Abstract
Genome-wide association studies (GWAS) have identified a large number of SNPs that are linked to human autoimmune diseases. However, the functional consequences of most of these genetic variations remain undefined. T cell protein tyrosine phosphatase (TCPTP, which is encoded by PTPN2) is a JAK/STAT and growth factor receptor phosphatase that has been linked to the pathogenesis of type 1 diabetes, rheumatoid arthritis, and Crohn’s disease by GWAS. In this issue of the JCI, Wiede and colleagues have generated a T cell–specific deletion of TCPTP and identified a novel role for this phosphatase as a negative regulator of TCR signaling. These data provide new insight as to how noncoding PTPN2 SNPs identified in GWAS could drive human autoimmune diseases.
Limitations of genome-wide association studies
Recent years have seen an explosion of genome-wide association studies (GWAS) designed to identify, in an unbiased manner, genetic loci associated with complex polygenic traits and diseases, including several autoimmune diseases (1). However, defining the functional consequences of disease-associated SNPs has been extremely challenging for several reasons. First, the majority of SNPs linked to polygenic autoimmune diseases individually confer a modest relative risk of developing disease (with odds ratios less than 1.5), suggesting that a complex genetic environment is required to manifest disease. Second, it is possible that some disease-associated SNPs are not causative, but instead act as genetic markers for rare disease-causing SNPs located nearby. Last, most disease-associated SNPs are noncoding, so it is likely that subtle and cell type–specific changes in transcript expression or splicing confer susceptibility to disease. Defining such subtle changes in gene expression has been difficult, and direct study of noncoding SNPs in model organisms or cell lines in order to define their effects on disease pathogenesis has not been possible.
Noncoding SNPs in and near the protein tyrosine phosphatase, non-receptor type 2 (PTPN2) gene have been associated with type 1 diabetes, Crohn’s disease, and rheumatoid arthritis (1–3). It is not well understood how these SNPs contribute to disease pathogenesis. In this issue of the JCI, Wiede and colleagues have generated mice with a T cell–specific deletion of Ptpn2 and used these animals to identify a novel role for the PTPN2 gene product T cell protein tyrosine phosphatase (TCPTP) as a negative regulator of TCR signaling (4). In doing so, they shed light on how disease-associated PTPN2 SNPs may drive human autoimmunity by dysregulating T cell development and homeostasis.
TCPTP is required to suppress systemic inflammation in mice
TCPTP is a ubiquitous non-receptor protein tyrosine phosphatase, with highest expression detectable in hematopoietic tissues and predominantly nuclear localization (2). Although TCPTP-deficient mice are born at Mendelian ratios, they become ill and die by three to five weeks of age (5). This early-onset disease is characterized by inflammatory infiltrates in multiple organs (6). Notably, these mice exhibit a dramatic increase in expression of proinflammatory cytokine transcripts in various tissues, including those encoding TNF-α, IFN-γ, and IL-12 (6). However, the site and stimulus for this increased cytokine production are unclear, as analysis of bone marrow chimeric mice indicates that TCPTP plays a critical regulatory role in both hematopoietic and non-hematopoietic cells (2).
Studies of substrate-trap mutants and TCPTP-deficient cells have identified TCPTP as a negative regulator of multiple cytokine signaling pathways. JAK1, JAK3, STAT1, STAT3, and STAT5a/b are all putative TCPTP targets downstream of cytokines such as IL-2, IL-6, IL-4, and IFN-γ (2, 7, 8). In addition to JAK/STAT signaling pathways, TCPTP negatively regulates signaling downstream of several growth factor receptors, including the insulin receptor, EGFR, PDGFR, and CSF-1R (2). More recently, it has been shown that TCPTP negatively regulates TNF-α–induced ERK phosphorylation by targeting TNF receptor–associated factors (TRAFs) and Src family kinases (SFKs), substantially expanding the range of putative TCPTP substrates (9).
What do we know about the role of TCPTP in human autoimmune disease?
Modeling the functional consequences of noncoding disease-associated SNPs has been challenging. Direct study of primary cells from patients or healthy controls harboring disease-associated SNPs offers a powerful approach. Recently, Buckner and colleagues reported a modest (25%) reduction in expression of PTPN2 transcripts in primary human T cells harboring an intronic PTPN2 SNP associated with type 1 diabetes (10). They went on to probe the IL-2 signaling pathway and reported subtly impaired STAT5 phosphorylation in CD25+ regulatory T cells and CD45RO+ memory T cells bearing the disease-associated PTPN2 SNP. By contrast, it has been previously shown that TCPTP-deficient mouse T cells exhibit enhanced JAK/STAT phosphorylation upon IL-2 stimulation (7, 8). Reconciling these disparate results will be important for our understanding of how the PTPN2 locus contributes to disease pathogenesis. Importantly, TCPTP is ubiquitously expressed, and disease-associated SNPs may affect expression or splicing in many other tissues as well. It is possible, for example, that PTPN2 polymorphisms contribute to type 1 diabetes not only through immunologic pathways, but also by direct regulation of insulin receptor signaling (2).
