Abstract
Sexually transmitted infections (STIs) have plagued humans for millennia and can result in chronic disease, pregnancy complications, infertility, and even death. Recent technological advances have led to a better understanding of the causative agents for these infections as well as aspects of their pathogenesis that might represent novel therapeutic targets. The articles in this Review Series provide excellent updates on the recent advances in understanding of the pathogenesis of some very important and persistent STIs and discuss the importance of considering each pathogen in the broader context of the environment of the individual who it infects.
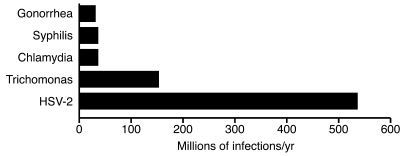
With the discovery of antibiotics, the development of vaccines and effective disease control programs, and the availability of condoms, why are sexually transmitted infections (STIs) still with us and thriving? Certainly sex is necessary for species survival and is a basic drive, but why should STIs still present as a possible result of the activity? Why haven’t we treated our way out of the high prevalence rates of some STIs? Globally, congenital syphilis is still a leading cause of stillbirth and neonatal death (1). Quinolone-resistant gonorrhoea is a worldwide problem (2). Indeed, five out of the top ten reportable diseases in the US are STIs. There are over 19 million new cases of STIs reported each year in the US, and global rates are staggering (Figure 1). Direct and indirect costs to the US for STIs are estimated to be $10–$17 billion dollars a year (3). Given the rapidly expanding genomic, proteomic, and metabolomic databases, why haven’t we produced more and more effective vaccines? Perhaps the answer is simply that we don’t have all the pieces to solve these puzzles yet. To forge ahead we need first to return to the basics — determine what we know, what we need to know, and where we go next.
Figure 1. Global estimates of prevalent cases of STIs in 2005.
At any point in 2005, there were approximately 318 million prevalent cases of curable STIs (syphilis, gonorrhea, chlamydia, and trichomonas) and 536 million estimated HSV-2 infections (based on data from ref. 35).
The articles in this Review Series focus on three sexually transmissible microbes and also describe the microbiome of the female reproductive tract, which may play a leading role in their infectivity and pathogenesis. The authors review the diseases associated with or caused by the pathogens, new discoveries of their pathogenesis, and the contributions of molecular technologies and genomic advances to this knowledge and offer suggestions regarding where our future research needs to focus regarding vaccine development and prevention efforts.
Shining a new light on an old and persistent disease
In the first review of this series (4), Emily L. Ho and Sheila A. Lukehart describe the complex challenges presented by Treponema pallidum subsp. pallidum, the agent of syphilis, and review recent progress in the application of modern molecular techniques toward understanding its pathogenesis, improving diagnosis, and developing better treatment strategies. Understanding syphilis infection has been a challenge for researchers, in part, because T. pallidum cannot be cultured or genetically manipulated. Consequently, the publication and analysis of the T. pallidum genome has enabled a better understanding of the molecular basis of syphilis pathogenesis (5). The application of new molecular techniques may uncover why this ancient disease persists and sometimes flourishes.
Recognizing clinical manifestations of syphilis, explaining how it vacillates between active disease and latent infection, and achieving accurate diagnoses has always been challenging. Syphilis has been described clinically as the “great imitator,” because of its protean clinical manifestations (6). Untreated infection may evolve through several stages: the primary stage manifests classically as a painless ulcer referred to as a “chancre;” the secondary stage is the bacteremic phase, accompanied by protean systemic symptoms such as rash, generalized lymphadenopathy, malaise, and low-grade fever; the latent phase has no clinical manifestations and may persist as such for life; and the tertiary stage presents with signs and symptoms of end organ destruction. To complicate matters, the disease does not always march through consecutive clinical stages. An infected individual may present in the latent stage with no recollection of prior symptoms. Furthermore, the stages of primary and secondary infection, if clinically evident, occur relatively early after the infection, within weeks to months of inoculation, whereas the time interval between secondary and tertiary syphilis can be years, if that progression occurs at all. Predicting who is at greatest risk of developing the clinical manifestations of neurosyphilis has been particularly challenging. Discovering the nature of the host-parasite interaction may help solve some of these clinical conundrums.
