Abstract
Tissue engineering strategies for intervertebral disc repair have focused on the use of autologous disc-derived chondrocytes. Difficulties with graft procurement, harvest site morbidity, and functionality, however, may limit the utility of this cell source. We used an in vivo porcine model to investigate allogeneic non-disc-derived chondrocytes and allogeneic mesenchymal stem cells (MSCs) for disc repair. After denucleation, lumbar discs were injected with either fibrin carrier alone, allogeneic juvenile chondrocytes (JCs), or allogeneic MSCs. Discs were harvested at 3, 6, and 12 months, and cell viability and functionality were assessed qualitatively and quantitatively. JC-treated discs demonstrated abundant cartilage formation at 3 months, and to a lesser extent at 6 and 12 months. For the carrier and MSC-treated groups, however, there was little evidence of proteoglycan matrix or residual notochordal/chondrocyte cells, but rather a type I/II collagen-enriched scar tissue. By contrast, JCs produced a type II collagen-rich matrix that was largely absent of type I collagen. Viable JCs were observed at all time points, whereas no evidence of viable MSCs was found. These data support the premise that committed chondrocytes are more appropriate for use in disc repair, as they are uniquely suited for survival in the ischemic disc microenvironment.
Introduction
Low back pain is the most common health problem for men and women between the ages of 20 and 50, resulting in approximately 13 million doctor visits in the United States each year and costing $28 billion in annual productivity losses.1,2 Although the exact cause of most cases of low back pain remains unknown, it is understood that degenerative changes in the intervertebral disc (IVD) plays a central role in the pathogenic mechanism leading to pain.3 Current treatment modalities for disc-related back pain (medication; selective nerve root blocks; surgical discectomy and fusion) are costly therapies aimed only at alleviating symptoms rather than addressing the underlying pathophysiologic processes of degeneration.
Disc degeneration includes decreases in cell density within the nucleus pulposus (NP),4,5 as well as alterations in NP cell function that include reduced proteoglycan synthesis6 and increased proteolytic enzyme production.7,8 This detrimental cell behavior has adverse effects on disc matrix, such as Type II collagen denaturation9 and cleavage of aggregating proteoglycan,8 and significantly alter the biomechanical behavior of the IVD, perpetuating the degenerative process. Pathological findings of an apparent ingrowth of nerve fibers and blood vessels into the normally aneural and avascular tissues of excised painful human discs suggest that either the breakdown or loss of anti-angiogenic/neuronogenic factors produced within the disc may orchestrate molecular changes that promote discogenic pain.10,11 Consequently, the development of new therapeutic strategies to augment damaged tissues within degenerate discs may offer new hope to alleviating chronic low back pain.
The concept of biological disc repair has grown in recent years out of an improved understanding of the cellular and molecular events involved in disc aging and degeneration. Recent cell-based approaches have focused on the use of autologous disc chondrocytes.12 For instance, Meisel et al.13 have shown that implantation of culture-expanded autologous disc chondrocytes reduces back pain and may improve disc height in patients after lumbar discectomy for symptomatic disc herniation. Nevertheless, this cell source does have three main practical limitations in the clinical setting:
Graft procurement: The procurement of autologous disc chondrocytes, whether accomplished by image-guided aspiration or open surgical collection, is an invasive process. This is particularly problematic for those patients without a symptomatic disc herniation requiring surgical discectomy who would have to undergo an additional procedure to collect autologous disc cells.
Harvest-site morbidity: The harvesting of disc chondrocytes from a healthy disc can potentially accelerate degeneration at that level. On the other hand, harvesting disc cells from an already degenerated level requires adequate time after the procedure to allow for both expansion of the cells in culture, as well as for healing of the annulus fibrosus in order to ensure containment of the cells after injection.
Functionality: NP cells from a degenerated disc may not be functionally ideal for re-implantation. Kluba et al.14 observed that NP cells from patients with degenerative disc disease expressed lower levels of Type II collagen compared to those from patients with idiopathic scoliosis.
Non-disc-derived chondroctyes and bone marrow-derived mesenchymal stem cells (MSCs) are two cell types whose procurement and expansion avoid these drawbacks while maintaining the potential to produce NP-like extracellular matrix (ECM).15–17 Rahmat et al.18 demonstrated that autologous non-disc chondrocytes from costal cartilage remain viable, continue to produce disc-like matrix, and possibly prevent disc degeneration 3 months after implantation in a sheep model of IVD injury. Gorensek et al.17 observed that autologous chondrocytes from auricular cartilage survived and produced hyaline-like cartilage 6 months after transplantation into injured rabbit discs. With regard to MSCs, autologous MSCs transplanted into degenerated rabbit discs have been shown to not only proliferate and differentiate into NP-like cells,19 but also to improve disc height, magnetic resonance imaging signal intensity, and proteoglycan content.20 Although these data are promising, no study to date has assessed the ability of either allogeneic non-disc chondrocytes or allogeneic MSCs to promote disc repair after experimental injury.
The purpose of the current study was to assess and compare the viability and functionality of transplanted allogeneic porcine juvenile articular chondroctyes (JCs) and allogeneic MSCs after IVD injury and treatment in an adult mini-pig model. We hypothesized that, as JCs are fully differentiated chondrocytes harvested from a relatively hypoxic microenvironment (articular surface), their survival and functionality in vivo after transplantation into an injured disc would be superior to naïve MSCs.
Materials and Methods
Thirty adult female miniature swine (Sinclair Research Center, Inc.; 48.7±8 kg) were included in this study, which was approved by the committee on animal research at our institution. Within each animal, four lumbar levels (L1/2, L2/3, L3/4, and L4/5) were randomized into separate treatment groups: (1) control/no disc injury; (2) nuclectomy with fibrin carrier alone; (3) nuclectomy with allogeneic male JCs in fibrin carrier; and (4) nuclectomy with allogeneic male MSCs in fibrin carrier. Ten animals each were assigned to analysis at 3, 6, and 12 months post-treatment. After euthanasia, the lumbar spines were harvested and the study levels analyzed for gross disc morphology. Each disc was then randomly assigned to either histological analysis of morphology and matrix proteoglycan (H&E, Safranin-O/fast green) or biochemical analysis of water, proteoglycan, protein, and DNA content. Fluorescence in situ hybridization (FISH) was used to distinguish injected Y-chromosome-positive male cells from female host cells.
