Abstract
Amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig's disease) is a debilitating and universally fatal neurodegenerative disease that devastates upper and lower motor neurons. The causes of ALS are poorly understood. A central role for RNA-binding proteins and RNA metabolism in ALS has recently emerged. The RNA-binding proteins TDP-43 and FUS are principal components of cytoplasmic inclusions found in motor neurons of ALS patients and mutations in TDP-43 and FUS are linked to familial and sporadic ALS. Pathology and genetics also connect TDP-43 and FUS with frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). It was unknown whether mechanisms of FUS aggregation and toxicity were similar or different to those of TDP-43. To address this issue, we have employed yeast models and pure protein biochemistry to define mechanisms underlying TDP-43 and FUS aggregation and toxicity, and to identify genetic modifiers relevant to human disease. We have identified prion-like domains in FUS and TDP-43 and provide evidence that these domains are required for aggregation. Our studies have defined key similarities as well as important differences between the two proteins. Collectively, our findings lead us to suggest that FUS and TDP-43, though similar RNA-binding proteins, likely aggregate and confer disease phenotypes via distinct mechanisms.
Key words: TDP-43, FUS/TLS, yeast, ALS, FTLD-U, prion
Introduction
As human life expectancy continues to increase, neurodegenerative diseases are increasing in prevalence, posing a clear and present danger to public health worldwide.1 Thus novel treatments and therapeutic interventions are desperately needed. These truly disastrous disorders include Alzheimer disease, Huntington disease, Parkinson disease and amyotrophic lateral sclerosis (ALS). Although these are very different diseases—some affecting memory but sparing movement, others robbing movement while preserving cognitive function—many of them share a common theme in the accumulation of insoluble protein aggregates in the brain.2–4
Many of the proteins found aggregated in pathological inclusions (e.g., Aβ, α-synuclein, polyglutamine, Prion Protein) are expressed broadly,5–10 some even ubiquitously, yet they seem to only aggregate in certain neurons and not others. Moreover, although protein aggregation defines neurodegenerative diseases broadly, there is also remarkable cell type specificity.11 For example, dopaminergic neurons are preferentially affected in Parkinson disease, whereas striatal neurons are primarily affected in Huntington disease and motor neurons are selectively lost in ALS. Therefore, an in depth understanding of the molecular and cell biological underpinnings of protein inclusions and the cellular pathways with which the aggregated proteins interact normally as well as during pathogenesis will provide critical insight into disease mechanisms.
In this Review, we discuss the emerging role of two RNA-binding proteins, TDP-43 and FUS/TLS, in ALS and highlight our recent studies to elucidate mechanisms of aggregation and toxicity. These studies have allowed us to define key similarities and differences between TDP-43 and FUS/TLS, which will likely be of therapeutic significance and have led to the discovery of a common genetic risk factor for ALS.
Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis (ALS)
Frontotemporal lobar degeneration (FTLD) is the second leading cause of dementia in people under 65, exceeded by only Alzheimer disease.12 FTD is characterized by severe changes in personality and abnormal social behavior.13 Motor defects have also been associated with FTLD.14 FTLDs comprise a heterogeneous class consisting of several subtypes, based on pathological characteristics.15,16 Some forms lack ubiquitinated inclusions and others contain tau pathology. But the most common subtype is frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). This subtype contains tau- and α-synuclein-negative cytoplasmic inclusions.17 Though mostly a sporadic disorder, several familial forms of FTLD-U have been linked to chromosomes 9 and 17 and recently, mutations in the gene encoding the secreted factor progranulin have been identified.18,19 Since progranulin protein is not part of the pathological inclusions, it suggested that there must be another disease protein associated with FTLD-U (see below).
Amyotrophic lateral sclerosis (ALS) is a devastating adult-onset neurodegenerative disease that wreaks havoc on motor neurons. A progressive and inexorably fatal muscle paralysis ensues, which typically causes death within 2 to 5 years of disease onset. ALS is mostly a sporadic disease (SALS) but approximately 10% of ALS cases are familial (FALS). Mutations in SOD1, the gene encoding Cu/Zn superoxide dismutase, have been identified in ∼20% of FALS cases,20 for an overall incidence of ∼2%. Because of clinical similarities between SALS and FALS, it is likely that studying the FALS cases will provide insight into the more common SALS forms.21 Indeed, a majority of the ALS research over the last 10–15 years has focused on SOD1. Neurons from patients harboring mutations in SOD1 are characterized by cytotoxic SOD1 aggregates that are ubiquitin-positive22 and transgenic mouse models expressing mutant SOD1 have provided enormous insight into disease mechanisms.23–32 However, neurons of non-SOD1 ALS patients contain ubiquitin-positive inclusions that are SOD1-negative,33 suggesting the presence of additional pathological protein(s). Therefore, the recent identification of TDP-43 as a major component of these inclusions in non-SOD1 sporadic and familial ALS has opened up a completely new area of focus for ALS research.
TDP-43 is the Major Disease Protein in FTLD-U and ALS
Though mutations in several genes, including progranulin (PGRN), have been linked to FTLD-U, the identity of the major protein(s) in these inclusions remained elusive. Biochemical and immunohistochemical approaches were used to raise monoclonal antibodies against the insoluble high-molecular weight material from FTLD-U disease brains.34 These novel antibodies were then used to identify and subsequently purify the antigen, which was revealed to be the TAR-DNA-binding protein 43 (TDP-43).35 TDP-43 was confirmed to be the major disease protein because TDP-43-specific monoclonal antibodies marked FTLD-U inclusions and a TDP-43 biochemical disease “signature” was present in FTLD-U and ALS, but not control brains. This signature included truncated, hyperphosphorylated and ubiquitinated TDP-43. Interestingly, while TDP-43 is normally a nuclear protein, pathological inclusions contained predominantly cytoplasmic TDP-43 aggregates, suggesting that altered subcellular localization is important for disease pathogenesis. However, whether this is because of loss of nuclear function, a gain of toxic function or some combination of both remains to be determined.
