Abstract
Human umbilical cord mesenchymal stem cells (hUCMSCs) avoid the invasive procedure required to harvest bone marrow MSCs. The addition of collagen fibers into self-setting calcium phosphate cement (CPC) may increase the scaffold strength, and enhance cell attachment and differentiation. The objectives of this study were to develop a novel class of collagen-CPC composite scaffolds, and to investigate hUCMSC attachment, proliferation, and osteogenic differentiation on collagen-CPC scaffolds for the first time. Collagen fibers in CPC improved the load-bearing capability. Flow cytometry showed that the hUCMSCs expressed cell surface markers characteristic of MSCs, and were negative for hematopoietic and endothelial cell markers. hUCMSCs proliferated rapidly in all CPC composite scaffolds, with cell number increasing by sevenfold in 8 days. Cellular function was enhanced with collagen fibers in CPC scaffolds. Cell density increased from (645±60) cells/mm2 on CPC with 0% collagen, to (1056±65) cells/mm2 on CPC with 8% collagen (p<0.05). The actin stress fibers inside the hUCMSCs were stained, and the fluorescence intensity was doubled when the collagen in CPC was increased by 0% to 8%. RT-PCR showed that hUCMSCs on CPC with collagen had higher osteogenic expression than those on CPC without collagen. Alizarin Red S staining revealed a great increase in mineralization by hUCMSCs on CPC with collagen than that without collagen. In conclusion, hUCMSCs showed excellent proliferation, differentiation, and synthesis of bone minerals in collagen-CPC composite scaffolds for the first time. The novel hUCMSC-seeded collagen-CPC construct with superior cell function and load-bearing capability is promising to enhance bone regeneration in a wide range of orthopedic and craniofacial applications.
Introduction
Stem cell-based approaches are highly promising for tissue engineering and regenerative medicine.1–6 Bone is the most implanted tissue after blood.7 Nearly seven million bone fractures occur annually in the United States alone.8 These numbers are predicted to increase dramatically as the world population ages.9–11 Bone marrow-derived mesenchymal stem cells (BMSCs) have good potential for bone and other tissue regenerations.1,3,12,13 The two caveats of human BMSCs (hBMSCs) are that they require an invasive procedure to harvest, and their potency decreases due to aging and disease. Human umbilical cord mesenchymal stem cells (hUCMSCs) are a relatively new stem cell source with multipotent characteristics, and can differentiate into adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells.14–19 hUCMSCs have several advantages: (a) Umbilical cords are a medical waste, can be collected at a low cost, and are inexhaustible; (b) they can be harvested without an invasive procedure required for hBMSCs, and without the ethical controversies of human embryonic stem cells (hESCs); (c) hUCMSCs are a primitive MSC population that express certain hESC markers and exhibit high plasticity and developmental flexibility16; and (d) hUCMSCs appear to cause no immunorejection and are not tumorigenic in animal studies.16,20 Recently, a few studies have investigated hUCMSCs for bone tissue engineering, in which hUCMSCs were cultured with polystyrene,17 polymer scaffolds,19 collagen scaffolds,21 nanofibers,22 and calcium phosphate (CaP) scaffolds.23,24
Scaffolds are needed to maintain the volume and to support external stresses. CaP bioceramics such as hydroxyapatite (HA) are important for bone repair because of their similarity to the bone minerals.25–29 Bone is comprised of collagen (mainly type I) and nano-HA crystals. Hence, synthetic collagen-HA composites are promising in mimicking and replacing bone.30–33 Collagen-HA composites can potentially combine the best of both worlds, with the HA ceramic providing bioactivity and mechanical stiffness; whereas with the collagen fibers improving toughness and cell adhesion. The collagen-CaP composites in previous studies are usually performs that require implantation, and are not moldable or injectable.31–33 Efforts were also made to incorporate collagen into calcium phosphate cements (CPCs).34,35
CPCs are injectable and moldable, and can set in situ to provide intimate adaptation to complex defects.36–39 CPC is comprised of a mixture of tetracalcium phosphate [TTCP: Ca4(PO4)2O] and dicalcium phosphate anhydrous (DCPA: CaHPO4), which forms resorbable HA.36,40,41 Due to its excellent osteoconductivity, CPC was approved in 1996 by the Food and Drug Administration for repairing craniofacial defects, thus becoming the first CPC available for clinical use.40 However, the brittleness of CPC limits its use to only nonload-bearing areas.40,41 Several studies incorporated collagen into CPCs.42–45 In one study, a CPC-collagen composite showed good resorption and bone integration in vivo in minipigs.44 Another study fabricated collagen-CPC scaffolds for the nonviral delivery of a plasmid encoding osteoinductive protein bone morphogenetic proteins such as BMP-7.45 In a recent study, type I bovine collagen was incorporated into an injectable CPC, which not only improved the CPC toughness, but also increased the MC3T3-E1 osteoblast attachment.35 However, stem cells have not been seeded on the collagen-CPC construct. Osteogenic differentiation and mineralization of cells on collagen-CPC have not been investigated.
The objectives of this study were to develop collagen-CPC-hydrogel microbead scaffold, and to investigate the effects of collagen volume fraction in CPC on hUCMSC proliferation, osteogenic differentiation, and mineral synthesis for the first time. Alginate microbeads were incorporated into the collagen-CPC paste, because the microbeads could potentially deliver growth factors, and the microbeads could subsequently degrade and create macropores in CPC. The hypotheses were as follows: (1) Collagen fibers will strengthen and toughen the CPC-microbead scaffold; (2) Collagen in CPC will enhance hUCMSC attachment and proliferation; (3) Collagen in CPC will facilitate the osteogenic differentiation and bone mineral synthesis of hUCMSCs.
Materials and Methods
hUCMSC culture
hUCMSCs were purchased from ScienCell Laboratories. They were derived from the Wharton's Jelly in umbilical cords of healthy babies and harvested as previously described.14,19 The use of hUCMSCs was approved by the University of Maryland. Cells were cultured in a low-glucose Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Invitrogen) (control media). At 80%–90% confluence, hUCMSCs were detached and passaged. Passage 4 cells were used for all experiments. The osteogenic media contained 100 nM dexamethasone, 10 mM β-glycerophosphate, 0.05 mM ascorbic acid, and 10 nM 1α,25-Dihydroxyvitamin (Sigma).19,46
Immunophenotyping of hUCMSCs
To analyze the cell-surface expression of characteristic mesenchymal protein markers, surface antigens were detected by using the flow cytometry method.47 Cells were harvested using a TrypLE™ Express (Gibco, Invitrogen), and 10,000 cells were suspended in 50 μL phosphate buffer saline (PBS) containing 0.1% bovine serum albumin (BSA) (Sigma). The cells were separately labeled with antibodies for 30 min at 4°C in dark and washed with PBS containing 0.1% BSA. Antibodies against the following cell surface markers were used: fluorescein isothiocyanate-conjugated rat anti-human antigen for CD29, CD31, CD34, CD44, CD45, CD105, HLA-class 1, HLA-DR (Molecular Probes, Invitrogen), and CD34 (BD Biosciences). The acquisition was performed on an FACScan flow cytometer (Becton Dickinson). The data were analyzed with FlowJo software (TreeStar). hUCMSCs with no labeling were used as control.