Although studies of primary human cells offer one approach to define the effects of noncoding SNPs, sample size, tissue access, and genetic heterogeneity limit both assays and conclusions. Animal studies therefore remain a critical and complementary strategy to investigate the functional consequences of disease-associated SNPs. The Tremblay group has studied Ptpn2+/– mice in order to model the effect of a subtle and ubiquitous reduction in TCPTP expression (11). These animals do not develop spontaneous disease on the C57BL/6 genetic background (5). However, in response to DSS-induced damage to gut epithelium, the mice respond with excess cytokine production and exaggerated gut inflammation (11). Although the damage and subsequent disease elicited by DSS is distinct from that observed in patients with Crohn’s disease, it may reasonably model how gut injury or impaired gut epithelial homeostasis interacts with commensal flora on a susceptible proinflammatory genetic background. Ptpn2+/– mice may therefore prove to be a useful system to model how the Crohn’s disease–associated PTPN2 SNP drives disease.
Defining the function of TCPTP in T cells sheds light on the pathogenesis of human autoimmunity
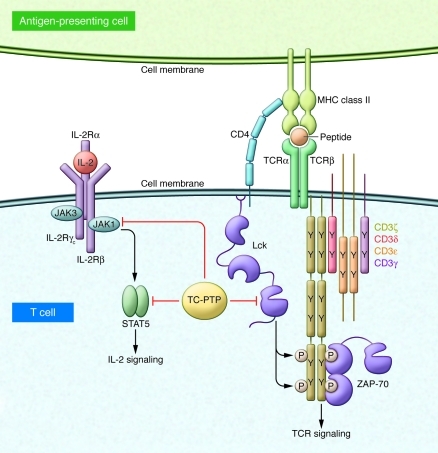
The studies described in the previous section suggest how subtle alterations in TCPTP expression in T cells and other tissues could account for the disease association of noncoding SNPs in PTPN2. However, the precise function of TCPTP in T cells, key effectors in the pathogenesis of many autoimmune diseases, has not been well studied, in part because of the inflammation and mortality associated with global TCPTP deficiency in mice (5). In this issue of the JCI, Wiede and colleagues address this issue by generating mice in which Ptpn2 is conditionally deleted in T cells (4). Analysis of these mice has unmasked a cell-intrinsic function for TCPTP as a negative regulator of TCR signaling (Figure 1). Using substrate-trap mutants of TCPTP, Wiede and colleagues identified the T cell SFKs Lck and Fyn as direct substrates of TCPTP, although the mechanism by which the predominantly nuclear TCPTP is recruited to the membrane-associated SFKs remains to be determined. SFKs are critical mediators of TCR signaling and are themselves tightly regulated by inhibitory and activation loop tyrosine phosphorylation (12). By dephosphorylating the activation loop tyrosine of the SFKs, TCPTP presumably inhibits SFK enzymatic activity and thereby impairs TCR signaling. Wiede and colleagues found that in vitro, TCPTP-deficient T cells exhibited enhanced activation and proliferation upon TCR stimulation (4). In vivo, conditional deletion of TCPTP in T cells was found to drive enhanced TCR-dependent selection of T cells in the thymus and accumulation of memory phenotype T cells in the periphery. Importantly, regulatory Foxp3+ T cells, critical suppressors of systemic autoimmunity, were unperturbed. Taken together, these observations of Wiede and colleagues suggest that an altered threshold for T cell activation may contribute to abnormal T cell homeostasis in the T cell–specific TCPTP-deficient mice. However, TCPTP is a critical negative regulator of JAK/STAT signaling pathways downstream of cytokines such as IL-2, which has an important function in T cell activation (2, 7, 8). Therefore, it remains possible that abnormal T cell homeostasis in these mice is not driven exclusively by dysregulated TCR signaling (Figure 1).
Figure 1. Dual regulatory functions for TCPTP in T cells.
