Abstract
To establish whether there is new evidence to inform changes to WHO 2003 recommendations for micronutrient intake in persons with HIV/AIDS, we conducted a narrative review of the literature published from 2003 to 2010. Although the review focused on new randomized controlled trials of multiple micronutrients in HIV-infected adults, including pregnant and lactating women, we also considered randomized trials of single micronutrients. The review found that there are few published randomized controlled trials of micronutrients in HIV-infected persons and that most trials used high-dose multiple micronutrient supplementation. The trials were heterogeneous with respect to the composition and dose of micronutrients used and the target population studied. Despite this heterogeneity, 5 of 6 trials that used high-dose multiple micronutrients showed benefits in terms of either improved CD4 cell counts or survival. However, many of these trials were small and of short duration, and therefore the long-term risks and benefits of high-dose multiple micronutrients are not established. The current WHO recommendation for an intake of micronutrients at Recommended Dietary Allowance amounts continues to be a reasonable target for persons with clinically stable HIV infection. In light of new data that show adverse effects of high-dose vitamin A, the current recommendation for a single high dose of vitamin A in HIV-infected women within 6 wk of delivery should be reviewed.
INTRODUCTION
In May 2003 the WHO issued the report of a technical consultation on nutrient requirements for persons living with HIV/AIDS (1). The report noted that many populations did not achieve dietary intakes of micronutrients at the Recommended Dietary Allowance (RDA). The report outlined recommendations for micronutrient intake in persons with HIV/AIDS as follows: “To ensure micronutrient intakes at RDA levels, HIV-infected adults and children are encouraged to consume healthy diets. Nevertheless, dietary intake of micronutrients at RDA levels may not be sufficient to correct nutritional deficiencies in HIV-infected individuals. There is evidence that some micronutrient supplements, eg, vitamin A, zinc and iron, can produce adverse outcomes in HIV-infected populations.” (1, p 7).
At the time the recommendations were formulated there were few data describing the effect of micronutrients on HIV-related outcomes. The data that were available suggested a high prevalence of serum micronutrient deficiencies among persons infected with HIV and an association of either poor dietary intake or low serum micronutrient concentrations with unfavorable outcomes (2–7). These data were largely from observational studies. However, observational studies can only provide statistical associations. They cannot establish whether poor nutrition is a cause or a consequence of HIV progression, and they cannot establish the potential benefits and risks of micronutrient supplementation. The safety and efficacy of micronutrient supplementation in HIV can be determined only by randomized controlled trials, and data from such trials were lacking at the time the recommendations were formulated.
The purpose of this review was to summarize new evidence published from 2003 to 2010 on the relation between micronutrient supplementation and HIV-related outcomes in adolescents and adults. The focus is on data derived from randomized controlled trials on the premise that new data from observational studies cannot provide the quality of evidence needed to inform changes to the current WHO recommendations. Given that the current recommendations encompass a wide range of micronutrients, this review emphasizes the published randomized trials of multiple micronutrients.
REVIEW STRATEGY AND SEARCH RESULTS
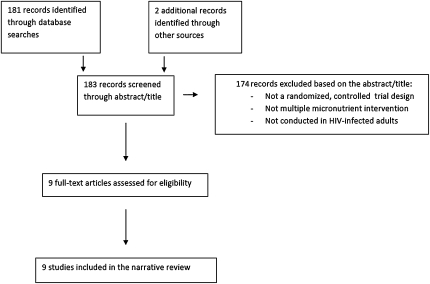
The review was conducted with a search of PUBMED (US National Library of Medicine, NIH, Bethesda, MD) and MEDLINE (US National Library of Medicine, Bethesda, MD) with the use of the OVID interface (http://gateway.ovid.com) and, finally, a search in Google (www.google.com) for theses and other foreign manuscripts not covered by PUBMED or MEDLINE. The search terms used were a combination of the key text words “micronutrients,” “vitamins,” and “HIV.” Selected articles included all identified randomized controlled trials of multiple micronutrients in adults with HIV infection published between January 2003 and 30 June 2010, regardless of the primary study outcome. We examined each article for citations. In addition, we learned of other randomized trials that were not identified in our initial search from colleagues who are experts in the area of HIV and nutrition.
Each study was examined for possible sources of bias, including possible confounding (a concern in small trials), lack of adherence to the intervention, and loss-to-follow-up bias. Of 9 identified randomized controlled trials of multiple micronutrients in HIV-infected adults, none were excluded, and all 9 published trials were considered of high-enough quality to be included in the review (Figure 1).
FIGURE 1.
Flowchart of citations included in the narrative review of multiple micronutrient trials in HIV-infected adolescents and adults.
MULTIPLE MICRONUTRIENT INTERVENTION TRIALS IN HIV-INFECTED ADULTS
Since the WHO issued its recommendations in 2003, ≥9 randomized controlled trials of multiple micronutrient supplements in HIV-infected adults have been published in the peer-reviewed literature. Of these 9 trials, 7 were conducted in sub-Saharan Africa (8–14), 1 was conducted in Thailand (15), and 1 was conducted in the United States (16). In addition to differences in geographic location, the participants in these trials varied in characteristics. For example, one study involved only pregnant women (8), another involved only nonpregnant women (9), and 3 studies included HIV/tuberculosis coinfected adults initiating tuberculosis treatment (10, 11, 14). The only trial conducted in persons receiving stable antiretroviral therapy (ART) was confined to HIV-infected adults with distal symmetrical polyneuropathy who were receiving stavudine- and/or didanosine-based ART (16). Other salient differences between the participants in these 9 trials are shown in Table 1. Most of the trials conducted a limited evaluation of serum micronutrient status at baseline, and the results of testing indicated a wide range in the prevalence of deficiencies. It is noteworthy that the average BMI of the participants was in the normal range, except for 2 studies that were conducted in HIV/tuberculosis coinfected subjects (10, 11) in which the subjects were underweight on average. Follow-up was of short duration in most trials and ranged from 6 wk to 71 mo.