TDP-43 Mutations Linked to ALS
Despite the widespread TDP-43 pathology seen in nearly every non-SOD1 ALS case,35–38 it was still unclear whether TDP-43 aggregation was a cause or consequence of neurodegeneration associated with ALS. It was questioned if TDP-43 was a bona fide disease protein or perhaps just a marker of disease.39 Thus, it was a true breakthrough when five independent groups reported the identification of TDP-43 mutations in sporadic and familial ALS patients.40–44 In one fell swoop, a connection between genetics and pathology became apparent and TDP-43 joined the ranks of tau, α-synuclein, Aβ, prion protein (PrP) and polyglutamine as a truly important human neurodegenerative disease protein. Indeed, a new disease classification was born: the TDP-43 proteinopathy.45
TDP-43 is a highly conserved, ubiquitously expressed protein, initially identified by virtue of its ability to bind the HIV-1 TAR DNA element and act as a transcriptional repressor.46 In addition to a glycine-rich C-terminal region, TDP-43 contains two RNA recognition motifs (RRM1 and RRM2) and is able to bind UG-repeats in RNA.47,48 Some reports suggest TDP-43 might play a role in regulating splicing,49 while others propose it can act as a kind of bridge for nuclear bodies via an interaction with the survival motor neuron (SMN) protein.50 Mouse knockouts of TDP-43 result in very early embryonic lethality51,52 and knock-down of TDP-43 function by siRNA in human cell culture results in defects in nuclear shape, cell cycle abnormalities and apoptosis, owing to a failure to effectively repress transcription of cyclin-dependent kinase 6.53 Nothing is known about how one or more of these functions or perhaps a pathological gain-of-function might contribute to disease.
TDP-43 Proteinopathies: A Molecular Link Between Diverse Neurodegenerative Diseases
The identification of TDP-43 in ALS and FTLD-U, not only ushered in a new research field (TDP-43 neurodegenerative proteinopathies), but also provided the long sought after molecular link between these two diseases. Interestingly, though traditionally considered a motor neuron disease, some patients with ALS can also exhibit symptoms of FTLD and the cytoplasmic ubiquitinated inclusions resemble those of FTLD-U.14 Thus, the clinicopathological features of ALS and FTLD-U suggest perhaps a similar underlying pathogenic mechanism converging on TDP-43. Emerging evidence indicates TDP-43 might also play a broader role because ubiquitinated TDP-43-positive inclusions have also been found in a significant proportion of Alzheimer disease and inclusion body myopathy and Paget disease of bone cases.54–56
Can Yeast Teach Us About Neurodegenerative Diseases?
The baker's yeast Saccharomyces cerevisiae has emerged as a powerful model system to study the molecular mechanisms underpinning protein-misfolding diseases.57 Admittedly, it seems like an unusual approach—studying a complicated brain disease using a single-celled microorganism. However, surviving cellular stresses caused by misfolded proteins is an ancient problem that all cells struggle with and many of the mechanisms employed to deal with protein misfolding are conserved from yeast to man. Hence, we and others have been using yeast cells to model the key cellular events associated with protein misfolding in human neurodegenerative diseases and to perform high-throughput genome-wide screens to elucidate the basic cellular mechanisms of neurodegeneration.57–80 While at first it may seem implausible that simple yeast cells can provide insight into mechanisms of complicated neurodegenerative disease, one should remember that almost everything we know today about cancer biology has, as its foundation, basic studies begun in yeast to define how the cell cycle and cell division are regulated.81,82
These yeast models provide a unique opportunity to observe and understand protein folding and misfolding in real time as it occurs in a living cell. Importantly, the discoveries made using yeast are not specific to yeast and there are several examples in which these have been extended and validated in animal models,62,67,70,83,84 and even recently extended to human, identifying a novel genetic risk factor for ALS74 (see below).
Yeast Studies Reveal Insight into TDP-43 Pathogenesis
We have been using the budding yeast, Saccaharomyces cerevisiae, as a model system to study TDP-43. We generated a yeast TDP-43 proteinopathy model, which recapitulated two key aspects of TDP-43 seen in disease: cytoplasmic aggregation and toxicity. We next used this yeast model to define the regions of TDP-43 that are sufficient and necessary for aggregation and toxicity. We discovered that the glycine-rich C-terminal domain of TDP-43 is absolutely required for aggregation and toxicity.68 To this date, over forty ALS-linked mutations in TDP-43 have been reported—all but one of them are located in the C-terminal domain—underscoring the power of the yeast model to predict key features of TDP-43, with direct relevance to human disease. Interestingly, the C-terminal domain was not sufficient for TDP-43 toxicity, because an RNA-recognition motif was also required, indicating that RNA binding, in addition to aggregation, is a component of TDP-43 toxicity.
We next sought to determine the mechanism by which these ALS-linked TDP-43 mutations could contribute to disease. We combined the yeast model and in vitro biochemistry to analyze the effects of ALS-linked TDP-43 mutations on aggregation and toxicity. Some ALS-linked mutations (e.g., G294A) neither accelerated aggregation nor increased toxicity,73 suggesting they might contribute to disease by affecting other pathways. Importantly, we discovered that some ALS-linked mutations (e.g., M337V and Q331K) can accelerate TDP-43 aggregation and increase toxicity, providing a mechanism by which they might cause disease.73 These findings are reminiscent of the effects of Parkinson disease associated mutations in the α-synuclein gene.85,86 Importantly, we also validated our findings in a Drosophila TDP-43 model and found that the Q331K mutation in TDP-43, which is much more toxic than WT TDP-43 in yeast, is also more toxic in flies.74 Several other laboratories have also seen similar effects of ALS-linked mutations on TDP-43 in diverse experimental systems ranging from cell culture, flies, chicken embryos, mouse and rat.42,88–93 Thus, ALS-linked mutations in TDP-43 might cause disease by a toxic gain-of-function mechanism. These studies show that the toxicity and aggregation properties of TDP-43 seen in human ALS patients are reflected in yeast. This indicates that the yeast genetics armamentarium can now be used to define mechanisms of TDP-43 toxicity by screening for genes that suppress or enhance its toxicity to yeast.
Yeast Genetic Screen Identifies Novel Genetic Risk Factor for ALS
We reasoned that if we could identify yeast genes that could suppress or enhance TDP-43 toxicity, it would provide insight into the specific cellular functions and pathways affected by TDP-43 aggregation. We performed two high-throughput genetic screens to define the cellular pathways that are affected by TDP-43 aggregation. We identified 41 potent modifiers of TDP-43 toxicity. These hits include RNA-binding proteins, kinases, phosphatases and other proteins, many with human homologs, which will hopefully provide new insight into TDP-43 aggregation and toxicity74 (Gitler AD, unpublished). One of the hits from these yeast TDP-43 toxicity genetic modifier screens was PBP1, which is the homolog of a human neurodegenerative disease protein, ataxin 2. We have validated this genetic interaction in the fly nervous system and used biochemistry to show TDP-43 and ataxin 2 physically associate in a manner that appears to depend on RNA in mammalian cells.74 We also found that the ataxin 2 protein is mislocalized in ALS patient spinal cord neurons. Ataxin 2 is a polyglutamine (polyQ) disease protein. The polyQ tract of ataxin 2 is normally 22 or 23 Qs and polyQ expansions >34 cause spinocerebellar ataxia 2 (SCA2).
