Abstract
The transporter associated with antigen processing (TAP) translocates antigenic peptides from the cytosol into the lumen of the endoplasmic reticular and plays a critical role in the major histocompatibility complex (MHC) class I molecule-mediated antigenic presentation pathway. In this study, the porcine TAP1 gene was mapped to the pig chromosome 7 (SSC7) and was closely linked to the marker SSC2B02 (retention fraction=43%, LOD=15.18). Subcellular localization of TAP1 by transient transfection of PK15 cells indicated that the TAP1 protein might be located in the endoplasmic reticulum (ER) in pig kidney epithelial cells (PK-15). Gene expression analysis by semi-quantitative RT-PCR revealed that TAP1 was selectively expressed in some immune and immune-related tissues. Quantitative real-time PCR (qRT-PCR) analysis revealed that this gene was up-regulated after treatments that mimic viral and bacterial infection (polyriboinosinic-polyribocytidylic acid (poly(I:C)) and lipopolysaccharide (LPS), respectively). In addition, elevated TAP1 expression was detected after porcine reproductive and respiratory syndrome virus (PRRSV) infection in porcine white blood cells (WBCs). One single nucleotide polymorphism (SNP) in exon 3 of TAP1 was detected in a Landrace pig population by Bsp143I restriction enzyme digestion. Different genotypes of this SNP had significant associations (P<0.05) with the red blood cell distribution width (RDW) of 1-day-old (1 d) pigs (P=0.0168), the PRRSV antibody level (PRRSV Ab) (P=0.0445) and the absolute lymphocyte count (LYM#) (P=0.024) of 17 d pigs. Our results showed that the TAP1 gene might have important roles in swine immune responses, and these results provide useful information for further functional studies.
Keywords: Pig, TAP1, Localization, Expression, PRRSV, Association analyses
Introduction
The transporter associated with antigen processing (TAP), which is located in the MHC II region, is a heterodimer composed of the TAP1 and TAP2 subunits. Both subunits belong to the ATP-binding cassette family of transporters. TAP1 functions by providing a supply of candidate peptides to the MHC I molecules within the peptide loading complex 1 and by transporting antigen peptides from the cytoplasm into the endoplasmic reticulum (ER) 2. It has become evident that TAP is involved in immune responses in both humans and pigs. In humans, increased expression of TAP1 and MHC I pathway-related genes has a pivotal role in the processing and presentation of viral antigens and the clearance of viruses within infected cells 3. In pigs, Garcia-Borges et al. first cloned the complete coding sequence of the porcine TAP1 gene, and an alternatively spliced variant of this gene has been proposed to be associated with host resistance mechanisms by inhibiting the expression of the immediate-early protein ICP47 of herpes simplex virus-type 1 (HSV-1)4. TAP is also a target for viral escape strategies 1. Some inhibitors of TAP, such as herpes simplex virus (HSV) 5-6, Epstein-Barr virus (EBV) 7, cytomegalovirus (CMV) 8 and varicella virus 9 can inhibit the MHC class I antigen presentation pathway by inhibiting TAP, which translocates peptides across the endoplasmic reticulum membrane. Similarly, an early pseudorabies virus protein induces the down-regulation of swine leukocyte antigen class I (SLA I) by interfering with the TAP genes in swine 10.
TAP1 is expressed in many immune cell types, including human Hu-H7 hepatocytes, macrophages and porcine PK-15 epithelial cells 3-11-12, and is strongly induced by some cytokines. TAP1 is up-regulated by 20-fold and 10-fold, respectively, at the mRNA and protein levels within 12 h by IFN-γ in both endothelial and HeLa cells 13. In vitro IFN-γ and TNF-α stimulation up-regulates TAP1 in a fraction of tumor samples 14. Treatment with IFN-α induces TAP1 expression primarily in human macrophages 15. Both IFN-γ and LPS can synergistically increase TAP1 transcription, which may be mediated by STAT1 16. Up-regulation of TAP expression may strengthen MHC Ι processing and CTL killing efficiency. It has been reported that TAP1 could also be strongly induced by the p53 and p73 tumor suppressors, which leads to the activation of the MHC class I pathway 17. In contrast, a reduction of TAP expression and function has been observed in murine tumor cell lines expressing IL-10 18.
PRRSV, a single positive-stranded RNA virus belonging to the family Arteriviridae 19-20, is the most economically significant infectious disease threat to the swine industry worldwide. It causes high mortality of piglets, reproductive failure in pregnant gilts and sows and respiratory disease in pigs of all ages 21-22. It has been reported that PRRSV reduces the antigen-presenting ability of monocyte-derived dendritic cells (Mo-DC) and results in reduced expression of MHC I and MHC II 23, which might be involved in viral immune evasion during host defense. In this study, we completed new molecular characterization of the porcine TAP1 gene and localization analysis at both the gene level and the protein level. A thorough expression analysis including tissue distribution and immunostimulation by poly(I:C) and LPS was completed as well. In vivo PRRSV infection and association analysis between SNPs and immune traits highlighted the role of porcine TAP1 gene in PRRS and pig genetics.
Materials and methods
Gene mapping of porcine TAP1 gene
The INRA-University of Minnesota porcine radiation hybrid (IMpRH) panel was used to map the porcine TAP1 gene to a porcine chromosome 24. The PCR primers (map-F/map-R in Table 1) were designed to amplify a 329-base-pair (bp) genomic fragment spanning intron 3, exon 4 and intron 4 of the porcine TAP1 gene. PCR reaction was performed as described previously 25. Subsequently, the result was analyzed on the website http://www.toulouse.inra.fr/lgc/pig/RH/ for RH mapping 26.
Table 1.