Fabrication of collagen-CPC composite scaffolds
TTCP was synthesized using equimolar amounts of DCPA and calcium carbonate (J.T. Baker). The reactant was ground in a ball mill (Retsch PM4) and sieved to obtain TTCP particles of 1 to 80 μm, with a median of 17 μm, following previous studies.36,40,48 DCPA was ground in the ball mill to obtain a median particle size of 1 μm. TTCP and DCPA powders were mixed to form the CPC powder. Chitosan lactate (VANSON) was mixed with water at a chitosan/(chitosan+water) mass fraction of 15% to form the liquid.48 To reinforce the CPC scaffold, a resorbable suture (Vicryl, polyglactin 910; Ethicon) was cut to filaments of 3 mm length and mixed with CPC paste at a fiber volume fraction (polyglactin fiber volume/total specimen volume) of 25%.
Alginate hydrogel microbeads were fabricated and mixed with the CPC paste. These microbeads were used, because they could potentially encapsulate and deliver growth factors. The present study focused on fabricating the collagen-CPC-microbead scaffold, its mechanical properties, and hUCMSC response. The incorporation of growth factors in the microbeads would be the topic of a separate study. A 1.2% sodium alginate solution was prepared by dissolving alginate (ProNova) in saline.46 The alginate solution was fed to a bead-generating device (Var J1; Nisco), which produced alginate microbeads with a mean diameter of 207 μm.46 The microbeads were mixed with CPC paste at a microbead volume fraction (microbead volume/total specimen volume) of 40%.
Type I collagen fibers obtained from bovine Achilles tendon (Sigma) were incorporated into CPC. A previous study measured the same bottle of collagen fibers using scanning electron microscopy (SEM), and reported collagen fiber diameters ranging from ∼0.1 to 3 μm, and lengths from about 20 to 100 μm.35 It also reported that the exact length was difficult to measure, because the collagen fibers were flexible and zigzag.35 Three different collagen volume fractions were used in CPC (collagen volume/total specimen volume): 0%, 4%, and 8%. The collagen fibers were randomly mixed into the CPC paste using a spatula. The CPC powder to liquid mass ratio of 2:1 yielded a flowable paste. Hence, the collagen fibers were well mixed with the paste. Collagen volume fraction of 10% or higher were not used, because they yielded pastes that were relatively dry and not suitable for injectable applications. The powder, liquid, and fiber mixing procedures were the same as those in a previous study, which showed relatively uniform and random collagen fiber distributions in CPC based on SEM observations.35
In this study, all CPC specimens contained 25% polyglactin fibers and 40% alginate microbeads, in addition to various collagen fiber volume fractions. For abbreviation, CPC containing 25% polyglactin fiber and 40% microbeads is referred to as CPC composite; when collagen is added, the material is referred to as collagen-CPC composite.
Scaffold mechanical properties
The collagen-CPC paste was placed into a mold of 3×4×25 mm. The specimens were incubated at 37°C for 4 h, and then the set specimens were demolded and immersed in distilled water at 37°C for 20 h before testing, following previous studies.36,46,48 It should be noted that although 4 h used to be on the safe side for demolding the specimen, a previous study reported a CPC setting time of being ∼8 min when the chitosan liquid was used.48 The specimens were then fractured in three-point flexure with a span of 20 mm at a crosshead speed of 1 mm/min on a Universal Testing Machine (100 N capacity load cell; 5500R, MTS). Flexural strength, elastic modulus, and work-of-fracture (toughness) were measured.48 The specimen was tested immediately after being taken out of the immersion and was thoroughly wet when it was fractured. Separate specimens were immersed in the osteogenic media for 1, 8, and 21 days, and then their mechanical properties were measured.
hUCMSC attachment, viability, and morphology
The collagen-CPC composite paste was filled into a circular Teflon mold with a diameter of 12 mm and a thickness of 1.5 mm. The specimens were incubated at 37°C for 4 h, and then demolded and immersed in water at 37°C for 20 h. The disk specimens were sterilized in an ethylene oxide sterilizer (Andersen) for 12 h and then degassed for 7 days.
Each well of a 12-well plate containing a scaffold disk received 150,000 cells in 2 mL of osteogenic media. The media was changed every 2 days. After 1, 4, and 8 days, the constructs were washed in Tyrode's Hepes buffer, stained with a live/dead kit (Invitrogen), and viewed by epifluorescence microscopy (TE2000S; Nikon). Three randomly chosen fields of view were photographed from each disk. Five disks yielded 15 photos for each material at each time point. NLive is the number of live cells, and NDead is the number of dead cells. The percentage of live cells is P=NLive/(NLive+NDead). Live cell density, D, is the number of live cells attached to the specimen divided by the surface area A: D=NLive/A.35
hUCMSCs cultured on CPC composite scaffolds were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer pH 7.4, dehydrated with a graded series of ethanol (25%–100%), rinsed with hexamethyldisilazane, sputter-coated with gold, and examined under the SEM. The working distance was 9.7 mm, and the voltage was 5.0 kV.
Immunofluorescence of actin fibers in hUCMSCs attached on scaffolds
The actin fibers in the cell cytoskeleton were examined to determine whether the addition of collagen in CPC would enhance cell attachment and increase the amount of actin stress fibers. hUCMSC constructs after 1 day culture were washed with PBS, fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 for 5 min, and blocked with 0.1% BSA for 30 min.49 An actin cytoskeleton and focal adhesion staining kit (Chemicon) was used, which stained actin fibers into a red color. After incubating the construct with diluted (1:400) TRITC-conjugated phalloidin, cell nuclei were labeled with 4′,6-diamidino-2-phenylindole to reveal the nuclei in blue. Fluorescence microscopy (TE2000S; Nikon) was used to examine the specimens. The actin fluorescence intensity is increased when there is a higher density of actins stress fibers. The fluorescence intensity of actin fibers in hUCMSCs was measured via a NIS-Elements BR software (Nikon). Measurements were made at a total of 30 positions on each scaffold for the CPC-microbead scaffolds with 0%, 4% and 8% collagen, to obtain mean and standard deviation values.
hUCMSC osteogenic differentiation
Quantitative real-time reverse transcription polymerase chain reaction measurement (qRT-PCR, 7900HT; Applied Biosystems) was performed. The constructs were cultured in osteogenic media for 1, 4, and 8 days.50 Six disks were used for each material at each time point (n=6). The total cellular RNA on the scaffolds were extracted with TRIzol reagent (Invitrogen) and reverse transcribed into cDNA. TaqMan gene expression kits were used to measure the transcript levels of the proposed genes on human alkaline phosphatase (ALP, Hs00758162_m1), osteocalcin (OC, Hs00609452_g1), collagen type I (Coll I, Hs00164004), Runx2 (Hs00231692_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Hs99999905). Relative expression for each target gene was evaluated using the 2−ΔΔCt method.51 The Ct values of target genes were normalized by the Ct of the TaqMan human housekeeping gene GAPDH to obtain the ΔCt values. These values were subtracted by the Ct value of the hUCMSCs cultured on tissue culture polystyrene (TCPS) in the control media for 1 day (the calibrator) to obtain the ΔΔCt values.46,50
hUCMSC mineralization
Staining of the minerals synthesized by hUCMSCs was performed to further confirm osteogenic differentiation. First, hUCMSCs were seeded on TCPS, and cultured in control media or osteogenic media for 14 days. Alizarin Red S (ARS) staining was used to visualize bone mineralization.47 The adherent cells were washed with PBS, fixed with 10% formaldehyde, and stained with ARS (Millipore) for 5 min, which stained calcium-rich deposits by cells into a red color. After staining, the cell-scaffold construct was washed with deionized water for about twenty times until the used water was clear with no dye extraction from the sample. Each wash was done with gentle rocking in a rotator (model 4630; Barnstead) for 5 min. The purpose of this step was to establish the ARS staining for hUCMSCs on TCPS.
