Abstract
Adenosine deaminases acting on RNA (ADARs) catalyze adenosine to inosine editing within double-stranded RNA (dsRNA) substrates. Inosine is read as a guanine by most cellular processes and therefore these changes create codons for a different amino acid, stop codons or even a new splice-site allowing protein diversity generated from a single gene. We are reviewing here the current structural and molecular knowledge on RNA editing by the ADAR family of protein. We focus especially on two types of nucleic acid binding domains present in ADARs, namely the double-stranded RNA and Z-DNA binding domains.
1. Introduction
The published sequence of the human, mouse and rat genomes (Venter et al. 2001; Baltimore 2001) revealed a surprisingly small number of genes, estimated to be around 26,000. Such a small number cannot fully account for the expected molecular complexity of these species and it is now well appreciated that such a complexity is likely to come from the multitude of protein variants created by alternative-splicing and editing of pre-mRNA (Graveley 2001; Pullirsch and Jantsch 2010). For example, the sole paralytic gene (a Drosophila sodium channel) can generate up to one million mRNA isoforms by combining its 13 alternative exons and its 11 known RNA editing sites (Hanrahan et al. 2000). Moreover, alternatively spliced and edited mRNAs are particularly abundant in the neurons. The finely regulated population of the different isoforms of most neurotransmitter receptors, ion channels, neuronal cell-surface receptors and adhesion molecules ensure proper brain function. Any imbalance of the gene expression can impair neurological functions and lead to severe diseases such as brain cancer, schizophrenia or neuromuscular and neurodegenerative syndromes (Maas et al. 2006).
RNA editing is a postranscriptional modification of pre-mRNA (Gott and Emeson 2000). Editing occurs via insertion or deletion of poly-U sequence (seen in Trypanosome mitochondria (Benne et al. 1986)), or via a single base conversion by deamination, cytidine to uridine (C→U) or adenosine to inosine (A→I) (seen from protozoa to man) (Gott and Emeson 2000). These changes can create a codon for a different amino acid, a stop codon or even a new splice-site allowing protein diversity to be created from a single gene (Gott and Emeson 2000; Keegan et al. 2001; Bass 2002). A→I editing occurs by hydrolytic deamination of the adenine base (Fig. 1a). Because inosine base-pairs with cytidine (Fig. 1b), inosine is read as a guanine by most cellular processes. RNA editing by adenosine deamination is catalyzed by members of an enzyme family known as adenosine deaminases that act on RNA (ADAR) (Bass et al. 1997).
Fig. 1.
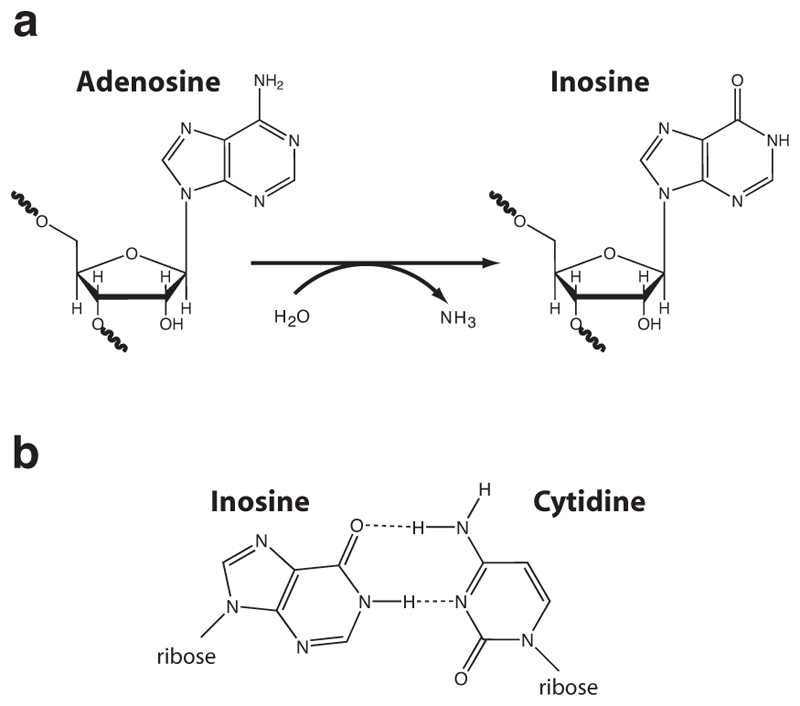
Deamination of adenosine to inosine by ADAR. a) Hydrolytic deamination converts adenosine to inosine. b) Inosine base-pairs with cytidine and is thus read as a guanine by most cellular processes.
We are reviewing here the current structural and molecular knowledge of RNA editing by the ADAR family of proteins. More comprehensive reviews on ADAR functions are available elsewhere (Gott and Emeson 2000; Keegan et al. 2001; Bass 2002; Wulff and Nishikura 2010; Nishikura 2010). We are focusing here on the structures of RNA substrates and how these structures are recognized by the double-stranded RNA binding domains (dsRBDs also refer to as dsRBMs for double-stranded RNA binding motifs) present in the ADAR family of protein. We are also reviewing the current structural knowledge on another type of nucleic acid binding domain present in ADARs, namely the Z-DNA binding domains.
2. ADAR Family Members and Their Domain Organization
ADAR proteins were first discovered in Xenopus laevis (Rebagliati and Melton 1987; Bass and Weintraub 1987, 1988) and have now been characterized in nearly all metazoa from worm to man (Tonkin et al. 2002; Palladino et al. 2000; Slavov et al. 2000; Herbert et al. 1995; Melcher et al. 1996b; O’Connell et al. 1995; Kim et al. 1994; Palavicini et al. 2009), but not in plants, yeast, or fungi. In vertebrates, two functional enzymes (ADAR1 and ADAR2) and one inactive enzyme (ADAR3 (Melcher et al. 1996a; Chen et al. 2000)) have been characterized. ADAR3 most likely originated from ADAR2 to which it is most similar in sequence and domain organization (Fig. 2a). In C. elegans, two active ADARs (CeADAR1 and CeADAR2) have been found whereas in D. melanogaster, a single ADAR2-like protein (dADAR) was found (Fig. 2a).
Fig. 2.
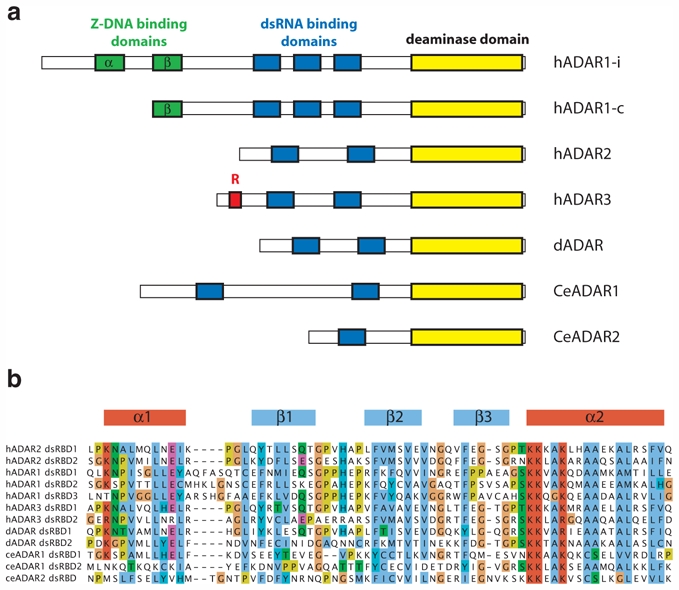
Domain organization of the ADAR family members. a) The ADAR family members are represented with their domain structure organization. Three ADARs are found in vertebrates (ADAR1—3). One ADAR is found in D. melanogaster (dADAR) and two in C. elegans (CeADAR1—2). ADARs have a conserved C-terminal deaminase domain (in yellow) and diverse numbers of dsRNA binding domains (in blue). In addition ADAR1 has one or two copies of Z-DNA binding domains (in green). The long isoform of ADAR1 is interferon-inducible (ADAR1-i) whereas the short isoform is constitutively expressed (ADAR1-c). ADAR3 has an arginine-rich R domain (in red). b) Sequence alignment of dsRBDs from the ADAR family members. The alignment is colored by amino acid conservation and properties. hADAR2 dsRBD1 secondary structure elements are shown on top of the alignment.
ADARs from all organisms have a common modular domain organization that includes from one to three copies of a dsRNA binding domain (dsRBD) in their N-terminal region followed by a C-terminal adenosine deaminase catalytic domain (Fig. 2a). For detailed information regarding the structure and the catalytic activity of the C-terminal domain, please refer to the chapter by Beal and coworkers.
In addition to this common feature, ADAR1 exhibit Z-DNA binding domains in its most N-terminal part, Zα and Zβ (Herbert et al. 1997). This renders it unique among the members of ADAR protein family (Fig. 2a). Actually, ADAR1 is expressed in two isoforms: the interferon-inducible ADAR1-i (inducible; 150 kDa) and the constitutively expressed ADAR1-c (constitutive; 110 kDa) which is initiated from a downstream methionine as the result of alternative splicing and skipping of the exon containing the upstream methionine (Patterson and Samuel 1995; Patterson et al. 1995; Kawakubo and Samuel 2000). As a consequence, the short-version of ADAR1 lacks the N-terminal Z-DNA binding domain (Fig. 2a). It is important to notice that only Zα but not Zβ has the ability to bind Z-DNA, the left-handed form of DNA (Athanasiadis et al. 2005).