MSC preparation
Juvenile male Yorkshire farm pigs (approximately 3 to 4 months old and weighing 47.5±5 kg) were used for MSC preparation. The bilateral humerus, femur, and tibia were removed from the euthanized pigs. Bone marrow blood was flushed and diluted 1:1 with Mg2+/Ca2+-free phosphate-buffered saline (PBS) containing ethylenediaminetetraacetic acid (EDTA) (1gm/L). Bone marrow blood was filtered, washed, and centrifuge at 1500 rpm for 8 min at room temperature. The cell pellet was then incubated with 5 mL of lysis buffer to remove red blood cells and centrifuged at 1200 rpm for 5 min. Cell number was estimated using a hemocytometer, and the cells were washed with PBS and spun down before resuspension in fresh MSC basal medium supplemented with mesenchymal cell growth supplement (Lonza/Cambrex), L-Glutamine and Pen/Strep (Cambrex), as well as additional 0.5% antibiotic and antimycotic and 1% fungizone (Cell culture facility at parent institution). Cells were cultured onto 225 cm2 T flasks (Corning) and maintained at 37°C with 5% CO2 in air.
After 3 days in culture, nonadherent cells were rinsed away in fresh medium and the attached cells were allowed to expand for an additional 12 days, with twice-weekly medium exchange. On day 15 the enriched population of MSCs was harvested using 0.25% trypsin-EDTA (Cambrex) for 7 min at 37°C and then counted. For further expansion of the isolated cells, this population was re-plated at approximately 4000 cells/cm2 in T-225 flasks and maintained as before, with twice-weekly medium exchange. A final population doubling of 8000 cells/cm2 was achieved at passage two with a doubling time of 3.5 days. To confirm chondrogenic potential, a sample of the harvested MSCs were pelleted by centrifugation (approximately 500,000 cells per pellet) and cultured in chondrogenic medium (Cambrex) for 2 weeks. These pellets were processed for paraffin histologic analysis with Safranin-O/fast green staining that demonstrated proteoglycan matrix accumulation, and cellular morphologic features consistent with chondrogenic differentiation. The remaining MSCs were resuspended in 0.5 mL of CryoStor CS5 (Biolife Solutions, Inc) at a concentration of 50 million cells/mL. Cryovials were stored in liquid nitrogen until use.
JC preparation
Articular cartilage was dissected from the condyles of the distal femur of a single 7-day-old male miniature pig (Sinclair Research Center, Inc.). The cartilage was treated to isolate chondrocytes according to a modified method of Adkisson et al.21 Briefly, the cartilage pieces were washed 3×and subsequently digested using a purified mixture of collagenase/neutral protease (Liberase Blendzyme 2®; Roche Applied Science) in HL-1 Complete Serum-free Medium (Lonza) at a concentration of 1.6 WU/mL. This mixture was incubated at 37°C for no more than 9 h, and dissociated chondrocytes were filtered through a 70 μm nylon strainer. JCs were pelleted and washed in fresh HL-1 medium before estimating total cell yield and viability (ViaCount; Guava Technologies, Inc).
To expand the primary population, a cell suspension (3.33×104 cells/cm2) was inoculated into T-150 culture flasks containing expansion medium formulated to retain chondrocyte hyaline phenotype after expansion. The expansion medium was comprised of HL-1 Complete Serum-Free Medium, containing gentamicin, L-glutamine, FGF-2, TGF beta-1, hyaluronic acid, and L-ascorbate. Culture medium was changed every 3–4 days and the cells harvested at day 21 using a solution of Liberase Blenzyme 2. Chondrocytes were washed and counted before resuspension to a density of 50×106 cells/mL in CryoStor CS5 cryopreservation solution, containing 5% DMSO. Next, 0.5 mL of the cell suspension was aliquoted into cryovials, frozen at a rate of −1°C/min with the use of a controlled rate freezer (Cryomed; ThermoForma), and stored in liquid nitrogen for future use.
The viability and functional activity of thawed JCs was assessed in vitro by growing the cells in the presence of fibrin carrier for 2 weeks. Viability of the embedded chondrocytes was determined by confocal fluorescence microscopy using a commercially available assay (Live/Dead Viability Cytotoxicity Kit; Molecular Probes L-3224). Biochemical measurement of sulfated glycosaminoglycan (S-GAG), hydroxyproline, and DNA content of the fibrin/chondrocyte constructs was performed to confirm that native chondrocyte phenotype was retained by chondrocytes that had undergone 5.29 population doublings in vitro. Cell viability was estimated to be greater than 80% at 2 weeks, confirming that the carrier vehicle (fibrin) was not cytotoxic. Furthermore, biochemical composition analysis of the fibrin matrix containing newly synthesized cartilage macromolecules confirmed that culture-expanded JCs retained their biosynthetic activity after cryopreservation and growth in the designated carrier.
Fibrinogen component preparation
One month before surgery, whole blood from a single male Sinclair mini-pig was collected in Vacutainer tubes containing 3.2% sodium citrate. This whole blood was centrifuged to yield approximately thirty 25 mL aliquots of plasma, which were frozen at −80°C for 48 h. Each aliquot was thawed at 2°C–8°C for 24 h and centrifuged at 3000 rpm for 15 min to collect fibrinogen cryoprecipitate that was subsequently resuspended in 10 mL of supernatant plasma. This fibrinogen component solution was stored at −80°C until the day of surgery, at which time it was warmed to 37°C immediately before use (fibrinogen stored frozen at the above temperature is stable for up to 1 year). A single aliquot was used for each animal in the study. About 20,000 IU of bovine thrombin (Thrombin–JMI from King Pharmaceutical) was reconstituted in 20 mL of saline for a final concentration of 1000 IU/mL. This solution was maintained at room temperature until use.
Treatment preparation
The thawed fibrinogen component (0.8 cc) was drawn into a single 3 cc syringe as part of a FibriJet® applicator assembly (Micromedics). Next, 0.5 mL of either thawed MSCs or JCs in CryoStor solution was mixed with 0.3 mL of thrombin solution. This mixture, containing 25 million cells, was then drawn into another syringe taken from the FibriJet apparatus. Once both syringes were connected onto the applicator assembly, 0.3 cc from each syringe was dispensed to prime the assembly cannula. The remaining 1.0 mL was used for injection; however, only approximately 0.5–0.75 mL of the cell suspension was typically accepted by a denucleated disc. Therefore, the number of cells injected into treated discs ranged from 7 to 10 million cells, assuming 90% viability as demonstrated in bench-top studies using allogeneic pig JCs.