Given the striking interactions between ataxin 2 and TDP-43, we hypothesized that polyQ expansions in ataxin 2, which were longer than normal but not long enough to cause SCA2, might be associated with ALS. We analyzed the ataxin 2 gene in 915 individuals with ALS and 980 healthy controls and, remarkably, this revealed a significant association of ataxin-2 intermediate-length polyQ tract expansions with ALS (27-33Q, 4.7% of cases, p = 3.6 × 10−5).74,94 This connection between ataxin 2 polyQ expansions and risk for ALS is being validated in independent patient populations worldwide.95–99 These studies suggest that ataxin 2 is a new and potentially common ALS susceptibility gene. Because inhibiting ataxin 2 function in yeast or fly reduces TDP-43 toxicity, we propose that the ataxin 2/TDP-43 interaction could be a potential therapeutic target. Furthermore, these results also point to a similar molecular mechanism underpinning two seemingly distinct diseases—SCA2 and ALS.99 Future studies will be aimed at defining the role of ataxin 2/TDP-43 interactions and the mechanism by which polyQ expansions in ataxin 2 contribute to ALS pathogenesis.
It is, perhaps, inspiring that from a seemingly simple yeast genetic screen we have identified the most common genetic risk factor for ALS discovered to date74,94 and by so doing, identified potential targets for therapy. Some of the additional 40 genes from this yeast TDP-43 toxicity modifier screen (Gitler AD, unpublished) will hopefully provide even more insight into TDP-43 pathogenesis and its role in ALS.
FUS, Another RNA-Binding Protein Implicated in ALS
Shortly following the identification of mutations in TDP-43 in ALS, two groups reported mutations in another gene, the FUS (fused in sarcoma) gene, in familial ALS patients.100,101 Additional mutations in FUS have recently been identified in sporadic ALS cases and in some rare FTLD-U cases.102–109 Intriguingly, FUS is also an RNA-binding protein with domain structure remarkably similar to that of TDP-43: FUS also has an RRM and a glycine-rich domain. The identification of two proteins with similar domain architecture whose mutation is associated with ALS poses an emerging concept that RNA metabolic pathways may play a major role in the pathogenesis of ALS.110
FUS is normally localized to the nucleus, but mutant FUS proteins associated with ALS accumulate in the cytoplasm of motor neurons of ALS patients.101 Interestingly, these neurons do not have TDP-43 aggregates.101 Many of the ALS linked mutations in FUS, cluster in the extreme C-terminal domain, which harbors a conserved PY-motif. This domain can function as a non-canonical nuclear localization signal (NLS) that is decoded by karyopherin beta2 (also called transportin 2 or importin 3),111,112 and ALS-linked mutations in this region can disrupt proper FUS nuclear localization.87,113 Certain ALS-linked mutations (e.g., P525L) seem to have more dramatic effects on FUS nuclear localization and result in more severe clinical phenotypes, suggesting a mechanism by which some FUS mutations might contribute to ALS.
Identifying Prion-Like Domains in TDP-43 and FUS
TDP-43 and FUS are both RNA-binding proteins that can aggregate in ALS and mutations in the genes encoding these proteins cause rare forms of ALS. In addition to sharing a role in ALS, they both harbor several key structural features.110 Interestingly, using a bioinformatics approach, we and others recently discovered a novel “prion-like” domain in FUS and TDP-43 (reviewed in ref. 4, 114 and 115 and Fig. 1A). Like prion domains found in yeast prion proteins (e.g., Sup35, Ure2 and Rnq1), this domain is enriched in uncharged polar amino acids (such as asparagine, glutamine and tyrosine) and glycine.116,117 Remarkably, by using this algorithm to score 27,879 human proteins, FUS and TDP-43 ranked 15th and 69th, respectively. The FUS prion-like domain is located in the N-terminal region of the protein (residues 1–239, with an additional region in the first RGG domain: residues 391–405), whereas the TDP-43 prion-like domain is at the C-terminal end (residues 277–414).4 Notably, several other human RNA-binding proteins also contain predicted prion-like domains, raising the intriguing possibility that perhaps there will be additional aggregation-prone RNA-binding proteins bearing prion-like domains that can contribute to ALS or related disorders. However, in contrast to modular prion domains from known yeast prions,118 a portion of the predicted prion-like domain of FUS (residues 1–167) when fused to ‘MC’ of Sup35 does not appear to be sufficient to confer a heritable [PSI+] prion phenotype in yeast.79 However, this region of FUS does not aggregate in isolation in yeast and so perhaps this result was expected.77 Indeed, it is more likely that the full N-terminal prion-like domain of FUS (residues 1–239) is needed, as well as determinants in the second prion-like region in the first RGG domain (residues 391–405).4,77 Regardless, the prion-like domains of TDP-43 and FUS appear to play critical roles in driving aggregation68,73,77–79,115 and toxicity, and are thus very likely to be relevant to disease pathogenesis. TDP-43 and FUS can now be added to the expanding list of neurodegenerative disease proteins with prion-like properties that may be important for pathogenesis, given the ‘prionoid’ aggregation propensity of many proteins associated with human neurodegenerative disease.119 Indeed, it is tempting to speculate that a prionoid might underpin the spread of pathology between contiguous regions of the brain and the involvement of multiple cell types in ALS.114,120
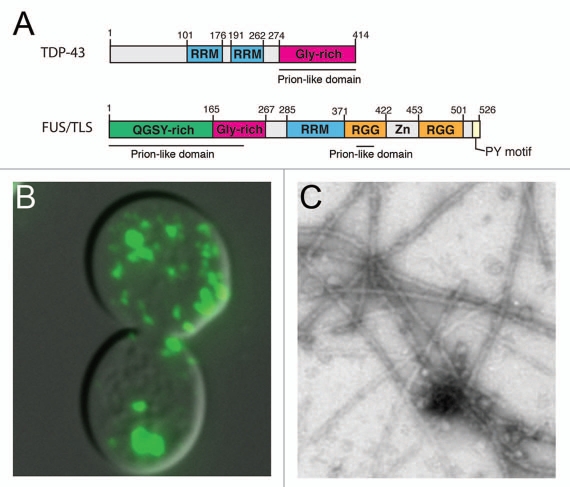
Figure 1.
Prion-like domains of ALS disease proteins TDP-43 and FUS contribute to aggregation. (A) Domain architecture of TDP-43 and FUS with the location of the prion-like domains indicated. Both proteins also contain RNA-recognition motifs (RRMs) and glycine-rich regions (Gly-rich). (B) FUS and TDP-43 form cytoplasmic aggregates when expressed in yeast cells. Shown is a representative example of a yeast cell expressing a FUS-YFP fusion protein. (C) TDP-43 and FUS spontaneously aggregate in vitro. Shown is an example of filamentous aggregates formed by FUS (1–422) in vitro.