ADAR1 and ADAR2 are expressed in human in most tissues and function as homodimers (Cho et al. 2003). In contrast, ADAR3 expresses only in the central nervous system and does not dimerize (Chen et al. 2000) which could explain its inactivity. Moreover, ADAR3 acts as a repressor of ADAR1 and ADAR2 activity, most probably by sequestering their potential substrates without editing them (Chen et al. 2000). ADAR3 contains also an arginine-rich RNA binding domain (R-domain) in its N-terminal region. It has been shown to be responsible for the binding of ADAR3 to single-stranded RNA (Chen et al. 2000). However, there is no structure of this domain in complex with ssRNA that would reveal the molecular basis of RNA recognition. Interestingly, a recent study showed that an R-domain is also present in a minor splicing variant of ADAR2 (Maas and Gommans 2009).
After the presentation of ADARs editing substrates, the structure and function of the Z-DNA binding domains and the dsRNA binding domains of ADAR will be described in the remaining sections.
3. RNA Editing Substrate
3.1. Specificity of Editing
Adenosine deaminases that act on RNA (ADARs) convert adenosine to inosine in cellular and viral RNA transcripts containing either perfect or imperfect regions of double-stranded RNA (dsRNA) (Gott and Emeson 2000; Bass 2002; Nishikura 2010). A→I modification is nonspecific within perfect dsRNA substrates, deaminating up to 50 % of the adenosine residues (Polson and Bass 1994; Nishikura et al. 1991). The nonspecific reaction occurs as long as the double-stranded architecture of the RNA substrate is maintained since ADARs unwind dsRNA by changing A-U base-pairs to I-U mismatches (Bass and Weintraub 1988; Wagner et al. 1989). Such modifications can modulate gene silencing triggered by intramolecular structures in mRNA (Tonkin and Bass 2003), nuclear retention of RNA transcripts (Zhang and Carmichael 2001), or antiviral responses by extensive modification of viral transcripts (Wong et al. 1991). The majority of nonselective editing occurs in untranslated regions (UTRs) and introns where large regular duplexes are formed between inverted repeats of Alu and LINE (Long Interspersed Nucleotides Element in primates) or SINE domains (Small Interspersed Nucleotides Elements found in mouse) (Levanon et al. 2004; Athanasiadis et al. 2004; Osenberg et al. 2010). It is estimated that this constitutes about 15,000 editing events in about 2000 human genes. The biological function of this major A→I editing event is not fully understood yet (Hundley and Bass 2010).
A→I editing can also be highly specific within imperfect dsRNA regions in modifying a single or limited set of adenosine residues (Gott and Emeson 2000; Bass 2002). Selective editing within pre-mRNAs has been shown to affect the primary amino acid sequence of the resultant protein therefore producing multiple isoforms from a single gene. For example, editing by ADARs produced functionally important isoforms of numerous proteins involved in synaptic neurotransmission, including ligand and voltage-gated ion channels and G-protein coupled receptors. The pre-mRNA encoding the B-subunit of the 3-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) subtype of glutamate receptor (GluR-B) is probably the most extensively studied mRNA editing substrate (Seeburg et al. 1998). It is edited at multiple sites and one of these locations is the R/G site, where a genomically-encoded AGA is modified to IGA, resulting in an arginine-to-glycine change (the ribosome interprets I as G due to its similar base-pairing properties – Fig. 1b). The R/G site of the GluR-B pre-mRNA is often used as a model system for A→I editing studies as it forms a small and well conserved 70 nucleotide stem-loop containing three mismatches (Aruscavage and Bass 2000), referred to as the R/G stem-loop (Fig. 3).
Fig. 3.
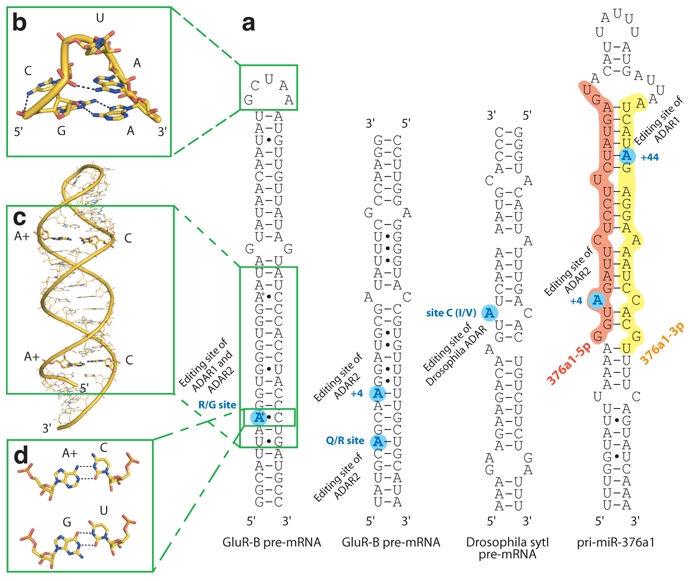
Structures of various ADAR editing substrates. a) Secondary structure of ADAR editing substrates: GluR-B R/G and Q/R sites, Drosophila sytI I/V site and pri-miR-376a1. b) Structure of the GluR-B GCUAA apical pentaloop (PDB code 1YSV). c) Structure of the RNA helix surrounding the GluR-B R/G site revealing two particular A+•C wobble base-pairs (PDB code 2L2J). d) Hydrogen bond pattern in an A+•C wobble base-pair and comparison with a G•U wobble base-pair.
More recently, specific editing of many pri-miRNAs, pre-miRNA and miRNAs have been discovered suggesting a crosstalk between the RNA editing and RNA interference machineries (Nishikura 2006; Ohman 2007). MicroRNA editing can regulate miRNA expression by affecting pri-microRNA and pre-miRNA processing (Kawahara et al. 2008; Kawahara et al. 2007a; Heale et al. 2009). MiRNA editing can also affect gene targeting when the seed sequence of the miRNA is edited. This later editing event allows an extension of the number of genes targeted by the miRNAs (Kawahara et al. 2007b). Examples of editing site in miRNA are shown in Figure 3. For comprehensive informations on the modulation of micro-RNA function by ADAR please refer to the chapter by Nishikura and coworkers.
3.2. What Makes a Good Editing Site?
What characterizes a specific A→I RNA editing site is a major and longstanding question in the field. It is clear that the targeted adenosine has to be embedded in an RNA stem, and that both the sequence around the adenine and the secondary structure elements present in the RNA stem will have a major impact on the efficiency and the selectivity of editing. The terms preferences and selectivity are used to describe the properties that enable ADAR proteins to modify a specific adenosine among others (Polson and Bass 1994).
3.2.1. Preferences
Even though ADAR dsRBDs are thought to bind unspecifically to any dsRNA, ADARs have small sequence preferences for deaminating particular adenosines among others. Detailed inspection of the editing ability of ADAR1 and ADAR2 on the GluR-B R/G and Q/R sites revealed that these enzymes have overlapping but distinct preferences (Lai et al. 1997; Melcher et al. 1996b; Gerber et al. 1997; Maas et al. 1996). Xenopus and human ADAR1 have a similar preference for A = U > C > G at the 5′ of the edited adenosine (Polson and Bass 1994; Lehmann and Bass 2000). Human ADAR2 has also a similar but distinct preference for the 5′ neighbor of the edited adenosine (U ≈ A > C = G) (Lehmann and Bass 2000). In addition, human ADAR2 has also a 3′ neighbor preference (G = U > C = A) (Lehmann and Bass 2000). These initial preference rules were further confirmed and optimized in subsequently discovered targets (Kawahara et al. 2008; Riedmann et al. 2008; Li et al. 2009; Wulff et al. 2011). Chimeric ADARs containing the dsRBDs of ADAR2 and the catalytic domain of ADAR1 and vice versa suggested that the nearest-neighbour preferences come from the deaminase domain (Wong et al. 2001) but recent structures suggest that dsRBDs could also play a role (Stefl et al. 2010). The nucleotide base-pairing with the target adenosine can also drastically influence editing with a preference for a cytidine (forming a AC mismatch which is then converted to a matching I-C pair, like in the GluR-B R/G site, Fig. 3) (Levanon et al. 2004; Athanasiadis et al. 2004; Riedmann et al. 2008; Blow et al. 2004; Wong et al. 2001) over a uridine (like in the GluR-B Q/R site, Fig. 3). Purines are not favored and a guanosine in some case can severely impaired editing (Wong et al. 2001; Kallman et al. 2003; Ohlson et al. 2007). This discrimination between various pairing partners is also determined by the catalytic domain rather than the dsRBDs (Wong et al. 2001).
3.2.2. Selectivity
Obviously, the slight preferences for the identity of neighbouring nucleotides cannot explain the acute specificity observed in some ADAR substrates, like in the GluR-B R/G or Q/R sites, where adenines in good sequence context (as defined by 5′ and 3′ neighbour and pairing partner preferences) remain not edited. The property of having adenines in good sequence context that remain not edited defines the concept of selectivity. Ultimately, this can result in having a few and even a single edited adenine in an entire dsRNA structure, which one describes as specificity. One can easily notice that sites of highly specific editing events are never long and perfectly base-paired dsRNA (Fig. 3). The presence of secondary structure elements like terminal loops, internal loops, bulges and mismatches is very frequent in such substrate. These secondary structured elements are highly conserved during evolution (Aruscavage and Bass 2000; Dawson et al. 2004; Reenan 2005) indicating that the RNA structure is important for the specificity of editing (Aruscavage and Bass 2000; Dawson et al. 2004; Reenan 2005; Ohman et al. 2000; Lehmann and Bass 1999). For example, the presence of internal loops has been shown to increase the selectivity of editing by uncoupling and decreasing the effective length of individual helices which then reduces to a minimum the many ways of binding of ADAR to these substrates (Ohman et al. 2000). However, RNA sequences around highly specific editing sites are also particularly conserved (Aruscavage and Bass 2000; Niswender et al. 1998), and this cannot be explained if only secondary structured elements would define the selectivity of editing. Thus, both the structure and the sequence of the RNA editing site determine the selectivity of editing by ADAR. In contrast to their preferences, ADARs selectivity comes most probably from the binding selectivity of their dsRBDs.