Surgical procedure
Animals were placed under general anesthesia. Briefly, pigs were injected with telazol for induction, intubated, ventilated, and placed on isoflorane maintenance. Once under sufficient anesthesia, the right flank was shaved and aseptically prepped. An approximately 20 cm-long oblique incision was made along the right flank along the edge of the external oblique muscle between the ribs and the pelvis. A retroperitoneal dissection was then performed to expose the spine and the anterolateral portion of the L1/2, L2/3, L3/4, and L4/5 IVDs. Intraoperative radiographs were taken to confirm the levels, and a nylon suture was placed in the uppermost level to assist in identification of treatment levels at necropsy. Using a no. 11 surgical blade, a small incision approximately 1 cm was made horizontally in the center of the disc. The annular incision was then temporarily distracted using a modified retractor. Next, a SpineJet™ MicroResector (HydroCision, Inc.) was inserted into the incision for 1 min and manipulated to remove as much NP as possible. Once the NP was removed, fibrin carrier alone, JCs in fibrin carrier, or MSCs in fibrin carrier were injected at a volume of 0.5–0.75 cc.
The annular incision remained non-sutured at closure. The wound was closed in the appropriate manner. Following the procedure, the animal was awoken from anesthesia and recovered. The total time for this procedure was approximately 1 h.
Tissue harvest
Pig spines were harvested en bloc and were X-rayed to confirm treatment levels. Treatment and control levels were isolated by blunt dissection to remove spinal musculature and soft tissues, using the nylon suture as a landmark to positively catalog harvested treatment levels. Adjacent vertebrae were trimmed within 5 mm of the vertebral endplates using a precision saw. Specimens designated for histological assessment were placed in neutral buffered formalin. Specimens designated for biochemical analysis were photographed then sectioned horizontally along the mid height. The opened disc was photographed, and the nucleus was collected based on morphological features and placed in pre-weighed vials for storage at −20°C.
Tissue biochemistry
NP samples were processed using previously reported protocols.5 Briefly, the samples were completely dried in a 37°C incubator; then, the tare weight was subtracted from the final weight to determine sample dry weight and water content. Dried NP samples were then subjected to papain (Sigma P4762) digestion. The completely digested samples were then centrifuged to collect both supernatant and the pellet. The pellet was next reconstituted with TE buffer. Both supernatant and the pellet of each sample were tested for DNA content, S-GAG content, and protein content. The final result of each sample in each assay was pooled from the both supernatant and the pellet. S-GAG and protein data were normalization by DNA content using results from DNA quantification.
DNA content
PicoGreen assay (Molecular Probes) was used to determine DNA content from each group according to the manufacturer's directions. Cell density was calculated using 6 pg of DNA per cell.
S-GAG content
Proteoglycan levels in each group was measured using a colorimetric reaction to detect S-GAG content (DMMB assay) based on a modified method of Farndale et al.22 Briefly, 21 mg of DMMB (Sigma) reagent was stirred with 5 mL of absolute ethanol and 2 g sodium formate (Sigma). The final volume was adjusted to 800 mL with distilled water and concentrated formic acid was added to make stock solution (pH 3.5). Bovine cartilage chondroitin sulfate A (Sigma) was used as a standard. Absorbance at 525 nm was read using a SpectraMax M5 microplate reader (Molecular Device).
Protein content
Protein content was determined by OD280 reading.
Histology
Specimens were fixed for 1 to 2 weeks in 10% neutral buffered formalin, and then decalcified in formic acid until a radiographic endpoint test demonstrated complete decalcification. They were stored in 70% ethyl alcohol for 2 to 7 days, passed through an ascending series of alcohols, cleared in Clearite (Richard-Allan Scientific), and infiltrated with paraffin using a vacuum apparatus. Six-micrometer-thick sections were stained with either H&E or Safranin-O/Fast Green.
Immunohistochemical staining
Paraffin sections were adhered to charged slides (Fisher Brand ProbeOn Plus) previously treated with Biobond™ Tissue Section Adhesive (Ted Pella, Inc). Slides were then baked for 20 min at 60°C, dewaxed using EZ DeWax™ Solution (BioGenex Laboratories) and immersed in a descending series of ethanol. Unmasking of antigens within the cartilaginous ECM was achieved using pepsin solution (2 mg/mL in 0.1 HCl; 37°C for 20 min) made fresh daily. Tissue sections were then washed and permeabilized in 0.03% triton X-100 and rinsed in PBS. Endogenous peroxidase activity was blocked with 0.3% H2O2 in PBS and nonspecific staining was blocked with 2.5% horse sera. After washing, slides were incubated with primary antibody (Table 1) overnight at room temperature using a covered HybriWell™ Sealing System (Grace Bio-Labs) to minimize the volume of primary reagent used. The antibody was aspirated away and sections were washed and incubated at room temperature for 30 min with horseradish peroxidase (HRP)-labeled secondary antibodies (HRP-labeled goat anti-rabbit [1:400 dilution] and HRP-labeled anti-mouse/rabbit [undiluted] Vector Labs ImmPress Reagent Kit). Colorimetric development (10 min at room temp) was performed with diaminobenzadine reagent, according to manufacture's directions (Vector Labs). Samples were then washed and mounted in CC/Mount aqueous medium and cover slipped.
Table 1.
Primary Antibodies Used in Immunohistochemical Analyses
| Description | Dilution | Source |
|---|---|---|
| Type I collagen mouse monoclonal | 1:25 | Calbiochem |
| Mature Type II collagen rat anti-human | 1:200 | Gift from Linda Sandell |
| Link Protein mouse monoclonal | 1:10 | University of Iowa Development Studies Hybridoma Bank |
| Chondromodulin-I rabbit polyclonal | 1:200 | Santa Cruz Biotech |
Fluorescent in situ hybridization
Formalin-fixed, paraffin-embedded tissue sections were dewaxed in xylene, followed by 70% ethanol wash. Pepsin digestion (2 mg/mL in 0.1 M HCl) was performed to permeabilize tissue at 37°C for 20 min. Tissue sections were hybridized with the first probe (Genedetect.com) after the post fixation, quench both endogenous peroxidase and endogenous autofluorescence activities; block endogenous biotin and pre-hybridization steps. The satellite probe (SATgsbiot-1) was designed to target DNA repeats present in pig genomic DNA and will serve as a general control identifying cells of both male and female origin. The SRY probe (SRY39gsbiot-1) was designed to target the Y chromosome-specific SRY gene present only in the injected male JCs and MSCs. Each of these probes was purchased from GENEDETECT.com. An HRP-streptavidin-conjugated secondary antibody and tyramide signal amplification process was then used to stain tissue sections. These probes were purchased from Invitrogen Molecular Probes. The general distribution of injected JCs and MSCs in treated discs was observed under fluorescence microscopy (Leica, Leitz DM RB), and compared with H&E and Safranin-O/fast green stains from serial sections.