TAP1 primer sequences used in this study.
| Gene name | Primer name | Primer sequences(5'-3') | Primer location | PCR(Tm)( °C) | Size(bp) |
|---|---|---|---|---|---|
| SNP-F | tcggaaatgtggataagagca | intron1 | 56 | 790 | |
| SNP-R | aaacagacggataatgaaagagg | intron3 | |||
| TAP1 | map-F | acctggctcattgctcgtc | intron3 | 61 | 329 |
| map-R | tctgggatgagggtcagtgtag | intron4 | |||
| exp-F | ccagatccagttcaccgaagc | exon6 | 61 | 190 | |
| exp-R | cgggtaggcaaaggagacatt | exon7 | |||
| pEGFP-F | ccgctagccgacctcgtacgccaatg | 5'UTR | 60 | 2274 | |
| pEGFP-R | ccggatcccttaactcaggagcatct | 3'UTR | |||
| β-actin | Actb-F | ggacttcgagcaggagatgg | 61 | 130 | |
| Actb-R | gcaccgtgttggcgtagagg | ||||
Subcellular localization of TAP1 in PK-15 cells
The 2241-bp open reading frame (ORF) encoding porcine TAP1 protein was amplified and subcloned into the NheI-BamHI site of the pEGFP-N1 vector (Clontech, USA) to yield the mammalian expression plasmid pEGFP-TAP1. PK-15 cells were transiently transfected with the pEGFP-TAP1 vector, and the empty pEGFP-N1 vector was used as a negative control. The primer pair pEGFP-F and pEGFP-R for porcine TAP1 is listed in Table 1. PK-15 cells were seeded on coverslips in 6-well plates and washed three times with phosphate-buffered saline (PBS). After a 6 h transient transfection using LipofectamineTM 2000 (Invitrogen, USA), the medium was replaced with fresh challenge media with or without poly (I:C) (Sigma, USA) at 10 µg/ml, following by 18 h transient transfection. Cells were fixed in 4% polyformaldehyde in PBS for 15 min at room temperature, permeabilized with pre-warmed 0.1% Triton X-100 in PBS for 5 min at 37 °C, and then stained with 40 μg/ml tetramethylrhodamine-conjugated ConA (Invitrogen, USA) in PBS at 37 °C for 20 min. All fluorescence images were generated using an LSM 510 META confocal microscope (ZEISS, Germany). LSM Image Examiner was used to generate images of individual fluorescent markers and to overlay pictures to demonstrate the relative distribution of the fusion protein. All images were obtained at 63× optical zoom.
RNA extraction and expression pattern of the TAP1 gene
Total RNA from 18 porcine organs (brain, heart, liver, spleen, stomach, lung, kidney, lymph node, fat, bone marrow, small intestines, muscle, testis, epididymis, bladder, uterus, ovary and skin) was isolated with an RNA prep pure Tissue Kit (Tiangen Biotech (Beijing) Co., Ltd). Total RNA was then quantified using a NanoDropTM 1000 Spectrophotometer (Thermo Fisher Scientific Inc., USA). The quality of the RNA was evaluated by formaldehyde denaturing gel electrophoresis in 1.2% agarose gels, which showed dispersed bands (28S and 18S) without any obvious smearing patterns that would indicate degradation. A total of 2 μg RNA was used for reverse transcriptase-polymerase chain reaction (RT-PCR) using the TransScript First-Strand cDNA Synthesis SuperMix according to the manufacturer's instructions (Tiangen Biotech (Beijing) Co., Ltd). Semiquantitative RT-PCR (30 cycles) was employed to analyze the tissue distributions using the gene-specific primers exp-F and exp-R (Table 1). The expression level of β-actin (Table 1) was used as the internal control.
Immunostimulation with poly (I:C) and LPS in PK-15 cells
PK-15 cells were seeded in 6-well plates at a concentration of 2.5×105 cells/well and were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented 10% (v/v) fetal bovine serum in humidified air containing 5% CO2 at 37 °C. When ~50% confluence was observed, the medium was replaced with fresh challenge media with or without poly(I:C) at 10 µg/ml or LPS (Sigma, USA) at 1 µg/ml. Total RNA was harvested at 0, 6, 12, 24 and 48 hour post infection (hpi) using TRIzol reagent.
PRRSV infection, white blood cell isolation and qRT-PCR
All animal sample collection was performed according to guidelines approved by the Animal Care and Use Committee of Guangdong Province, China. A total of 72 PRRSV-negative local Chinese hybrid pigs (30 d∼40 d old) were used for the detection of porcine TAP1 gene expression in response to PRRSV infection. The pigs were divided into 2 groups and were housed in isolation facilities. The infection group consisted of 49 pigs and the control group consisted of 23 pigs, which were littermates of the pigs in the infection group. After 7 d of acclimation, pigs in the infection group were inoculated with 2 ml of 105 50% tissue culture infectious doses/ml (TCID50) per 10 kg body weight of PRRSV strain TPP6 by cervical muscle injection, while the control group was inoculated with 2 ml of DMEM culture medium. Blood was collected from the jugular vein of pigs using a 5 ml tube with EDTA-K2 anticoagulant at 0, 4, 7, 14, 21, 28, 35 and 42 day post infection (dpi). Red blood cell (RBC) lysis buffer was added to the blood samples and lysis was allowed to proceed for 5 min at room temperature. White blood cells (WBCs) were isolated by centrifugation at 2800 revolutions per minute (rpm) for 5 min, following by WBC RNA extraction using RNA prep pure Blood Kit (Tiangen Biotech, Beijing). Three pigs at each time point from the infection and control groups were selected to evaluate the mRNA expression of the porcine TAP1 gene.
Poly(I:C)-, LPS- and PRRSV-induced expression of the porcine TAP1 gene was determined by qRT-PCR using a standard SYBR Green PCR kit (Toyobo, Japan) and a Bio-Rad iQ5 Real-Time PCR Detection System. Reactions were prepared in a total volume of 20 µl containing SYBR® Green Real-time PCR Master Mix, gene-specific primers (exp-F/exp-R in Table 1) and template cDNA. The cycling conditions consisted of an initial, single cycle for 3 min at 95°C followed by 40 cycles of 30 s at 95°C, 30 s at 61°C and 15 s at 72°C. Three independent samples at each time point/condition were analyzed. Porcine β-actin was used as the internal control, and all reactions were run in triplicate. The ΔΔCt method was used to determine the different expression levels 27.
