Abstract
A case of Creutzfeldt-Jakob disease (CJD) with a rare mutation of the prion protein (PrP) gene (PRNP) at codon 208 (R208H), while the codon 129 was a methionine homozygous genotype is reported. The patient initial displayed hand tremor, dizziness and progressive cognitive dysfunction. Subsequently, other symptoms gradually appeared, including cerebellar ataxia and mental disorder. No periodic activity was recorded at electroencephalography (EEG) and 14-3-3 protein in cerebrospinal fluid was negative. Total clinical course was about four months. Retrospective investigation of this family across seven generations did not figure out clear family history. However, genetic analyses revealed six first-degree family members with the R208H allele.
Key words: creutzfeldt-Jakob disease, PRNP, R208H
Introduction
Creutzfeldt-Jakob disease (CJD) is a rapidly progressive, uniformly fatal, transmissible spongiform encephalopathy (TSE) characterized by the accumulation of an abnormal isoform (PrPSc) of the host encoded cellular prion protein (PrPC) in the brain. CJD is usually classified into three types: sporadic CJD (sCJD), iatrogenic CJD (iCJD) and genetic or familiar CJD (gCJD or fCJD). gCJD accounts for approximately 10–15% of human prion diseases that are caused by mutations of the prion protein gene (PRNP). Up to now, more than 55 mutations have been identified in the development of human genetic TSE, with a variety of geographical distributions and frequencies. Here we report the first Chinese patient suffered from gCJD of R208H mutation in PRNP, who initially exhibited clinical signs of typical sCJD. PRNP sequence analyses of 29 family members revealed six of his lineal relatives carrying R208H allele, but remaining healthy.
Case Presentation
The patient was a 45-year-old man, who complained hand tremor, dizziness and vertigo, as well as progressive cognitive dysfunction for more than one month. About 40 days before admission, he started to have hand tremor, especially the right hand, without any definite inducement, so that he was unable to tie his shoelace and buttons. Almost meanwhile, he complained dizziness and vertigo, gradually appeared walking unsteadiness. The symptom aggravated quickly that he appeared slurred speech, incontinence of feces and urine. He was gradually unable to recognize his families half month prior to the admission. After hospitalization, he quickly displayed dementia, mental disorder, loss of appetite, disturbances of sleep-wakefulness rhythm. His body temperature and blood pressure were at the normal range. Neurological examination revealed a lead pipe-like increased muscle tension of limb accompany with body tremor, especially in resting situation. Bilateral pathological reflexes were positive. Routine hematological and blood biochemical examinations did not revealed any abnormality. During hospitalization, his psychotic symptoms were remissive, but other neurologic symptoms worsened progressively. He discharged from hospital half month later. At the terminus of the clinical course, he appeared severe narcoma. He died at hometown two months after discharge. Total clinical course was about 4 months.
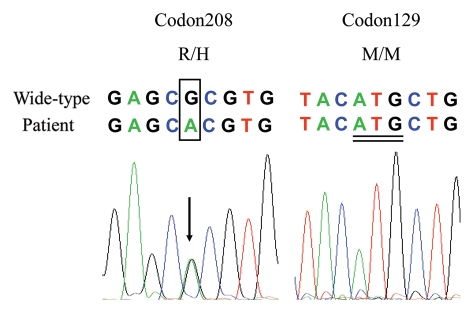
During hospitalization, electroencephalography (EEG) revealed moderate abnormality, but without periodic activity. Magnetic resonance imaging (MRI) did not identify special abnormality. About 45 days after onset, CSF was collected by lumbar puncture. Routine CSF examinations showed slightly increased glucose (7.66 mM/L) and total protein (614.9 mM/L), compared with the normal values (CSF-Glu, 2.5–4.5 mM/L, CSF-Pro, 150–450 mM/L). Western blot of protein 14-3-3 in CSF was negative. His genomic DNA was extracted from peripheral blood leukocytes and the coding region of PRNP was amplified by PCR. Sequencing of the PCR products revealed a methionine homozygous genotype at codon 129 of PRNP. A missense mutation guanine (G) to adenine (A) at the position of nt 623 in one PRNP allele was identified, leading to a change from argnine (R) to histidine (H) mutation at residue 208 (Fig. 1). No other mutation was observed in the rest of the PRNP gene. No brain autopsy was obtained.
Figure 1.
(A) DNA sequencing of the PRNP gene revealed an adenine substitution for guanine at the second position of codon 208, which results in the substitution of a histidine for an arginine (R208H). Different sources of sequences are indicated above the sequencing graph. The mutation bases are bracketed by a black frame and the corresponding peak is signaled by arrow. (B) A methionine homozygous genotype at codon 129 of PRNP. Double underlined indicates the nucleotide sequence of code 129.