3.3. Structures of Editing Substrates
Structural information on A→I RNA editing substrates has been limited so far to the GluR-B R/G site. The GluR-B R/G site is embedded within a 71 nt RNA stem-loop containing three base-pair mismatches and capped with a GCUAA pentaloop. The solution structure of the long human R/G stem-loop has been determined in two fragments by solution NMR.
In the first structure, the apical part of the stem-loop containing the GCU(A/C)A pentaloop has revealed a rigid pentaloop fold, novel for this time (Stefl and Allain 2005). The fold is stabilized by a complex interplay of hydrogen-bonds and stacking interactions (Fig. 3b). The structure of the GCUAA pentaloop explains well the phylogenetic conservation of GCUMA (where M is A/C) (Aruscavage and Bass 2000). The UNCG tetraloops (Cheong et al. 1990; Allain and Varani 1995; Ennifar et al. 2000) and the GCUAA pentaloop are structurally similar. This is particularly interesting considering that the pre-mRNA encoding the R/G site of subunit C of the glutamate receptor that is also specifically edited by ADAR2 has a UCCR tetraloop (Aruscavage and Bass 2000). When the size of the GCUAA pentaloop is changed or the loop is deleted, the level of editing is reduced (Stefl et al. 2006) indicating that this structural element plays an important role in the recognition processes of ADAR2. The role of the loop was subsequently confirmed by using a high throughput method (Pokharel and Beal 2006).
In the second structure, the RNA helix surrounding the editing site that contains two A-C mismatches has revealed an unexpected regular A-form helix (Fig. 3c) (Stefl et al. 2010). Indeed, adenine C2 chemical shifts (a sensitive probe to monitor the protonation of adenine N1) have shown that these two adenines involved in A-C mismatches were protonated at pH below 7.0 and were thus forming a so-called A+•C wobble base pair similar in its hydrogen bonding pattern to a G•U wobble base pair (Fig. 3d). Thus A+•C wobble base pairs generate only little deformation of the helical properties of the stem.
Overall, these structures together with the chemical-shift analysis of the 71 nt R/G site (Stefl et al. 2006; Stefl et al. 2010) revealed a rigid RNA stem-loop throughout the sequence. Indeed, the terminal loop is structured (Stefl and Allain 2005) and the three mismatches in the stem (two AC and one GG; Fig. 3) are forming non Watson-Crick pairs leading to rigid and rather regular RNA helix (Stefl et al. 2006; Stefl et al. 2010). This is particularly interesting in the context of the selectivity of editing, since it is largely believed that mismatches contribute to the ADAR selectivity. Even if a A+•C wobble base pair might be more deformable than a regular A-U pair, it seems unlikely that the sole shape recognition of such mismatches could allow the editing of the R/G site to be selective.
4. ADAR Z-DNA Binding Domains
In the late 1970s, the first atomic resolution structure of DNA, solved by X-ray crystallography was surprisingly different from the expected right-handed B-form helix (Wang et al. 1979). This structure indeed showed a left-handed double helix in which the bases alternate in anti- and syn-conformations along one strand. As a consequence, there was a zigzag arrangement of the backbone of the molecule. This property gave its name to the Z-DNA conformation observed in this crystal (Wang et al. 1979). During fifteen years, many people felt that Z-DNA was a nonfunctional conformation of DNA, and as a consequence its study rapidly declined (Rich and Zhang 2003). However, the discovery in 1995 of a protein binding specifically and tightly to Z-DNA (Herbert et al. 1995), and two years later the isolation of a small domain responsible for this activity (Herbert et al. 1997), brought back Z-DNA in the limelight. The first Z-DNA binding property was discovered in the vertebrate ADAR1 protein. And so far, even if other proteins have been shown to bind Z-DNA, ADAR1 stays the best-characterized member of the Z-DNA binding protein family. ADAR1 has two related Z-DNA binding domains named Zα and Zβ (Fig. 2), the latter one having no binding capacity for Z-DNA. In this section we review the structural knowledge on ADAR1 Z-DNA binding domains and especially on the binding of Zα to Z-DNA.
4.1. Z-DNA Binding Domain: Structure and Substrate Recognition
The first structural information on Z-DNA binding domains and the molecular basis of the recognition of Z-DNA were revealed together by the crystal structure of the human Zα domain of ADAR1 complexed to DNA (Schwartz et al. 1999b). The Zα domain has a compact α/β fold containing a three-helix bundle (α1 to α3) flanked on one side by a twisted antiparallel β-sheet (β1 to β3) with a αβααββ topology (Fig. 4). This arrangement of three α-helices and β-strands is known as the helix-turn-helix β-sheet fold (or α+ βHTH fold). This fold differs from the related helix-turn-helix (HTH) fold in the fact that it has an additional C-terminal β-sheet packed against the core formed by the α-helices. The interaction of HTH proteins with right-handed B-DNA has been well characterized (Harrison and Aggarwal 1990). The mode of interaction of Zα with Z-DNA has noticeable differences arising from the differences between Z- and B-DNA (Schwartz et al. 1999b). The bound DNA duplex shows a left-handed helix structure with the typical zigzag backbone conformation of the Z-DNA (Fig. 4a). The Zα domain makes contact to only one strand of the DNA molecule in a single continuous recognition surface formed by helix α3 and the C-terminal β-hairpin (Fig. 4a,c). Interestingly, this surface is complementary to the DNA backbone in terms of shape and electrostatic properties. Indeed, all the polar interactions involve direct or water-mediated contacts to the sugar-phosphate backbone of the DNA. This implies that the interaction between Zα and Z-DNA is conformation or shape specific rather than sequence specific. This was further confirmed by recent structures of Zα bound to other DNA molecules of various sequences (Ha et al. 2009). These structures showed almost identical structures regardless of the sequence, confirming that the mode of binding of Zα to Z-DNA is a well conserved shape-specific mode of binding.
Fig. 4.
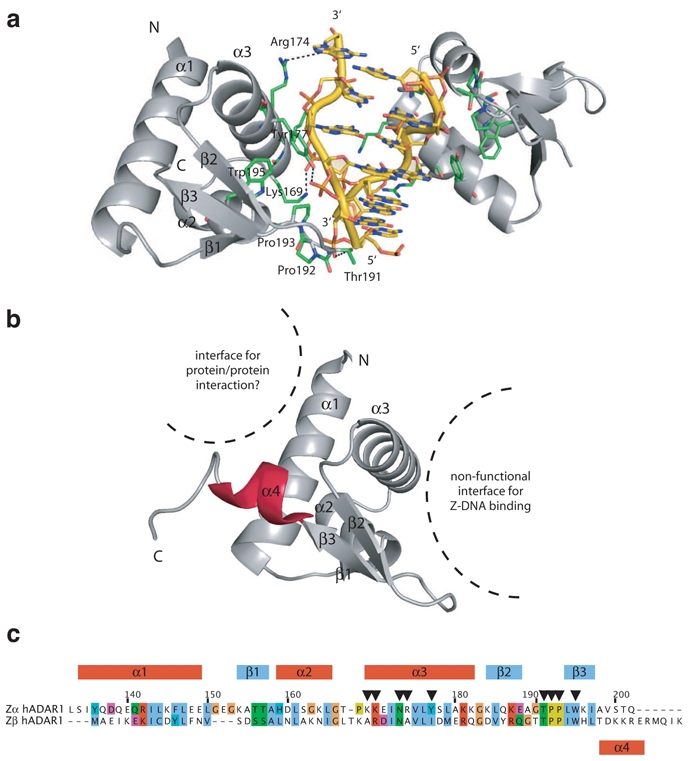
Structures of ADAR Z-DNA binding domains. a) Structure of the Zα domain of ADAR1 in complex with Z-DNA (CG)3 showing contacts with the phosphate backbone via helix α1 and beta hairpin β2-β3. b) Structure of the Zβ domain of ADAR1 in its free state with a nonfunctional Z-DNA binding surface and a potential protein/protein interaction surface. The additional helix α4 is shown in red. c) Sequence alignment of hADAR1 Zα and Zβ domains. The alignment is colored by amino acid conservation and properties. Common secondary structure elements are shown on top of the alignment. The position of the additional helix α4 is shown below. Residues of Zα involved in direct or water-mediated contacts with Z-DNA are reported with black arrows. Some of these residues are not conserved in Zβ. Residue numbers correspond to the one of Zα.
4.2. A Role for ADAR1 Zα Domain?
Although the biological role of Z-DNA binding by Zα has not been clearly defined yet, one possible function might be to direct ADAR1 at actively transcribing genes. Indeed, Z-DNA is stabilized by negative supercoiling (Peck et al. 1982) which is formed transiently upstream of an active RNA polymerase (Liu and Wang 1987). Localizing ADAR1 to the site of transcription would then allow it to efficiently act upon the RNA prior to splicing.