Perhaps one of the biggest challenges, given that T. pallidum cannot be cultured in vitro, is confirming the diagnosis of active, incident, or even persistent infection. Currently marketed diagnostic tests for syphilis rely on detection of IgG and IgM antibodies directed against the pathogen’s proteins TpN17 and TpN47, which may also be produced in persons with periodontal disease due to oral treponemes (7). Therefore, teasing through the array of polypeptides predicted to be in the T. pallidum proteome will help in identifying more specific antibodies for screening and diagnosis. Furthermore, the currently available assays cannot distinguish between recent or remote syphilis infections or among the various stages of infection and cannot be used reliably with cerebral spinal fluid to diagnose neurosyphilis.
Untangling the pathogenesis and discovering an accurate diagnostic for neurosyphilis is yet another persistent challenge. Invasion of the nervous system by T. pallidum occurs early in infection, within days to weeks of inoculation (6). Although some patients appear to control or clear CNS infection, in others T. pallidum “escapes” immune detection to cause persistent and advanced disease. This evasion may be due to antigenic variation of bacterial surface proteins. Bioinformatic approaches have identified several outer membrane proteins, but these can differ substantially among and within individual strains, and molecular studies show that new variants arise by segmental gene conversion, which may enable the organism to evade clearance and persist (8). Why some individuals have persistent yet chronic latent infection and others reactivate to tertiary disease is unknown.
Since T. pallidum can be cultured only transiently in vitro in rabbit epithelial cells, assessment of antibiotic sensitivity profiles cannot be done. Parenteral penicillin is the recommended treatment, based on over 50 years of treatment success, which is defined as clinical resolution and prevention of sexual transmission (9). When a penicillin allergy arises in nonpregnant persons, macrolides and tetracyclines have been used. However, clinical failure has been documented with use of macrolides, and this resistance is attributed to an adenine-to-guanine mutation at the position cognate to A2058 in the E. coli 23S rRNA gene (10). This resistance seems widespread, with scattered reports from many continents (11–13). Molecular strain typing may uncover important information, which not only documents antibiotic resistance, but also aids our understanding of transmission dynamics within sexual networks as well as identification of unique strains prone to neuroinvasion and may lead the way to vaccine development.
Human papillomavirus — vaccine success for all?
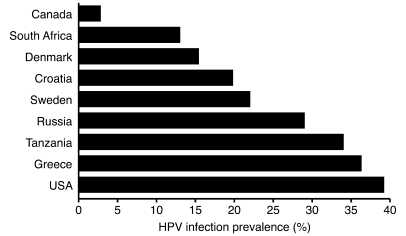
With the discovery that certain high-risk strains of human papillomavirus (HPV) cause nearly 100% of invasive cervical cancer (14), followed by the development of the highly efficacious HPV vaccines, we can assume that the major challenges in HPV control and cervical cancer prevention have been met (15). Or can we? There is no doubt that the vaccine, if given to girls and boys before sexual debut, is extremely effective. In the Review by Patti Gravitt (16), some remaining challenges are discussed. HPV is a very common infection (Figure 2), but what is the natural history of HPV infection across the lifespan, particularly in older women? How do we reliably determine incident HPV infection? Can infected women truly clear HPV, or do they immunologically control persistent infection? Do serum antibodies detected after natural infection confer protection against reinfection with the same HPV genotype? The answers to these questions may (and should) influence policy debates regarding best practices for vaccine implementation. A major limitation of predictive models is the lack of a true measure of HPV infection status; Gravitt discusses three possible pathways for the natural history of HPV infection in women and presents the data supporting each possibility. Typically, HPV DNA-positive women are considered “infected,” and HPV DNA-negative women are considered “uninfected;” however, natural history studies of HPV infection are based on HPV DNA measures repeated at four, six, and twelve month intervals, and more frequent sampling is needed to determine whether women clear infection or merely transition from active viral shedding to latency.
Figure 2. HPV, prevalence studies among female populations, 1995–2001 (reproduced with permission from ref. 36).
Difficulty also exists in determining whether serum antibodies developed against HPV are protective against reinfection. A major issue here is that different serological assays are used to detect antibodies in various studies. The most intriguing data come from the placebo arm of the quadrivalent HPV vaccine trial on women aged 24–45 (protocol 019), which showed that HPV-seropositive younger women appear to have protection against new HPV infection, but older women do not (17). This may be due to waning immunity, acquiring new sexual partners and thus increased chance of exposure, or merely a transition from undetectable to detectable DNA from a latent HPV infection. Clarification of these issues is important, because the difference between new acquisition and reactivation goes beyond the bench and may lead literally to the bedside, causing questions of sexual infidelity in relationships.