TCPTP, the protein encoded by the PTPN2 gene, has previously been shown to target JAK1/3 and STAT5a/b downstream of IL-2 receptor signaling as well as other cytokine pathways. In this issue of the JCI, Wiede and colleagues identify a new role for TCPTP as a negative regulator of TCR signaling through its ability to dephosphorylate the activation loop tyrosine of the SFK Lck (Y394) (4).
Wiede and colleagues also demonstrated that conditional deletion of TCPTP in T cells was sufficient to drive an inflammatory disease on the C57BL/6 background, with onset by one year of age (4). This disease occurs much later than in mice completely deficient in TCPTP (5), suggesting that TCPTP plays a critical negative regulatory role in other cell types to suppress inflammation. The disease observed by Wiede and colleagues in mice with T cell–specific TCPTP deficiency (4) was characterized by increased serum levels of proinflammatory cytokines such as IL-6, TNF-α, and IFN-γ, as well as lung and liver immune cell infiltrates. In addition, these mice exhibited evidence of non-cell-autonomous dysregulation of B cell homeostasis, including autoantibody production and spontaneous germinal center formation. Whether the disease described by Wiede and colleagues is driven by dysregulation of TCR signaling exclusively, or may also be due to derepression of cytokine signaling pathways in T cells, remains unclear. Because both T cell development and T cell function are affected in these mice, it is uncertain whether selection of an altered TCR repertoire during thymic development or inappropriate peripheral T cell activation drives disease.
Dysregulated TCR signaling is implicated in human autoimmune disease
The conditional T cell deletion of Ptpn2 reported by Wiede and colleagues (4) suggests that dysregulated TCR signaling may contribute to autoimmunity in patients harboring disease-associated PTPN2 SNPs (1–3). Although Wiede et al. did not directly probe this pathway in patient samples, the TCR signaling pathway has been previously implicated in autoimmune disease pathogenesis by GWAS (13). A particularly well-studied and relevant example is the PTPN22 gene, which encodes Lyp (murine ortholog, Pep), a hematopoietically expressed cytoplasmic tyrosine phosphatase that is structurally unrelated to TCPTP. A single coding SNP in this gene (PTPN22 C1858T) has been associated with multiple autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, and type 1 diabetes (1, 14–16). Lyp/Pep, like TCPTP, negatively regulates TCR signaling, at least in part by dephosphorylating the activation loop tyrosine of SFKs (17). Indeed, Pep-deficient mice recapitulate a subset of phenotypes identified by Wiede and colleagues in their T cell–specific TCPTP-deficient mice (4), including enhanced thymic positive selection, accumulation of effector/memory phenotype T cells, and enhanced spontaneous germinal center formation (18). However, the disease that develops spontaneously in the T cell–specific TCPTP-deficient mice does not occur in Pep-deficient mice. This may be due to the distinct potency and temporal requirement of the two phosphatases during TCR signaling. Alternatively, this may suggest that dysregulation of cytokine signaling pathways in T cell–specific TCPTP-deficient mice contribute to late-onset inflammatory disease.
Unlike PTPN2 noncoding SNPs, the PTPN22 C1858T SNP results in a coding mutation (LypR620W) that has rendered it particularly amenable to study in model systems. It has been shown that this single residue change impairs constitutive association of Lyp with its binding partner c-Src tyrosine kinase (Csk) (16, 19). Csk serves to recruit Lyp to the proximity of its substrates and cooperates to inhibit TCR signaling by phosphorylating the inhibitory tyrosine of the SFKs (17). Although this mutation might thus be predicted to impair Lyp function, studies in cell lines and primary human cells have yielded conflicting results (19–22). Most recently, Zhang et al. have generated a knock-in mouse harboring the disease-associated PTPN22 SNP (23). They show that T cells from these animals as well as from patients homozygous for the risk allele exhibit hyperresponsive TCR signaling that is at least partly due to degradation of the Lyp/Pep R620W variant. These data strongly suggest that the PTPN22 risk allele produces a hypomorph and may have functional consequences similar to those of noncoding SNPs in PTPN2. However, as with PTPN2, function of the disease-associated PTPN22 C1858T SNP in cell lineages other than T cells may also be important (21, 23).
Although the PTPN2 and PTPN22 SNPs may have similar functional consequences for TCR signaling and exhibit overlapping human disease association for type 1 diabetes and rheumatoid arthritis, some important differences stand out. In particular, the PTPN22 C1858T allele is associated with systemic lupus erythematosus, but the PTPN2 genetic locus is not (15). Conversely, while the PTPN2 locus is linked to Crohn’s disease, the disease-associated PTPN22 C1858T allele subtly lowers risk for this condition (1, 24). This suggests that distinct pathways and/or distinct cell types may be regulated by the PTPN2 and PTPN22 SNPs.