SNP identification, allele frequencies and association analysis
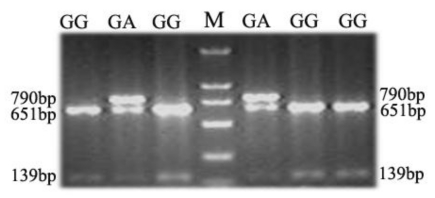
An A/G polymorphism in exon 3 of the porcine TAP1 gene, which had been reported based on genome sequencing of different breeds of pigs was detected in our Landrace population by Bsp143I restriction analysis 28. PCR restriction fragment length polymorphism (PCR-RFLP) analysis with the primer pair SNP-F/SNP-R (Table 1) was employed to genotype the SNP polymorphic sites in this study. In total, 306 DNA samples of Landrace piglets, 17 DNA samples of sires and 36 DNA samples of dams were genotyped. The blood parameters and levels of antibodies to PRRSV, CSFV and PRV were measured for all piglets at 1 d, 17 d and 32 d. As the A/G polymorphism identified creates a polymorphic Bsp143I restriction site, allele A (in which the restriction site is absent) is characterized by the presence of one fragment 790 bp in length, whereas for allele G (which possesses the polymorphic restriction site), the amplicon is cut into fragments of about 651 and 139 bp (Fig. 5). Subsequently, deviation from Hardy-Weinberg equilibrium was examined with the Fisher's exact test (probability test) using PopGene 3.2 29. A total of 18 blood parameters were analyzed and association analysis was performed as described previously 30.
Fig 5.
PCR-RFLP analysis of a porcine TAP1 polymorphism. The genotypes are shown on the top of the lanes (M, molecular weight marker).
Results
Chromosome mapping and subcellular localization of TAP1 in PK-15 cells
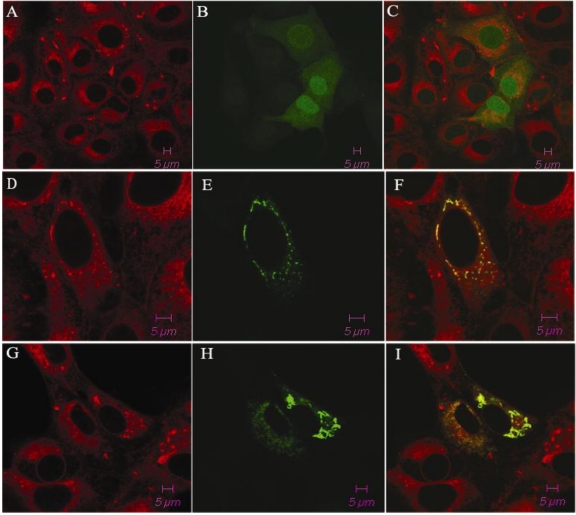
The porcine TAP1 gene was found to be located on chromosome 7 (SSC7) using IMpRH, and the two-point analysis from RH mapping showed that porcine TAP1 is closely linked to the marker SSC2B02 (retention fraction=43%, LOD=15.18). We also completed subcellular localization analysis of the porcine TAP1 protein by fluorescence and confocal analysis. With pEGFP-N1 empty vector transfected EGFP was observed throughout the entire cell (Fig. 1C). The green fluorescence of pEGFP-TAP1 overlapped with the red fluorescence of an ER marker, indicating that TAP1 might be localized subcellularly on the ER (Fig. 1F). Under poly(I:C) stimulation, the subcellular location of porcine TAP1 did not change (Fig. 1I).
Fig 1.
The subcellular localization of the pEGFP-TAP1 fusion protein in PK-15 cells. Cells expressing pEGFP-TAP1 (D, E, F), pEGFP-TAP1 (with poly(I:C) stimulation, G, H, I) and pEGFP-N1 empty vector (A, B, C) are displayed in the panel. The cells were stained with tetramethylrhodamine-conjugated ConA (excited at 550 nm; red, A, D, G) and the expression of the green fluorescent protein is displayed in the center of the panel (excited at 488 nm; B, E, H).The fluorescent signals were analyzed by confocal microscopy. The overlay images were generated by merging two signals (C, F, I).
Tissue distribution analysis and poly(I:C)- or LPS-induced expression of TAP1 mRNA in PK-15 cells
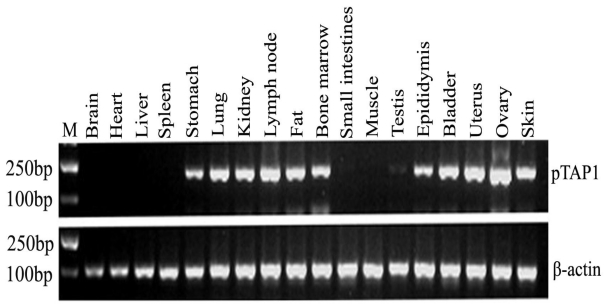
To investigate the general tissue distributions of porcine TAP1, RT-PCR was used to analyze TAP1 transcripts in RNA prepared from various tissues. Our results showed that the porcine TAP1 gene is selectively expressed in certain tissues including the lymph nodes, bone marrow, lungs, fat, skin, stomach, kidneys, epididymis, bladder, uterus and ovaries (Fig. 2). There was low or no expression in the testis, brain, heart, liver, spleen, small intestines and muscle.
Fig 2.
Tissue distribution analysis of porcine TAP1. β-actin was amplified as the internal control. Lengths of the PCR products (pTAP1: 190 bp; β-actin: 233 bp) are marked in the figure. Markers for mw (M): DL2000 DNA marker (2000 bp, 1000 bp, 750 bp, 500 bp, 250 bp, 100 bp).