Defining Domains of FUS Necessary and Sufficient for Aggregation and Toxicity in Yeast
In addition to defective localization of mutant FUS, there are emerging examples of mislocalized wild-type FUS contributing to additional neurodegenerative diseases, including juvenile ALS,121 basophilic inclusion body disease,122 and almost all of the remaining TDP-43-negative FTLD-U cases (now called FTLD-FUS123). Moreover, FUS aggregates have been reported in several polyglutamine disorders, including Huntington disease and the spinocerebellar ataxias.124,125 Because both WT and mutant FUS likely play prominent roles in neurodegeneration, it will be important to define the mechanisms of FUS aggregation.
To address these deficits, and given our recent results modeling disease relevant aspects of TDP-43 aggregation in yeast, we next sought to determine sequence features that were sufficient and necessary for FUS aggregation and toxicity in yeast.77 Ju et al. Fushimi et al. and Kryndushkin et al. have used similar approaches to define FUS aggregation mechanisms in yeast.78–80 Like TDP-43, FUS expression in yeast resulted in cytoplasmic aggregation and toxicity.77 We first performed a structure/function analysis to determine the domains of FUS that were required for aggregation and toxicity.
We generated a series of FUS truncations and expressed each of these constructs in yeast as YFP-fusions. We determined which FUS constructs aggregated and which were toxic (Fig. 1B). For TDP-43, we have previously found that the C-terminal prion-like domain and an RNA recognition motif (RRM) are sufficient for toxicity68 but for FUS, we found that the N-terminal prion-like domain and the RRM (amino acids 1–373) resulted in an entirely nuclear localized protein, which was not toxic.77 Addition of the first RGG domain (to generate FUS amino acids 1–422) was sufficient to confer cytoplasmic aggregation and toxicity, albeit not as toxic as full-length FUS. Remarkably, the first RGG domain of FUS also contains a stretch that resembles a yeast prion domain,4 opening the possibility that the two prion-like portions of FUS communicate to promote aggregation.77 Having established the minimal region of FUS sufficient for aggregation and toxicity, we next sought to define the regions of FUS that were required. Deletion of portions of the N-terminal prion-like domain of FUS (amino acids 1–239) completely prevented aggregation. Thus, in contrast to TDP-43, which requires its C-terminal prion-like domain and a portion of RRM2 for aggregation and toxicity in yeast,68 for FUS, the N-terminal prion domain, RRM and first RGG domain are required.77
TDP-43 and FUS are both aggregation-prone RNA-binding proteins with similar structural and sequence features. However, our studies have defined several key differences, which will likely have important implications for the design of therapeutic strategies aimed at preventing or reversing aggregation.
FUS Aggregates Formed in vitro Resemble FUS Aggregates in Degenerating Neurons of ALS Patients
FUS forms cytoplasmic inclusions in motor neurons of some ALS patients as well as in several other neurodegenerative disease situations. However, it is unclear if FUS itself is aggregation-prone or if it co-aggregates with other proteins in disease. We have recently developed in vitro aggregation assays to demonstrate TDP-43 is aggregation-prone and to determine that ALS-linked TDP-43 mutations can accelerate aggregation.73 We therefore used a similar approach to test if FUS is intrinsically prone to aggregation. We purified bacterially expressed recombinant FUS under native conditions. FUS aggregated extremely rapidly. Importantly, the aggregates formed by FUS did not react with the amyloid-diagnostic dye Thioflavin-T. Thus, pure FUS forms aggregates that are likely non-amyloid in nature, just like the aggregated species of FUS observed in ALS and FTLD-U patients.45,126,127
We used electron microscopy (EM) to show that pure full-length FUS rapidly formed filamentous structures, which were strikingly similar to the FUS aggregates observed in the degenerating motor neurons of ALS patients (reviewed in ref. 121 and 128 and Fig. 1C). We also observed small pore-shaped FUS oligomers that would cluster adjacent to the FUS filaments, reminiscent of the granular structures observed in association with filamentous FUS aggregates in motor neurons of ALS patients.121,128 Importantly, the domain requirements for the aggregation of pure FUS matched those defined in yeast.77 Clearly, FUS is an intrinsically aggregation-prone protein capable of forming aggregated structures very similar to those observed in motor neurons of ALS patients.
ALS-Linked FUS Mutations Do Not Affect Aggregation or Toxicity
Another interesting difference we observed between TDP-43 and FUS in yeast is the effect of ALS-linked mutations. We have previously found that some ALS-linked TDP-43 mutations (e.g., Q331K and M337V) increase TDP-43 aggregation in vitro and in yeast cells and are more toxic than WT TDP-43 in yeast cells and in Drosophila.73,74 We next used the yeast model to test the effects of ALS-linked mutations on FUS aggregation and toxicity. We found that C-terminal, ALS-linked FUS mutations do not promote FUS aggregation in yeast or in our in vitro aggregation assay.77 Given the recent evidence demonstrating that some ALS-linked mutations can disrupt FUS nuclear localization,87,113 these C-terminal ALS-linked FUS mutations likely promote pathological events that are upstream of aggregation and toxicity.129 Thus, even though FUS and TDP-43 are related RNA-binding proteins, the mechanisms by which ALS-linked mutations contribute to disease might be different for each protein.
Yeast Genetic Screens Identify Modifiers of FUS Toxicity
Given the recent successes in identifying new genes and pathways with direct relevance to human ALS by performing genetic modifier screens with the yeast TDP-43 model,74 we next sought to perform similar screens to investigate FUS toxicity. We also reasoned that if the types of hits that modified FUS toxicity were different from those that affected TDP-43 toxicity, it would suggest that the proteins contribute to pathogenesis by distinct mechanism. Conversely, similar modifier genes would suggest similar pathogenic mechanisms. We performed two unbiased yeast genetic modifier screens to identify genes that could enhance or suppress FUS toxicity.
First, we performed a plasmid overexpression screen. We identified 24 genes that suppressed and 10 genes that enhanced FUS toxicity when overexpressed. The largest functional class enriched in the screen included RNA-binding proteins and proteins involved in RNA metabolism, emphasizing the role of RNA metabolic pathways in FUS pathogenesis. The human homologs of several suppressors isolated in our yeast screen buffered against FUS toxicity in mammalian cell culture.74 Surprisingly, out of the 41 yeast genes that we found modify TDP-43 toxicity74 (Gitler AD, unpublished observations), only two affected FUS, strongly suggesting that despite being similar proteins, the mechanisms by which FUS and TDP-43 contribute to disease are likely very different.