Data analysis
All statistical analyses were performed using JMP statistical software (V 8.0; SAS). For the biochemistry data, standard analysis of variance procedures were used to compare group means and to estimate the effect of specimen variables (treatment type and harvest time point entered as a categorical predictors) on the measured parameters of interest (DNA content, ng/mL; S-GAG content, mg/mL; and protein content, μg/mL, were entered as continuous variables). When indicated, a Tukey post-hoc test was performed to identify statistically significant group differences.
Results
Animals tolerated the surgical procedure without complications or neurological deficit, surviving to each pre-determined endpoint.
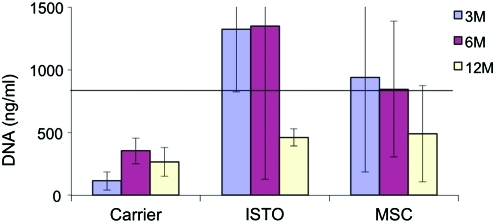
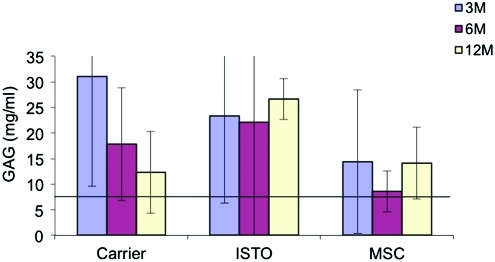
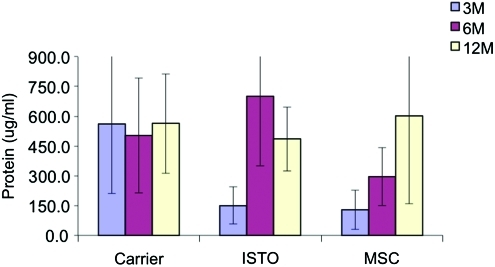
Quantitative differences in the biochemical composition of cell-treated and control discs were observed (Figs. 1–3). The DNA content for the JC-treated discs was significantly higher than the fibrin-only discs at 3 weeks (1324±501 ng/mL vs. 114±271 ng/mL; p=0.02; Fig. 1). By 12 months, DNA content in both the JC and fibrin-only discs was significantly less than controls (462±70 and 265±116 respectively vs. 797±500 ng/mL; p=0.002). There were no significant differences in the S-GAG content at 3 and 6 months (Fig. 2). However, by 12 months the S-GAG content for the JC-treated discs was more than double that for all other groups (p<0.0001). The total protein content of the nucleus samples was significantly higher in the fibrin-only group than the JC- and MSC-treated groups at 3 months (562±351 μg/mL vs. 151±95 and 129±100 μg/mL respectively; p=0.02; Fig. 3). By 12 months, all three treatment groups were comparable and significantly greater than control (p<0.001).
FIG. 1.
DNA content per wet weight. Horizontal line represents DNA value for control level. DNA content for JC-treated discs was greater than carrier-only at 3 weeks. By 12 months, the DNA content for all three treatment groups was significantly less than control. JC, juvenile chondrocyte; MSC, mesenchymal stem cell. Color images available online at www.liebertonline.com/tea
FIG. 3.
Sulfated GAG content per wet weight. Horizontal line represents value for control level. By 12 months the GAG content for the JC-treated discs was significantly greater than all other groups. GAG, glycosaminoglycan. Color images available online at www.liebertonline.com/tea
FIG. 2.
Protein content per wet weight. Protein content for the JC-treated and MSC-treated levels increased after 3 months, become comparable to carrier at the 12-month time point. Color images available online at www.liebertonline.com/tea
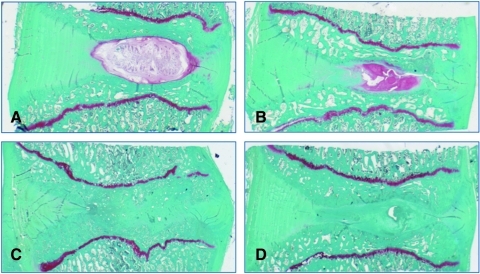
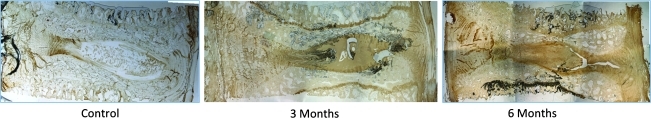
Histologically, no inflammatory response was observed in any treatment group. Annular surgical defects were still apparent at 3 months, with significant granulation tissue that extended into the nucleus. For the carrier and MSC-treated groups, the nuclear region was filled with collagen with little evidence of proteoglycan matrix or residual notochordal/chondrocyte cells, as demonstrated by the absence of Safranin-O metachromasia (Fig. 4). By contrast, five of seven JC-treated discs presented at 3 months with abundant regions of ECM demonstrating intense Safranin-O staining, and to a lesser extent at 6 and 12 months (Fig. 4). Within groups, the extent of degenerative change appeared to relate to the degree of endplate damage that had occurred as a result of the denucleation procedure. This was particularly evident in the 6- and 12-month groups, where endplate disruption was apparent in 8 of 13 discs and 9 of 14 discs, respectively.
FIG. 4.
At 3 months abundant cartilage formation was observed for JC-treated discs (B). Safranin-O staining highlights collagen (green) and proteoglycan (red). Control (A), JC-treated disc (B), carrier-treated disc (C), MSC-treated disc (D).