TABLE 1.
Characteristics of participants in randomized controlled trials of multiple micronutrients in HIV-infected adults1
| First author (reference) | |||||||||
| Fawzi (8) | Jaimton (15) | McClelland (9) | Range (10) | Semba (11) | Kelly (12) | Kaiser (16) | Villamor (14) | Kawai (13) | |
| Total n | 1051 | 481 | 400 | 181 | 829 | 161 | 40 | 471 | 272 |
| Country | Tanzania | Thailand | Kenya | Tanzania | Malawi | Zambia | USA | Tanzania | Tanzania |
| Females (%) | 100 | 61 | 100 | 41 | 59 | 68 | 13 | 42 | 100 |
| BMI (kg/m2) | NR | 21.4 | 22.1 | 18.2 | 18.2 | 22.5 | NR | 19.5 | 24.9 |
| ART use | No | <3% | No | No | No | Variable | Yes | No | No |
| Follow-up (wk) | Variable | 48 | 6 | 32 | 96 | 172 | 12 | 75 | 10 |
| Comorbid conditions | NR | NR | NR | TB | TB | NR | Distal symmetrical polyneuropathy | TB | NR |
| Other | Pregnant | NR | NR | Initiating TB treatment | Initiating TB treatment | NR | All on D4T and/or DDI-based ART | Initiating TB treatment | Pregnant |
| Serum micronutrient deficiency (%) | |||||||||
| Vitamin A | 502 | NR | NR | NR | 60 | 10 | NR | NR | NR |
| Vitamin E | NR | 12 | 5 | NR | NR | NR | NR | NR | NR |
| Selenium | NR | <1 | 30 | NR | 73 | NR | NR | NR | NR |
| Zinc | NR | NR | NR | NR | NR | 17 | NR | NR | NR |
ART, antiretroviral therapy; DDI, didanosine; D4T, stavudine; NR, not reported; TB, tuberculosis.
Reported in reference 17.
The composition and dose of micronutrients used in the trials expressed as multiples of the RDA or, in the absence of an RDA, as Adequate Intake are shown in Table 2 (18). The supplement formulations used in these trials varied widely in both composition and dose. Six of the trials used multiple micronutrients at high doses (8–10, 14–16), 2 of the trials used doses at or close to the RDA (11, 12), and 1 trial compared high and low doses (13). The doses of micronutrients ranged from <1 RDA to >1000 times the RDA. Where given, the rationale for the use of supplementation at high amounts was the hypothesis that persons with HIV have greater requirements for micronutrients than do healthy persons (19). Alternatively, the rationale was based on benefits seen in an earlier trial of high-dose multiple micronutrients (17). Whereas the composition of the supplements varied widely, all trials included ≥1 RDA of vitamins B-1 (thiamine), B-2 (riboflavin), B-6, and B-12. Vitamins C and E, folic acid, and selenium were also used in several of the supplement formulations tested.
TABLE 2.
Composition and dose of supplements used in randomized controlled trials of multiple micronutrients in HIV-infected adults1
| First author (reference) |
|||||||||
| Micronutrient in multiples of RDA | Fawzi (8) | Jiamton (15) | McClelland (9) | Range (10) | Semba (11) | Kelly (12) | Kaiser (16) | Villamor (14) | Kawai (13) |
| Thiamin, B-1 | 18 | 20 | 18 | 17 | 1 | 1 | 50 | 17 | 1 vs >10 |
| Riboflavin, B-2 | 18 | 12 | 18 | 15 | 1 | 1 | 46 | 15 | 1 vs >10 |
| Vitamin B-6 | 19 | 31 | 19 | 19 | 2 | 1 | 153 | 19 | 2 vs >10 |
| Vitamin B-12 | 21 | 13 | 21 | 21 | 3 | 1 | 1041 | 21 | 3 vs >10 |
| Vitamin C | 7 | 4 | 7 | 2 | 6 | <1 | 20 | 6 | 1 vs 7 |
| Folic acid | 2 | <1 | 2 | 2 | 1 | 1 | 2 | 2 | <1 vs 2 |
| Niacin | 7 | No | 7 | 3 | 1 | 1 | 4 | 6 | 1 vs 6 |
| Vitamin E | 2 | 5 | 2 | 4 | 9 | No | 36 | 13 | 1 vs 3 |
| Iron | No | 1 | No | No | No | 4 | 2 | No | No |
| Vitamin A | No | 3 | No | 2 | 3 | No | 3 | 2 | No |
| β-Carotene | No | 6 mg | No | No | No | 5 mg | 12 mg | No | No |
| Vitamin D3 | No | 1 | No | 1 | 2 | 1 | 2 | No | No |
| Copper | No | 3 | No | 6 | No | 2 | 2 | No | No |
| Zinc | No | 3 | No | No | 1 | 1 | 3 | No | No |
| Selenium | No | 7 | 3 | 4 | 1 | 1 | 4 | 2 | No |
| Iodine | No | 2 | No | No | 1 | 1 | 1 | No | No |
| Panthothenic acid2 | No | 5 | No | No | No | No | 12 | No | No |
| Magnesium | No | <1 | No | No | No | No | 174 | No | No |
| Manganese2 | No | 4 | No | No | No | No | 4 | No | No |
| Chromium2 | No | 5 | No | No | No | No | <1 | No | No |
| Cystine | No | Yes | No | No | No | No | No | No | No |
| Vitamin K2 | No | 2 | No | No | No | No | No | No | No |
| N-Acetyl cysteine | No | No | No | No | No | No | No | No | |
| Acetyl l-carnitine | No | No | No | No | No | No | Yes | No | No |
| α-Lipoic acid | No | No | No | No | No | No | Yes | No | No |
| Inositol | No | No | No | No | No | No | Yes | No | No |