Future directions
Mouse and human data suggest that reduced expression of TCPTP might drive human autoimmune disease by enhancing signaling downstream of the TCR, cytokines, or growth factors to produce a proinflammatory cytokine milieu (2, 4, 5, 10, 11). Importantly, disease may reflect abnormalities in immune cell development as well as mature immune cell function. Because TCPTP is ubiquitously expressed and regulates multiple signaling pathways, dissecting the functional consequences of human SNPs in the gene encoding this protein will be challenging.
To clarify both the function of TCPTP in immune homeostasis and its role in human disease, several key questions remain to be addressed. The identification of SFKs as substrates of TCPTP in T cells by Wiede and colleagues (4, 9) raises the possibility that TCPTP regulates SFK-dependent signaling pathways in other cell types. To better clarify the contribution of the PTPN2 genetic locus to human autoimmune diseases, extensive resequencing of PTPN2 to identify rare coding SNPs will be important, as will defining PTPN2 transcript expression in human subjects harboring disease-associated SNPs. Further evaluation of JAK/STAT, growth factor receptor, and SFK-dependent signaling pathways in primary human cells may help define the functional consequences of these SNPs. Finally, modeling disease in mice will continue to be critical. Focus on heterozygous rather than knockout animals in the context of susceptible genetic backgrounds or environmental provocation will be informative. As this work proceeds, the mice bearing a floxed PTPN2 allele generated by Wiede and colleagues and described in this issue of the JCI (4) will prove to be a very important tool.
Acknowledgments
This work was supported by the Rosalind Russell Medical Research Foundation Bechtel Award (to J. Zikherman), Arthritis National Research Foundation (to J. Zikherman), and NIH K08 AR059723 (to J. Zikherman).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(12):4618–4621. doi:10.1172/JCI60001
See the related article beginning on page 4758.
References
- 1.Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doody KM, Bourdeau A, Tremblay ML. T-cell protein tyrosine phosphatase is a key regulator in immune cell signaling: lessons from the knockout mouse model and implications in human disease. Immunol Rev. 2009;228(1):325–341. doi: 10.1111/j.1600-065X.2008.00743.x. [DOI] [PubMed] [Google Scholar]
- 3.Todd JA, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39(7):857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiede F, et al. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J Clin Invest. 2011;121(12):4758–4774. doi: 10.1172/JCI59492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You-Ten KE, et al. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J Exp Med. 1997;186(5):683–693. doi: 10.1084/jem.186.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinonen KM, et al. T-cell protein tyrosine phosphatase deletion results in progressive systemic inflammatory disease. Blood. 2004;103(9):3457–3464. doi: 10.1182/blood-2003-09-3153. [DOI] [PubMed] [Google Scholar]
- 7.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol. 2002;12(6):446–453. doi: 10.1016/S0960-9822(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 8.Aoki N, Matsuda T. A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus. Mol Endocrinol. 2002;16(1):58–69. doi: 10.1210/mend.16.1.0761. [DOI] [PubMed] [Google Scholar]
- 9.van Vliet C, et al. Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat Immunol. 2005;6(3):253–260. doi: 10.1038/ni1169. [DOI] [PubMed] [Google Scholar]
- 10.Long SA, et al. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4(+) T cells. Genes Immun. 2011;12(2):116–125. doi: 10.1038/gene.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan SW, Doody KM, Hardy S, Uetani N, Cournoyer D, Tremblay ML. Increased susceptibility to dextran sulfate sodium induced colitis in the T cell protein tyrosine phosphatase heterozygous mouse. PLoS One. 2010;5(1):e8868. doi: 10.1371/journal.pone.0008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228(1):288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zikherman J, Weiss A. Antigen receptor signaling in the rheumatic diseases. Arthritis Res Ther. 2009;11(1):202. doi: 10.1186/ar2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottini N, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36(4):337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 15.Kyogoku C, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75(3):504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Begovich AB, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75(2):330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189(1):111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303(5658):685–689. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- 19.Vang T, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37(12):1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 20.Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol. 2009;182(7):4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arechiga AF, et al. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182(6):3343–3347. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007;179(7):4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43(9):902–907. doi: 10.1038/ng.904. [DOI] [PubMed] [Google Scholar]
- 24. Diaz-Gallo LM, et al. Differential association of two PTPN22 coding variants with Crohn’s disease and ulcerative colitis [published online ahead of print February 1, 2011].Inflamm Bowel Dis . doi:10.1002/ibd.21630 . [DOI] [PubMed] [Google Scholar]