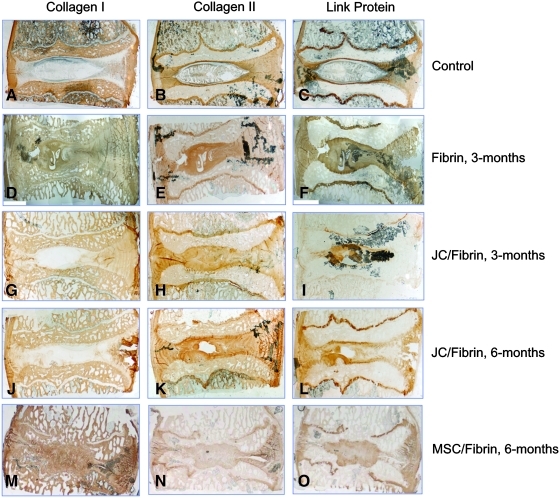
To further explore the nature of the ECM formed within JC-treated discs, immunohistochemical (IHC) staining was performed. Unoperated control levels were characterized by the presence of immunoreactivity for types I and II collagen in the annulus, with the greatest concentration of type II collagen localized to the cartilage endplates (Fig. 5B). Likewise, immunoreactivity for link protein was found localized to the cartilaginous endplates and the neighboring growth plates (Fig. 5C). As expected, the nucleus contained a reduced concentration of type II collagen immunoreactivity relative to both the intact end plate cartilage and the annulus and was devoid of immunoreactivity for type I collagen. By contrast, discs receiving carrier alone (fibrin) presented with newly formed tissue in the nucleus that largely showed uniform immunoreactivity to collagen types I and type II. This histopathologic feature was clearly distinguishable from native nonoperated discs and discs receiving JCs in fibrin carrier (Fig. 5I). The absence of type I collagen from the nucleus of JC-treated discs was observed equally for the 3-, 6-, and 12-month treatment groups, with the exception of a single disc (12 month) that demonstrated significant reactive bone presumably arising from damage to the bony endplates during denucleation.
FIG. 5.
Immunolocalization of type I collagen, type II collagen, and link protein for controls (A–C), fibrin-treated (D–F), JC-treated (G–L), and MSC-treated (M–O) discs.
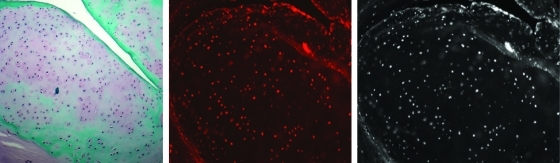
To demonstrate that newly formed cartilage identified in the nucleus of treated discs derived from the injected cells as opposed to an intrinsic healing response originating from female host cells, FISH analysis of Y-chromosome-positive male cells was performed. There was unequivocal proof that allogeneic JCs injected into denucleated healthy discs remain viable and contribute directly to the production of Safranin-O positive ground substance identified in the disc space 3, 6, and 12 months post-operatively (Fig. 6). By contrast, no positive staining of male cells was identified in any of the tissue sections prepared from the MSC-carrier group, suggesting that very few of these cells survived injection to 3 months.
FIG. 6.
FISH localization demonstrating the presence of donor cells at 3 months (Safranin-O stained section, left; FISH localization with fluorescent filter, middle; FISH localization without fluorescent filter, right). FISH, fluorescence in situ hybridization.
Knowing that the disc environment is avascular and that this leads to enhanced synthesis by chondrocytes of anti-angiogenic proteins, including chondromodulin-I (ChM-I), an inhibitory angiogenic factor produced by chondrocytes,23 the tissues were stained using a commercially available antibody. Strong, relatively uniform immunoreactivity for ChM-I was observed for JC-treated discs at 3 and 6 months, whereas staining for this protein in the nucleus of un-operated control levels was unremarkable. Only those fibrin carrier discs showing fibrocartilage scar formation likewise demonstrated positive immunoreactivity for ChM-I (Fig. 7).
FIG. 7.
Chondromodulin-I immunostaining of JC-treated discs. Uniform staining was observed in the nucleus for JC-treated discs at 3 and 6 months.
Discussion
We questioned whether allogeneic JCs and/or allogeneic MSCs survive and synthesize relevant matrix within the IVD environment of a large animal model. Success in this regard requires overcoming several hurdles that include surgical removal of host nucleus, delivery and retention of donor cells, and subsequent cell survival, proliferation, differentiation, and synthesis of an appropriate ECM. Our data demonstrate that JCs can survive up to 12 months and synthesize abundant cartilage-like matrix. By contrast, viable MSCs were not observed at any of the follow-up time points. Taken together, these data highlight the challenges of IVD tissue engineering, and indicate that appropriately differentiated cells can survive and synthesize matrix in situ.
The IVD is the largest avascular tissue in the body, and as such nuclear cells are severely challenged to maintain matrix so as to sustain appropriate biomechanical properties.24 It is not surprising, therefore, that cellular dysfunction is considered an underlying factor in disc degeneration. In support of this, several groups have reported the increased presence of senescence-associated beta-galactosidase in cells from aged or degenerate discs.25–27 These cells demonstrate slower growth kinetics, a reduced capacity to proliferate, and increased secretion of enzymes linked to disc degeneration.26,28 Consequently, tissue engineering approaches for disc repair may require introduction of an invigorated cell population capable of synthesizing relevant matrix.
This sub-optimal cell population may be triggered by the hostile biological environment epitomized by the IVD. Nuclear cells rely on diffusive transport through thick tissue to adjacent vertebral capillaries (as far as 8 mm in human lumbar discs).29 Consequently, nutrients and oxygen are scarce, and cells utilize anaerobic glycolysis for energy metabolism, producing lactic acid as a byproduct. Low oxygen and pH levels, along with reduced glucose, have been shown to have deleterious effects on disc cell function.30–32 Recently, Roberts and coworkers presented data suggesting that the mechanism of disc cell dysfunction secondary to poor transport was not glucose or oxygen deprivation per se, but rather withdrawal of other, as-yet characterized factors present in serum.33 Regardless, the disc nucleus is a challenging environment in which to place cells aimed at repairing degenerate disc matrix.