Second, hUCMSCs were seeded on CPC composite disks with 0%, 4%, and 8% collagen. The cells on the disks were cultured in osteogenic media for 14 and 21 days and then stained with ARS to visualize mineralization by the hUCMSCs. In addition, an osteogenesis assay kit (Millipore) was used to extract the stained minerals and measure the Alizarin Red concentration at OD405, following the manufacturer's instructions. Briefly, the cell-scaffold construct was incubated in 10% acetic acid for 30 min with shaking. Then, the cells and acetic acid were collected, heated at 85°C for 10 min, kept on ice for 5 min, and centrifuged for 15 min. Ammonium hydroxide was added to the supernatant to obtain a pH in the range of 4.1 to 4.5, and Alizarin Red concentration was measured at OD405. Alizarin Red standard curve was done with a known concentration of the dye. Control scaffolds with the same compositions, but without hUCMSC seeding, were also measured. The control's Alizarin Red concentration was subtracted from the Alizarin Red concentration of the corresponding scaffold with hUCMSCs, to yield the net mineral concentration synthesized by the cells. The purpose was to examine the effect of collagen content in CPC on mineral synthesis by the hUCMSCs.
One-way and two-way ANOVA were performed to detect significant (α=0.05) effects of the variables. Tukey's multiple comparison procedures were used to group and rank the measured values, and Dunn's multiple comparison tests were used on data with non-normal distribution or unequal variance, both at a family confidence coefficient of 0.95.
Results
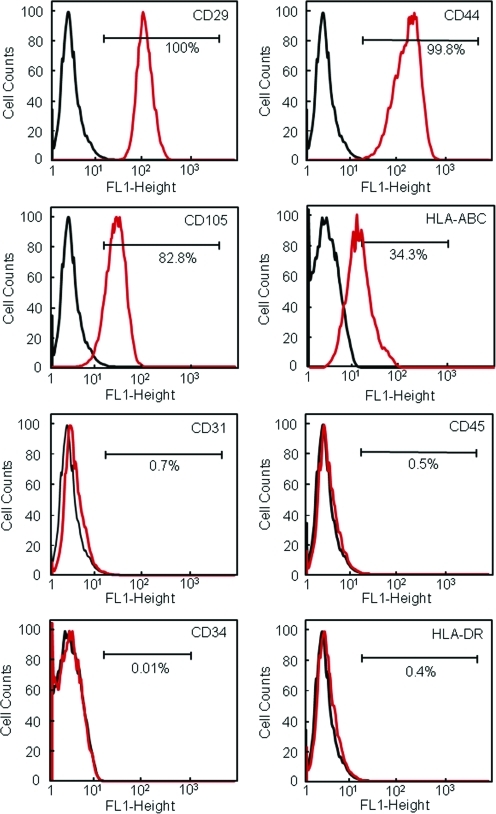
To characterize the hUCMSC population, the expression of surface markers was investigated by flow cytometry (Fig. 1). hUCMSCs expressed high levels of adhesion markers (CD29 and CD44) and MSC-specific antigen CD105 (also called SH2). Cells were positive for HLA-class I (HLA-ABC), and negative for HLA-Class II (HLA-DR). This is characteristic for MSCs. Cells did not express endothelial marker (CD31) or hematopoietic linage markers (CD34 and CD45). Hence, the hUCMSCs expressed a number of cell surface markers characteristic of MSCs, and were negative for typical hematopoietic and endothelial cell markers.
FIG. 1.
Immunophenotyping of hUCMSCs. Flow cytometry showed that the hUCMSCs expressed a number of cell surface markers characteristic of MSCs, and were negative for typical hematopoietic and endothelial cell markers. hUCMSC, human umbilical cord mesenchymal stem cell. Color images available online at www.liebertonline.com/tea
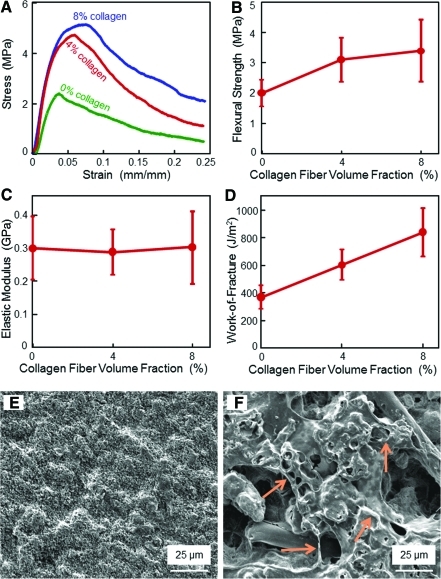
CPC composite scaffolds had a noncatastrophic failure, with the stress increasing with collagen fibers (Fig. 2A). In (B), flexural strength at 8% collagen was (3.4±1.0 MPa), higher than (2.0±0.4) MPa at 0% collagen (p<0.05). Elastic moduli in (C) were similar with each other (p>0.1). In (D), work-of-fracture (toughness) was increased from (368±80) J/m2 at 0% collagen, to (840±173) J/m2 at 8% collagen (p<0.05). A typical CPC surface with 0% collagen is shown in (E), and a rougher surface with 8% collagen is shown in (F). Arrows in (F) indicate the increased roughness, and these features appeared to be collagen fibers coated or mingled with CPC paste. These features were absent in specimens without collagen fibers.
FIG. 2.
Mechanical properties versus collagen fibers in CPC scaffold: (A) Stress-strain curves, (B) flexural strength, (C) elastic modulus, (D) work-of-fracture (toughness) (mean±sd; n=6). CPC composite scaffolds showed a noncatastrophic failure, with the load-bearing capability increasing with more collagen fibers. (E) SEM micrograph of the surface of CPC with 0% collagen. (F) Surface features of CPC composite containing 8% collagen fibers, with arrows, indicate features that were absent in specimens without collagen fibers. CPC composite specimens with collagen fibers had noticeably rougher surfaces than those without collagen fibers. CPC, calcium phosphate cement; SEM, scanning electron microscopy. Color images available online at www.liebertonline.com/tea
Immersion of the specimens in the osteogenic media for 21 days did not significantly degrade the mechanical properties of the composites. The flexural strength for CPC composite with 0% collagen (mean±sd; n=6) was (2.0±0.4) MPa at 1 day, similar to (2.1±0.3) at 8 days, and (1.8±0.5) MPa at 21 days (p>0.1). With 8% collagen in CPC, the strength was (3.4±1.0) MPa at 1 day, similar to (3.4±0.8) at 8 days, and (3.0±0.1) MPa at 21 days (p>0.1). The strength for CPC with 8% collagen fibers was significantly higher than that without collagen (p<0.05).