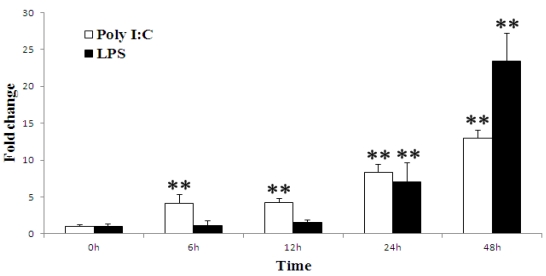
To determine whether the porcine TAP1 gene is responsive to viral or bacterial infections, stimulation experiments in which PK-15 cells were treated with poly(I:C) and LPS and analyzed by qRT-PCR were performed. The immunostimulation results show that porcine TAP1 was up-regulated following both types of stimulation (Fig. 3). Significant increases can be seen from 6 hpi(P=0.0045) to 48 hpi(P=0.0005) and from 24 hpi(P=0.0022) to 48 hpi(P=0.0005) following poly(I:C) and LPS stimulations, respectively.
Fig 3.
Porcine TAP1 mRNA levels in poly(I:C)- or LPS-stimulated PK-15 cells. Cells were harvested at the times indicated; RNA was prepared and RT-PCR was performed with β-actin amplified as the internal control. The mean results±s.e.m. of three samples are represented in each graph. Significant differences in the expression compared to the untreated control group (0 h) were calculated using two-tailed paired Student's t-tests. P-value<0.01 were considered to be dramatically significant vs. 0 h and are indicated with (**).
Elevated expression of the TAP1 gene in PRRSV-infected pigs
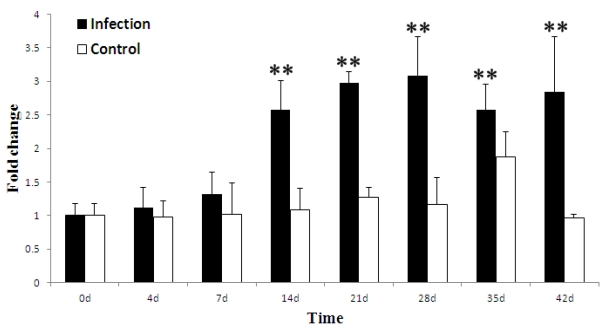
To investigate whether the porcine TAP1 gene is responsive to viral infections in vivo, stimulation experiments in which health pigs were infected PRRSV and analyzed by qRT-PCR were performed. The ability of PRRSV to induce the expression of the porcine TAP1 gene in WBCs is shown in Fig. 4. Our results demonstrate that the level of TAP1 mRNA in WBCs was higher than baseline from 4 to 42 d, peaking at 28 d and decreasing from 35 d. The expression of TAP1 mRNA was maintained at a constant level from 0 to 42 days in the control group.
Fig 4.
Porcine TAP1 mRNA levels in WBCs after PRRSV infection (in vivo stimulation) with for 0, 4, 7, 14, 21, 28, 35 and 42 d. mRNA levels of β-actin were used as internal control. The mean results±s.e.m. of three individual animals are presented in the graph. The black symbols indicate the infection group and white symbols indicate the control group. (Calculated methods and significance levels are as noted for Fig. 3).
Polymorphism detection and association analysis
To determine whether the genetic variation of porcine TAP1 is associated with immunological traits in pigs, two genotypes, GG (homozygote) and heterozygote GA, were detected by PCR-RFLP for the SNP (A/G polymorphism in exon 3) (Fig. 5). The numbers of samples with the genotypes GA and GG were 31 and 275, respectively. The TAP1-Exon III-Bsp143I G allele is the predominant allele, with a frequency 0.9493. Analysis in PopGene showed that the observed genotypic values of 306 piglets were not statistically different from the expected values based on the Hardy-Weinberg equilibrium (P=0.3589, P>0.05, Table 2). These results indicate that different genotypes of the SNP in TAP1-Exon III are significantly associated (P<0.05) with RDW at 1 d (P=0.0168), PRRSV Ab at 17 d (P=0.0445) and LYM# at 17 d (P=0.024) (Table 3). Moreover, the associations of RDW (1 d) and LYM# (17 d) with the GA genotype and of PRRSV Ab (17 d) with the GG genotype were the strongest (Table 3). The homozygote AA was not detected in this population. Pigs with the genotype AA may be rejected during long-term selection because of the disadvantage of this allele.
Table 2.
Distribution of PCR-RFLP-TAP1 polymorphism in 306 Landrace piglets.
| Biomass | Genotype | Allele frequence | Chi-squre | Pdf=1 | ||
|---|---|---|---|---|---|---|
| GA | GG | A | G | |||
| 306 | 31 | 275 | 0.0507 | 0.9493 | 0.841741 | 0.358899 |
Table 3.
Association analysis of the TAP1SNP with immune response and growth traits.
| Trait | Genotype(n) | Lsmesn±SE | P-value |
|---|---|---|---|
| 1-day RDW | GA(28) | 15.0211±0.3691 | 0.0168* |
| GG(255) | 14.3952±0.2740 | ||
| 17-day PRRSV Ab | GA(23) | 0.7845±0.1875 | 0.0445* |
| GG(236) | 0.9723±0.1650 | ||
| 17-day LYM# | GA(21) | 8.6247±0.8105 | 0.024* |
| GG(205) | 7.0456±0.4669 |
*: significant difference level at P<0.05 level.
Discussion
Previous studies of TAP1 have mainly focused on humans and mice. However, little is known about the potential role of TAP1 in porcine immune responses and infectious diseases. Studies on important genes involved in immune responses and various genetic markers are necessary to improve the genetic resistance to infectious disease 31-32, which prompts us to complete further investigations of TAP1 in swine.