| Calcium | No | No | No | No | No | No | <1 | No | No |
| Boron | No | No | No | No | No | No | Yes | No | No |
| Potassium2 | No | No | No | No | No | No | <1 | No | No |
| Biotin2 | No | No | No | No | No | No | 2 | No | No |
| Molybdenum | No | No | No | No | No | No | 7 | No | No |
| Choline2 | No | No | No | No | No | No | 9 | No | No |
| Bioflavonoid complex | No | No | No | No | No | No | Yes | No | No |
| l-Glutamine | No | No | No | No | No | No | Yes | No | No |
| Betaine HCL | No | No | No | No | No | No | Yes | No | No |
No, micronutrient not given; RDA, Recommended Dietary Allowance; Yes, micronutrients given (no RDA exists).
Expressed as Adequate Intake (reference 18).
All except for one of the trials (13) had a placebo control group, and 2 trials used a 2 × 2 factorial design to compare the effects of a multiple micronutrient with or without an additional supplement: vitamin A (8, 17) or zinc (10). All trials appeared to be conducted with appropriate blinding of treatment allocation and statistical control of potential confounding, in cases in which this was a concern. Loss-to-follow-up rates were ≤16%, although not reported in one of the trials (17). Details of the individual trials follow.
In an earlier study of HIV-infected pregnant Tanzanian women, Fawzi et al (17) found that, compared with placebo, a high-dose formulation of multiple micronutrients (Table 2) resulted in significantly greater increases in CD4, CD8, and CD3 cell counts during and after pregnancy (30 wk postpartum), as well as better birth outcomes. Slightly improved weight gain during pregnancy was also noted (20). This was the first published randomized controlled trial to show a potential benefit of micronutrients in persons with HIV infection. Further follow-up of the same women over ∼6 y showed continued benefits of multiple micronutrients on T cell counts, lower viral loads, slower HIV progression, and improved overall survival (8). The same study later reported a beneficial effect of micronutrients on maternal wasting (21), maternal and child hemoglobin status (22), and maternal depression and quality of life (23) and other beneficial outcomes in both mother and child likely associated with the slower disease progression and improved HIV survival observed previously in the supplemented group.
In Thailand, Jiamton et al (15) examined the effect of a 48-wk course of high-dose multiple micronutrients compared with placebo in 481 HIV-infected adults. Micronutrients had no effect on CD4 count or viral load. However, in the subgroup of participants with low CD4 cell counts, all-cause mortality was reduced by 63% in those with baseline CD4 cell counts < 200/μL and by 74% in those with baseline CD4 cell counts <100 /μL.
In a study in 400 Kenyan women, designed to examine the effect of multivitamins over 6 wk on genital HIV shedding, McClelland et al (9) noted a modest, but significant increase in CD4 (+23 cells/μL) and CD8 (+74 cells/μL) cell counts but no effect on HIV viral load. Contrary to the study hypothesis, micronutrients were associated with increased genital shedding of HIV, even in the subgroup that had no genital shedding at baseline. Although the reasons for this observation are not clear, these data raise concerns that supplementation could lead to increased HIV transmission.
Tuberculosis coinfection is common in HIV and, like HIV, has devastating effects on nutritional status. With the use of a 2 × 2 factorial design, Range et al (10) examined the effect of an 8-mo course of high-dose micronutrients with and without zinc (given at 4 times the RDA) on the health of 181 Tanzanian HIV-infected adults initiating treatment of tuberculosis. A combination of micronutrients plus zinc resulted in a 71% lower risk of mortality, but neither micronutrients alone nor zinc alone had any effect on mortality. None of the interventions tested had an impact on CD4 cell counts or HIV viral load, although these outcomes were examined only within the first 2 mo of treatment.
Semba et al (11) conducted a similar study in Malawi, but the dose of micronutrient supplements used was close to the RDA. They found no differences in mortality between those receiving and those not receiving supplements over 2 y of follow-up. The authors did not report effects of micronutrients on CD4 cell counts or on HIV viral load.