In Figure 3, hUCMSCs attaching to CPC composite scaffolds proliferated well from 1 to 8 days (A–F). In (G) and (H), a noticeable difference was the presence of mineral nodules (indicated by arrows) in (H) with 8% collagen, but the absence of such nodules in (G) with 0% collagen, indicating the effect of collagen in CPC on cell differentiation and mineralization. Extensive meshwork of cytoplamic extensions was observed that firmly attached to the fibers (I and J). In general, the SEM observations revealed an increased formation of extracellular matrix by the hUCMSCs on CPC with collagen, compared with CPC without collagen.
FIG. 3.
hUCMSCs attaching to CPC-microbead composite with 0%, 4%, and 8% collagen. In (A–F), live cells were stained green, and live cell numbers increased from 1 to 8 days. Dead cells were stained red and were few on all scaffolds. In (G) and (H), hUCMSCs were cultured on CPC-microbead scaffold with 0% and 8% collagen fibers, respectively. Thick extracellular matrix with numerous mineral nodules were observed (arrows). The cells had developed long cytoplasmic extensions and cell-cell junctions, as shown in (I; indicated by arrows). Cells readily adhered to the fibers in CPC, as shown in (J). SEM observations revealed an increased formation of extracellular matrix by the hUCMSCs on CPC with collagen, compared with CPC without collagen. Color images available online at www.liebertonline.com/tea
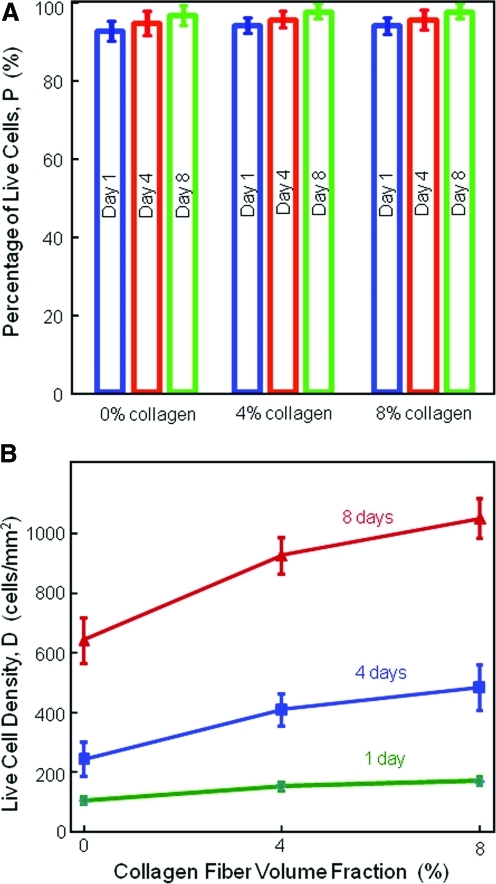
Figure 4 plots the percentage of live cells and cell density. In (A), the percentages of live cells were above 90%, suggesting a good cytocompatibility of CPC matrices. In (B), hUCMSCs increased in numbers on all CPC scaffolds. At 8% collagen, the cell density increased from (173±14) cell/mm2 at 1 day, to (487±77) cell/mm2 at 4 days, and (1056±65) cell/mm2 at 8 days (p<0.05). The cell number was increased by 7.7 times in 8 days. Increasing the collagen content in CPC increased cell proliferation. At 8 days, the cell density increased from (645±60) cells/mm2 at 0% collagen in CPC, to (1056±65) cells/mm2 at 8% collagen in CPC (p<0.05).
FIG. 4.
Percentage of live cells and cell density (mean±sd; n=5). In (A), the percentage of live cells was above 90% for all constructs at all time periods, suggesting a good cytocompatibility for CPC matrices. In (B), hUCMSCs proliferated well on all CPC scaffolds. The cell number was increased by 7.7 times in 8 days. Increasing the collagen content in CPC significantly increased the hUCMSC proliferation. Color images available online at www.liebertonline.com/tea
Examination of actin stress fibers that are related to initial cell attachment confirmed that collagen-CPC scaffold had greater cell attachment than the CPC without collagen (Fig. 5). The red fluorescence intensified when the collagen in CPC was increased from 0% to 8% (A–C), thus indicating an increased number of actin stress fibers. In (D–F), the cell nuclei were fluoresced blue. The actin fiber fluorescence intensity, proportional to the amount of actin fibers in the hUCMSCs, increased from (12.9±0.6) at 0% collagen to (22.2±0.9) at 8% collagen (p<0.05).
FIG. 5.
Fluorescence of actin stress fibers in hUCMSCs. In (A–C), the actin stress fibers in the hUCMSCs were stained red. The red color was increasingly brighter and denser when the collagen in CPC was increased from 0% to 8%. In (D–F), the cell nuclei (blue fluorescence) indicated the location and distribution of hUCMSCs on the scaffolds. A higher magnification photo in (G) shows the actin fibers as the red lines. (H) Actin fiber fluorescence intensity (mean±sd; n=5). Color images available online at www.liebertonline.com/tea
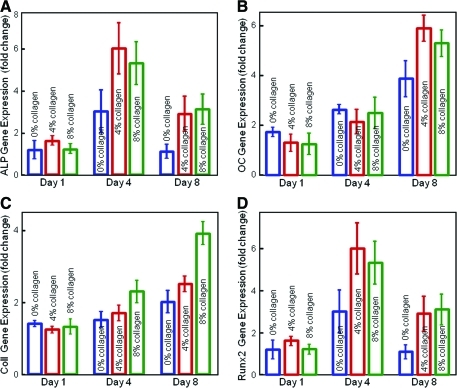
In Figure 6, the ALP gene expression was low at 1 day, peaked at 4 days, and then decreased at 8 days. The OC expression was greatly increased at 8 days. Collagen I also increased at 8 days, whereas Runx2 peaked at 4 days. At 4 and 8 days, hUCMSCs on CPC with collagen fibers had significantly higher osteogenic gene expressions than those on CPC without collagen (p<0.05).
FIG. 6.