Using the IMpRH panel, TAP1 was mapped to SSC7 and was found to be closely linked to the marker SSC2B02, which is located in the MHC region and is linked to DQA and DQB 33-34. In humans, the TAP1 gene has been mapped to chromosome 6p21.3 and is located in the MHC II region between HLA-DP and DQ 35. Comparative map analysis between humans and pigs (www.animalgenome.org/pigs/) further confirmed our chromosome mapping results; human chromosome 6p21 is homologous to pig 7p11 and q11-q14 36-37. At the protein level, porcine TAP1 was predicted to be located in the ER by bioinformatic analysis (http://wolfpsort.org/). To validate our prediction, the subcellular location of TAP1 was determined by fluorescence and confocal analysis of PK-15 cells transiently transfected with the pEGFP-TAP1 vector. The results were in agreement with the bioinformatics analysis. Consistent with porcine TAP1, the human homologue is located in the ER and the cis-Golgi 38-39. These data strongly support the notion that TAP1 might be located in the ER. Interestingly, our subcellular localization analysis of porcine TAP1 following poly(I:C) stimulation showed that the localization was the same as that under the normal conditions, indicating that the protein localization is not significantly affected by viral stimulation (Fig. 1I). Subcellular location of porcine TAP1 is also consistent with its function in transporting the antigen peptide from the cytosol into the lumen of the endoplasmic reticulum in living cells.
Under normal conditions, porcine TAP1 was selectively expressed in immune (lymph node and bone marrow) and immune-related tissues (lung, skin, stomach and fat) in addition to organs of the urogenital system (kidney, epididymis, bladder, uterus and ovary). The tissue distributions in pig are generally identical with that in both humans and mice 40. The porcine TAP1 expression pattern indicates that it may play a vital role in immune responses. Surprisingly, there was no TAP1 expression in the spleen under normal conditions, which may be associated with following two reasons: Firstly, the difference of breed and day in pigs may be responsible for the mRNA expression quantity of TAP1 in the some immune tissues; Secondly, the expression of TAP1 in some immune organs may be induced by other virus or bacterium infection. After immunostimulation mimicking viral or bacterial stimulation using poly(I:C) and LPS, we detected TAP1 up-regulation in PK-15 cells, indicating its wide roles during defenses in pigs. In humans, TAP1 expression could also be induced by LPS after treatment for 24 h in primary human macrophages 15; up-regulation of TAP expression might strengthen MHC I processing and CTL killing efficiency. It has been shown that porcine TAP1 expression was significantly induced at 24 hpi and 48 hpi in Salmonella choleraesuis-infected porcine lungs 41. In this study, we first observed the up-regulated expression of porcine TAP1 following PRRSV infection in vivo. We observed a gradual but not significant up-regulation of TAP1 gene expression in WBCs from 4 dpi (FC=1.1, P=0.6980) to 7 dpi (FC=1.3, P=0.3635) (Fig. 4). This up-regulation may imply that TAP1 production in response to PRRSV infection is slow and weak. Similarly, Suradhat et al. found that the IFN-γ gene expression was not significantly up-regulated from 5 dpi in PBMCs following by PRRSV infection. These results may be due to the weak innate immune response to PRRSV 42. However, up-regulation of IL-10 gene expression was observed previously in PBMC of PRRSV infected pigs from 5 dpi 43. Interestingly, Salazar-Onfray et al. demonstrated that IL-10 can decrease the expression and function of the TAP1/2 molecular complex44. Overall, this implies that the expression and effect of TAP1 may be fine-tuned by IL-10 during an innate immune response to PRRSV from blood. We also found that TAP1 expression was markedly up-regulated from 14 dpi (P=0.0050) to 42 dpi (P=0.0036), which occurs in the adaptive immune response stage of PRRSV infection. In this stage, much greater cytokine, interferon and antibody levels were produced to protect pigs against the PRRSV invasion; the role of these increased levels is not clear. Our result suggests a potential induction mechanism for the anti-virus immune response of porcine TAP1 in blood following PRRSV infection. In other species, modulations of viral infections, including hepatitis B virus (HBV) in HepG2 cells 45, single-stranded hepatitis A virus in hepatocytes 3, West Nile virus (WNV) infection in human skin fibroblasts (HFFs) 46 and Marek's disease virus (MDV) infection in chicken spleen 47 have also been reported.
The ultimate objective of modern breeding is to produce pigs with superior immune response and health traits; however, infectious disease has been the most costly and hazardous problem in breeding. Studies on various genetic improvement methods and important immune response genes are crucial to improve the genetic resistance to infectious disease 31-32. However, little information has been reported on the correlation between the TAP1 gene and pig infectious disease. One SNP (A/G polymorphism) in exon 3 of porcine TAP1 was found to be significantly associated with RDW, LYM# and the PRRSV Ab level. Blood parameters and antibody levels can reflect the immune competence of an individual or a population to some extent and may be used as potential indicators for disease or disease severity. RDW is essential diagnostic parameter that reflects the difference in the red cell count. Zou et al. found that there were 14 QTLs associated with RDW at 18, 46 and 240 d after white Duroc×Erhualian intercross resource population, and a QTL for RDW at 18 d was located in the KIT region 48. RDW is used as an inexpensive and powerful prognostic marker in heart failure and cardiovascular disease, which has been a hot research area in recent years 49-52. Lymphocytes are important components of the host immune response. The absolute lymphocyte number is also a prognostic indicator for survival in various hematological malignancies 53-56.