A point particularly interesting in the context of RNA editing is that Zα has also the property to bind to Z-RNA (Brown et al. 2000). This type of domains is thus also refers to as Z-DNA/Z-RNA binding domains. The structure of Zα bound to Z-RNA has revealed a well-conserved mode of interaction between Zα and the nucleic acid backbone of Z-DNA and Z-RNA (Placido et al. 2007). This property of Zα might thus help to target ADAR1 to specific sites that are prone to form Z-RNA. For example, the formation of Z-RNA is favoured by alternative purine-pyrimidine sequences, and especially guanosine and cytosine repeats (Herbert and Rich 1999). Interestingly, it was shown that A→I editing by ADAR1-i is substantially increased in a dsRNA substrate containing such Z-forming purine-pyrimidine repeats (Koeris et al. 2005). Moreover, such influence of Z-forming sequences on the level of A→I editing was not observed in the case of ADAR2 which does not contain Z-DNA/Z-RNA binding domain. This suggests a direct role of the Z-DNA/Z-RNA binding domain of ADAR1-i in the enhanced editing activity towards dsRNA with Z-forming sequences. Moreover, editing reactions conducted with ADAR1-i under short incubation time showed a clear positive correlation between the proximity of the edited adenosine to the Z-forming sequence and the number of editing event at those sites (Koeris et al. 2005). So, in contrast to Z-DNA binding, Z-RNA binding by the Zα domain of ADAR1-i has a clear biological role in the context of RNA editing, which is to target ADAR1-i to Z-forming sequences within dsRNA substrates. Such Z-forming RNA structure can be directly encoded in the RNA substrate, but Z-RNA can also be formed in the trail of transcription of RNA viruses, and would allow ADAR1 to more efficiently modify these RNA viruses. Indeed, ADAR1-i has been associated with RNA editing of a wide array of viral genomes (Cattaneo 1994; Horikami and Moyer 1995; Polson et al. 1996; Taylor et al. 2005), and in certain cases depending on virus-host combinations, displays an antiviral action (Samuel 2011).
The structures of Zα bound to Z-DNA and Z-RNA gave crucial understanding of the molecular basis of the zigzag backbone recognition by a specific set of side chains of Zα. However, the mechanism by which Zα converts a right-handed backbone structure (B-form DNA helix or A-form RNA helix) in a left-handed one (B to Z transition or A to Z transition, respectively) has been investigated only recently. The different possible mechanisms as well as the recent proposed model will be discussed in the next section.
4.3. How Does a Z-DNA Binding Domain Bind to Z-DNA?
So far only the B to Z transition of DNA induced by the binding of ADAR1 Zα domain has been experimentally investigated. Two different mechanisms for such a B to Z transition can be imagined (Kim et al. 2000): (1) a passive mechanism, in which Zα would bind to the small fraction of Z-DNA present in equilibrium with B-DNA, and because of its high affinity for Z-DNA will then pull the equilibrium towards the formation of Zα/Z-DNA complex; (2) an active mechanism, in which Zα would bind to B-DNA and will then actively convert it to Z-DNA.
The structure of the free Zα domain of ADAR1 has been determined in solution by NMR (Schade et al. 1999). The comparison of this structure with the one of Zα bound to Z-DNA (Schwartz et al. 1999b) has revealed that most Z-DNA contacting residues are pre-positioned in the free Zα domain to fit Z-DNA. Of the nine Zα side chains contacting Z-DNA in the crystal structure, seven are well-ordered and already pre-positioned in free Zα, which is thus pre-shaped to fit Z-DNA. Moreover, structural comparison of Zα with homologous proteins that bind B-DNA suggested that binding of Zα to B-DNA is disfavoured by steric hindrance (Schade et al. 1999). Altogether, these strongly suggest that binding of Zα would follow a passive mechanism.
However, recent NMR studies monitoring hydrogen exchange rates of imino protons have made possible the indirect observation of Zα bound to B-DNA in the pathway of binding in different DNA sequence context (Kang et al. 2009; Seo et al. 2010). These studies would strongly tend to validate an active mechanism, but the authors could nonetheless not clearly exclude a passive one to occur (Kang et al. 2009). The elucidation of the binding mechanism of Zα to Z-DNA would thus probably deserve further study. It would also be interesting to analyze similarly the binding mechanism to Z-RNA.
4.4. ADAR1 Zβ Domain: a Domain for Protein-Protein Interaction?
Zβ, the second Z-DNA binding domain of ADAR1, is present in both the inter-feron-induced ADAR1-i and the constitutively expressed ADAR1-c (Fig. 2). In contrast to Zα domain, the Zβ domain of ADAR1 does not interact with Z-DNA (Schwartz et al. 1999a). Nevertheless, the Zβ domain is highly conserved among ADAR1 which thus suggests that the two domains Zα and Zβ probably perform different functions. The Zβ structure has been solved by crystallography and consists of four →-helices and a three-stranded β-sheet with a αβααββ α topology (Fig. 4b,c) (Athanasiadis et al. 2005). This structure has revealed that Zβ is closely related in structure to Zα and belongs to the same α+ βHTH family. However, Zβ has an additional helix, helix α4 (Fig. 4b) and is also lacking several crucial residues important for Z-DNA binding (Fig. 4c). This latter point explains why Zβ does not bind to Z-DNA. Interestingly there is no steric clash that would prevent Zβ to bind to Z-DNA, and the partial restoration of a Zα sequence in Zβ results in weak Z-DNA binding (Kim et al. 2004). The mapping of Zβ amino acid conservation has revealed a distinct conserved surface involved in metal binding and dimerization (Athanasiadis et al. 2005). However, since no biochemical data support neither metal binding nor dimerization of Zβ, these properties observed in the crystal might have been influenced by packing forces. Nonetheless, the dimerization of Zβ is an appealing model, considering that ADAR proteins have been shown to be active as dimers (Cho et al. 2003; Gallo et al. 2003). For example, the N-terminal part of dADAR has been shown to be involved in dimerization (Gallo et al. 2003), but the site of dimerization for vertebrate ADARs remains to be established.
5. ADAR dsRNA Binding Domains
ADAR dsRNA binding domains are essential components for ADAR activity, since they are directly involved in dsRNA substrate recognition and binding. However, the molecular basis explaining how domains largely thought to bind dsRNA non-specifically are actually targeting very specific adenine positions in certain substrate (like the GluR-B R/G site – Fig. 3) have been a puzzling paradox for many years. Recent structures of ADAR2 dsRBDs bound to a natural substrate have given some critical insights into the sequence-specific recognition of ADAR substrates.
5.1. Structural Characteristics of a dsRNA Binding Domain
The dsRBD is a ~65–75 amino acids domain found in eukaryotic, prokaryotic and even viral proteins which have been shown to interact specifically with dsRNA. dsRBDs were first identified in Staufen, a protein responsible for mRNA localization in Drosophila and PKR, a dsRNA dependent protein kinase (St Johnston et al. 1992; McCormack et al. 1992; Green and Mathews 1992). Since the early 1990s, the list of dsRBD containing proteins has been growing and regroups proteins with a large variety of function as development, RNA interference, RNA transport, RNA processing and of course RNA editing (Fierro-Monti and Mathews 2000; Saunders and Barber 2003; Chang and Ramos 2005; Stefl et al. 2005). The structures of various dsRBDs have been determined uncovering a mixed α/β fold with a conserved αβββα topology in which the two α-helices are packed against the three-stranded anti-parallel β-sheet (Bycroft et al. 1995; Kharrat et al. 1995). In addition, structures of dsRBDs have been determined in complex with dsRNA, most of which with non-natural RNA duplexes (Ryter and Schultz 1998; Ramos et al. 2000; Gan et al. 2006; Wu et al. 2004). These structures have suggested that dsRBDs recognize A-form helix of dsRNA in a sequence-independent manner, since the majority of dsRBD-RNA interaction involve direct contacts with the 2′-hydroxyl groups of the ribose sugar rings and direct or water-mediated contacts with non-bridging oxygen of the phosphodiester backbone and that a subclass of dsRBDs prefer stem-loop over A-form helices (Ramos et al. 2000; Wu et al. 2004). More recently, the structures of the two dsRBDs of ADAR2 in complex with a natural dsRNA substrate have been determined (Stefl et al. 2010), revealing a sequence specific readout of the dsRNA minor groove. These structures will be discussed in the following section.
5.2. Sequence Specific Recognition with dsRBD
Recently, the structures of human ADAR2 dsRBD1 and dsRBD2 have been determined in complex with their respective RNA target on the GluR-B R/G site RNA helix (Stefl et al. 2010). These structures have confirmed the conserved mode of recognition of the A-form RNA helix (Ryter and Schultz 1998; Ramos et al. 2000; Gan et al. 2006; Wu et al. 2004) in which helix α1 and β1-β2 loop interact with the minor groove of the RNA helix at one turn of interval and in which conserved positively charged residues in the N-terminal end of helix α2 interact across the major groove with non-bridging oxygen of the phosphodiester backbone. Strikingly, a detailed inspection of the interaction regions revealed unexpected sequence-specific contacts of both dsRBD to the RNA minor grooves.
The RNA major groove is deep and narrow, and as a consequence bases are inaccessible to protein side chains. In contrast, the RNA minor groove is wide and shallow (Saenger 1984) but stereochemical considerations have suggested that discrimination of some base pairs would be difficult in the minor groove (Seeman et al. 1976; Steitz 1990). Discrimination in the minor groove can mostly arise from an appreciation of the group lying in the position 2 of purine rings, i.e. the amino NH2 group of a guanine which is a polar hydrogen bond donor, and the aromatic H2 proton of an adenine which is non polar and small and can thus accommodate hydrophobic side chains in its close vicinity whereas the amino group of a guanine would lead to steric clashes (Fig. 5c,d).
Fig. 5.