A second study from Tanzania included 471 adults coinfected with HIV and tuberculosis, who were randomly assigned at tuberculosis therapy initiation to receive high-dose multiple micronutrient supplement (without zinc) or placebo (14). ART was not available at the time of study. Acid-fast bacteria smear–positive patients were followed for a median of 30 mo. Tuberculosis recurrence, the primary outcome, was lower only for those who were acid-fast bacteria–negative at 1 mo. Mortality and HIV disease progression were unaffected, and there was no change in viral load or CD4 cell count over the period of observation. However, micronutrient supplementation resulted in 57% less peripheral neuropathy, 73% less extrapulmonary tuberculosis, and 90% fewer genital ulcers. The authors concluded that high-dose micronutrient supplements improve tuberculosis-related outcomes in HIV coinfected patients.
The ability of micronutrient supplementation to reduce the frequency and severity of diarrhea was examined by Kelly et al (12) in a Zambian community that included 161 persons infected with HIV. Anthropometric measures of nutritional status, CD4 counts, and mortality were secondary endpoints. Among the persons infected with HIV, micronutrient supplementation, which was given at a dose similar to the RDA, had no effect on the incidence of diarrhea, anthropometric measures, or CD4 counts. However, there was a 75% lower mortality in the supplemented group than in the placebo group.
These studies were all conducted in resource-poor environments where there is limited access to ART, and therefore they cannot tell us if micronutrients provide additional benefits to persons who are already receiving stable ART. Kaiser et al (16) published the first report of micronutrient supplementation in HIV-infected persons receiving stable ART. The participants selected were 40 HIV-infected adults who had distal symmetrical polyneuropathy and who were receiving stable stavudine- and/or didanosine-based highly active ART. After 12 wk the group receiving micronutrients had higher CD4 cell counts (+71 CD4 cells/μL) compared with the group receiving placebo. Micronutrients did not have a significant impact on HIV viral load, neuropathy scores, fasting serum glucose, insulin, lactate, or liver or renal function tests.
The results of these trials are summarized in Table 3. All trials, except for one (14) that used high-dose multiple micronutrients, reported a benefit compared with placebo in terms of either increased CD4 cell counts or reduced mortality. Thus, high-dose multiple micronutrients of varying composition were of benefit in HIV-infected persons from different geographic regions and with widely varying characteristics, including differing levels of micronutrient deficiency.
TABLE 3.
Effect of MMN compared with placebo on HIV-related outcomes in randomized controlled trials in HIV-infected adults1
| First author (reference) | Dose of micronutrients | CD4 cell count | Mortality |
| Fawzi (8) | High | ↑ | ↓30% |
| Jiamton (15) | High | No effect | ↓74% (in CD4 <100/μL) |
| McClelland (9) | High | ↑ | Not reported |
| Range (10) | High | No effect | ↓71%, MMN + Zn only |
| No effect, MMN or Zn alone | |||
| Semba (11) | RDA | Not reported | No effect |
| Kelly (12) | RDA | No effect | ↓75% |
| Kaiser (16) | High | ↑ | Not reported |
| Villamor (14) | High | No effect | No effect |
MMN, multiple micronutrient supplementation; RDA, Recommended Dietary Allowance; Zn, zinc supplementation; ↑, increased; ↓, decreased.
Two of the studies described above compared placebo with multiple micronutrients given at amounts close to the RDA: the community-based study by Kelly et al in Zambia (12) and the clinic-based study by Semba et al (11) in tuberculosis/HIV-infected subjects in Malawi. The former study showed a benefit on mortality of micronutrients at RDA doses, whereas the latter study did not. However, the data in Table 2 show that the HIV/tuberculosis coinfected subjects in Malawi were underweight on average and had a higher prevalence of vitamin A deficiency than did the HIV-infected subjects in Zambia. Thus, baseline nutritional status may explain the differing results in these trials.
This raises the question of whether multiple micronutrient supplements must be given at high doses to see a beneficial effect on HIV-related outcomes. Kawai et al (13) addressed this question with preliminary data from a trial in pregnant HIV-infected Tanzanian women. Women between 12 and 27 wk gestation were randomly assigned to receive single-RDA or multiple RDA doses of micronutrients from enrollment to 6 wk after delivery. The primary outcomes of the trial were related to pregnancy. T cell counts and HIV viral load were measured within 10 wk of the delivery in a subset of 272 of these women who were also participating in a randomized trial of selenium supplements (24). No differences between the high-dose and RDA-dose groups were seen in average CD4, CD8, or CD3 cell counts; in HIV viral load; or in hemoglobin concentration. There was no placebo group in this study, so the benefits relative to no supplement could not be measured. There was no difference between the high-dose and RDA-dose groups in birth outcomes, including birth weight or the risk of low birth weight, preterm birth, fetal death, or early infant death. These data are promising, but further studies are needed to address the important question of whether micronutrient intake at the RDA is of equivalent benefit to higher dose supplementation.
The impact of long-term micronutrient supplementation on chronic disease outcomes in persons with HIV/AIDS has rarely been considered. However, any future recommendations should acknowledge that the expanding access to ART in many of the most affected regions of the world and the consequent improvement in survival of persons with HIV/AIDS are occurring against a backdrop of an increasing prevalence of chronic disease in these regions. There is justifiable concern that long-term, high-dose micronutrient supplementation will increase the risk of chronic diseases in persons with HIV/AIDS, on the basis of studies that show unexpected increases in death due to lung cancer and cardiovascular disease in susceptible populations, such as smokers, receiving vitamin A and β-carotene supplements (25–27). Vitamin A and β-carotene supplementation at moderate doses may elevate serum cholesterol and triglycerides (28). Elevations in serum lipids may occur with other antioxidant combinations as well (29). Because persons infected with HIV have unfavorable lipid changes, including elevated serum triglycerides, due to actions of the virus and ART (30–32), and because there is a concern for premature atherosclerosis in this population (33), the ability of long-term micronutrient supplementation to increase the risk of cardiovascular disease by further elevating serum lipids needs to be considered a potential adverse effect. Recommendations may need to be tailored to specific populations. For example, high-dose multiple micronutrient supplements may be appropriate only for short-term nutritional rehabilitation in underweight and malnourished persons with HIV.