PRRS is an economically important disease. Because of the high variability in the PRRSV genome between North American and European isolates, no vaccines could offer effective protection against all PRRSV isolates currently existing 57-58. Alternative methods such as the characterization of host-resistance mechanisms using genetics strategies would be helpful to identify the genes involved in resistance to homologous or heterologous PRRSV infection 59. Therefore, more studies are now focusing on candidate genes that are associated with PRRS resistance during innate immune and adaptive immune responses. These genes include IFNs (IFN-α, IFN-γ), ILs (IL-8, IL-10, IL-1β), TLRs (TLR-3), CXCLs and the PRRSV receptor (SIGLEC1, CD163) 60-65. Interestingly, our association analysis showed that the porcine TAP1 gene was associated with the PRRSV antibody level. Proper antibody levels are important to protect pigs from virus infection. These results indicate that porcine TAP1 may not only play crucial roles in antigen presentation but may also significantly affect antibody levels during PRRSV infection.
In conclusion, we investigated TAP1 as a candidate gene for disease resistance in swine. We performed gene mapping and analyzed the genetic variation of porcine TAP1 gene, and we investigated the induced expression change in cells stimulated in vitro by poly(I:C)/LPS, or infected health pigs by PRRSV. Together with this, our results indicate that the porcine TAP1 gene is closely related with porcine immune responses; thus, this report provides useful information for further functional studies.
Acknowledgments
We appreciate Dr Martine Yerle of INRA, France, for providing the IMpRH panel. We thank Ping Zhou, Ling Sun in Huazhong Agriculture University and Yuxiu Deng, Xiaofei Luo in Guangdong Wen's Research Institute for technical assistance and sample collection. This research was supported by key project of National Natural Science Foundation of China (U0631005) and 863 project of china (2011AA100304).
References
- 1.Parcej D, Tampe R. ABC proteins in antigen translocation and viral inhibition. Nat Chem Biol. 2010;6(8):572–80. doi: 10.1038/nchembio.410. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell P, Bangia N, Dick T. et al. The nature of the MHC class I peptide loading complex. Immunol Rev. 1999;172:21–8. doi: 10.1111/j.1600-065x.1999.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Yanagi M, Mori-Aoki A. et al. Transfection of single-stranded hepatitis A virus RNA activates MHC class I pathway. Clin Exp Immunol. 2002;127(2):234–42. doi: 10.1046/j.1365-2249.2002.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Borges CN, Phanavanh B, Crew MD. Characterization of porcine TAP genes: alternative splicing of TAP1. Immunogenetics. 2006;58(5-6):374–82. doi: 10.1007/s00251-006-0103-8. [DOI] [PubMed] [Google Scholar]
- 5.Tomazin R, Hill AB, Jugovic P. et al. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15(13):3256–66. [PMC free article] [PubMed] [Google Scholar]
- 6.Jugovic P, Hill AM, Tomazin R. et al. Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47. J Virol. 1998;72(6):5076–84. doi: 10.1128/jvi.72.6.5076-5084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna R, Burrows SR, Moss DJ. et al. Peptide transporter (TAP-1 and TAP-2)-independent endogenous processing of Epstein-Barr virus (EBV) latent membrane protein 2A: implications for cytotoxic T-lymphocyte control of EBV-associated malignancies. J Virol. 1996;70(8):5357–62. doi: 10.1128/jvi.70.8.5357-5362.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halenius A, Momburg F, Reinhard H. et al. Physical and functional interactions of the cytomegalovirus US6 glycoprotein with the transporter associated with antigen processing. J Biol Chem. 2006;281(9):5383–90. doi: 10.1074/jbc.M510223200. [DOI] [PubMed] [Google Scholar]
- 9.Verweij MC, Lipinska AD, Koppers-Lalic D. et al. The capacity of UL49.5 proteins to inhibit TAP is widely distributed amongst members of the genus Varicellovirus. J Virol. 2011 Mar;85(5):2351–63. doi: 10.1128/JVI.01621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambagala AP, Hinkley S, Srikumaran S. An early pseudorabies virus protein down-regulates porcine MHC class I expression by inhibition of transporter associated with antigen processing (TAP) J Immunol. 2000;164(1):93–9. doi: 10.4049/jimmunol.164.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Flori L, Rogel-Gaillard C, Cochet M. et al. Transcriptomic analysis of the dialogue between Pseudorabies virus and porcine epithelial cells during infection. BMC Genomics. 2008;9:123. doi: 10.1186/1471-2164-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cramer LA, Klemsz MJ. Altered kinetics of Tap-1 gene expression in macrophages following stimulation with both IFN-gamma and LPS. Cell Immunol. 1997;178(1):53–61. doi: 10.1006/cimm.1997.1118. [DOI] [PubMed] [Google Scholar]
- 13.Ma W, Lehner PJ, Cresswell P. et al. Interferon-gamma rapidly increases peptide transporter (TAP) subunit expression and peptide transport capacity in endothelial cells. J Biol Chem. 1997;272(26):16585–90. doi: 10.1074/jbc.272.26.16585. [DOI] [PubMed] [Google Scholar]
- 14.Nagy N, Vanky F, Klein E. Tumor surveillance: expression of the transporter associated with antigen processing (TAP-1) in ex-vivo human tumor samples and its elevation by in vitro treatment with IFN-gamma and TNF-alpha. Immunol Lett. 1998;64(2-3):153–60. doi: 10.1016/s0165-2478(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 15.Schiffer R, Baron J, Dagtekin G. et al. Differential regulation of the expression of transporters associated with antigen processing, TAP1 and TAP2, by cytokines and lipopolysaccharide in primary human macrophages. Inflamm Res. 2002;51(8):403–8. doi: 10.1007/pl00000321. [DOI] [PubMed] [Google Scholar]