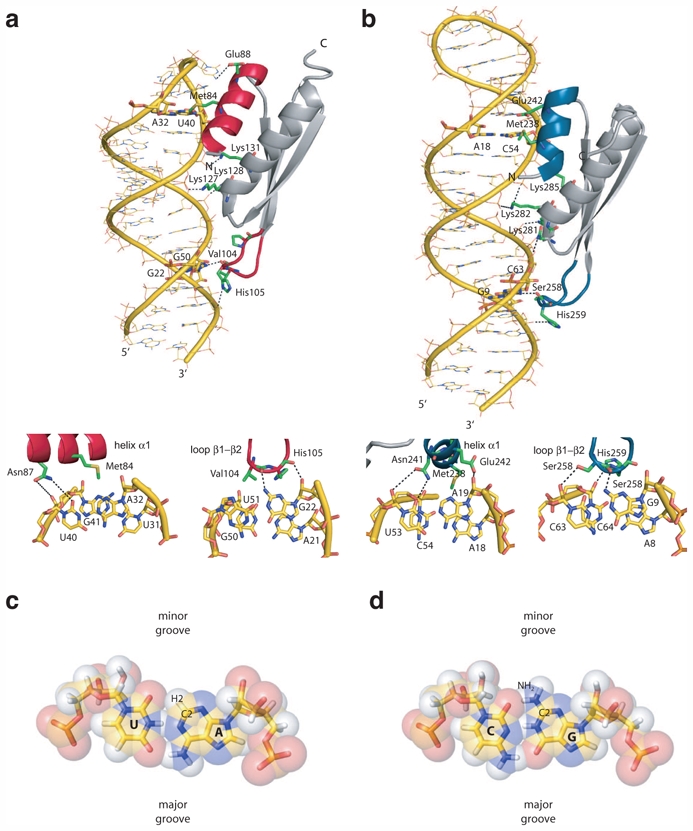
RNA recognition by ADAR2 dsRBDs through sequence specific readout of the minor groove. a) Structure of ADAR2 dsRBD1 in complex with the GluR-B R/G upper stem-loop (PDB code 2L3C). Overall structure (top) and close-up view of the minor groove sequence-specific recognitions mediated by helix α1 and the β1-β2 loop (bottom). b) Structure of ADAR2 dsRBD2 in complex with the GluR-B R/G lower stem-loop (PDB code 2L2K). Overall structure (top) and close-up view of the minor groove sequence-specific recognitions mediated by helix α1 and the β1-β2 loop (bottom). c) Chemical groups of an A-U pair lying in the major and minor grooves. d) Chemical groups of a G-C pair lying in the major and minor grooves. Discrimination in the minor groove relies on the appreciation of the group in position 2 of purine rings.
Two sequence specific contacts at two consecutive RNA minor grooves enable ADAR2 dsRBD1 to bind the GluR-B RNA upper stem-loop (USL) at a single register (Stefl et al. 2010). These specific contacts are on one hand a hydrogen bond to the amino group of G22 in the GG mismatch via the main chain carbonyl of Val104 in the β1-β2 loop and on the other hand a hydrophobic contact to the adenine H2 of A32 via the side chain of Met84 in helix α1 (Fig. 5a). Similarly, ADAR2 dsRBD2 recognizes the GluR-B RNA lower stem-loop via two sequence specific contacts at two consecutive RNA minor grooves: a hydrogen bond to the amino group of G9, located 3′ to the editing site, via the main chain carbonyl of Ser258 in the β1-β2 loop and a hydrophobic contact to the adenine H2 of A18 via the side chain of Met238 in helix α1 (Fig. 5b) (Stefl et al. 2010).
The importance of these contacts for the binding affinity of the dsRBDs with their respective RNA partner was further quantified in a solution binding assay and in an in vitro editing assay (Stefl et al. 2010). In mutating any of the bases that are recognized in a sequence-specific manner by the dsRBDs, the binding affinity is reduced compared to the wild-type. Furthermore, when replacing the GG mismatch or the two AC mismatches by Watson-Crick pairs that keep intact the specific contacts to the RNA, the binding affinity is less affected than when mutating the specifically recognized bases. In addition, the editing activity of protein mutants affected in the residues involved in sequence specific contacts (Met in helix α1 and β1-β2 loop) is reduced to less than 30 % of the wild-type protein editing activity. Altogether, this strongly supports the idea that the two dsRBDs of ADAR2 recognize primarily the sequence of the RNA helix rather than its shape.
These structures give the means to reconsider the common beliefs on dsRBDs, and to propose that binding of certain dsRBDs might occur sequence specifically.
5.3. How dsRBDs Are Positioned on Substrate?
Structural studies on full-length ADAR proteins in complex with their RNA substrates are essential for understanding the overall editing mechanism process. One interesting point in the field is the requirement of dimerization of ADAR to be active. In vitro studies have shown that editing activity of ADAR1 and ADAR2 (Cho et al. 2003) and of Drosophila dADAR (Gallo et al. 2003) needs dimerization. Dimerization of ADAR1 and ADAR2 have been confirmed in vivo by fluorescence and bioluminescence resonance energy transfer studies (Chilibeck et al. 2006; Poulsen et al. 2006). A source of discussion in the field is to know whether this dimerization is dependent on RNA binding (Cho et al. 2003; Gallo et al. 2003; Poulsen et al. 2006; Valente and Nishikura 2007). There are no structural data with a full length ADAR protein bound to a RNA substrate that would reveal the molecular basis for this dimerization. Nevertheless, the structure of the N-terminal domain of ADAR2 consisting of both dsRBDs have been solved by solution NMR in complex with the GluR-B R/G site RNA (Stefl et al. 2010). In the structure, the two dsRBDs bind one face of the RNA covering approximately 120 degrees of the space around the RNA helix (Fig. 6a,b). This structure is then perfectly in accordance with the dimerization model of ADARs, since an other ADAR molecule could bind to the other face of the RNA helix without steric hindrance. Moreover it is clear that in interacting with the guanosine 3′ to the edited adenosine (Fig. 6d), ADAR2 dsRBD2 brings the deaminase domain in close proximity to the editing site. When this precise positioning is impaired, specific editing of the GluR-B R/G site is nearly abolished which underlines the functional importance of sequence-specific recognition of RNA by dsRBDs for A→I editing (Stefl et al. 2010). Even with these new insights, structural aspects of substrate recognition by ADARs remain a source of questions in the editing field. For example, how the targeted adenosine would be flipped out to reach the catalytic domain, and how after dimerization of ADARs the two catalytic domains would be positioned relative to each other and to the dsRBDs, is of great interest and deserves further structural studies.
Fig. 6.
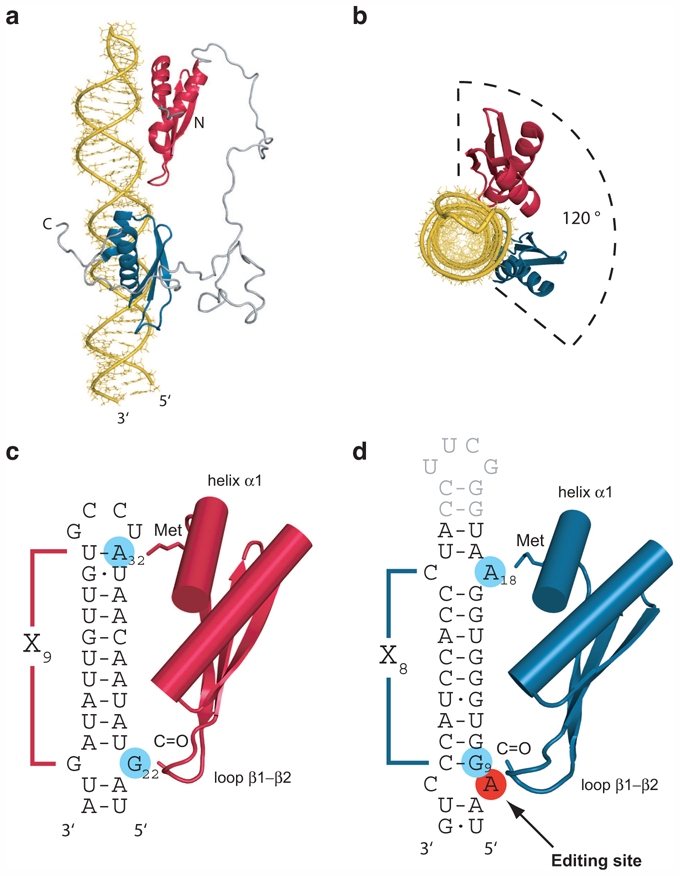
Spatial organization of the two dsRBDs of ADAR2 on the GluR-B R/G site. a) Side view of the complex (PDB code 2L3J). b) Top view of the complex showing the portion of the space covered by the two dsRBDs around the RNA helix. c) Schematic representation of the sequence specific contacts defining the binding register of ADAR2 dsRBD1. d) Schematic representation of the sequence specific contacts defining the binding register of ADAR2 dsRBD2.
5.4. Are the Binding Sites of ADAR dsRBDs Predictable?
An attractive idea would be to transpose the structural knowledge of ADAR2 bound to the GluR-B R/G site to predict the mode of binding of ADAR2 on other substrates. Even more challenging would be the prediction of other ADAR dsRBDs on their respective substrates. The structures of ADAR2 dsRBDs bound to the GluR-B R/G site have shown that the binding is achieve by a direct readout of the RNA sequence in the minor groove of the dsRNA substrate. The two dsRBDs use helix α1 and the β1-β2 loop as molecular rulers to find their binding register in the RNA minor groove (Fig. 5a,b). While dsRBD1 preferentially recognize G-X9-A (Fig. 6c), dsRBD2 binds the same sequence but with a different register length: G-X8-A (Fig. 6d). The length and the relative position of helix α1 relative to the dsRBD fold appear to be the key structural elements that determine the register length of these two dsRBDs (Stefl et al. 2010). Such binding sequence and register for ADAR2 dsRBD2 are present on GluR-B Q/R site (Fig. 3a), but are not always present on ADAR2 substrates (Fig. 3a), and one can thus not exclude that its dsRBDs would adopt a different mode of binding involving different side chains in helix α1 when bound to different substrate. In addition, sequence alignment of diverse ADAR dsRBDs could serve to anticipate similarities and discrepancies between dsRBDs regarding the preferred sequence and register of binding (Fig. 2b). For instance, ADAR1 dsRBDs appears to have a longer helix α1 and lack the ADAR2 equivalent of the methionine involved in the sequence specific contacts (Fig. 2b and Fig. 5). These could explain why ADAR1 and ADAR2 have different substrate specificities (Bass 2002; Lehmann and Bass 2000). Furthermore, dADAR dsRBD2 is very similar to ADAR2 dsRBD2, but whereas their helix α1 are extremely alike, the β1-β2 loop region is less conserved (Fig. 2b) and a prediction on the sequence and register of binding for this dsRBD remains difficult. Generally, the binding specificities of dsRBDs of other members of the ADAR family are still difficult to predict and would need more structural data involving various dsRBDs.