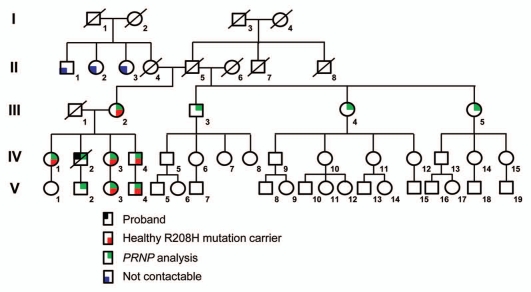
During hospitalization, retrospective investigation of his family members did not recall similar neurological disorders. His father (III1) died at the age of 49 with esophagus cancer and his mother (III2) was still alive with the age of 70. An extensive investigation was performed on his patrilineal and matrilineal relatives, covering 49 peoples of his patrilineal lineage in seven generations and 41 of his matrilineal lineage across five generations. The proband's matrilineal grandmother (II4) died at the age of 20 just after his mother was born. His step-grandmother (II6) delivered three children. No similar neurologic disease was recalled from either patrilineal or matrilineal families. Twenty-nine family members' blood samples were collected with informed consent. PRNP sequencing analyses revealed such mutation at codon 208 (R208H) in six proband's lineal relatives, including proband's mother (III2), elder sister (IV1), younger sister (IV3) and her daughter (V3), younger brother (IV4) and his son (V4, Fig. 2). All of those PRNP mutation carriers still remain healthy. The rest of the tested family members, including the proband's son (V2), was confirmed to contain wild-type PRNP genotype.
Figure 2.
The pedigree of the first Chinese gCJD (R208H) family. The pedigree of the proband's matrilineal lineage comprising 49 members across five generations. The circles or squares with black slash represent the death family members. The circle shaded with black at the top left corner represents proband. The circles or squares shaded with red at the lower right corner represent the healthy R208H mutation carriers. The circles or squares shaded with green at top right corner represent the persons analyzed with PRNP sequencing in this study. The circles or squares shaded with blue at the lower left corner represent the members not contactable. I to V in Roman numerals on the left indicate the different generations of the family.
Discussion
In this report, we have described the first Chinese R208H gCJD case. In addition to this case, four other R208H gCJD cases have been reported since 1996.1 The main clinical characteristics of those four cases and the case in this report are comparably summarized in Table 1. The onset ages of R208H gCJD range from 45–69 year-old (median: 60 year-old). Generally, the clinical manifestations of R208H gCJD are similar as that of sCJD. The initial presentations differ largely, Ataxia is observed in all five cases. The durations of R208H gCJD range from 4 to 12 months (median: 7 months), which are somewhat shorter than that of some kinds of gCJD, e.g., G114V and GSS. Two out of five cases have showed typical periodic sharp wave complexes (PSWC) in EEG in the later stage. CSF 14-3-3 is positive in two patients from four tested ones. All patients are homozygous at codon 129, including four Met/Met and one Val/Val.
Table 1.
Clinical, electroencephalographic, 14-3-3 and genetic features of patients with the PRNP R208H mutation
| Authors, year, country | Age at onset (years), gender | Initial clinical manifestation | Clinical symptom | 14-3-3 in CSF | Histology and Immunohistochemistry | EEG features | Codon 129 | Clinical course |
| Mastrianni JA et al, 1996, America | 60, Male | Rapidly progressive dementia, gait difficulty | Ataxia, anorexia with weight loss, progressive forgetfulness, myoclonus | ∅ | Severe spongiform changes, gliosis, loss of neurons, PrPSc deposition | PSWC | M/M | 7 months |
| Capellari S et al, 2005, Italy | 58, Female | Anorexia with weight loss, apathie behavior, decreased elocutions | Ataxia, Myoclonus, akinetic mutism | + | Severe spongiform changes, gliosis, loss of neurons, PrPSc deposition | PSWC | M/M | 7 months |
| Roeber et al, 2005, France | 69, Female | Acute weakness and clumsiness of the lefthand, dysarthria, gait disturbance. | mild cognitive impairment, ataxia | + | Severe spongiform changes, gliosis, loss of neurons, PrPSc and tau deposition | No periodic activity | M/M | 12 months |
| Celine Basset-Leobon et al, 2006, Germany | 61, Male | Diabetes mellitus, a long-standing history of memory loss and emotional disorders | Aggressiveness, eating disorder, delirium, cerebellar ataxia | − | Severe spongiform changes, gliosis, loss of neurons,PrPSc deposition, Kuru plaque | Slow activity | V/V | 7 months |
| Chen et al, current case, China | 45, Male | Anorexia, hand tremor, dizziness, progressive cognitive dysfunction | Cerebellar ataxia | − | No brain autopsy | No periodic activity | M/M | 4 months |
Key: CSF, cerebrospinal fluid; PSWC, periodic sharp wave complex; +, positive; −, negative; ∅, not available.