- 16.Cramer LA, Nelson SL, Klemsz MJ. Synergistic induction of the Tap-1 gene by IFN-gamma and lipopolysaccharide in macrophages is regulated by STAT1. J Immunol. 2000;165(6):3190–7. doi: 10.4049/jimmunol.165.6.3190. [DOI] [PubMed] [Google Scholar]
- 17.Zhu K, Wang J, Zhu J. et al. p53 induces TAP1 and enhances the transport of MHC class I peptides. Oncogene. 1999;18(54):7740–7. doi: 10.1038/sj.onc.1203235. [DOI] [PubMed] [Google Scholar]
- 18.Salazar-Onfray F, Charo J, Petersson M. et al. Down-regulation of the expression and function of the transporter associated with antigen processing in murine tumor cell lines expressing IL-10. J Immunol. 1997;159(7):3195–202. [PubMed] [Google Scholar]
- 19.Snijder EJ, Meulenberg JJ. The molecular biology of arteriviruses. J Gen Virol. 1998;79( Pt 5):961–79. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- 20.Allende R, Lewis TL, Lu Z. et al. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J Gen Virol. 1999;80( Pt 2):307–15. doi: 10.1099/0022-1317-80-2-307. [DOI] [PubMed] [Google Scholar]
- 21.Mengeling WL, Lager KM. A brief review of procedures and potential problems associated with the diagnosis of porcine reproductive and respiratory syndrome. Vet Res. 2000;31(1):61–9. doi: 10.1051/vetres:2000108. [DOI] [PubMed] [Google Scholar]
- 22.Neumann EJ, Kliebenstein JB, Johnson CD. et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005;227(3):385–92. doi: 10.2460/javma.2005.227.385. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Eaton M, Mayer M. et al. Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Arch Virol. 2007;152(2):289–303. doi: 10.1007/s00705-006-0857-1. [DOI] [PubMed] [Google Scholar]
- 24.Yerle M, Pinton P, Robic A. et al. Construction of a whole-genome radiation hybrid panel for high-resolution gene mapping in pigs. Cytogenet Cell Genet. 1998;82(3-4):182–8. doi: 10.1159/000015095. [DOI] [PubMed] [Google Scholar]
- 25.Ma G, Huang J, Sun N. et al. Molecular characterization of the porcine GBP1 and GBP2 genes. Mol Immunol. 2008;45(10):2797–807. doi: 10.1016/j.molimm.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Milan D, Hawken R, Cabau C. et al. IMpRH server: an RH mapping server available on the Web. Bioinformatics. 2000;16(6):558–9. doi: 10.1093/bioinformatics/16.6.558. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Chen HY, Wu ZF. Prospective of BF,DRB,DQB,TAP1 and IFN-γ genes used as porcine marker assisted disease resistance and breeding. Biotechnology bulletin. 2009;1:103–106. [Google Scholar]
- 29.Yeh FC, Yang RC, Boyle T. POPGENE VERSION 1.31, Quick User Guide. Canada: University of Alberta; 1999. pp. 103–106. [Google Scholar]
- 30.Liu XD, Chen HB, Tong Q. et al. Molecular characterization of caveolin-1 in pigs infected with Haemophilus parasuis. J Immunol. 2011;186(5):3031–46. doi: 10.4049/jimmunol.0902687. [DOI] [PubMed] [Google Scholar]
- 31.Genetic improvement of immune response and disease resistance: Past experience and a vision for the future; 2003. Bonnie A, Mallare BA, Bruce N, et al; http://www.nsif.com/Conferences/2003/pdf/GeneticImmuneResponse.pdf. [Google Scholar]
- 32.Shinkai H, Tanaka M, Morozumi T. et al. Biased distribution of single nucleotide polymorphisms (SNPs) in porcine Toll-like receptor 1 (TLR1), TLR2, TLR4, TLR5, and TLR6 genes. Immunogenetics. 2006;58(4):324–30. doi: 10.1007/s00251-005-0068-z. [DOI] [PubMed] [Google Scholar]
- 33.Genet C, Renard C, Cabau C. et al. In the QTL region surrounding porcine MHC, gene order is conserved with human genome. Mamm Genome. 2001;12(3):246–9. doi: 10.1007/s003350010261. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, Matsumoto T, Yanai S. et al. Conservation of the syntenies between porcine chromosome 7 and human chromosomes 6, 14 and 15 demonstrated by radiation hybrid mapping and linkage analysis. Anim Genet. 2003;34(4):255–63. doi: 10.1046/j.1365-2052.2003.00999.x. [DOI] [PubMed] [Google Scholar]
- 35.Spies T, Cerundolo V, Colonna M. et al. Presentation of viral antigen by MHC class I molecules is dependent on a putative peptide transporter heterodimer. Nature. 1992;355(6361):644–6. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- 36.Goureau A, Yerle M, Schmitz A. et al. Human and porcine correspondence of chromosome segments using bidirectional chromosome painting. Genomics. 1996;36(2):252–62. doi: 10.1006/geno.1996.0460. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa A, Demeure O, Urien C. et al. A physical map of large segments of pig chromosome 7q11-q14: comparative analysis with human chromosome 6p21. Mamm Genome. 2004;15(12):982–95. doi: 10.1007/s00335-004-3008-6. [DOI] [PubMed] [Google Scholar]
- 38.Kleijmeer MJ, Kelly A, Geuze HJ. et al. Location of MHC-encoded transporters in the endoplasmic reticulum and cis-Golgi. Nature. 1992;357(6376):342–4. doi: 10.1038/357342a0. [DOI] [PubMed] [Google Scholar]
- 39.Meyer TH, van Endert PM, Uebel S. et al. Functional expression and purification of the ABC transporter complex associated with antigen processing (TAP) in insect cells. FEBS Lett. 1994;351(3):443–7. doi: 10.1016/0014-5793(94)00908-2. [DOI] [PubMed] [Google Scholar]