6. dsRBDs and Z-DNA Binding Domains Act on the Subcellular Localization of ADARs
Whereas ADAR1-i is mostly detected in the cytoplasm (Patterson and Samuel 1995; Poulsen et al. 2001; Desterro et al. 2003), ADAR1-c localizes mainly in the nucleus (Desterro et al. 2003; Sansam et al. 2003). ADAR1-c is mostly located in nucleoli but constantly shuttles between nucleoli and the nucleoplasm, where most ADAR substrates are found (Sansam et al. 2003). A nuclear localization signal (NLS) has been identified in the third dsRBD of human ADAR1 (Eckmann et al. 2001; Strehblow et al. 2002; Poulsen et al. 2001). ADAR1-i harbors also a nuclear export signal (NES) within its most N-terminal Z-DNA binding domain (Zα) (Poulsen et al. 2001), and has the property to shuttle between the nucleus and the cytoplasm (Eckmann et al. 2001; Poulsen et al. 2001). As most RNA viruses are localizing in the cytoplasm, the unique cytoplasmic localization of ADAR1-i among ADARs, gives additional support for an antiviral function of this protein (George et al. 2011; Samuel 2011). Although lacking the Zα domain, ADAR1-c is also shuttling between the nucleus and the cytoplasm. Transportin-1 is a nuclear import factor for ADAR1, and dsRNA binding of the third dsRBD modulates its interaction with transportin-1 and exportin-5 and thus regulate the nucleocytoplasmic properties of ADAR1 (Fritz et al. 2009).
ADAR2 is localized exclusively in the nucleus, where similarly to ADAR1-c it resides mostly in nucleoli, but shuttles constantly to reach the nucleoplasm where ADAR substrates are located (Desterro et al. 2003; Sansam et al. 2003). Although the dsRBDs are not involved in the nuclear localization of ADAR2, they play a crucial role for targeting ADAR2 to the nucleolus, likely through their ability to bind rRNA (Sansam et al. 2003; Xu et al. 2006). However, the biological signification of the nucleolar localization of ADAR2 and ADAR1-c is largely unknown.
7. Concluding Remarks
During the last ten years, the ADAR family of protein has contributed to a great extent to our general understanding of the molecular basis of nucleic acid recognition for both Z-DNA binding domains and dsRNA binding domains. However, our current understanding of the molecular basis of substrate recognition by ADARs is unfortunately still incomplete. More structures of ADARs substrates alone and in complex with ADARs dsRBDs, would undoubtedly be of great value for the understanding of substrates recognition. In addition, a structure of a full-length ADAR protein revealing how the catalytically active dimer would assemble on an RNA substrate would be fantastic and essential for understanding the overall editing mechanism process. The relatively small amount of structural information obtained to date is a consequence of the demanding biochemical properties of ADAR proteins and also probably of the small number of groups in structural biology working on the editing field. However, pieces by pieces, we start having a better view on substrate recognition by ADARs. Hopefully, in the near future, more structural information would allow the prediction of ADARs mode of binding on RNA substrates with an increased confidence.
Acknowledgments
The authors would like to thank Richard Stefl, Florian Oberstrass, Lenka Skrisovska and Christophe Maris who have done the work performed in the Allain’s Lab described in this review. We sincerely apologize to the colleagues whose important work is not cited because of space limitation, or unfortunately because of our negligence. This work was supported by the Swiss National Science Foundation Nr. 31003-133134 and the SNF-NCCR structural biology. PB is supported by the Postdoctoral ETH Fellowship Program.
References
- Allain FH, Varani G. Structure of the P1 helix from group I self-splicing introns. J Mol Biol. 1995;250:333–353. doi: 10.1006/jmbi.1995.0381. [DOI] [PubMed] [Google Scholar]
- Aruscavage PJ, Bass BL. A phylogenetic analysis reveals an unusual sequence conservation within introns involved in RNA editing. RNA. 2000;6:257–269. doi: 10.1017/s1355838200991921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadis A, Placido D, Maas S, Brown BA, Lowenhaupt K, Rich A. The crystal structure of the Zbeta domain of the RNA-editing enzyme ADAR1 reveals distinct conserved surfaces among Z-domains. J Mol Biol. 2005;351:496–507. doi: 10.1016/j.jmb.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol. 2004;2:e391. doi: 10.1371/journal.pbio.0020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Our genome unveiled. Nature. 2001;409:814–816. doi: 10.1038/35057267. [DOI] [PubMed] [Google Scholar]
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BL, Nishikura K, Keller W, Seeburg PH, Emeson RB, O’Connell MA, Samuel CE, Herbert A. A standardized nomenclature for adenosine deaminases that act on RNA. RNA. 1997;3:947–949. [PMC free article] [PubMed] [Google Scholar]
- Bass BL, Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987;48:607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Bass BL, Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988;55:1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Benne R, Van den Burg J, Brakenhoff JP, Sloof P, Van Boom JH, Tromp MC. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986;46:819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- Blow M, Futreal PA, Wooster R, Stratton MR. A survey of RNA editing in human brain. Genome Res. 2004;14:2379–2387. doi: 10.1101/gr.2951204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BAn, Lowenhaupt K, Wilbert CM, Hanlon EB, Rich A. The zalpha domain of the editing enzyme dsRNA adenosine deaminase binds left-handed Z-RNA as well as Z-DNA. Proc Natl Acad Sci U S A. 2000;97:13532–13536. doi: 10.1073/pnas.240464097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft M, Grunert S, Murzin AG, Proctor M, St Johnston D. NMR solution structure of a dsRNA binding domain from Drosophila staufen protein reveals homology to the N-terminal domain of ribosomal protein S5. EMBO J. 1995;14:3563–3571. doi: 10.1002/j.1460-2075.1995.tb07362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Biased (A-->I) hypermutation of animal RNA virus genomes. Curr Opin Genet Dev. 1994;4:895–900. doi: 10.1016/0959-437x(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Chang K-Y, Ramos A. The double-stranded RNA-binding motif, a versatile macromolecular docking platform. FEBS J. 2005;272:2109–2117. doi: 10.1111/j.1742-4658.2005.04652.x. [DOI] [PubMed] [Google Scholar]
- Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA. 2000;6:755–767. doi: 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong C, Varani G, Tinoco I., Jr Solution structure of an unusually stable RNA hairpin, 5′GGAC(UUCG)GUCC. Nature. 1990;346:680–682. doi: 10.1038/346680a0. [DOI] [PubMed] [Google Scholar]
- Chilibeck KA, Wu T, Liang C, Schellenberg MJ, Gesner EM, Lynch JM, MacMillan AM. FRET analysis of in vivo dimerization by RNA-editing enzymes. J Biol Chem. 2006;281:16530–16535. doi: 10.1074/jbc.M511831200. [DOI] [PubMed] [Google Scholar]
- Cho D-SC, Yang W, Lee JT, Shiekhattar R, Murray JM, Nishikura K. Requirement of dimerization for RNA editing activity of adenosine deaminases acting on RNA. J Biol Chem. 2003;278:17093–17102. doi: 10.1074/jbc.M213127200. [DOI] [PubMed] [Google Scholar]
- Dawson TR, Sansam CL, Emeson RB. Structure and sequence determinants required for the RNA editing of ADAR2 substrates. J Biol Chem. 2004;279:4941–4951. doi: 10.1074/jbc.M310068200. [DOI] [PubMed] [Google Scholar]
- Desterro JMP, Keegan LP, Lafarga M, Berciano MT, O’Connell M, Carmo-Fonseca M. Dynamic association of RNA-editing enzymes with the nucleolus. J Cell Sci. 2003;116:1805–1818. doi: 10.1242/jcs.00371. [DOI] [PubMed] [Google Scholar]
- Eckmann CR, Neunteufl A, Pfaffstetter L, Jantsch MF. The human but not the Xenopus RNA-editing enzyme ADAR1 has an atypical nuclear localization signal and displays the characteristics of a shuttling protein. Mol Biol Cell. 2001;12:1911–1924. doi: 10.1091/mbc.12.7.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennifar E, Nikulin A, Tishchenko S, Serganov A, Nevskaya N, Garber M, Ehresmann B, Ehresmann C, Nikonov S, Dumas P. The crystal structure of UUCG tetraloop. J Mol Biol. 2000;304:35–42. doi: 10.1006/jmbi.2000.4204. [DOI] [PubMed] [Google Scholar]
- Fierro-Monti I, Mathews MB. Proteins binding to duplexed RNA: one motif, multiple functions. Trends Biochem Sci. 2000;25:241–246. doi: 10.1016/s0968-0004(00)01580-2. [DOI] [PubMed] [Google Scholar]
- Fritz J, Strehblow A, Taschner A, Schopoff S, Pasierbek P, Jantsch MF. RNA-regulated interaction of transportin-1 and exportin-5 with the double-stranded RNA-binding domain regulates nucleocytoplasmic shuttling of ADAR1. Mol Cell Biol. 2009;29:1487–1497. doi: 10.1128/MCB.01519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo A, Keegan LP, Ring GM, O’Connell MA. An ADAR that edits transcripts encoding ion channel subunits functions as a dimer. EMBO J. 2003;22:3421–3430. doi: 10.1093/emboj/cdg327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan J, Tropea JE, Austin BP, Court DL, Waugh DS, Ji X. Structural insight into the mechanism of double-stranded RNA processing by ribonuclease III. Cell. 2006;124:355–366. doi: 10.1016/j.cell.2005.11.034. [DOI] [PubMed] [Google Scholar]