Herpes simplex virus-2 — vaccine failure and where we go next
Herpes simplex virus-2 (HSV-2) is another ancient, persistent virus and remains a challenge to those working in vaccine development. While many vaccines have been studied in animal models, only a few have reached clinical trials, and these were not consistently effective. Perhaps, as Christine Johnston, David Koelle, and Anna Wald discuss in the third Review of this series (18), going back to basics and unravelling HSV pathogenesis might provide insight to new vaccine development.
HSV is one of the most prevalent sexually transmitted pathogens (19). Most infected persons are not aware of their infection and may transmit it during periods of subclinical viral shedding. Nonetheless, the morbidity associated with symptomatic disease is significant. Genital HSV has a high recurrence rate and can cause complications such as aseptic meningitis and neonatal transmission, with resulting fetal death or severe and lasting neurologic compromise. Regardless of whether it is symptomatic or asymptomatic, its association with increased HIV transmission and acquisition highlights concern for better individual and population prevention (20, 21).
It was previously thought that HSV infection vacillated between active disease with visible ulcerations and latency. Eloquent studies by the authors of this Review demonstrated that HSV and its host are in constant conflict, with frequent HSV reactivation and rapid clearance, most likely involving both innate and acquired immunologic processes (22–24). In fact, mathematical models and genital mapping studies suggest that a nearly constant, low quantity of HSV is likely to be released from sensory ganglia into the genital tract (25). Is this enough to transmit the infection? The relationship between the inoculum dose and other epithelial variables has not been established. Clearly, from discordant partner studies, we know that not all sexual acts between these couples transmit infection. The authors have, in fact, identified some HSV-2–seronegative persons in long-term HSV-2 discordant sexual relationships who have T-cell responses to HSV-2, and these responses may be weighted toward certain HSV-2 proteins (26). Such data are very important in defining future subunit vaccine composition. Another long-standing belief was that individuals infected with one strain of HSV-2 rarely became infected with another HSV-2 strain. Recent studies using more sensitive PCR techniques and newly identified variable regions in HSV-2 found multiple strain genital infections in 15% of healthy adults infected with genital HSV-1 and HSV-2 (27, 28). Due to increased knowledge of viral SNPs, these multi-strain HSV-2 infections can be more closely studied to determine whether immunity provoked by wild-type infection is a necessary component to a successful vaccine.
Understanding of the interplay between innate and cell-mediated immune responses to HSV infection is advancing. The authors review current discoveries in both pathways. While innate immune system agonists can lead to profound, local HSV resistance, classic vaccinology cannot yet achieve this response, and no clear acquired cellular immunity correlates of control of HSV reactivation or shedding have been elucidated in humans. Neutralizing antibodies to viral envelope glycoproteins are required for viral entry into cells and have been the focus of several failed glycoprotein gB and gB subunit vaccines (29, 30). However, crystallographic structures of HSV proteins with their ligands and dynamic structural changes during viral entry are available, and this may allow the development of a vaccine modeled on the success of the HPV vaccine. Clearer understanding of specific HSV immune evasion mechanisms may inform the creation of replication competent or incompetent virus-based vaccines.
The authors also stress that although the ideal HSV vaccine will prevent primary infection, a therapeutic vaccine to improve the clinical course in individual patients and potentially decrease HSV shedding and therefore transmission, could have a significant public health benefit. Successful outcomes for these vaccines would include a decrease in HSV shedding frequency and quantity. Regardless of which type of vaccine comes first, we are well on the way to its discovery.
The vaginal microbiome — what is it and how does it work?