- 40.Su AI, Cooke MP, Ching KA. et al. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99(7):4465–70. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao SH, Kuhar D, Lunney JK. et al. Gene expression profiling in Salmonella Choleraesuis-infected porcine lung using a long oligonucleotide microarray. Mamm Genome. 2006;17(7):777–89. doi: 10.1007/s00335-005-0155-3. [DOI] [PubMed] [Google Scholar]
- 42.Murtaugh MP, Xiao Z, Zuckermann F. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 2002;15(4):533–47. doi: 10.1089/088282402320914485. [DOI] [PubMed] [Google Scholar]
- 43.Suradhat S, Thanawongnuwech R, Poovorawan Y. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J Gen Virol. 2003;84(Pt 2):453–9. doi: 10.1099/vir.0.18698-0. [DOI] [PubMed] [Google Scholar]
- 44.Salazar-Onfray F, Charo J, Petersson M. et al. Down-regulation of the expression and function of the transporter associated with antigen processing in murine tumor cell lines expressing IL-10. J Immunol. 1997;159(7):3195–202. [PubMed] [Google Scholar]
- 45.Chen WH, Ding J, Gong WD. et al. [The mechanism for up-regulation of HLA-I expression on HepG2 cells by HBV] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2004;20(1):74–7. [PubMed] [Google Scholar]
- 46.Arnold SJ, Osvath SR, Hall RA. et al. Regulation of antigen processing and presentation molecules in West Nile virus-infected human skin fibroblasts. Virology. 2004;324(2):286–96. doi: 10.1016/j.virol.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Lian L, Qu LJ, Zheng JX. et al. Expression profiles of genes within a subregion of chicken major histocompatibility complex B in spleen after Marek's disease virus infection. Poult Sci. 2010;89(10):2123–9. doi: 10.3382/ps.2010-00919. [DOI] [PubMed] [Google Scholar]
- 48.Zou ZZ, Ren J, Yan XM. et al. Quantitative trait loci for porcine baseline erythroid traits at three growth ages in a White Duroc × Erhualian F2 resource population. Mamm Genome. 2008;19:640–646. doi: 10.1007/s00335-008-9142-9. [DOI] [PubMed] [Google Scholar]
- 49.Zalawadiya SK, Veeranna V, Niraj A. et al. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol. 2010;106(7):988–93. doi: 10.1016/j.amjcard.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Al-Najjar Y, Goode KM, Zhang J. et al. Red cell distribution width: an inexpensive and powerful prognostic marker in heart failure. Eur J Heart Fail. 2009;11(12):1155–62. doi: 10.1093/eurjhf/hfp147. [DOI] [PubMed] [Google Scholar]
- 51.Yaman H, Celik T, Akgul EO. et al. Red cell distribution width and acute coronary syndromes. Int J Cardiol. 2010;145(2):353. doi: 10.1016/j.ijcard.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Allen LA, Felker GM, Mehra MR. et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16(3):230–8. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siddiqui M, Ristow K, Markovic SN. et al. Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br J Haematol. 2006;134(6):596–601. doi: 10.1111/j.1365-2141.2006.06232.x. [DOI] [PubMed] [Google Scholar]
- 54.Kim DH, Baek JH, Chae YS. et al. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B-cell lymphoma. Leukemia. 2007;21(10):2227–30. doi: 10.1038/sj.leu.2404780. [DOI] [PubMed] [Google Scholar]
- 55.De Angulo G, Yuen C, Palla SL. et al. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: implications for risk stratification and future studies. Cancer. 2008;112(2):407–15. doi: 10.1002/cncr.23168. [DOI] [PubMed] [Google Scholar]
- 56.Huang JJ, Jiang WQ, Lin TY. et al. Absolute lymphocyte count is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. Ann Oncol. 2011;22(1):149–55. doi: 10.1093/annonc/mdq314. [DOI] [PubMed] [Google Scholar]
- 57.Plagemann PG. Porcine reproductive and respiratory syndrome virus: origin hypothesis. Emerg Infect Dis. 2003;9(8):903–8. doi: 10.3201/eid0908.030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bates JS, Petry DB, Eudy J. et al. Differential expression in lung and bronchial lymph node of pigs with high and low responses to infection with porcine reproductive and respiratory syndrome virus. J Anim Sci. 2008;86(12):3279–89. doi: 10.2527/jas.2007-0685. [DOI] [PubMed] [Google Scholar]
- 59.Lewis CR, Ait-Ali T, Clapperton M. et al. Genetic per spectives on host responses to porcine reproductive and respiratory syndrome (PRRS) Viral Immunol. 2007;20:343–358. doi: 10.1089/vim.2007.0024. [DOI] [PubMed] [Google Scholar]
- 60.Jafer O, Zhang S, Sargent C. et al. Identification of SNPs in porcine genes expressed during porcine respiratory and reproductive syndrome virus infection. Anim Genet. 2009;40(4):580–2. doi: 10.1111/j.1365-2052.2009.01879.x. [DOI] [PubMed] [Google Scholar]
- 61.Sang Y, Ross CR, Rowland RR. et al. Toll-like receptor 3 activation decreases porcine arterivirus infection. Viral Immunol. 2008;21(3):303–13. doi: 10.1089/vim.2008.0042. [DOI] [PubMed] [Google Scholar]
- 62.Calvert JG, Slade DE, Shields SL. et al. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J Virol. 2007;81(14):7371–9. doi: 10.1128/JVI.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Gorp H, Van Breedam W, Delputte PL. et al. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J Gen Virol. 2008;89(Pt 12):2943–53. doi: 10.1099/vir.0.2008/005009-0. [DOI] [PubMed] [Google Scholar]
- 64.Petry DB, Lunney J, Boyd P. et al. Differential immunity in pigs with high and low responses to porcine reproductive and respiratory syndrome virus infection. J Anim Sci. 2007;85(9):2075–92. doi: 10.2527/jas.2006-721. [DOI] [PubMed] [Google Scholar]
- 65.Lunney JK, Fritz ER, Reecy JM. et al. Interleukin-8, interleukin-1beta, and interferon-gamma levels are linked to PRRS virus clearance. Viral Immunol. 2010;23(2):127–34. doi: 10.1089/vim.2009.0087. [DOI] [PubMed] [Google Scholar]