- George C, Gan Z, Liu Y, Samuel C. Adenosine Deaminases Acting on RNA, RNA Editing, and Interferon Action. J Interferon Cytokine Res. 2011;31:99–117. doi: 10.1089/jir.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A, O’Connell MA, Keller W. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA. 1997;3:453–463. [PMC free article] [PubMed] [Google Scholar]
- Gott JM, Emeson RB. Functions and mechanisms of RNA editing. Annu Rev Genet. 2000;34:499–531. doi: 10.1146/annurev.genet.34.1.499. [DOI] [PubMed] [Google Scholar]
- Graveley BR. Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 2001;17:100–107. doi: 10.1016/s0168-9525(00)02176-4. [DOI] [PubMed] [Google Scholar]
- Green SR, Mathews MB. Two RNA-binding motifs in the double-stranded RNA-activated protein kinase, DAI. Genes Dev. 1992;6:2478–2490. doi: 10.1101/gad.6.12b.2478. [DOI] [PubMed] [Google Scholar]
- Ha SC, Choi J, Hwang H-Y, Rich A, Kim Y-G, Kim KK. The structures of non-CG-repeat Z-DNAs co-crystallized with the Z-DNA-binding domain, hZ alpha(ADAR1) Nucleic Acids Res. 2009;37:629–637. doi: 10.1093/nar/gkn976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan CJ, Palladino MJ, Ganetzky B, Reenan RA. RNA editing of the Drosophila para Na(+) channel transcript. Evolutionary conservation and developmental regulation. Genetics. 2000;155:1149–1160. doi: 10.1093/genetics/155.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SC, Aggarwal AK. DNA recognition by proteins with the helix-turn-helix motif. Annu Rev Biochem. 1990;59:933–969. doi: 10.1146/annurev.bi.59.070190.004441. [DOI] [PubMed] [Google Scholar]
- Heale BS, Keegan LP, McGurk L, Michlewski G, Brindle J, Stanton CM, Caceres JF, O’Connell MA. Editing independent effects of ADARs on the miRNA/siRNA pathways. EMBO J. 2009;28:3145–3156. doi: 10.1038/emboj.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A, Alfken J, Kim YG, Mian IS, Nishikura K, Rich A. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc Natl Acad Sci U S A. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A, Lowenhaupt K, Spitzner J, Rich A. Chicken double-stranded RNA adenosine deaminase has apparent specificity for Z-DNA. Proc Natl Acad Sci U S A. 1995;92:7550–7554. doi: 10.1073/pnas.92.16.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A, Rich A. Left-handed Z-DNA: structure and function. Genetica. 1999;106:37–47. doi: 10.1023/a:1003768526018. [DOI] [PubMed] [Google Scholar]
- Horikami SM, Moyer SA. Double-stranded RNA adenosine deaminase activity during measles virus infection. Virus Res. 1995;36:87–96. doi: 10.1016/0168-1702(94)00103-j. [DOI] [PubMed] [Google Scholar]
- Hundley HA, Bass BL. ADAR editing in double-stranded UTRs and other noncoding RNA sequences. Trends Biochem Sci. 2010;35:377–383. doi: 10.1016/j.tibs.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallman AM, Sahlin M, Ohman M. ADAR2 A->I editing: site selectivity and editing efficiency are separate events. Nucleic Acids Res. 2003;31:4874–4881. doi: 10.1093/nar/gkg681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y-M, Bang J, Lee E-H, Ahn H-C, Seo Y-J, Kim KK, Kim Y-G, Choi B-S, Lee J-H. NMR spectroscopic elucidation of the B-Z transition of a DNA double helix induced by the Z alpha domain of human ADAR1. J Am Chem Soc. 2009;131:11485–11491. doi: 10.1021/ja902654u. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Megraw M, Kreider E, Iizasa H, Valente L, Hatzigeorgiou AG, Nishikura K. Frequency and fate of microRNA editing in human brain. Nucleic Acids Res. 2008;36:5270–5280. doi: 10.1093/nar/gkn479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Zinshteyn B, Chendrimada TP, Shiekhattar R, Nishikura K. RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer-TRBP complex. EMBO Rep. 2007a;8:763–769. doi: 10.1038/sj.embor.7401011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007b;315:1137–1140. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo K, Samuel CE. Human RNA-specific adenosine deaminase (ADAR1) gene specifies transcripts that initiate from a constitutively active alternative promoter. Gene. 2000;258:165–172. doi: 10.1016/s0378-1119(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Keegan LP, Gallo A, O’Connell MA. The many roles of an RNA editor. Nat Rev Genet. 2001;2:869–878. doi: 10.1038/35098584. [DOI] [PubMed] [Google Scholar]
- Kharrat A, Macias MJ, Gibson TJ, Nilges M, Pastore A. Structure of the dsRNA binding domain of E. coli RNase III. EMBO J. 1995;14:3572–3584. doi: 10.1002/j.1460-2075.1995.tb07363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci U S A. 1994;91:11457–11461. doi: 10.1073/pnas.91.24.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-G, Lowenhaupt K, Oh D-B, Kim KK, Rich A. Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: Implications for development of a therapy for poxvirus infection. Proc Natl Acad Sci U S A. 2004;101:1514–1518. doi: 10.1073/pnas.0308260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Lowenhaupt K, Maas S, Herbert A, Schwartz T, Rich A. The zab domain of the human RNA editing enzyme ADAR1 recognizes Z-DNA when surrounded by B-DNA. J Biol Chem. 2000;275:26828–26833. doi: 10.1074/jbc.M003477200. [DOI] [PubMed] [Google Scholar]
- Koeris M, Funke L, Shrestha J, Rich A, Maas S. Modulation of ADAR1 editing activity by Z-RNA in vitro. Nucleic Acids Res. 2005;33:5362–5370. doi: 10.1093/nar/gki849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Chen CX, Carter KC, Nishikura K. Editing of glutamate receptor B subunit ion channel RNAs by four alternatively spliced DRADA2 double-stranded RNA adenosine deaminases. Mol Cell Biol. 1997;17:2413–2424. doi: 10.1128/mcb.17.5.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann KA, Bass BL. The importance of internal loops within RNA substrates of ADAR1. J Mol Biol. 1999;291:1–13. doi: 10.1006/jmbi.1999.2914. [DOI] [PubMed] [Google Scholar]
- Lehmann KA, Bass BL. Double-stranded RNA adenosine deaminases ADAR1 and ADAR2 have overlapping specificities. Biochemistry. 2000;39:12875–12884. doi: 10.1021/bi001383g. [DOI] [PubMed] [Google Scholar]
- Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, Olshansky M, et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol. 2004;22:1001–1005. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- Li JB, Levanon EY, Yoon JK, Aach J, Xie B, LeProust E, Zhang K, Gao Y, Church GM. Genome-Wide Identification of Human RNA Editing Sites by Parallel DNA Capturing and Sequencing. Science. 2009;324:1210–1213. doi: 10.1126/science.1170995. [DOI] [PubMed] [Google Scholar]
- Liu LF, Wang JC. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Gommans WM. Novel exon of mammalian ADAR2 extends open reading frame. PLoS One. 2009;4:e4225. doi: 10.1371/journal.pone.0004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Kawahara Y, Tamburro KM, Nishikura K. A-to-I RNA editing and human disease. RNA Biol. 2006;3:1–9. doi: 10.4161/rna.3.1.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Melcher T, Herb A, Seeburg PH, Keller W, Krause S, Higuchi M, O’Connell MA. Structural requirements for RNA editing in glutamate receptor pre-mRNAs by recombinant double-stranded RNA adenosine deaminase. J Biol Chem. 1996;271:12221–12226. doi: 10.1074/jbc.271.21.12221. [DOI] [PubMed] [Google Scholar]
- McCormack SJ, Thomis DC, Samuel CE. Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2 alpha protein kinase. Virology. 1992;188:47–56. doi: 10.1016/0042-6822(92)90733-6. [DOI] [PubMed] [Google Scholar]
- Melcher T, Maas S, Herb A, Sprengel R, Higuchi M, Seeburg PH. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J Biol Chem. 1996a;271:31795–31798. doi: 10.1074/jbc.271.50.31795. [DOI] [PubMed] [Google Scholar]
- Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996b;379:460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- Nishikura K. Editor meets silencer: crosstalk between RNA editing and RNA interference. Nat Rev Mol Cell Biol. 2006;7:919–931. doi: 10.1038/nrm2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem. 2010;79:321–349. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K, Yoo C, Kim U, Murray JM, Estes PA, Cash FE, Liebhaber SA. Substrate specificity of the dsRNA unwinding/modifying activity. EMBO J. 1991;10:3523–3532. doi: 10.1002/j.1460-2075.1991.tb04916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Sanders-Bush E, Emeson RB. Identification and characterization of RNA editing events within the 5-HT2C receptor. Ann N Y Acad Sci. 1998;861:38–48. doi: 10.1111/j.1749-6632.1998.tb10171.x. [DOI] [PubMed] [Google Scholar]