In the final Review in this series, Rebecca Brotman (31) considers STI prevention in the context of the vaginal microbiome. The vagina houses a unique microenvironment, which is both complex and dynamic. Nearly all women are vaginally colonized by obligately anaerobic gram-negative rods and cocci as well as several species of anaerobic and not-yet-named bacteria (32). The balance of bacteria is influenced by hormonal changes that occur throughout a woman’s lifetime and will be different during premenarche, reproductive years, and after menopause. Hydrogen-peroxide producing Lactobacillus species have been thought to be very important in maintaining an acidic environment and a low bacterial count. Disruption of this microbiome, termed bacterial vaginosis (BV), is associated with a loss of lactic acid–producing bacteria, an overgrowth of anaerobic bacteria, and a markedly increased risk for acquisition of STIs, including HIV, and the development of pelvic inflammatory disease. As a gynecologic condition, BV was described over 30 years ago by Amsel and was associated clinically with a homogeneous grayish-white vaginal discharge, bacterial overgrowth, elevated pH above 4.5, and an amine odor released upon addition of 10% KOH to the vaginal discharge fluid (33). Nugent et al. then quantitated the number and types of bacterial overgrowth observed in Gram-stained vaginal fluid and developed a Nugent Gram stain score (34). Using Nugent’s score, it has been noted that many women with high quantities of bacteria consistent with BV do not have symptoms. The factors leading to and the adverse outcomes resulting from symptomatic versus asymptomatic BV are not fully understood and are an active area of research.
More knowledge is needed regarding the dynamics of the vaginal ecosystem, with regards to documenting the frequency of fluctuations in the composition of vaginal communities and the effect of behavioral factors, such as intercourse, microbicides, or intrinsic and extrinsic hormones, on its fluctuations. As with HSV, more frequent self-collected sampling and behavioral diaries are needed to increase our understanding and allow researchers to build a database that we might mine for information. The NIH Human Microbiome Project, hopefully, will add much to these data.
To date we do not understand how protective vaginal microbiota are maintained, why frequent shifts in microbial composition occur, or how these shifts induce changes in the vaginal environment that relate to the dramatic increase in susceptibility to such a wide range of adverse health outcomes. However, new technologies are assisting in solving these mysteries.
Preventing STIs — where do we go from here?
The articles in this Review Series provide excellent updates of the recent advances in understanding the pathogenesis of some very important and persistent STIs. Furthermore, the review of the vaginal microbiome stresses the importance of considering each pathogen in the broader context of the environment of the individual who it infects. Even though we’ve come a long way toward understanding how these specific STIs invade our bodies and stimulate and also evade our immune system, we don’t have all the answers. Basic questions remain. Why does T. pallidum persist in some individuals and cause neurosyphilis? Is that persistence related to the organism or the individual’s unique response to the organism? How can we improve syphilis diagnosis? Will T. pallidum antibiotic resistance become a global issue, and how can we monitor it? Questions also persist with HPV and HSV. What is the next step in HSV vaccine development? Should we strive for a vaccine to prevent infection or alter the natural history of HSV infection? Both approaches could significantly impact HSV-2 transmission and acquisition. Although we have a very successful vaccine for HPV, should older women get it? Furthermore, when a woman tests positive for HPV DNA, do we really know if it is a new HPV infection or if it is a reactivation event? Finally, how does the individual’s microbiome influence the pathogenesis of STIs? Future research will shed light on this path to STI prevention, and, regardless of the direction, it will be an interesting journey. Furthermore, it is clear that to answer these questions we must begin at the beginning and go back to the basic biology of each pathogen, for only then might our path forward be clear.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(12):4580–4583. doi:10.1172/JCI61592.