RT-PCR of osteogenic differentiation of hUCMSCs. (A) ALP, (B) OC, (C) collagen I, and (D) Runx2 gene expressions (mean±sd; n=6). The ALP expression was low at 1 day, greatly increased at 4 days, and then decreased at 8 days. OC increased at 8 days. Collagen I also increased at 8 days, whereas Runx2 peaked at 4 days. hUCMSCs on CPC with collagen had higher gene expression peaks than those on CPC without collagen (p<0.05). ALP, alkaline phosphatase; OC, osteocalcin. Color images available online at www.liebertonline.com/tea
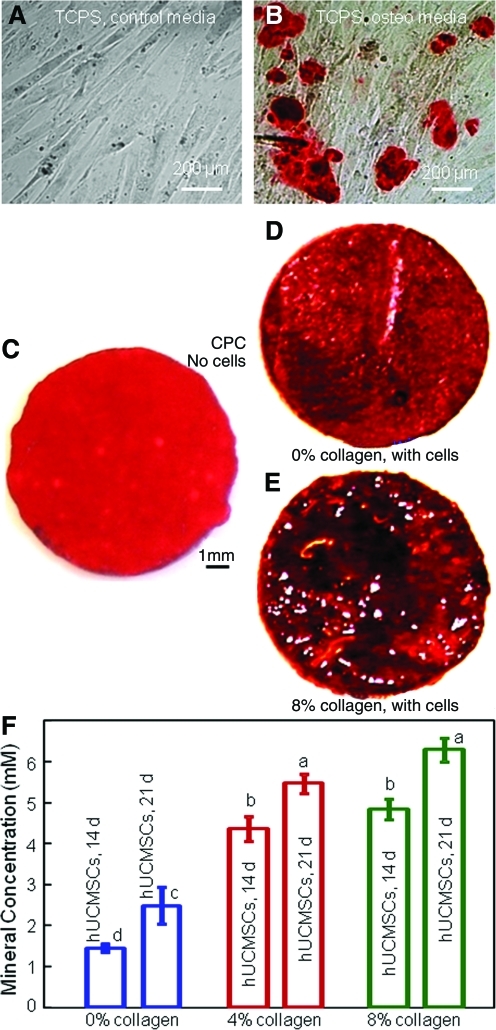
Figure 7A and B used control media and osteogenic media, respectively, for hUCMSCs on TCPS for 14 days. ARS stains calcium minerals into a red color. hUCMSCs in osteogenic media showed significant mineral staining, whereas that in control media had none. Hence, there was indeed mineral synthesis by the hUCMSCs in the osteogenic media.
FIG. 7.
Alizarin Red S (ARS) staining of mineral synthesis by hUCMSCs. hUCMSCs were cultured on TCPS for 14 days in (A) control media, and (B) osteogenic media. ARS stains minerals into a red color. (C) ARS staining of CPC control disk with no cells immersed in the osteogenic media for 21 days. ARS staining of hUCMSCs in osteogenic media on: (D) CPC with 0% collagen for 21 days, (E) CPC with 8% collagen for 21 days. The red staining became increasingly thicker and denser due to cell seeding and collagen fibers in CPC. (F) Calcium mineral concentration synthesized by the hUCMSCs was measured by an osteogenesis assay (mean±sd; n=3). Mineral concentration increased with increasing collagen content in CPC. Dissimilar letters at the bars indicate values that are significantly different (p<0.05). TCPS, tissue culture polystyrene. Color images available online at www.liebertonline.com/tea
As shown in Figure 7C, ARS staining yielded a red color for CPC immersed in the osteogenic media for 21 days with no cells, because CPC consisted of hydroxyapatite. However, when hUCMSCs were seeded on CPC with (D) 0%, and (E) 8% collagen and cultured in osteogenic media for 21 days, the red staining became much thicker and denser. There was a layer of new mineral matrix synthesized by the cells covering the CPC, and the mineral staining increased with increasing the collagen amount in CPC. The thick matrix mineralization formed in the cell-scaffold construct covered not only the top surface, but also the peripheral areas at the sides of the construct. In (F), the mineral synthesized by the hUCMSCs increased with collagen fraction in CPC (p<0.05). These results indicate that hUCMSCs seeded on collagen-CPC scaffolds successfully underwent osteogenic differentiation and mineralization, which were enhanced by CPC with 4% and 8% collagen than CPC with 0% collagen.
Discussion
The current study investigated hUCMSC seeding on a novel collagen-CPC scaffold containing hydrogel microbeads, and determined the effects of collagen in CPC on the attachment, viability, differentiation, and mineralization of hUCMSCs for the first time. Previous studies showed that collagen-HA composites had several advantages. Collagen-HA composites are biomimetic and enhance osteogenesis. One study showed that although HA and collagen alone enhanced osteoblast differentiation, when they were combined into a composite they accelerated osteogenesis.52 The specific amino-acid sequence (Arg-Gly-Asp) that type I collagen contains can be recognized by osteoblast membrane receptors such as integrins.7 Endothelial cells, needed to generate blood vessels, can also attach to collagen type I. Osteogenic cells adhered better to collagen surfaces than to poly-L-lactic acid (PLLA) and polyglycolic acid implants.53 Collagen fibers can toughen the brittle HA, whereas HA can offer rigidity to a spongy collagen that can lose shape under load. Further, collagen-HA composites are biocompatible in human and animal studies.30,54 Type I collagen triggers immune reactions in only a low percentage of people, and a simple serologic test can determine whether a patient has it.55,56 In addition, collagen and HA implants significantly inhibited the growth of bacteria pathogens that cause infections, compared with polylactic-co-glycolic acid implants.57 However, for a prefabricated bioceramic implants such as HA to fit into a bone cavity, the surgeon needs to machine the graft to the desired shape or carve the surgical site, thus leading to increases in bone loss, trauma, and surgical time.9 Although previous collagen-HA and other collagen-CaP implants are generally performs, the collagen-CPC paste of the current study is injectable, can be shaped, and then harden in situ, which can be especially advantageous for use in complex bone cavities and craniofacial repairs where easy contouring and esthetics are important.
There were only a few previous studies in which collagen was added to CPCs.34,35,42–45 One study showed a reduction in strength due to collagen addition,42 whereas another study obtained a decrease in cell activities in vitro after collagen incorporation.34 Further, previous studies on collagen addition in CPCs have not reported on stem cell seeding and osteogenic differentiation. This was investigated for the first time in the current study. The present study showed that adding collagen fibers significantly improved the mechanical properties of CPC. Flexural strength was increased by 70% with the addition of 8% collagen in CPC. The elastic modulus was not significantly increased. This was likely because the collagen fibers were flexible and not stiff, and, hence, the CPC matrix dominated the elastic modulus of the scaffold. Work-of-fracture (toughness) was more than doubled due to collagen reinforcement. A previous study showed that the collagen fibers were well mixed and bonded with the CPC matrix.35 The current study focused on scaffold reinforcement as well as hUCMSC attachment and osteogenic differentiation, without measuring the porosity; however, the porosity did appear to increase with collagen fiber addition into the CPC (Fig. 2F). Although the present study focused on mechanical properties and hUCMSC response, the microbeads in CPC could potentially encapsulate and deliver growth factors, which will be investigated in a separate study. The mechanical properties of CPC are important, because in vivo loading may result in scaffold failure, or a loss of growth factors by convective transport. In a previous study, the protein was rapidly purged out of a collagen carrier under stresses in vivo, because the collagen sponge had no load-bearing capability.58 Therefore, the mechanical properties of the collagen-CPC scaffold would be important to provide a matrix for cell function, while maintaining the volume and supporting the external stresses. For comparison, a previous study reported an injectable polymeric carrier for cell delivery that had a strength of 0.7 MPa.59 Hygrogels for cell delivery had a strength of about 0.1 MPa.60,61 Although these systems are useful for nonload-bearing applications, their strengths are much lower than the cancellous bone's tensile strength of 3.5 MPa.62 The novel collagen-CPC containing 40% microbeads had a flexural strength of 3.4 MPa, which may enable it to be used in a wide range of moderate load-bearing orthopedic and craniofacial applications.