In addition to the yeast plasmid overexpression screen, we also performed a deletion screen. We used synthetic genetic array (SGA) analysis130,131 to introduce a FUS expression plasmid into each non-essential yeast deletion strain by mating. We identified some yeast deletions that enhanced FUS toxicity and others that suppressed toxicity.77 Again, there was very little overlap between the genetic modifiers of TDP-43 and FUS toxicity. We indentified 36 deletions that suppressed FUS toxicity and 24 that enhanced toxicity. Deletions of yeast genes involved in RNA metabolic processes, ribosome biogenesis and cellular stress responses were enriched as hits and many of these had human homologs. This, together with our other in vitro and in vivo experiments, strongly suggest that the mechanisms underpinning FUS and TDP-43 toxicity are not as similar as everyone has presumed. A critical test of this hypothesis will come from a comprehensive comparison of the RNAs regulated by TDP-43 and FUS. The specific RNA targets of TDP-43 are beginning to be defined,132–135 using genome-wide approaches and similar strategies to define FUS targets are also underway. These studies, along with the development of new animal models to study FUS,136,137 will help clarify the similarities and differences of TDP-43 and FUS-regulated pathways in disease pathogenesis.
The FUS About Stress Granules and ALS
Several of the yeast genes that modified FUS toxicity contained human homologs, suggesting that perhaps pathways involved in FUS toxicity in yeast could be conserved to man. Interestingly, FUS has recently been shown to co-localize with stress granules in transfected cells and cytoplasmic FUS-positive inclusions in ALS and FTLD-U patients contain stress granule markers.87,113,138–140 Stress granules and processing bodies (P-bodies) are transient cytoplasmic structures containing RNAs and RNA-binding proteins, including translation initiation factors and the polyA-binding protein (PABP-1). They are sites where cells sequester mRNAs, during situations of stress, to inhibit translation initiation.141 In the plasmid overexpression screen, we identified two translation initiation factors (Tif2 and Tif3) and Pab1, the yeast homolog of human PABP-1, and a protein involved in stress granule assembly in yeast, as suppressors of FUS toxicity. In the deletion screen, we identified deletions of Pub1 (TIAL1 in human) and Lsm7 (LSM7 in human), components of stress granules and P-bodies, respectively, as potent suppressors of FUS toxicity. These findings support the emerging role of stress granule pathways in ALS, but also provide an important extension, strongly suggesting that, rather than simply being markers of FUS-positive inclusions in disease, perhaps stress granule components also play an important role in mediating FUS toxicity. Therefore, approaches aimed at manipulating stress granule assembly might be a novel avenue for therapeutic intervention.
TDP-43 and FUS: The Tip of an Iceberg?
The identification of key roles for TDP-43 and FUS in ALS is beginning to change our picture of the genetic landscape of the disease.126 Both of these are aggregation-prone RNA-binding proteins, both harbor prion-like domains, and both can be mutated in ALS, raising the intriguing possibility that they might sit at the tip of an iceberg for RNA-binding proteins in ALS. Could additional RNA-binding proteins, with properties similar to those of TDP-43 and FUS also contribute to the disease? Our bioinformatics analysis used to identify prion-like domains in TDP-43 and FUS4 also revealed clear prion-like domains in several other human RNA-binding proteins (Gitler AD and Shorter J, unpublished). Might these prion-like domain containing RNA-binding proteins also be poised to contribute to ALS? For FUS and TDP-43, certain ALS-linked mutations seem to have more severe effects on the protein and result in more severe clinical phenotypes than others.113 Therefore, it is possible that certain exceptionally deleterious mutations on their own (e.g., FUS P525L113) could be sufficient to cause disease, whereas other variants might be weaker and require additional hits to cause disease.129,142 These additional hits might involve environmental factors (stress, injury, environmental exposure, etc.,) or perhaps even genetic lesions in additional aggregation-prone RNA-binding proteins. In the future, next generation sequencing technologies will be routine, allowing for the elucidation of all of the genetic contributors to neurodegenerative diseases like ALS. Meanwhile, this concept of multiple aggregation prone RNA-binding proteins contributing to disease, on their own, via interactions with each other, or through interactions with the environment, will hopefully provide a conceptual framework for testing hypotheses about the role of RNA-binding proteins in pathogenesis.
Acknowledgments
This work was supported by a grant from the Packard Center for ALS Research at Johns Hopkins (A.D.G. and J.S.), NIH Director's New Innovator Awards 1DP2OD004417-01 (A.D.G.) and 1DP2OD002177-01 (J.S.), NIH R01 NS065317 (A.D.G.), NIH R21 NS067354-0110 (J.S.), a University of Pennsylvania Diabetes and Endocrinology Research Center Pilot and Feasibility grant (J.S.), a Bill and Melinda Gates Foundation Grand Challenges Explorations Award (J.S.) and an Ellison Medical Foundation New Scholar in Aging Award (J.S.). A.D.G. is a Pew Scholar in the Biomedical Sciences, supported by The Pew Charitable Trusts.