SINGLE MICRONUTRIENT INTERVENTION TRIALS IN HIV-INFECTED ADULTS
There have also been several randomized controlled trials of single micronutrient supplements published since 2003. These trials have been conducted in widely disparate populations, making generalizations difficult. In HIV-infected persons undergoing routine clinical care, mixed carotenoids plus multivitamins improved overall mortality compared with multivitamins alone (34). In a similar setting, chromium showed some benefit on metabolic abnormalities (35), and a positive serum response to selenium supplementation was associated with a lower viral load in HIV-infected persons who were not frankly selenium deficient (36). In HIV-infected pregnant Tanzanian women who were already taking high-dose multiple micronutrients, selenium reduced diarrhea-related morbidity (24) but had no effect on CD4 count, viral load, or mortality (37); zinc, however, had a negative impact on maternal hemoglobin (39), increased the risk of wasting (39), and had no benefit on T cell counts, viral load, or pregnancy outcomes (38, 39). Zinc had no effect on the persistence of diarrhea in one study (40) but reduced the risk of diarrhea in another study that included only HIV-infected persons with low plasma zinc (41). In this latter study (41), zinc given at a dose similar to the RDA resulted in a 76% reduction in the risk of immunologic failure, defined as CD4 <200 cells/mm3, but did not affect mortality over 18 mo of follow-up. The conflicting results underline the importance of knowing the baseline micronutrient status of the target population. Whereas these data are insufficient to make recommendations for the use of single micronutrients, the potential benefits of zinc, chromium, and selenium in some HIV-infected populations deserve further examination.
Concern about possible untoward effects of iron prompted 2 studies that examined the effect of iron supplementation on HIV viral load (42, 43). Semba et al (42) found that an RDA dose of iron added to a regimen of multiple micronutrients improved anemia and iron status in hepatitis C–infected women, with no evidence of untoward effects on HIV or hepatitis C viral load. Similar results were obtained in a small retrospective analysis of a randomized controlled trial of 4-mo of low-dose iron in 32 Kenyan adults (43), although statistical power was lacking in that study.
VITAMIN A IN POSTPARTUM WOMEN
In 2003 the WHO also issued recommendations for micronutrients in HIV-infected postpartum women as follows (1): “In areas of endemic vitamin A deficiency, WHO recommends that a single high-dose of vitamin A (200,000 IU) be given to women as soon as possible after delivery, but no later than 6 weeks after delivery.” (p 9). This recommendation was based on the observation that high-dose vitamin A supplements were beneficial to HIV-uninfected postpartum women and their infants (44, 45).
To the authors’ knowledge, only one published randomized trial has addressed this recommendation. Humphrey et al (46) conducted a complex randomized, placebo- controlled trial of high-dose vitamin A supplementation in 4495 mother-infant pairs in a 2 × 2 factorial design. The mothers received 400,000 IU vitamin A or placebo postpartum, and the infants received 50,000 IU units vitamin A or placebo. Thus, there were 4 treatment groups: vitamin A in both mothers and infants; vitamin A in mothers, placebo in infants; placebo in mothers, vitamin A in infants; placebo in both mothers and infants. The study showed no effect of vitamin A on postpartum mother-to-child transmission of HIV. Vitamin A supplementation in either the mother or the infant had no effect on mortality in the subgroup of infants who were HIV-positive at birth. In the subgroup of infants who were HIV-negative at birth and HIV-positive at 6 wk, infant vitamin A supplementation (with or without maternal supplementation) reduced mortality by 28%, but there was no effect of maternal vitamin A supplementation. However, vitamin A given to the mother, the infant, or both mother and infant doubled the risk of mortality in the subgroup of infants who were still HIV-negative at 6 wk postpartum. Vitamin A had no effect on mortality in the mothers (47) or on anemia in the infants (48). These data underscore prior concerns about the safety of vitamin A, which were reflected in the WHO 2003 recommendations. In light of these data, the current WHO recommendations for vitamin A supplementation in HIV-infected postpartum women should be reviewed.
There is a need for more randomized controlled trials designed for specific populations, including tuberculosis and hepatitis C–coinfected populations and those receiving stable ART. Trials are needed to establish whether micronutrients at multiples of the RDA are superior to micronutrients at the RDA and, if so, in which populations (eg, underweight compared with normal weight). There should be an effort to use a standardized dose and a combination of micronutrients on the basis of consensus in all future trials. Treatment of common gastrointestinal infections should be part of any evaluation of the effect of micronutrient supplements. Serum lipid concentrations should be monitored. Because serum micronutrient deficiencies are difficult to interpret in the presence of HIV infection, there may be value in identifying high-risk populations on the basis of the prevalence of dietary inadequacy and serum deficiencies in HIV-uninfected members of the same population.