Incorporation of collagen fibers in CPC enhanced the hUCMSC attachment. hUCMSCs are a relatively new MSC source that avoids the invasive procedure needed to harvest bone marrow MSCs. Flow cytometry analysis of cell surface protein markers confirmed that the hUCMSCs had surface markers characteristic of MSCs, and without expressing surface markers for hematopoietic and endothelial progenitor cells. hUCMSCs seeded on collagen-CPC composite showed excellent viability. The addition of collagen in CPC increased the synthesis of the extracellular matrix with the formation of numerous mineral nodules (Fig. 3H). The addition of collagen increased the cell attachment and proliferation, thus resulting in a cell density at 8 days being 70% higher on CPC with 8% collagen, than that on CPC with 0% collagen. Actin stress fibers were numerous in hUCMSCs on CPC scaffolds. These actin stress fibers anchor to the cell membrane at locations that are frequently connected to the extracellular matrix or the scaffold substrate. These connection sites are called focal adhesions.49,63,64 The focal adhesions are associated with the ends of the actin stress fibers. The attachment of cells to the substrate is via focal adhesions that link the actin cytoskeleton to the extracellular matrix, and the actin stress fibers are closely associated with the focal adhesion sites.63,64 Hence, a higher density of actin stress fibers in the cell cytoskeleton indicates a firmer and stronger link between the cells and the substrate. A higher density of actin stress fibers is beneficial, because it indicates an enhanced morphological stability of the cell cytoskeleton and better adhesion and motility of the cells, which, in turn, indicates a greater suitability of the scaffold matrix for cell attachment and function. Further, the actin cytoskeleton organization helps regulate cellular signal transduction, cell differentiation, and gene expression.63,64 Therefore, a high actin fiber density indicates a well-established communication between the cells and the substrate. In the current study, the fluorescence intensity of the actin stress fibers of hUCMSCs on CPC was nearly doubled when the collagen in CPC was increased from 0% to 8%. Therefore, adding collagen fibers in CPC could enhance the cell attachment and the link between the cells and the scaffold.
Further, adding collagen to CPC enhanced the hUCMSC differentiation and mineral synthesis. At 4 days, the ALP and Runx2 gene expressions of CPC composite with collagen were higher than that on CPC with 0% collagen. ALP and OC are well-known bone markers.17,19,28,50 Runx2 (runt-related transcription factor 2) regulates skeletal development, and it is a crucial transcription gene within the osteogenic phenotype.65 At 8 days, ALP, OC, collagen I, and Runx2 were all higher for hUCMSCs on CPC with 8% collagen than that on CPC without collagen. The time periods of these results are consistent with previous studies. For example, one study showed that the ALP gene expression was minimal at 1 day, peaked at 4 days, and then decreased at 8 days.50 In addition, the previous study showed that the OC expression peaked at 8 days, later than the ALP peak.50 During osteogenic differentiation, the genetic expressions of bone markers such as ALP and OC are upregulated at an early stage of differentiation. This sets off a cascade of events, which lead to the production of the extracellular matrix including bone minerals. Hence, mineralization occurs later than gene expression. Indeed, previous studies found a large increase in calcium content in the in vitro cell cultures between 12 and 21 days.66 Hence, the current study performed ARS staining at 14 and 21 days. The staining color became a darker/thicker red when the collagen in CPC was increased from 0% to 8%. The mineral synthesis by hUCMSCs on CPC with collagen was 2–3-fold higher than that on CPC without collagen. However, it should be noted that there was little difference in the RT-PCR data and mineralization between 4% and 8% of collagens. This indicates that the enhancement of cell differentiation and mineralization may be saturated with 4% of collagen in CPC. In addition, a previous study examined the substance synthesized by the cells via x-ray diffraction. The x-ray pattern matched those of a standard HA.67 A chemical analysis of the cell-synthesized substance yielded a Ca/P molar ratio of 1.35, which was consistent with previously reported Ca/P ratio of 1.39 to 1.41 of mineralization by cells.67 Adding collagen into CPC enhanced the osteogenic differentiation of the hUCMSCs, likely due to two reasons. First, the Arg-Gly-Asp sequence of type I collagen enhanced cell adhesion and function.7 Hence, adding collagen fibers in CPC provided a better environment for the cells to improve cell attachment and proliferation. This better environment could enhance osteogenic differentiation once the cells were stimulated with an osteogenic media. Second, the addition of collagen fibers into CPC also rendered the CPC scaffold surface rougher with a higher surface area (Fig. 2F). These two factors likely contributed to the enhanced osteogenic differentiation and mineralization due to the incorporation of collagen fibers in CPC. The second factor is consistent with a recent study that showed threefolds more bone ingrowth in defects containing carbon-nanotube nanocomposite scaffold, compared with control polymer scaffolds without nanotubes.68 The authors attributed this increase to the high surface area and roughness that may have enhanced cell attachment and stimulated the cells to synthesize the extracellular matrix.68 Regarding cell seeding and macropore formation in CPC, one approach would be to encapsulate the cells in degradable hydrogel microbeads to protect the cells from the paste mixing forces and the CPC setting reaction.46 After setting, the microbeads could degrade to release the cells throughout the CPC scaffold, while concomitantly creating macropores for cell migration and tissue ingrowth. Animal studies are needed to investigate the efficacy of collagen-CPC scaffold with stem cell delivery for bone tissue regeneration.
Conclusions
hUCMSCs were seeded on in situ-setting collagen-CPC composite scaffolds for the first time, and showed a significant increase in attachment, viability, osteogenic differentiation, and mineralization with increasing collagen volume fraction in CPC. The novel collagen-CPC-hydrogel microbead paste can be injected and contoured, and then harden in the bone cavity to form a load-bearing scaffold. Its mechanical strength approached that of cancellous bone, and was much higher than previous injectable polymeric and hydrogel carriers for cell delivery. Collagen incorporation in CPC enhanced hUCMSC attachment, increased actin stress fiber density, and increased cell proliferation and osteogenic differentiation. hUCMSCs showed excellent differentiation with elevated ALP, OC, collagen I, and Runx2 gene expressions, as well as synthesis of bone minerals. Hence, hUCMSC appeared to be a viable stem cell source for bone tissue engineering, which avoids the invasive procedure required to harvest hBMSCs. The hUCMSC-seeded collagen-CPC composite construct with excellent cell function is promising to enhance bone regeneration in a wide range of orthopedic, dental, and craniofacial applications.