References
- 1.Trojanowski JQ. PENN neurodegenerative disease research—in the spirit of Benjamin Franklin. Neurosignals. 2008;16:5–10. doi: 10.1159/000109753. [DOI] [PubMed] [Google Scholar]
- 2.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nature Medicine. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 3.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 4.Cushman M, Johnson BS, King OD, Gitler AD, Shorter J. Prion-like disorders: blurring the divide between transmissibility and infectivity. J Cell Sci. 2010;123:1191–1201. doi: 10.1242/jcs.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 6.Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 7.Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 8.DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 9.Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- 10.Weissmann C. Molecular biology of prion diseases. Trends Cell Biol. 1994;4:10–14. doi: 10.1016/0962-8924(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 11.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 13.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 14.Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–1079. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- 15.Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 16.Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josephs KA, Jones AG, Dickson DW. Hippocampal sclerosis and ubiquitin-positive inclusions in dementia lacking distinctive histopathology. Dement Geriatr Cogn Disord. 2004;17:342–345. doi: 10.1159/000077168. [DOI] [PubMed] [Google Scholar]
- 18.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 19.Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 20.Valentine JS, Hart PJ. Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2003;100:3617–3622. doi: 10.1073/pnas.0730423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 22.Shibata N, Hirano A, Kobayashi M, Sasaki S, Kato T, Matsumoto S, et al. Cu/Zn superoxide dismutase-like immunoreactivity in Lewy body-like inclusions of sporadic amyotrophic lateral sclerosis. Neurosci Lett. 1994;179:149–152. doi: 10.1016/0304-3940(94)90956-3. [DOI] [PubMed] [Google Scholar]
- 23.Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 24.Cleveland DW. From Charcot to SOD1: mechanisms of selective motor neuron death in ALS. Neuron. 1999;24:515–520. doi: 10.1016/s0896-6273(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 25.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 26.Williamson TL, Corson LB, Huang L, Burlingame A, Liu J, Bruijn LI, et al. Toxicity of ALS-linked SOD1 mutants. Science. 2000;288:399. doi: 10.1126/science.288.5465.399a. [DOI] [PubMed] [Google Scholar]
- 27.Subramaniam JR, Lyons WE, Liu J, Bartnikas TB, Rothstein J, Price DL, et al. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat Neurosci. 2002;5:301–307. doi: 10.1038/nn823. [DOI] [PubMed] [Google Scholar]
- 28.Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, et al. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc Natl Acad Sci USA. 2008;105:7594–7599. doi: 10.1073/pnas.0802556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, et al. Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Pasinelli P, Houseweart MK, Brown RH, Jr, Cleveland DW. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2000;97:13901–13906. doi: 10.1073/pnas.240305897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Lillo C, Jonsson PA, Vande Velde C, Ward CM, Miller TM, et al. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 34.Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 36.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 37.Seelaar H, Schelhaas HJ, Azmani A, Kusters B, Rosso S, Majoor-Krakauer D, et al. TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain. 2007;130:1375–1385. doi: 10.1093/brain/awm024. [DOI] [PubMed] [Google Scholar]
- 38.Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, et al. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol. 2007;113:535–542. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]
- 39.Rothstein JD. TDP-43 in amyotrophic lateral sclerosis: pathophysiology or patho-babel? Ann Neurol. 2007;61:382–384. doi: 10.1002/ana.21155. [DOI] [PubMed] [Google Scholar]
- 40.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, et al. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 42.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoseki A, Shiga A, Tan CF, Tagawa A, Kaneko H, Koyama A, et al. TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol. 2008;63:538–542. doi: 10.1002/ana.21392. [DOI] [PubMed] [Google Scholar]
- 45.Forman MS, Trojanowski JQ, Lee VM. TDP-43: a novel neurodegenerative proteinopathy. Curr Opin Neurobiol. 2007;17:548–555. doi: 10.1016/j.conb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol. 1995;69:3584–3596. doi: 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- 48.Strong MJ, Volkening K, Hammond R, Yang W, Strong W, Leystra-Lantz C, et al. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol Cell Neurosci. 2007;35:320–327. doi: 10.1016/j.mcn.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Ayala YM, Pagani F, Baralle FE. TDP43 depletion rescues aberrant CFTR exon 9 skipping. FEBS Lett. 2006;580:1339–1344. doi: 10.1016/j.febslet.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 50.Wang IF, Reddy NM, Shen CK. Higher order arrangement of the eukaryotic nuclear bodies. Proc Natl Acad Sci USA. 2002;99:13583–13588. doi: 10.1073/pnas.212483099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiang PM, Ling J, Jeong YH, Price DL, Aja SM, Wong PC. Deletion of TDP-43 downregulates Tbc1d1, a gene linked to obesity, and alters body fat metabolism. Proc Natl Acad Sci USA. 2010;107:16320–16324. doi: 10.1073/pnas.1002176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu LS, Cheng WC, Hou SC, Yan YT, Jiang ST, Shen CK. TDP-43, a neuro-pathosignature factor, is essential for early mouse embryogenesis. Genesis. 2010;48:56–62. doi: 10.1002/dvg.20584. [DOI] [PubMed] [Google Scholar]
- 53.Ayala YM, Misteli T, Baralle FE. TDP-43 regulates retinoblastoma protein phosphorylation through the repression of cyclin-dependent kinase 6 expression. Proc Natl Acad Sci USA. 2008;105:3785–3789. doi: 10.1073/pnas.0800546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, et al. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol. 2007;66:152–157. doi: 10.1097/nen.0b013e31803020b9. [DOI] [PubMed] [Google Scholar]
- 57.Gitler AD. Beer and Bread to Brains and Beyond: Can Yeast Cells Teach Us about Neurodegenerative Disease? Neurosignals. 2008;16:52–62. doi: 10.1159/000109759. [DOI] [PubMed] [Google Scholar]
- 58.Krobitsch S, Lindquist S. Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc Natl Acad Sci USA. 2000;97:1589–1594. doi: 10.1073/pnas.97.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ. Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein. Science. 2003;302:1769–1772. doi: 10.1126/science.1090389. [DOI] [PubMed] [Google Scholar]