Some important questions that remain to be answered include the following:
What are the relative benefits of high-dose supplementation or RDA supplementation compared with no supplementation?
Can the same benefits be achieved with food alone rather than with the use of supplements?
Are the micronutrient needs equivalent in persons receiving ART to persons not receiving ART?
Are the micronutrient needs equivalent in persons coinfected with tuberculosis or hepatitis C?
Is it safe to give micronutrient supplements to HIV-infected persons with liver disease from hepatitis C coinfection or alcohol abuse?
Is the need for supplements the same for persons in resource-rich compared with resource-poor environments or for persons living in different regions?
To what extent is benefit dependent on the presence or degree of baseline deficiency?
What are the long-term effects of supplementation on chronic disease outcomes?
As with all reviews, this study is limited by the risk that biases in the studies reviewed were not completely reported, possible incomplete retrieval of published studies, and the potential for publication bias favoring trials with positive results.
In conclusion, this review found that there are few randomized controlled trials of multiple micronutrient supplementation in persons with HIV/AIDS published since the WHO recommendations were formulated in 2003, and that the published trials are highly heterogeneous and many are of short duration. This impedes the ability to make broad recommendations about the use of micronutrients in persons living with HIV/AIDS. There continues to be concern for adverse consequences of some micronutrients, including vitamin A and zinc in some populations. Overall, dietary intake of micronutrients at RDA amounts remains a reasonable recommendation for persons with clinically stable disease. However, high-dose multiple micronutrient supplementation may benefit some persons with HIV/AIDS in the short term. Considerations include the underlying nutritional status, immune status, the presence of coinfections, and whether the supplementation is intended for short-term nutrition rehabilitation or for long-term health maintenance.
Acknowledgments
We thank Alice Tang for her helpful comments on the content of the manuscript. We also thank Saskia de Pee and Richard Semba for sharing with us their background paper for the World Food Program entitled “Nutrition and HIV Infection.”
The authors’ responsibilities were as follows—JEF: conducted the research, wrote the manuscript, and has final responsibility for the content; and KS: contributed to the research and writing of the manuscript. Neither of the authors had a conflict of interest to report.
REFERENCES
- 1.WHO Nutrient requirements for people living with HIV/AIDS: report of a technical consultation, World Health Organization, Geneva, 13-15 May 2003. Available from: http://www.who.int/nutrition/publications/Content_nutrient_requirements.pdf (cited 5 July 2010)
- 2.Semba RD, Graham NM, Caiaffa WT, Margolick JB, Clement L, Vlahov D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Arch Intern Med 1993;153:2149–54 [PubMed] [Google Scholar]
- 3.Baum MK, Shor-Posner G, Lu Y, Rosner B, Sauberlich HE, Fletcher MA, Szapocznik J, Eisdorfer C, Buring JE, Hennekens CH. Micronutrients and HIV-1 disease progression. AIDS 1995;9:1051–6 [DOI] [PubMed] [Google Scholar]
- 4.Tang AM, Graham NM, Saah AJ. Effects of micronutrient intake on survival in human immunodeficiency virus type 1 infection. Am J Epidemiol 1996;143:1244–56 [DOI] [PubMed] [Google Scholar]
- 5.Tang AM, Graham NM, Semba RD, Saah AJ. Association between serum vitamin A and E levels and HIV-1 disease progression. AIDS 1997;11:613–20 [DOI] [PubMed] [Google Scholar]
- 6.Tang AM, Graham NM, Chandra RK, Saah AJ. Low serum vitamin B-12 concentrations are associated with faster human immunodeficiency virus type 1 (HIV-1) disease. J Nutr 1997;127:345–51 [DOI] [PubMed] [Google Scholar]
- 7.Baum MK, Shor-Posner G, Lai S, Zhang G, Lai H, Fletcher MA, Sauberlich H, Page JB. High risk of HIV-related mortality is associated with selenium deficiency. J Acquir Immune Defic Syndr Hum Retrovirol 1997;15:370–4 [DOI] [PubMed] [Google Scholar]
- 8.Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, Mwakagile D, Mugusi F, Hertzmark E, Essex M, et al. A randomized trial of micronutrient supplements and HIV disease progression and mortality. N Engl J Med 2004;351:23–32 [DOI] [PubMed] [Google Scholar]
- 9.McClelland RS, Baeten JM, Overbaugh J, Richardson BA, Mandaliya K, Emery S, Lavreys L, Ndinya-Achola JO, Bankson DD, Bwayo JJ, et al. Micronutrient supplementation increases genital tract shedding of HIV-1 in women: results of a randomized trial. J Acquir Immune Defic Syndr 2004;37:1657–63 [DOI] [PubMed] [Google Scholar]
- 10.Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multi-vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br J Nutr 2006;95:762–70 [DOI] [PubMed] [Google Scholar]