References
- 1. World Health Organization. The Global Elimination Of Congenital Syphilis: Rationale And Strategy For Action. Geneva, Switzerland: WHO Press; 2007. [Google Scholar]
- 2.Tapsall JW. Neisseria gonorrhoeae and emerging resistance to extended spectrum cephalosporins. Curr Opin Infect Dis. 2009;22(1):87–91. doi: 10.1097/QCO.0b013e328320a836. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Surveillance 2009. Atlanta, Georgia, USA: US Department of Health and Human Services; 2010. [Google Scholar]
- 4.Ho EL, Lukehart SA. Syphilis: using modern approaches to understand an old disease. J Clin Invest. 2011;121(12):4584–4592. doi: 10.1172/JCI57173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser CM, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. . Science. 1998;281(5375):375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 6.Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510–1514. doi: 10.1001/jama.290.11.1510. [DOI] [PubMed] [Google Scholar]
- 7.Riviere GR, et al. Identification of spirochetes related to Treponema pallidum in necrotizing ulcerative gingivitis and chronic periodontitis. . N Engl J Med. 1991;325(8):539–543. doi: 10.1056/NEJM199108223250803. [DOI] [PubMed] [Google Scholar]
- 8.LaFond RE, Molini BJ, Van Voorhis WC, Lukehart SA. Antigenic variation of TprK V regions abrogates specific antibody binding in syphilis. Infect Immun. 2006;74(11):6244–6251. doi: 10.1128/IAI.00827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Workowski KA, Berman S, Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 10.Lukehart SA, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. . N Engl J Med. 2004;351(2):154–158. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 11.Matejkova P, et al. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J Med Microbiol. 2009;58(pt 6):832–836. doi: 10.1099/jmm.0.007542-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhou P, et al. Azithromycin treatment failure among primary and secondary syphilis patients in Shanghai. Sex Transm Dis. 2010;37(11):726–729. doi: 10.1097/OLQ.0b013e3181e2c753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florindo C, Reigado V, Gomes JP, Azevedo J, Santo I, Borrego MJ. Molecular typing of treponema pallidum clinical strains from Lisbon, Portugal. J Clin Microbiol. 2008;46(11):3802–3803. doi: 10.1128/JCM.00128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walboomers JM, et al. Humanpapilloma virus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Romanowski B. Long term protection against cervical infection with the human papillomavirus: Review of currently available vaccines. Hum Vaccin. 2011;7(2):161–169. doi: 10.4161/hv.7.2.13690. [DOI] [PubMed] [Google Scholar]
- 16.Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121(12):4593–4599. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velicer C, Zhu X, Vuocolo S, Liaw KL, Saah A. Prevalence and incidence of HPV genital infection in women. Sex Transm Dis. 2009;36(11):696–703. doi: 10.1097/OLQ.0b013e3181ad25ff. [DOI] [PubMed] [Google Scholar]
- 18.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest. 2011;121(12):4600–4609. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86(10):805–812. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald A, Link K. Risk of human immunodeficiency virus infection in herpessimplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185(1):45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 21.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta–analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 22.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333(12):770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 23.Crespi CM, Cumberland WG, Wald A, Corey L, Blower S. Longitudinal study of herpes simplex virus type 2 infection using viral dynamic modelling. Sex Transm Infect. 2007;83(5):359–364. doi: 10.1136/sti.2006.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tronstein E, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011;305(14):1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiffer JT, et al. Frequent release of low amounts of herpes simplex virus from neurons:results of a mathematical model. Sci Transl Med. 2009;1(7):7ra16. doi: 10.1126/scitranslmed.3000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posavad CM, et al. Detailed characterization of T cell responses to herpes simplex virus-2 in immune seronegative persons. J Immunol. 2010;184(6):3250–3259. doi: 10.4049/jimmunol.0900722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roest RW, et al. Genotypic analysis of sequential genital herpes simplex virus type 1 (HSV-1) isolates of patients with recurrent HSV-1 associated genital herpes. J Med Virol. 2004;73(4):601–604. doi: 10.1002/jmv.20132. [DOI] [PubMed] [Google Scholar]
- 28.Roest RW, Maertzdorf J, Kant M, van der Meijden WI, Osterhaus AD, Verjans GM. High incidence of genotypic variance between sequential herpes simplex virus type 2 isolates from HIV-1-seropositive patients with recurrent genital herpes. J Infect Dis. 2006;194(8):1115–1118. doi: 10.1086/507683. [DOI] [PubMed] [Google Scholar]
- 29.Stanberry LR, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Painful failure of promising genital herpes vaccine. Science. 2010;330(6002):304–304. doi: 10.1126/science.330.6002.304. [DOI] [PubMed] [Google Scholar]
- 31.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011;121(12):4610–4617. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hillier SL, Holmes KK, Marrazzo JM. Bacterial vaginosis. In: Holmes KK, et al., eds.Sexually Transmitted Diseases . New York, New York, USA: McGraw–Hill, Health Professions Division; 2008:737–768. [Google Scholar]
- 33.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 34.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization, Department of Reproductive Health and Research.Prevalence And Incidence Of Selected Sexually Transmitted Infections . Geneva, Switzerland: WHO Press; 2011. [Google Scholar]
- 36. Toskin I. Sexually transmitted infections epidemiology. Paper presented at: Training Course in Sexual and Reproductive Health Research, July 8, 2011; Geneva, Switzerland. http://www.gfmer.ch/SRH-Course-2011/sti/STI-Epidemiology-Toskin-2011.htm . Accessed October 26, 2011. [Google Scholar]