- 61.Giorgini F, Guidetti P, Nguyen Q, Bennett SC, Muchowski PJ. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet. 2005;37:526–531. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duennwald ML, Jagadish S, Giorgini F, Muchowski PJ, Lindquist S. A network of protein interactions determines polyglutamine toxicity. Proc Natl Acad Sci USA. 2006;103:11051–11056. doi: 10.1073/pnas.0604548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duennwald ML, Jagadish S, Muchowski PJ, Lindquist S. Flanking sequences profoundly alter polyglutamine toxicity in yeast. Proc Natl Acad Sci USA. 2006;103:11045–11050. doi: 10.1073/pnas.0604547103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, Roark M, et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Hum Mol Genet. 2006;15:2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- 66.Witt SN, Flower TR. alpha-Synuclein, oxidative stress and apoptosis from the perspective of a yeast model of Parkinson's disease. FEMS Yeast Res. 2006;6:1107–1116. doi: 10.1111/j.1567-1364.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- 67.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, et al. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson BS, McCaffery JM, Lindquist S, Gitler AD. A yeast TDP-43 proteinopathy model: Exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc Natl Acad Sci USA. 2008;105:6439–6444. doi: 10.1073/pnas.0802082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soper JH, Roy S, Stieber A, Lee E, Wilson RB, Trojanowski JQ, et al. {alpha}-Synuclein Induced Aggregation of Cytoplasmic Vesicles in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:1093–1103. doi: 10.1091/mbc.E07-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Ham TJ, Breitling R, Swertz MA, Nollen EA. Neurodegenerative diseases: Lessons from genome-wide screens in small model organisms. EMBO Mol Med. 2009;1:360–370. doi: 10.1002/emmm.200900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeger-Lotem E, Riva L, Su LJ, Gitler AD, Cashikar AG, King OD, et al. Bridging high-throughput genetic and transcriptional data reveals cellular responses to alpha-synuclein toxicity. Nat Genet. 2009;41:316–323. doi: 10.1038/ng.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braun RJ, Sommer C, Carmona-Gutierrez D, Khoury CM, Ring J, Buttner S, et al. Neurotoxic 43-kDa TAR DNA-binding Protein (TDP-43) Triggers Mitochondrion-dependent Programmed Cell Death in Yeast. J Biol Chem. 2011;286:19958–19972. doi: 10.1074/jbc.M110.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Konopka CA, Locke MN, Gallagher PS, Pham N, Hart MP, Walker CJ, et al. A yeast model for polyalanine-expansion aggregation and toxicity. Mol Biol Cell. 2011;22:1971–1984. doi: 10.1091/mbc.E11-01-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Z, Diaz Z, Fang X, Hart MP, Chesi A, Shorter J, et al. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol. 2011;9:1000614. doi: 10.1371/journal.pbio.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ju S, Tardiff DF, Han H, Divya K, Zhong Q, Bosco DA, et al. A Yeast model of FUS/TLS-dependent cytotoxicity. PLoS Biol. 2011;9:1001052. doi: 10.1371/journal.pbio.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kryndushkin D, Wickner RB, Shewmaker F. FUS/TLS forms cytoplasmic aggregates, inhibits cell growth and interacts with TDP-43 in a yeast model of amyotrophic lateral sclerosis. Protein Cell. 2011;2:223–236. doi: 10.1007/s13238-011-1525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fushimi K, Long C, Jayaram N, Chen X, Li L, Wu JY. Expression of human FUS/TLS in yeast leads to protein aggregation and cytotoxicity, recapitulating key features of FUS proteinopathy. Protein Cell. 2011;2:141–149. doi: 10.1007/s13238-011-1014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hartwell LH. Nobel Lecture. Yeast and cancer. Biosci Rep. 2002;22:373–394. doi: 10.1023/a:1020918107706. [DOI] [PubMed] [Google Scholar]
- 82.Nurse P. The Nobel Prize and beyond: an interview with Sir Paul Nurse. Interview by Susan R. Owens. EMBO Rep. 2002;3:204–206. doi: 10.1093/embo-reports/kvf060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lo Bianco C, Shorter J, Regulier E, Lashuel H, Iwatsubo T, Lindquist S, et al. Hsp104 antagonizes alpha-synuclein aggregation and reduces dopaminergic degeneration in a rat model of Parkinson disease. J Clin Invest. 2008;118:3087–3097. doi: 10.1172/JCI35781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vacher C, Garcia-Oroz L, Rubinsztein DC. Overexpression of yeast hsp104 reduces polyglutamine aggregation and prolongs survival of a transgenic mouse model of Huntington's disease. Hum Mol Genet. 2005;14:3425–3433. doi: 10.1093/hmg/ddi372. [DOI] [PubMed] [Google Scholar]
- 85.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nature medicine. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 86.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ, Jr, et al. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2010;19:4160–4175. doi: 10.1093/hmg/ddq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barmada SJ, Skibinski G, Korb E, Rao EJ, Wu JY, Finkbeiner S. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci. 2010;30:639–649. doi: 10.1523/JNEUROSCI.4988-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kabashi E, Lin L, Tradewell ML, Dion PA, Bercier V, Bourgouin P, et al. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum Mol Genet. 2010;19:671–683. doi: 10.1093/hmg/ddp534. [DOI] [PubMed] [Google Scholar]
- 90.Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci USA. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Ray P, Rao EJ, Shi C, Guo W, Chen X, et al. A Drosophila model for TDP-43 proteinopathy. Proc Natl Acad Sci USA. 2010;107:3169–3174. doi: 10.1073/pnas.0913602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ritson GP, Custer SK, Freibaum BD, Guinto JB, Geffel D, Moore J, et al. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J Neurosci. 2010;30:7729–7739. doi: 10.1523/JNEUROSCI.5894-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo W, Chen Y, Zhou X, Kar A, Ray P, Chen X, et al. An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity. Nat Struct Mol Biol. 2011;18:822–830. doi: 10.1038/nsmb.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lagier-Tourenne C, Cleveland DW. Neurodegeneration: An expansion in ALS genetics. Nature. 2010;466:1052–1053. doi: 10.1038/4661052a. [DOI] [PubMed] [Google Scholar]
- 95.Lee T, Li YR, Ingre C, Weber M, Grehl T, Gredal O, et al. Ataxin-2 intermediate-length polyglutamine expansions in European ALS patients. Hum Mol Genet. 2011;20:1697–1700. doi: 10.1093/hmg/ddr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Damme P, Veldink JH, van Blitterswijk M, Corveleyn A, van Vught PW, Thijs V, et al. Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2. Neurology. 2011;76:2066–2072. doi: 10.1212/WNL.0b013e31821f445b. [DOI] [PubMed] [Google Scholar]
- 97.Ross OA, Rutherford NJ, Baker M, Soto-Ortolaza AI, Carrasquillo MM, Dejesus-Hernandez M, et al. Ataxin-2 repeat-length variation and neurodegeneration. Hum Mol Genet. 2011;20:3207–3212. doi: 10.1093/hmg/ddr227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corrado L, Mazzini L, Oggioni GD, Luciano B, Godi M, Brusco A, et al. ATXN-2 CAG repeat expansions are interrupted in ALS patients. Hum Genet. 2011 doi: 10.1007/s00439-011-1000-2. in press. [DOI] [PubMed] [Google Scholar]
- 99.Fischbeck KH, Pulst SM. Amyotrophic lateral sclerosis and spinocerebellar ataxia 2. Neurology. 2011;76(24):2050–2051. doi: 10.1212/WNL.0b013e31821f4498. [DOI] [PubMed] [Google Scholar]
- 100.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 101.Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Belzil VV, Valdmanis PN, Dion PA, Daoud H, Kabashi E, Noreau A, et al. Mutations in FUS cause FALS and SALS in French and French Canadian populations. Neurology. 2009;73:1176–1179. doi: 10.1212/WNL.0b013e3181bbfeef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blair IP, Williams KL, Warraich ST, Durnall JC, Thoeng AD, Manavis J, et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry. 2009;81:639–645. doi: 10.1136/jnnp.2009.194399. [DOI] [PubMed] [Google Scholar]