Our data demonstrate that JCs are capable of surviving and producing cartilage matrix within the IVD environment. Indeed, chondrocytes may be uniquely prepared to survive the ischemic disc microenvironment. Mobasheri et al. recently provided a dual model of oxygen and glucose sensing in chondrocytes that enables these cells to undergo glycolytic metabolism under harsh environmental conditions.34 This model incorporates the hypoxia inducible factor alpha (HIF-1alpha) as an oxygen sensor and the hypoxia responsive facilitative glucose transporters, GLUT1 and GLUT3, as putative components of the glucose-sensing apparatus in chondrocytes. Recent studies confirm that GLUT1 and GLUT3 are expressed by chondrocytes35–37 and that the transcription factor HIF-1alpha plays a pivotal role in regulating chondrocyte survival38 and ECM synthesis.39
While MSCs have the potential to differentiate into cartilage under certain controlled conditions in vitro, this was not observed in vivo in the present study, suggesting that undifferentiated cells do not have proper environmental cues to form cartilage spontaneously within the disc. This is likely due to their poor ability to function under conditions of serum deprivation,40 and high osmolarity, disc-like conditions.41 Additionally, it may be that even when MSCs undergo chondrogenic differentiation they are inherently inferior to chondrocytes in their matrix synthesizing capacity.42 Yet, Yang and coworkers have reported that MSCs prevent degenerative changes in the mouse model that is attributed to chondrocytic differentiation and/or paracrine signaling of host notochordal cells.43 However, given the mouse disc's small size, avascularity and transport restrictions likely do not encumber the increased anabolism of synthetically active cells, as may be the case in the mini-pig that has discs closer in size to human. It may also be that poor MSC performance was due to the source of our MSCs (Yorkshire farm pigs) being a different pig strain than the recipients (Sinclair mini-pigs). However, we do not believe this was the case since MSCs have special immunological properties, and several groups have reported successful xenogeneic transplantation of these cells.44,45 Additionally, our observations are consistent with those of others who report that pre-differentiated cells integrate and function better in vivo when compared to undifferentiated cells in tissue engineering applications.46
The rationale for the cell number chosen for study comes from two different labs in which it was learned that nutrients, glucose in particular, play a critical role in chondrocyte survival and matrix synthesis. Nutrient diffusion into the disc nucleus is driven by concentration gradients between the blood plasma within the vertebral endplates and the nucleus matrix, and represents a balance between supply (capillary density) and demand (cell density and metabolic rate). Using an in vitro growth chamber with a diffusion distance reproducing the average height of adult human discs, Horner et al. have shown that glucose gradients play a critical role in regulating the viability of cultured bovine nucleus cells.31 While low levels of glucose caused cellular necrosis, it was reported that 5×106 to 10×106 cells/mL survive (95% viability) at a diffusion distance that is comparable to that of the normal adult human disc (5–8 mm). These data suggest that relatively intact adult human discs could support 4×106 to 8×106 cells/mL, which is in agreement with cell densities reported to be present in human discs (5×106 cells/mL).47
The surgical procedure to denucleate the host nucleus was traumatic to the annulus, and on occasion the endplate, given the relatively narrow porcine disc height. Disposable SpineJet probes used in the study were designed to accommodate human lumbar discs for which the disc height is significantly greater than that of the mini-pig. Discs with endplate damage tended to degenerate more, suggesting that an inflammatory environment triggered by iatrogenic disc damage may be deleterious to subsequent repair efforts. Previous studies exploring JC survival after injection into the rat tail disc produced a cartilaginous matrix that was similar in its appearance to what was observed at 3 months in the mini-pig lumbar disc.48 However, this model is not considered to be as harsh as the present mini-pig model for two reasons: the nucleus was not mechanically removed, and the reduced disc height creates less of a nutrient diffusion barrier. Importantly, the rat study showed a marked increase in T2-weighted signal for JC-treated discs as compared to native discs and discs treated with carrier alone. Moreover, there was no loss of injectate from treated rat-tail discs.
While significant animal-to-animal variability was observed in the present study, there were some apparent histologic trends. New cartilage formation in the JC-treated group was more apparent at 3 months than at 6 or 12 months, based on Safranin-O staining. Further analysis of collagen composition by IHC revealed that the nucleus of JC-treated discs was devoid of type I collagen immunoreactivity, containing predominantly type II collagen at 3, 6, and 12 months. Some response variability is likely due to surgical technique, as we noted endplate damage in the more degenerated discs likely induced by the SpineJet instrumentation used for nucleus removal. A second factor relates to the large incision that was created in the annulus to gain access to the nucleus for removal. No attempt was made to close the incision after injection of carrier alone or cells in carrier. It is highly likely that a portion of the injectate may have extruded from the 10 mm incision, as no sutures were used to close this large incision. Another potential factor that may contribute to variability in the healing response between groups is the lack of gravity loading associated with the quadruped spine, and will be discussed later.
Assessment of donor cell viability using FISH confirmed that male JCs survived injection into the denucleated disc space and that these cells synthesized a type II collagen-rich repair tissue that had integrated with the host annulus. Based upon positive staining identified in most 3-, 6-, and 12-month samples, any lack of staining for levels receiving JC treatment suggests that the material may have seeped out before clotting or have been extruded. The fact that MSC-treated discs had no evidence of proteoglycan synthesis histologically at either time point suggests that these cells had either failed to survive injection or that this material was extruded. It is unlikely that the contents of MSC-carrier discs were preferentially extruded over JC-carrier discs, providing supportive evidence that undifferentiated MSCs are unable to survive the avascular environment of the disc.
The demonstration that JC-treated discs produced high levels of ChM-I in the newly formed ECM and that expression of this protein persisted for at least 12 months postinjection is significant in light of the propensity for degenerative discs to show an advancement of neural structures and blood vessels into the inner third of the annulus.11,49 We speculate that catabolic destruction of aggregating proteoglycan and loss of ChM-I expression coupled with senescence of nucleus chondrocytes may enable pathological nuclear ingrowth of these structures as demonstrated historically. Independent studies have shown that aggrecan and ChM-I function to inhibit neural and endothelial cell adhesion, migration, and tubule formation.23,50 Therefore, it is hypothesized that a biologic approach to augmenting the disc nucleus via administration of viable chondrocytes capable of producing anti-angiogenic/neuronogenic factors may cause a regression or block neural ingrowth and thereby reduce potential pain generators in the degenerate disc. Our data show that allogeneic JCs retain the ability to express ChM-I after serial expansion in vitro and subsequent intradiscal injection.
A variety of animal models have been developed to study the impact that experimental injury has on the various structures comprising the IVD.51 Such models permit an improved understanding of the biochemical and biomechanical consequences of disc injury, facilitating the development of novel therapeutic approaches to alleviate back pain associated with disc injury and/or degenerative disease.52 However, there are several issues to consider in relation to using animals as model systems for studying human disease that may limit direct translation of experimental outcomes to the human clinical setting. These include but are not limited to the persistence of notochord cells in the adult nucleus of many (quadruped) species, differences in disc geometry and tissue composition, differences in biomechanical properties, and the inability to measure pain thresholds in large animals. Consequently, the porcine model introduces several limitations into the current study. First, it does not replicate the human situation in general, and the low back pain patient in particular. The porcine disc differs from human in several respects such as the maintenance of a growth plate, the notochordal nucleus cell phenotype, and the relatively narrow disc height. The narrow disc height clearly contributed to the production of endplate injury during SpineJet nucleus removal. In addition, quadruped spines do not experience significant gravity loading. It is generally accepted that articular chondrocytes respond to dynamic compression by enhancing matrix synthesis both in vitro and in vivo and that static compression leads to a decrease in newly synthesized matrix proteoglycan by chondrocytes.53,54 In fact, past experience exploring the fate of neocartilage implants generated in vitro from JCs yielded similar results when viable tissue grafts were implanted into deep osteochondral defects, which prevented transmission of mechanical signals to chondrocytes contained within the implant.55 Consequently, rather than establishing efficacy (particularly since healthy, non-painful discs were treated), this study tests the potential for JCs and MSCs to survive in vivo in the ischemic disc space and secrete collagen and non-collagen macromolecules indigenous to cartilage.