Acknowledgments
The authors thank Dr. Michael D. Weir for experimental help and discussions in making the scaffolds. They are indebted to Dr. Ferenc Livak for helping with the flow cytometry analyses, which were performed at the University of Maryland Greenbaum Cancer Center Shared Flow Cytometry Facility. They also thank Drs. L. C. Chow and S. Takagi at the Paffenbarger Research Center, and Dr. Carl G. Simon at the National Institute of Standards and Technology for discussions and help. This study was supported by NIH R01 grants DE14190 and DE17974 (H.H.K.X.), Maryland Stem Cell Fund (H.H.K.X.), and the University of Maryland Dental School.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lavik E. Langer R. Tissue engineering: current state and perspectives. Appl Microbiol Biotech. 2004;65:1. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- 2.Mikos A.G. Herring S.W. Ochareon P. Elisseeff J. Lu H.H. Kandel R. Schoen F.J. Toner M. Mooney D. Atala A. van Dyke M.E. Kaplan D. Vunjak-Novakovic G. Engineering complex tissues. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson P.C. Mikos A.G. Fisher J.P. Jansen J.A. Strategic directions in tissue engineering. Tissue Eng. 2007;13:2827. doi: 10.1089/ten.2007.0335. [DOI] [PubMed] [Google Scholar]
- 4.Mao J.J. Vunjak-Novakovic G. Mikos A.G. Atala A. Regenerative Medicine: Translational Approaches and Tissue Engineering, Chapters 1–3. Boston and London: Artech House; 2007. [Google Scholar]
- 5.Chatterjee K. Lin-Gibson S. Wallace W.E. Parekh S.H. Lee Y.J. Cicerone M.T. Young M.F. Simon C.G., Jr The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials. 2010;31:5051. doi: 10.1016/j.biomaterials.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huebsch N. Arany P.R. Mao A.S. Shvartsman D. Ali A.O. Bencherif S.A. Rivera-Feliciano J. Mooney D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahl D.A. Czermuszka J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43. doi: 10.22203/ecm.v011a06. [DOI] [PubMed] [Google Scholar]
- 8.Praemer A. Furner S. Rice D.P. Musculoskeletal Conditions in the United States. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1999. [Google Scholar]
- 9.Laurencin C.T. Ambrosio A.M.A. Borden M.D. Cooper J.A. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 10.Meinel L. Karageorgiou V. Fajardo R. Snyder B. Shinde-Patil V. Zichner L. Kaplan D.L. Langer R. Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells; effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 11.Benoit D.S.W. Nuttelman C.R. Collins S.D. Anseth K.S. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Yao J. Radin S. Reilly G. Leboy P.S. Ducheyne P. Solution-mediated effect of bioactive glass in poly (lactic-co-glycolic acid)-bioactive glass composites on osteogenesis of marrow stromal cells. J Biomed Mater Res A. 2005;75:794. doi: 10.1002/jbm.a.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao J.J. Giannobile W.V. Helms J.A. Hollister S.J. Krebsbach P.H. Longaker M.T. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H.S. Hung S.C. Peng S.T. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 15.Sarugaser R. Lickorish D. Baksh D. Hosseini M.M. Davies J.E. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 16.Can A. Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 17.Baksh D. Yao R. Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 18.Bailey M.M. Wang L. Bode C.J. Mitchell K.E. Detamore M.S. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 19.Wang L. Singh M. Bonewald L.F. Detamore M.S. Signaling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. J Tissue Eng Regen Med. 2009;3:398. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 20.Weiss M.L. Medicetty S. Bledsoe A.R. Rachakatla R.S. Choi M. Merchav S. Luo Y. Rao M.S. Velagaleti G. Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 21.Schneider R.K. Puellen A. Kramann R. Raupach K. Bornemann J. Knuechel R. Pérez-Bouza A. Neuss S. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodeling in three-dimensional collagen scaffolds. Biomaterials. 2010;3:467. doi: 10.1016/j.biomaterials.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 22.Gauthaman K. Venugopal J.R. Yee F.C. Biswas A. Ramakrishna S. Bongso A. Osteogenic differentiation of human Wharton's jelly stem cells on nanofibrous substrates in vitro. Tissue Eng A. 2011;17:71. doi: 10.1089/ten.TEA.2010.0224. [DOI] [PubMed] [Google Scholar]
- 23.Jäger M. Degistirici O. Knipper A. Fischer J. Sager M. Krauspe R. Bone healing and migration of cord blood-derived stem cells into a critical size femoral defect after xenotransplantation. J Bone Miner Res. 2007;22:1224. doi: 10.1359/jbmr.070414. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L. Burguera E.F. Xu H.H.K. Amin N. Ryou H. Arola D.D. Fatigue and human umbilical cord stem cell seeding characteristics of calcium phosphate–chitosan–biodegradable fiber scaffolds. Biomaterials. 2010;31:840. doi: 10.1016/j.biomaterials.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducheyne P. Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20:2287. doi: 10.1016/s0142-9612(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 26.Foppiano S. Marshall S.J. Marshall G.W. Saiz E. Tomsia A.P. The influence of novel bioactive glasses on in vitro osteoblast behavior. J Biomed Mater Res A. 2004;71:242. doi: 10.1002/jbm.a.30159. [DOI] [PubMed] [Google Scholar]
- 27.Deville S. Saiz E. Nalla R.K. Tomsia A.P. Freezing as a path to build complex composites. Science. 2006;311:515. doi: 10.1126/science.1120937. [DOI] [PubMed] [Google Scholar]
- 28.Reilly G.C. Radin S. Chen A.T. Ducheyne P. Differential alkaline phosphatase responses of rat and human bone marrow derived mesenchymal stem cells to 45S5 bioactive glass. Biomaterials. 2007;28:4091. doi: 10.1016/j.biomaterials.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel M. Patel K.J. Caccamese J.F. Coletti D.P. Sauk J.J. Fisher J.P. Characterization of cyclic acetal hydroxyapatite nanocomposites for craniofacial tissue engineering. J Biomed Mater Res A. 2010;94:408. doi: 10.1002/jbm.a.32683. [DOI] [PubMed] [Google Scholar]
- 30.Parenteau-Bareil R. Gauvin R. Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863. [Google Scholar]
- 31.Brodie J.C. Goldie E. Connel G. Merry J. Grant M.H. Osteoblast interactions with calcium phosphate ceramics modified by coating with type I collagen. J Biomed Mater Res A. 2005;73:409. doi: 10.1002/jbm.a.30279. [DOI] [PubMed] [Google Scholar]
- 32.Keeney M. van den Beucken J.J. van der Kraan P.M. Jansen J.A. Pandit A. The ability of collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF165. Biomaterials. 2010;31:2893. doi: 10.1016/j.biomaterials.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Ghanaati S.M. Thimm B.W. Unger R.E. Orth C. Kohler T. Barbeck M. Muller R. Kirkpatrick C.J. Collagen-embedded hydroxyapatite-beta-tricalcium phosphate-silicon dioxide bone substitute granules assist rapid vascularization and promote cell growth. Biomed Mater. 2010;5:1. doi: 10.1088/1748-6041/5/2/025004. [DOI] [PubMed] [Google Scholar]
- 34.Hempel U. Reinstorf A. Poppe M. Fischer U. Gelinsky M. Pompe W. Wenzel K.W. Proliferation and differentiation of osteoblasts on Biocement D modified with collagen type I and citric acid. J Biomed Mater Res B. 2004;71:130. doi: 10.1002/jbm.b.30082. [DOI] [PubMed] [Google Scholar]