Besides the proband, six first-degree relatives of the proband are confirmed to have R208H mutation in PRNP, without gender difference. Surprisingly, not only his mother (III2), but also all his brother and sisters, as well as two of three tested persons in next generation carry R208H genotype. It may highlight a higher distributing frequency of R208H in this family. However, extensively retrospective investigation fails to figure out any disease-related family history. Reviews of the reported other four R208H cases have identified the same phenomenon that lacks of disease associated family history.2–4 Genetic examination of some relatives of the first American case has identified a family member with R208H mutation who was less than 50-year but still remained healthy at that time.1 It seems that the linkage between the genotype of R208H and the phenotype of gCJD is not as close as other mutations in PRNP, e.g., G114V with gCJD, D178N with FFI. Some other unknown elements may involve in the pathogenesis of R208H gCJD.
Based on the consanguinity map of this family, the R208H mutated allele seems to succeed from proband's matrilineal grandmother (II4), since all uncles and aunts from his patrilineal side or from his half-matrilineal side do not have such mutation. Since the siblings of proband's matrilineal grandmother have not contact each other for long time, the distribution of R208H mutation in his matrilineal family is unable to be addressed. Nevertheless, long-term follow-up of this family may help to address the linkage between the genotype of this mutation and the phenotype of gCJD.
We do not have brain autopsy of the Chinese R208H gCJD case. However, from the published data of the other four cases with autopsy, severe spongiform changes, gliosis, loss of neurons and PrPSc deposition are commonly observed. Moreover, peculiar tau protein pathology in the CA1 region of the hippocampus and an unusual 17-KD fragment of PrP are observed in a R208H/M129M case,3 and kuru-like plaques in the cerebellum, notably in the molecular layer, a type 2 pattern of PrPSc are detected in a R208H/V129V case.4 It indicates that like sCJD and other gCJDs, polymorphism of codon 129 in PRNP may influence the disease phenotype of R208H gCJD.
Conclusion
We have reported here the first Chinese R208H gCJD case that is the fifth one worldwide. Such gCJD cases are sporadic without clear family history. The onset ages of the disease seem to be earlier and durations are relatively shorter. The clinical manifestations are somehow like that of sCJD.
Acknowledgments
This work was supported by China Mega-Project for Infectious Disease (2009ZX10004-101), Chinese National Natural Science Foundation Grants 30771914 and 30800975, Institution Technique R&D Grant (2008EG150300), National Basic Research Program of China (973 Program) (2007CB310505) and SKLID Development Grant (2008SKLID102, 2011SKILD211 and 2011SKILD204).
References
- 1.Mastrianni JA, Iannicola C, Myers RM, DeArmond S, Prusiner SB. Mutation of the prion protein gene at codon 208 in familial Creutzfeldt-Jakob disease. Neurology. 1996;47:1305–1312. doi: 10.1212/wnl.47.5.1305. [DOI] [PubMed] [Google Scholar]
- 2.Capellari S, Cardone F, Notari S, Schinina ME, Maras B, Sita D, et al. Creutzfeldt-Jakob disease associated with the R208H mutation in the prion protein gene. Neurology. 2005;64:905–907. doi: 10.1212/01.WNL.0000152837.82388.DE. [DOI] [PubMed] [Google Scholar]
- 3.Roeber S, Krebs B, Neumann M, Windl O, Zerr I, Grasbon-Frodl EM, Kretzschmar HA. Creutzfeldt-Jakob disease in a patient with an R208H mutation of the prion protein gene (PRNP) and a 17-kDa prion protein fragment. Acta Neuropathol. 2005;109:443–448. doi: 10.1007/s00401-004-0978-0. [DOI] [PubMed] [Google Scholar]
- 4.Basset-Leobon C, Uro-Coste E, Peoc'h K, Haik S, Sazdovitch V, Rigal M, et al. Familial Creutzfeldt-Jakob disease with an R208H-129V haplotype and Kuru plaques. Arch Neurol. 2006;63:449–452. doi: 10.1001/archneur.63.3.449. [DOI] [PubMed] [Google Scholar]