Despite these limitations, our data demonstrate that allogeneic JCs appear to be an appropriate cell source for IVD repair. These cells were observed to synthesize cartilage-like matrix and survive for up to 12 months with no apparent adverse effects. Allogeneic MSCs, on the other hand, were not observed to survive at any time point post-injection. Juvenile chondrocytes displaying a committed phenotype, therefore, may be the more suitable choice for use in tissue engineering strategies for IVD repair compared to undifferentiated cells. It is intriguing to speculate that a nucleus repair tissue comprising type II collagen may prove to be more durable than scar tissue formed from a mix of type I and type II collagen, much like what has been reported clinically for microfacture repair of joint articular cartilage.55,56–58
Acknowledgments
Study supported by NIH AR052712 and ISTO Technologies.
Disclosure Statement
Lotz is consultant for ISTO Technologies, which partially funded the study. Authors Adkisson, Maloney, and Milliman are employees of ISTO Technologies.
References
- 1.Andersson G.B. Epidemiology of low back pain. Acta Orthop Scand Suppl. 1998;281:28. doi: 10.1080/17453674.1998.11744790. [DOI] [PubMed] [Google Scholar]
- 2.Maetzel A. Li L. The economic burden of low back pain: a review of studies published between 1996 and 2001. Best Pract Res Clin Rheumatol. 2002;16:23. doi: 10.1053/berh.2001.0204. [DOI] [PubMed] [Google Scholar]
- 3.Benneker L.M. Heini P.F. Alini M. Anderson S.E. Ito K. 2004 young investigator award winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine. 2005;30:167. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- 4.Boos N. Weissbach S. Rohrbach H. Weiler C. Spratt K.F. Nerlich A.G. Classification of age-related changes in lumbar intervertebral discs: 2002 volvo award in basic science. Spine. 2002;27:2631. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou J. Steffen T. Nelson F. Winterbottom N. Hollander A.P. Poole R.A. Aebi M. Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckwalter J.A. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Melrose J. Ghosh P. Taylor T.K. Neutral proteinases of the human intervertebral disc. Biochim Biophys Acta. 1987;923:483. doi: 10.1016/0304-4165(87)90058-4. [DOI] [PubMed] [Google Scholar]
- 8.Roberts S. Caterson B. Menage J. Evans E.H. Jaffray D.C. Eisenstein S.M. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hollander A.P. Heathfield T.F. Liu J.J. Pidoux I. Roughley P.J. Mort J.S. Poole A.R. Enhanced denaturation of the alpha (ii) chains of type-ii collagen in normal adult human intervertebral discs compared with femoral articular cartilage. J Orthop Res. 1996;14:61. doi: 10.1002/jor.1100140111. [DOI] [PubMed] [Google Scholar]
- 10.Freemont A.J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 11.Freemont A.J. Peacock T.E. Goupille P. Hoyland J.A. O'Brien J. Jayson M.I. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 12.Meisel H.J. Ganey T. Hutton W.C. Libera J. Minkus Y. Alasevic O. Clinical experience in cell-based therapeutics: Intervention and outcome. Eur Spine J. 2006;15(Suppl 3):S397. doi: 10.1007/s00586-006-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meisel H.J. Siodla V. Ganey T. Minkus Y. Hutton W.C. Alasevic O.J. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation a treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Kluba T. Niemeyer T. Gaissmaier C. Grunder T. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine. 2005;30:2743. doi: 10.1097/01.brs.0000192204.89160.6d. [DOI] [PubMed] [Google Scholar]
- 15.Mackay A.M. Beck S.C. Murphy J.M. Barry F.P. Chichester C.O. Pittenger M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 16.Barry F. Boynton R.E. Liu B. Murphy J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 17.Gorensek M. Jaksimovic C. Kregar-Velikonja N. Gorensek M. Knezevic M. Jeras M. Pavlovcic V. Cor A. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett. 2004;9:363. [PubMed] [Google Scholar]
- 18.Rahmat R. Moore R.J. Nikoloff S. NMatsacos D. Oakes B.W. Fraser R.D. Autologous chondrocyte implantation in an ovine model of disc degeneration. J Bone Joint Surg Proc. 2003;86-B:283. [Google Scholar]
- 19.Sakai D. Mochida J. Iwashina T. Watanabe T. Nakai T. Ando K. Hotta T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: Potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005;30:2379. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 20.Sakai D. Mochida J. Iwashina T. Hiyama A. Omi H. Imai M. Nakai T. Ando K. Hotta T. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 21.Adkisson H.D. Gillis M.P. Davis E.C. Maloney W. Hruska K.A. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001;391(Suppl):S280. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 22.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 23.Kusafuka K. Hiraki Y. Shukunami C. Kayano T. Takemura T. Cartilage-specific matrix protein, chondromodulin-I (ChM-I), is a strong angio-inhibitor in endochondral ossification of human neonatal vertebral tissues in vivo: Relationship with angiogenic factors in the cartilage. Acta Histochem. 2002;104:167. doi: 10.1078/0065-1281-00642. [DOI] [PubMed] [Google Scholar]
- 24.Urban J.P. Smith S. Fairbank J.C. Nutrition of the intervertebral disc. Spine. 2004;29:2700. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 25.Gruber H.E. Ingram J.A. Norton H.J. Hanley E.N., Jr Senescence in cells of the aging and degenerating intervertebral disc: Immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine. 2007;32:321. doi: 10.1097/01.brs.0000253960.57051.de. [DOI] [PubMed] [Google Scholar]