- 104.Corrado L, Del Bo R, Castellotti B, Ratti A, Cereda C, Penco S, et al. Mutations of FUS gene in sporadic amyotrophic lateral sclerosis. J Med Genet. 2010;47:190–194. doi: 10.1136/jmg.2009.071027. [DOI] [PubMed] [Google Scholar]
- 105.Dejesus-Hernandez M, Kocerha J, Finch N, Crook R, Baker M, Desaro P, et al. De novo truncating FUS gene mutation as a cause of sporadic amyotrophic lateral sclerosis. Hum Mutat. 2010;31:E1377–E1389. doi: 10.1002/humu.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drepper C, Herrmann T, Wessig C, Beck M, Sendtner M. C-terminal FUS/TLS mutations in familial and sporadic ALS in Germany. Neurobiol Aging. 32:548.e1–548.e4. doi: 10.1016/j.neurobiolaging.2009.11.017. 2009.” to “2011. [DOI] [PubMed] [Google Scholar]
- 107.Hewitt C, Kirby J, Highley JR, Hartley JA, Hibberd R, Hollinger HC, et al. Novel FUS/TLS mutations and pathology in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol. 2010;67:455–461. doi: 10.1001/archneurol.2010.52. [DOI] [PubMed] [Google Scholar]
- 108.Broustal O, Camuzat A, Guillot-Noel L, Guy N, Millecamps S, Deffond D, et al. FUS mutations in frontotemporal lobar degeneration with amyotrophic lateral sclerosis. J Alzheimers Dis. 2010;22:765–769. [PubMed] [Google Scholar]
- 109.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19:46–64. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee BJ, Cansizoglu AE, Suel KE, Louis TH, Zhang Z, Chook YM. Rules for nuclear localization sequence recognition by karyopherin beta2. Cell. 2006;126:543–558. doi: 10.1016/j.cell.2006.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suel KE, Gu H, Chook YM. Modular organization and combinatorial energetics of proline-tyrosine nuclear localization signals. PLoS Biol. 2008;6:137. doi: 10.1371/journal.pbio.0060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 2010;29:2841–2857. doi: 10.1038/emboj.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Udan M, Baloh RH. Implications of the prion-related Q/N domains in TDP-43 and FUS. Prion. 2011;5:1–5. doi: 10.4161/pri.5.1.14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fuentealba RA, Udan M, Bell S, Wegorzewska I, Shao J, Diamond MI, et al. Interaction with polyglutamine aggregates reveals a Q/N-rich domain in TDP-43. J Biol Chem. 2010;285:26304–26314. doi: 10.1074/jbc.M110.125039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137:146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet. 2005;6:435–450. doi: 10.1038/nrg1616. [DOI] [PubMed] [Google Scholar]
- 118.Li L, Lindquist S. Creating a protein-based element of inheritance. Science. 2000;287:661–664. doi: 10.1126/science.287.5453.661. [DOI] [PubMed] [Google Scholar]
- 119.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 120.Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality and spread: deconstructing motor neuron degeneration. Neurology. 2009;73:805–811. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang EJ, Zhang J, Geser F, Trojanowski JQ, Strober JB, Dickson DW, et al. Extensive FUS-Immunoreactive Pathology in Juvenile Amyotrophic Lateral Sclerosis with Basophilic Inclusions. Brain Pathol. 2011;20:1069–1076. doi: 10.1111/j.1750-3639.2010.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Munoz DG, Neumann M, Kusaka H, Yokota O, Ishihara K, Terada S, et al. FUS pathology in basophilic inclusion body disease. Acta Neuropathol. 2009;118:617–627. doi: 10.1007/s00401-009-0598-9. [DOI] [PubMed] [Google Scholar]
- 123.Urwin H, Josephs KA, Rohrer JD, Mackenzie IR, Neumann M, Authier A, et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathol. 2010;120:33–41. doi: 10.1007/s00401-010-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doi H, Koyano S, Suzuki Y, Nukina N, Kuroiwa Y. The RNA-binding protein FUS/TLS is a common aggregate-interacting protein in polyglutamine diseases. Neurosci Res. 2010;66:131–133. doi: 10.1016/j.neures.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 125.Woulfe J, Gray DA, Mackenzie IR. FUS-immunoreactive intranuclear inclusions in neurodegenerative disease. Brain Pathol. 2010;20:589–597. doi: 10.1111/j.1750-3639.2009.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 2010;6:211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kwong LK, Uryu K, Trojanowski JQ, Lee VM. TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis. Neurosignals. 2008;16:41–51. doi: 10.1159/000109758. [DOI] [PubMed] [Google Scholar]
- 128.Baumer D, Hilton D, Paine SM, Turner MR, Lowe J, Talbot K, et al. Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations. Neurology. 2010;75:611–618. doi: 10.1212/WNL.0b013e3181ed9cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dormann D, Haass C. TDP-43 and FUS: a nuclear affair. Trends Neurosci. 2011 doi: 10.1016/j.tins.2011.05.002. in press. [DOI] [PubMed] [Google Scholar]
- 130.Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- 131.Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- 132.Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14:459–468. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sephton CF, Cenik C, Kucukural A, Dammer EB, Cenik B, Han Y, et al. Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J Biol Chem. 2011;286:1204–1215. doi: 10.1074/jbc.M110.190884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao S, Sanelli T, Dib S, Sheps D, Findlater J, Bilbao J, et al. RNA targets of TDP-43 identified by UV-CLIP are deregulated in ALS. Mol Cell Neurosci. 2011;47:167–180. doi: 10.1016/j.mcn.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 136.Lanson NA, Jr, Maltare A, King H, Smith R, Kim JH, Taylor JP, et al. A Drosophila model of FUS-related neurodegeneration reveals genetic interaction between FUS and TDP-43. Hum Mol Genet. 2011;20:2510–2523. doi: 10.1093/hmg/ddr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang C, Zhou H, Tong J, Chen H, Liu YJ, Wang D, et al. FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. PLoS Genet. 2011;7:1002011. doi: 10.1371/journal.pgen.1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ito D, Seki M, Tsunoda Y, Uchiyama H, Suzuki N. Nuclear transport impairment of amyotrophic lateral sclerosis-linked mutations in FUS/TLS. Ann Neurol. 2011;69:152–162. doi: 10.1002/ana.22246. [DOI] [PubMed] [Google Scholar]
- 139.Kino Y, Washizu C, Aquilanti E, Okuno M, Kurosawa M, Yamada M, et al. Intracellular localization and splicing regulation of FUS/TLS are variably affected by amyotrophic lateral sclerosis-linked mutations. Nucleic Acids Res. 2011;39:2781–2798. doi: 10.1093/nar/gkq1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gal J, Zhang J, Kwinter DM, Zhai J, Jia H, Jia J, et al. Nuclear localization sequence of FUS and induction of stress granules by ALS mutants. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.06.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pesiridis GS, Tripathy K, Tanik S, Trojanowski JQ, Lee VM. A “Two-hit” Hypothesis for Inclusion Formation by Carboxyl-terminal Fragments of TDP-43 Protein Linked to RNA Depletion and Impaired Microtubule-dependent Transport. J Biol Chem. 2011;286:18845–18855. doi: 10.1074/jbc.M111.231118. [DOI] [PMC free article] [PubMed] [Google Scholar]