- 35.Moreau J.L. Weir M.D. Xu H.H.K. Self-setting collagen-calcium phosphate bone cement: mechanical and cellular properties. J Biomed Mater Res A. 2009;91:605. doi: 10.1002/jbm.a.32248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown W.E. Chow L.C. A new calcium phosphate water setting cement. In: Brown P.W., editor. Cements Res Progress. Westerville, OH: American Ceramic Society; 1986. p. 352. [Google Scholar]
- 37.Barralet J.E. Gaunt T. Wright A.J. Gibson I.R. Knowles J.C. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J Biomed Mater Res B. 2002;63:1. doi: 10.1002/jbm.1074. [DOI] [PubMed] [Google Scholar]
- 38.Bohner M. Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Habraken W.J. Liao H.B. Zhang Z. Wolke J.G. Grijpma D.W. Mikos A.G. Feijen J. Jansen J.A. In vivo degradation of calcium phosphate cement incorporated into biodegradable microspheres. Acta Biomater. 2010;6:2200. doi: 10.1016/j.actbio.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 40.Friedman C.D. Costantino P.D. Takagi S. Chow L.C. BoneSource hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res (Appl Biomater) 1998;43:428. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Shindo M.L. Costantino P.D. Friedman C.D. Chow L.C. Facial skeletal augmentation using hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 1993;119:185. doi: 10.1001/archotol.1993.01880140069012. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto Y. Ishikawa K. Takechi M. Toh T. Yuasa T. Nagayama M. Suzuki K. Basic properties of calcium phosphate cement containing atelocollagen in its liquid or powder phases. Biomaterials. 1998;19:707. doi: 10.1016/s0142-9612(97)00186-5. [DOI] [PubMed] [Google Scholar]
- 43.Reinstorf A. Ruhnow M. Gelinsky M. Pompe W. Hempel U. Wenzel K.W. Simon P. Phosphoserine-a convenient compound for modification of calcium phosphate bone cement collagen composites. J Mater Sci Mater Med. 2004;15:451. doi: 10.1023/b:jmsm.0000021119.14870.3d. [DOI] [PubMed] [Google Scholar]
- 44.Mai R. Reinstorf A. Pilling E. Hlawitschka M. Jung R. Gelinsky M. Schneider M. Loukota R. Pompe W. Eckelt U. Stadlinger B. Histologic study of incorporation and resorption of a bone cement-collagen composite: an in vivo study in the minipig. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e9. doi: 10.1016/j.tripleo.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Perez R.A. Ginebra M.P. Spector M. Cell response to collagen-calcium phosphate cement scaffolds investigated for nonviral gene delivery. J Mater Sci Mater Med. 2011;22:887. doi: 10.1007/s10856-011-4308-5. [DOI] [PubMed] [Google Scholar]
- 46.Zhao L. Weir M.D. Xu H.H.K. An injectable calcium phosphate—alginate hydrogel—umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31:6502. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varghese S. Hwang N.S. Ferran A. Hillel A. Theprungsirikul P. Canver A.C. Zhang Z. Gearhart J. Elisseeff J. Engineering musculoskeletal tissues with human embryonic germ cell derivatives. Stem Cells. 2010;28:765. doi: 10.1002/stem.325. [DOI] [PubMed] [Google Scholar]
- 48.Xu H.H.K. Takagi S. Quinn J.B. Chow L.C. Fast-setting and anti-washout calcium phosphate scaffolds with high strength and controlled macropore formation rates. J Biomed Mater Res A. 2004;68:725. doi: 10.1002/jbm.a.20093. [DOI] [PubMed] [Google Scholar]
- 49.Thein-Han W.W. Shah J. Misra R.D. Superior in vitro biological response and mechanical properties of an implantable nanostructured biomaterial: nanohydroxyapatite-silicone rubber composite. Acta Biomater. 2009;5:2668. doi: 10.1016/j.actbio.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 50.Kim K. Dean D. Mikos A.G. Fisher J.P. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromol. 2009;10:1810. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 52.Xie J. Baumann M.J. McCabe L.R. Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J Biomed Mater Res A. 2004;71:108. doi: 10.1002/jbm.a.30140. [DOI] [PubMed] [Google Scholar]
- 53.El-Amin S.F. Lu H.H. Khan Y. Burems J. Mitchell J. Tuan R.S. Laurencin C.T. Extracellular matrix production by human osteoblasts cultured on biodegradable polymers applicable for tissue engineering. Biomaterials. 2003;24:1213. doi: 10.1016/s0142-9612(02)00451-9. [DOI] [PubMed] [Google Scholar]
- 54.Scabbia A. Trombelli L. A comparative study on the use of a HA/collagen/chondroitin sulphate biomaterial (Biostite) and a bovine-derived HA xenograft (Bio-Oss) in the treatment of deep intra-osseous defects. J Clin Periodontol. 2004;31:348. doi: 10.1111/j.1600-051X.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 55.Charriere G. Bejot M. Schnitzler L. Ville G. Hartmann D.J. Reactions to a bovine collagen implant: clinical and immunologic study in 705 patients. J Am Acad Dermatol. 1989;21:1203. doi: 10.1016/s0190-9622(89)70330-3. [DOI] [PubMed] [Google Scholar]
- 56.Eaglstein W.H. Alvarez O.M. Auletta M. Leffel D. Rogers G.S. Zitelli J.A. Norris J.E. Thomas I. Irondo M. Fewkes J. Hardin-Young J. Duff R.G. Sabolinski M.L. Acute excisional wounds treated with a tissue-engineered skin (Apligraf) Dermatol Surg. 1999;25:195. doi: 10.1046/j.1524-4725.1999.08186.x. [DOI] [PubMed] [Google Scholar]
- 57.Carlson G.A. Dragoo J.L. Samimi B. Bruckner D.A. Bernard G.W. Hedrick M. Benhaim P. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem Biophys Res Commun. 2004;321:472. doi: 10.1016/j.bbrc.2004.06.165. [DOI] [PubMed] [Google Scholar]
- 58.Martin G.J. Boden S.D. Marone M.A. Moskovitz P.A. Posterolateral intertransverse process spinal arthrodesis with rhBMP-2 in a nonhuman primate: important lessons learned regarding dose, carrier, and safety. J Spinal Disord. 1999;12:179. [PubMed] [Google Scholar]
- 59.Shi X. Sitharaman B. Pham Q.P. Liang F. Wu K. Billups W.E. Wilson L.J. Mikos A.G. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials. 2007;28:4078. doi: 10.1016/j.biomaterials.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuo C.K. Ma P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part I. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 61.Drury J.L. Dennis R.G. Mooney D.J. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Damien C.J. Parsons J.R. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 63.Amano M. Chihara K. Kimura K. Fukata Y. Nakamura N. Matsuura Y. Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by rho-kinase. Science. 1997;275:1308. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 64.Grosheva I. Vittitow J.L. Goichberg P. Gabelt B.T. Kaufman P.L. Borras T. Geiger B. Bershadsky A.D. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp Eye Res. 2006;82:945. doi: 10.1016/j.exer.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Viereck V. Siggelkow H. Tauber S. Raddatz D. Schutze N. Hüfner M. Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J Cell Biochem. 2002;86:348. doi: 10.1002/jcb.10220. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y.H. Liu Y. Maye P. Rowe D.W. Examination of mineralized nodule formation in living osteoblastic cultures using fluorescent dyes. Biotechnol Prog. 2006;22:1697. doi: 10.1021/bp060274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H.H.K. Zhao L. Weir M.D. Stem cell-calcium phosphate constructs for bone engineering. J Dent Res. 2010;89:1482. doi: 10.1177/0022034510384623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sitharaman B. Shi X. Walboomers X.F. Liao H. Cuijpers V. Wilson L.J. Mikos A.G. Jansen J.A. In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering. Bone. 2008;43:362. doi: 10.1016/j.bone.2008.04.013. [DOI] [PubMed] [Google Scholar]