research and development office | 3 (2) |
| Confidentiality issues | 4 (3) | Confirmation of locally incurred costs | 3 (2) |
| Exclusions for subjects already in research | 2 (2) | ||
| Maximum number to be recruited from local site | 1 (1) | ||
| Researchers' credentials to be supplied | 1 (1) | ||
| Local research ethics committee number to be written on local documents | 1 (1) |
Footnotes
Funding: JT and NN were funded by the Meningitis Research Foundation and RB was funded by the Wellcome Trust.
Competing interests:None declared.
References
- 1.Busby A, Dolk H. Local research ethics committees' approval in a national population study. J R Coll Physicians Lond. 1998;32:142–145. [PMC free article] [PubMed] [Google Scholar]
- 2.Harries UJ, Fentem PH, Tuxworth W, Hoinville GW. Local reseach ethics committees' widely differing responses to a national survey protocol. J R Coll Physicians Lond. 1994;28:150–154. [PMC free article] [PubMed] [Google Scholar]
- 3.While AE. Ethics committees: impediments to research or guardians of ethical standards? BMJ. 1995;311:661. doi: 10.1136/bmj.311.7006.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stacey TE. How should an LREC handle an MREC approved application: interim guidance circular letter. 1-3. 28 Sep, 1998.
- 5.Foster C, Holley S. Ethical review of multi-centre research: a survey of multi-centre researchers in the South Thames region. J R Coll Physicians Lond. 1998;32:242–245. [PMC free article] [PubMed] [Google Scholar]
- 6.Holley S, Foster C. Ethical review of multi-centre research: a survey of local research ethics committees in the South Thames region. J R Coll Physicians Lond. 1998;32:238–241. [PMC free article] [PubMed] [Google Scholar]
- 7.Stacey TE. Ethical review of research in the NHS: the need for change. J R Coll Physicians Lond. 1998;32:190–192. [PMC free article] [PubMed] [Google Scholar]