- 11.Semba RD, Kumwenda J, Zijlstra E, Ricks MO, van Lettow M, Whalen C, Clark TD, Jorgensen L, Kohler J, Kumwenda N, et al. Micronutrient supplements and mortality of HIV-infected adults with pulmonary TB: a controlled clinical trial. Int J Tuberc Lung Dis 2007;11:854–9 [PubMed] [Google Scholar]
- 12.Kelly P, Katubulushi M, Todd J, Banda R, Yambayamba V, Fwoloshi M, Zulu I, Kafwembe E, Yavwa F, Sanderson IR, et al. Micronutrient supplementation has limited effects on intestinal infectious disease and mortality in a Zambian population of mixed HIV status: a cluster randomized trial. Am J Clin Nutr 2008;88:1010–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai K, Kupka R, Mugusi F, Aboud S, Okuma J, Villamor E, Spiegelman D, Fawzi WW. A randomized trial to determine the optimal dosage of multivitamin supplements to reduce adverse pregnancy outcomes among HIV-infected women in Tanzania. Am J Clin Nutr 2010;91:391–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villamor E, Mugusi F, Urassa W, Bosch RJ, Saathoff E, Matsumoto K, Meydani SN, Fawzi WW. A trial of the effect of micronutrient supplementation on treatment outcome, T cells counts, morbidity, and mortality in adults with pulmonary tuberculosis. J Infect Dis 2008;197:1499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiamton S, Pepin J, Suttent R, Filteau S, Mahakkanukrauh B, Hanshaoworakul W, Chaisilwattana P, Suthipinittharm P, Shetty P, Jaffar S. A randomized trial of the impact of multiple micronutrient supplementation on mortality among HIV-infected individuals living in Bangkok. AIDS 2003;17:2461–9 [DOI] [PubMed] [Google Scholar]
- 16.Kaiser JD, Campa AM, Ondercin JP, Leoung GS, Pless RF, Baum MK. Micronutrient supplementation increases CD4 count in HIV-infected individuals on highly active antiretroviral therapy: a prospective, double-blinded, placebo-controlled trial. J Acquir Immune Defic Syndr 2006;42:523–8 [DOI] [PubMed] [Google Scholar]
- 17.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, Antelman G, Mbise R, Herrera G, Kapiga S, et al. Randomized trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1 infected women in Tanzania. Lancet 1998;351:1477–82 [DOI] [PubMed] [Google Scholar]
- 18.Dietary Reference (DRIs): recommended intakes for individuals. Food and Nutrition Board, Institute of Medicine, National Academies, 2004. Available from: http://www.iom.edu/Global/News%20Announcements/∼/media/Files/Activity%20Files/Nutrition/DRIs/DRISummaryListing2.ashx (cited 3 September 2010)
- 19.Semba RD, Tang AM. Micronutrients and the pathogenesis of human immunodeficiency virus infection. Br J Nutr 1999;81:181–9 [DOI] [PubMed] [Google Scholar]
- 20.Villamor E, Msamanga G, Spiegelman D, Antelman G, Peterson KE, Hunter DJ, Fawzi WW. Effect of multivitamin and vitamin a supplements on weight gain during pregnancy among HIV-1-infected women. Am J Clin Nutr 2002;76:1082–90 [DOI] [PubMed] [Google Scholar]
- 21.Villamor E, Saathoff E, Manji K, Msamanga G, Hunter DJ, Fawzi WW. Vitamin supplements, socioeconomic status, and morbidity events as predictors of wasting in HIV-infected women from Tanzania. Am J Clin Nutr 2005;82:857–65 [DOI] [PubMed] [Google Scholar]
- 22.Fawzi WW, Msamanga GI, Kupka R, Spiegelman D, Villamor E, Mugusi F, Wei R, Hunter D. Multivitamin supplementation improves hematogic status in HIV-infected women and their children in Tanzania. Am J Clin Nutr 2007;85:1335–43 [DOI] [PubMed] [Google Scholar]
- 23.Smith Fawzi MC, Kaaya SF, Mbwambo J, Msamanga GI, Antelman G, Wei R, Hunter DJ, Fawzi WW. Multivitamin supplementation in HIV-positive pregnant women: impact on depression and quality of life in a resource poor setting. HIV Med 2007;8:203–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kupka R, Mugusi F, Aboud S, Hertzmark E, Spiegelman D, Fawzi WW. Effect of selenium supplements on hemoglobin concentration and morbidity among HIV-1-infected Tanzanian women. Clin Infect Dis 2009;48:1475–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Alpha Tocopherol Beta-carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029–35 [DOI] [PubMed] [Google Scholar]
- 26.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150–5 [DOI] [PubMed] [Google Scholar]
- 27.Törnwall ME, Virtamo J, Korhonen PA, Virtanen MJ, Taylor PR, Albanes D, Huttunen JK. Effect of alpha-tocopherol and beta carotene supplementation on coronary heart disease during the 6-year post-trial follow-up in the ATBC study. Eur Heart J 2004;25:1171–8 [DOI] [PubMed] [Google Scholar]
- 28.Cartmel B, Dziura J, Cullen MR, Vegso S, Omenn GS, Goodman GE, Redlich CA. Changes in cholesterol and trigyceride concentrations in the Vanguard population of the Carotene and Retinol Efficacy Trial (CARET). Eur J Clin Nutr 2005;59:1173–80 [DOI] [PubMed] [Google Scholar]
- 29.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant supplementation in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002;360:23–3312114037 [Google Scholar]