- 26.Le Maitre C.L. Freemont A.J. Hoyland J.A. Accelerated cellular senescence in degenerate intervertebral discs: A possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts S. Evans E.H. Kletsas D. Jaffray D.C. Eisenstein S.M. Senescence in human intervertebral discs. Eur Spine J. 2006;15(Suppl 3):S312. doi: 10.1007/s00586-006-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Maitre C.L. Freemont A.J. Hoyland J.A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson G.B. Schultz A. Nathan A. Irstam L. Roentgenographic measurement of lumbar intervertebral disc height. Spine. 1981;6:154. doi: 10.1097/00007632-198103000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Bibby S.R. Jones D.A. Ripley R.M. Urban J.P. Metabolism of the intervertebral disc: Effects of low levels of oxygen, glucose, and ph on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 2005;30:487. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 31.Horner H.A. Urban J.P. 2001 volvo award winner in basic science studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Grunhagen T. Wilde G. Soukane D.M. Shirazi-Adl SA. Urban J.P. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(Suppl 2):30. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 33.Johnson W.E. Stephan S. Roberts S. The influence of serum, glucose and oxygen on intervertebral disc cell growth in vitro: Implications for degenerative disc disease. Arthritis Res Ther. 2008;10:R46. doi: 10.1186/ar2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mobasheri A. Richardson S. Mobasheri R. Shakibaei M. Hoyland J.A. Hypoxia inducible factor-1 and facilitative glucose transporters glut1 and glut3: Putative molecular components of the oxygen and glucose sensing apparatus in articular chondrocytes. Histol Histopathol. 2005;20:1327. doi: 10.14670/HH-20.1327. [DOI] [PubMed] [Google Scholar]
- 35.Ohara H. Tamayama T. Maemura K. Kanbara K. Hayasaki H. Abe M. Watanabe M. Immunocytochemical demonstration of glucose transporters in epiphyseal growth plate chondrocytes of young rats in correlation with autoradiographic distribution of 2-deoxyglucose in chondrocytes of mice. Acta Histochem. 2001;103:365. doi: 10.1078/0065-1281-00604. [DOI] [PubMed] [Google Scholar]
- 36.Shikhman A.R. Brinson D.C. Valbracht J. Lotz M.K. Cytokine regulation of facilitated glucose transport in human articular chondrocytes. J Immunol. 2001;167:7001. doi: 10.4049/jimmunol.167.12.7001. [DOI] [PubMed] [Google Scholar]
- 37.Mobasheri A. Neama G. Bell S. Richardson S. Carter S.D. Human articular chondrocytes express three facilitative glucose transporter isoforms: Glut1, glut3 and glut9. Cell Biol Int. 2002;26:297. doi: 10.1006/cbir.2001.0850. [DOI] [PubMed] [Google Scholar]
- 38.Schipani E. Ryan H.E. Didrickson S. Kobayashi T. Knight M. Johnson R.S. Hypoxia in cartilage: Hif-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfander D. Cramer T. Schipani E. Johnson R.S. Hif-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci. 2003;116(Pt 9):1819. doi: 10.1242/jcs.00385. [DOI] [PubMed] [Google Scholar]
- 40.Potier E. Ferreira E. Meunier A. Sedel L. Logeart-Avramoglou D. Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007;13:1325. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- 41.Wuertz K. Godburn K. Neidlinger-Wilke C. Urban J. Iatridis J.C. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine. 2008;33:1843. doi: 10.1097/BRS.0b013e31817b8f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauck R.L. Yuan X. Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Yang F. Leung V.Y. Luk K.D. Chan D. Cheung K.M. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther. 2009;17:1959. doi: 10.1038/mt.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grinnemo K.H. Mansson A. Dellgren G. Klingberg D. Wardell E. Drvota V. Tammik C. Holgersson J. Ringden O. Sylven C. Le Blanc K. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127:1293. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 45.Niemeyer P. Vohrer J. Schmal H. Kasten P. Fellenberg J. Suedkamp N.P. Mehlhorn A.T. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10:784. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]
- 46.MacLaren R.E. Pearson R.A. MacNeil A. Douglas R.H. Salt T.E. Akimoto M. Swaroop A. Sowden J.C. Ali R.R. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 47.Maroudas A. Stockwell R.A. Nachemson A. Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J. Anat. 1975;120:113. [PMC free article] [PubMed] [Google Scholar]
- 48.Kim A.J. Adkisson H.D. Wenland M. Seyedin M. Berven S. Lotz J.C. Juvenile chondrocytes may facilitate disc repair. Open Tissue Eng Regen Med J. 2010;3:28. [Google Scholar]
- 49.Johnson W.E. Evans H. Menage J. Eisenstein S.M. El Haj A. Roberts S. Immunohistochemical detection of schwann cells in innervated and vascularized human intervertebral discs. Spine (Phila Pa 1976) 2001;26:2550. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- 50.Lemons M.L. Sandy J.D. Anderson D.K. Howland D.R. Intact aggrecan and chondroitin sulfate-depleted aggrecan core glycoprotein inhibit axon growth in the adult rat spinal cord. Exp Neurol. 2003;184:981. doi: 10.1016/S0014-4886(03)00383-2. [DOI] [PubMed] [Google Scholar]
- 51.Lotz J.C. Animal models of intervertebral disc degeneration: Lessons learned. Spine. 2004;29:2742. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- 52.Masuda K. Lotz J.C. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng Part B Rev. 2010;16:147. doi: 10.1089/ten.teb.2009.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fermor B. Weinberg J.B. Pisetsky D.S. Misukonis M.A. Banes A.J. Guilak F. The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. J Orthop Res. 2001;19:729. doi: 10.1016/S0736-0266(00)00049-8. [DOI] [PubMed] [Google Scholar]
- 54.Ragan P.M. Badger A.M. Cook M. Chin V.I. Gowen M. Grodzinsky A.J. Lark M.W. Down-regulation of chondrocyte aggrecan and type-ii collagen gene expression correlates with increases in static compression magnitude and duration. J Orthop Res. 1999;17:836. doi: 10.1002/jor.1100170608. [DOI] [PubMed] [Google Scholar]
- 55.Adkisson H.D., 4th Martin J.A. Amendola R.L. Milliman C. Mauch K.A. Katwal A.B. Seyedin M. Amendola A. Streeter P.R. Buckwalter J.A. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38:1324. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buckwalter J.A. Mankin HJ. Articular cartilage 2. Degeneration and osteoarthrosis, repair, regeneration and transplantation. J Bone Joint Surg Am. 1997;79:612. [Google Scholar]
- 57.Knutsen G. Drogset J.O. Engbretsen L. Grontveldt T. Isaksen V, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89:2105. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 58.Mithoefer K. McAdams T. Williams R.S. Kreuz P.C. Mandelbaum BR. Clinical efficacy of microfracture technique for articular cartilage repair in the knee. An evidence-based systematic analysis. Am J Sports Med. 2009;37:2053. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]