- 30.Grunfeld C, Kotler DP, Hamadeh R, Tierney A, Wang J, Pierson RN. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med 1989;86:27–31 [DOI] [PubMed] [Google Scholar]
- 31.Grunfeld C, Pang M, Doerrier W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992;74:1045–52 [DOI] [PubMed] [Google Scholar]
- 32.El-Sadr WM, Mullin CM, Carr A, Gibert C, Rappoport C, Visnegarwala F, Grunfeld C, Raghavan SS. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naïve cohort. HIV Med 2005;6:114–21 [DOI] [PubMed] [Google Scholar]
- 33.Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G, Fiocchi F, Lattanzi A, Rossi R, Modena MG, et al. Coronary aging in HIV-infected patients. Clin Infect Dis 2009;49:1756–62 [DOI] [PubMed] [Google Scholar]
- 34.Austin J, Singhal N, Voigt R, Smaill F, Gill MJ, Walmsley S, Salit I, Gilmour J, Schlech WF 3rd, Choudhri S, et al. A community randomized controlled clinical trial of mixed carotenoids and micronutrient supplementation of patients with acquired immunodeficiency syndrome. Eur J Clin Nutr 2006;60:1266–76 [DOI] [PubMed] [Google Scholar]
- 35.Aghdassi E, Arendt BM, Salit IE, Mohammed SS, Jalali P, Bondar H, Allard JP. In patients with HIV-infection, chromium supplementation improves insulin resistance and other metabolic abnormalities: a randomized, double-blind, placebo controlled trial. Curr HIV Res 2010;8:113–20 [DOI] [PubMed] [Google Scholar]
- 36.Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, et al. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med 2007;167:148–54 [DOI] [PubMed] [Google Scholar]
- 37.Kupka R, Mugusi F, Aboud S, Msamanga GI, Finkelstein JL, Spiegelman D, Fawzi WW. Randomized, double-blind, placebo-controlled trial of selenium supplements among HIV-infected pregnant women in Tanzania: effects on maternal and child outcomes. Am J Clin Nutr 2008;87:1802–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fawzi WW, Villamor E, Msamanga GI, Antelman G, Aboud S, Urassa W, Hunter D. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV-1-infected women in Tanzania. Am J Clin Nutr 2005;81:161–7 [DOI] [PubMed] [Google Scholar]
- 39.Villamor E, Aboud S, Koulinska IN, Kupka R, Urassa W, Chaplin B, Msamanga G, Fawzi WW. Zinc supplementation to HIV-1-infected pregnant women: effects on maternal anthropometry, viral load, and early mother-to-child transmission. Eur J Clin Nutr 2006;60:862–9 [DOI] [PubMed] [Google Scholar]
- 40.Cárcamo C, Hooton T, Weiss NS, Gilman R, Wener MH, Chavez V, Meneses R, Echevarria J, Vidal M, Holmes KK. Randomized controlled trial of zinc supplementation or persistent diarrhea in adults with HIV-1 infection. J Acquir Immune Defic Syndr 2006;43:197–201 [DOI] [PubMed] [Google Scholar]
- 41.Baum MK, Lai S, Sales S, Page JB, Campa A. Randomized, controlled clinical trial of zinc supplementation to prevent immunological failure in HIV-infected adults. Clin Infect Dis 2010;50:1653–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semba RD, Ricketts EP, Mehta S, Netski D, Thomas D, Kirk G, Wu AW, Vlahov D. Effect of micronutrients and iron supplementation on hemoglobin, iron status, and plasma hepatitis C and HIV RNA levels in female injection drug users a controlled clinical trial. J Acquir Immune Defic Syndr 2007;45:298–303 [DOI] [PubMed] [Google Scholar]
- 43.Olsen A. Mwaniki D, Krarup H, Friis H. Low-dose iron supplementation does not increase HIV viral load. J Acquir Immune Defic Syndr 2004;36:637–8 [DOI] [PubMed] [Google Scholar]
- 44.Stoltzfus RJ, Hakimi M, Miller KW, Rasmussen KM, Dawiesah S, Habicht JP, Dibley MJ. High dose vitamin A supplementation of breast feeding Indonesian mothers: effects on the vitamin A status of mother and infant. J Nutr 1993;123:666–75 [DOI] [PubMed] [Google Scholar]
- 45.Rice AL, Stoltzfus RJ, de Francisco A, Chakraborty J, Kjolhede CL, Wahed MA. Maternal vitamin A or beta-carotene supplementation in lactating Bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J Nutr 1999;129:356–65 [DOI] [PubMed] [Google Scholar]
- 46.Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Iliff PJ. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis 2006;193:860–71 [Published erratum appears in J Infect Dis 2008;197(10):1485.] [DOI] [PubMed] [Google Scholar]
- 47.Zvandasara P, Hargrove JW, Ntozini R, Chidawanyika H, Mutasa K, Iliff PJ, Moulton LH, Mzengeza F, Malaba LC, Ward BJ; the ZVITAMBO Study Group Mortality and morbidity among postpartum HIV-positive and HIV-negative women in Zimbabwe: risk factors, causes, and impact of single-dose postpartum vitamin A supplementation. J Acquir Immune Defic Syndr 2006;43:107–16 [DOI] [PubMed] [Google Scholar]
- 48.Miller MF, Stoltzfus RJ, Iliff PJ, Malaba LC, Mbuya NV, Zimbabwe Vitamin A for Mothers and Babies Project (ZVITAMBO) Study Group, Humphrey JH. Effect of maternal and neonatal vitamin A supplementation and other postnatal factors on anemia in Zimbabwean infants: a prospective, randomized study. Am J Clin Nutr 2006;84:212–22 [DOI] [PubMed] [Google Scholar]