- O’Connell MA, Krause S, Higuchi M, Hsuan JJ, Totty NF, Jenny A, Keller W. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol Cell Biol. 1995;15:1389–1397. doi: 10.1128/mcb.15.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson J, Pedersen JS, Haussler D, Ohman M. Editing modifies the GABA(A) receptor subunit alpha3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman M. A-to-I editing challenger or ally to the microRNA process. Biochimie. 2007;89:1171–1176. doi: 10.1016/j.biochi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Ohman M, Kallman AM, Bass BL. In vitro analysis of the binding of ADAR2 to the pre-mRNA encoding the GluR-B R/G site. RNA. 2000;6:687–697. doi: 10.1017/s1355838200000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osenberg S, Paz Yaacov N, Safran M, Moshkovitz S, Shtrichman R, Sherf O, Jacob-Hirsch J, Keshet G, Amariglio N, Itskovitz-Eldor J, Rechavi G. Alu sequences in undifferentiated human embryonic stem cells display high levels of A-to-I RNA editing. PLoS One. 2010;5:e11173. doi: 10.1371/journal.pone.0011173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palavicini JP, O’Connell MA, Rosenthal JJ. An extra double-stranded RNA binding domain confers high activity to a squid RNA editing enzyme. RNA. 2009;15:1208–1218. doi: 10.1261/rna.1471209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA. 2000;6:1004–1018. doi: 10.1017/s1355838200000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol Cell Biol. 1995;15:5376–5388. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Thomis DC, Hans SL, Samuel CE. Mechanism of interferon action: double-stranded RNA-specific adenosine deaminase from human cells is inducible by alpha and gamma interferons. Virology. 1995;210:508–511. doi: 10.1006/viro.1995.1370. [DOI] [PubMed] [Google Scholar]
- Peck LJ, Nordheim A, Rich A, Wang JC. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982;79:4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placido D, Brown BAn, Lowenhaupt K, Rich A, Athanasiadis A. A left-handed RNA double helix bound by the Z alpha domain of the RNA-editing enzyme ADAR1. Structure. 2007;15:395–404. doi: 10.1016/j.str.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokharel S, Beal PA. High-throughput screening for functional adenosine to inosine RNA editing systems. ACS Chem Biol. 2006;1:761–765. doi: 10.1021/cb6003838. [DOI] [PubMed] [Google Scholar]
- Polson AG, Bass BL. Preferential selection of adenosines for modification by double-stranded RNA adenosine deaminase. EMBO J. 1994;13:5701–5711. doi: 10.1002/j.1460-2075.1994.tb06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson AG, Bass BL, Casey JL. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature. 1996;380:454–456. doi: 10.1038/380454a0. [DOI] [PubMed] [Google Scholar]
- Poulsen H, Jorgensen R, Heding A, Nielsen FC, Bonven B, Egebjerg J. Dimerization of ADAR2 is mediated by the double-stranded RNA binding domain. RNA. 2006;12:1350–1360. doi: 10.1261/rna.2314406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen H, Nilsson J, Damgaard CK, Egebjerg J, Kjems J. CRM1 mediates the export of ADAR1 through a nuclear export signal within the Z-DNA binding domain. Mol Cell Biol. 2001;21:7862–7871. doi: 10.1128/MCB.21.22.7862-7871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullirsch D, Jantsch MF. Proteome diversification by adenosine to inosine RNA editing. RNA Biol. 2010;7:205–212. doi: 10.4161/rna.7.2.11286. [DOI] [PubMed] [Google Scholar]
- Ramos A, Grunert S, Adams J, Micklem DR, Proctor MR, Freund S, Bycroft M, St Johnston D, Varani G. RNA recognition by a Staufen double-stranded RNA-binding domain. EMBO J. 2000;19:997–1009. doi: 10.1093/emboj/19.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati MR, Melton DA. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987;48:599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Reenan RA. Molecular determinants and guided evolution of species-specific RNA editing. Nature. 2005;434:409–413. doi: 10.1038/nature03364. [DOI] [PubMed] [Google Scholar]
- Rich A, Zhang S. Timeline: Z-DNA: the long road to biological function. Nat Rev Genet. 2003;4:566–572. doi: 10.1038/nrg1115. [DOI] [PubMed] [Google Scholar]
- Riedmann EM, Schopoff S, Hartner JC, Jantsch MF. Specificity of ADAR-mediated RNA editing in newly identified targets. RNA. 2008;14:1110–1118. doi: 10.1261/rna.923308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenger W. Principles of nucleic acid structure. Springer; 1984. [Google Scholar]
- Samuel CE. Adenosine deaminases acting on RNA (ADARs) are both antiviral and proviral. Virology. 2011 doi: 10.1016/j.virol.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansam CL, Wells KS, Emeson RB. Modulation of RNA editing by functional nucleolar sequestration of ADAR2. Proc Natl Acad Sci U S A. 2003;100:14018–14023. doi: 10.1073/pnas.2336131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LR, Barber GN. The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J. 2003;17:961–983. doi: 10.1096/fj.02-0958rev. [DOI] [PubMed] [Google Scholar]
- Schade M, Turner CJ, Kuhne R, Schmieder P, Lowenhaupt K, Herbert A, Rich A, Oschkinat H. The solution structure of the Zalpha domain of the human RNA editing enzyme ADAR1 reveals a prepositioned binding surface for Z-DNA. Proc Natl Acad Sci U S A. 1999;96:12465–12470. doi: 10.1073/pnas.96.22.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T, Lowenhaupt K, Kim YG, Li L, Brown BAn, Herbert A, Rich A. Proteolytic dissection of Zab, the Z-DNA-binding domain of human ADAR1. J Biol Chem. 1999a;274:2899–2906. doi: 10.1074/jbc.274.5.2899. [DOI] [PubMed] [Google Scholar]
- Schwartz T, Rould MA, Lowenhaupt K, Herbert A, Rich A. Crystal structure of the Zalpha domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science. 1999b;284:1841–1845. doi: 10.1126/science.284.5421.1841. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res Brain Res Rev. 1998;26:217–229. doi: 10.1016/s0165-0173(97)00062-3. [DOI] [PubMed] [Google Scholar]
- Seeman NC, Rosenberg JM, Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci U S A. 1976;73:804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y-J, Ahn H-C, Lee E-H, Bang J, Kang Y-M, Kim H-E, Lee Y-M, Kim K, Choi B-S, Lee J-H. Sequence discrimination of the Zalpha domain of human ADAR1 during B-Z transition of DNA duplexes. FEBS Lett. 2010;584:4344–4350. doi: 10.1016/j.febslet.2010.09.036. [DOI] [PubMed] [Google Scholar]
- Slavov D, Clark M, Gardiner K. Comparative analysis of the RED1 and RED2 A-to-I RNA editing genes from mammals, pufferfish and zebrafish. Gene. 2000;250:41–51. doi: 10.1016/s0378-1119(00)00174-8. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Brown NH, Gall JG, Jantsch M. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci U S A. 1992;89:10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefl R, Allain FH-T. A novel RNA pentaloop fold involved in targeting ADAR2. RNA. 2005;11:592–597. doi: 10.1261/rna.7276805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefl R, Oberstrass FC, Hood JL, Jourdan M, Zimmermann M, Skrisovska L, Maris C, Peng L, Hofr C, Emeson RB, Allain FH-T. The solution structure of the ADAR2 dsRBM-RNA complex reveals a sequence-specific readout of the minor groove. Cell. 2010;143:225–237. doi: 10.1016/j.cell.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefl R, Skrisovska L, Allain FH-T. RNA sequence- and shape-dependent recognition by proteins in the ribonucleoprotein particle. EMBO Rep. 2005;6:33–38. doi: 10.1038/sj.embor.7400325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefl R, Xu M, Skrisovska L, Emeson RB, Allain FH-T. Structure and specific RNA binding of ADAR2 double-stranded RNA binding motifs. Structure. 2006;14:345–355. doi: 10.1016/j.str.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Steitz TA. Structural studies of protein-nucleic acid interaction: the sources of sequence-specific binding. Q Rev Biophys. 1990;23:205–280. doi: 10.1017/s0033583500005552. [DOI] [PubMed] [Google Scholar]
- Strehblow A, Hallegger M, Jantsch MF. Nucleocytoplasmic distribution of human RNA-editing enzyme ADAR1 is modulated by double-stranded RNA-binding domains, a leucine-rich export signal, and a putative dimerization domain. Mol Biol Cell. 2002;13:3822–3835. doi: 10.1091/mbc.E02-03-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DR, Puig M, Darnell ME, Mihalik K, Feinstone SM. New antiviral pathway that mediates hepatitis C virus replicon interferon sensitivity through ADAR1. J Virol. 2005;79:6291–6298. doi: 10.1128/JVI.79.10.6291-6298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin LA, Bass BL. Mutations in RNAi rescue aberrant chemotaxis of ADAR mutants. Science. 2003;302:1725. doi: 10.1126/science.1091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin LA, Saccomanno L, Morse DP, Brodigan T, Krause M, Bass BL. RNA editing by ADARs is important for normal behavior in Caenorhabditis elegans. EMBO J. 2002;21:6025–6035. doi: 10.1093/emboj/cdf607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente L, Nishikura K. RNA binding-independent dimerization of adenosine deaminases acting on RNA and dominant negative effects of nonfunctional subunits on dimer functions. J Biol Chem. 2007;282:16054–16061. doi: 10.1074/jbc.M611392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Wagner RW, Smith JE, Cooperman BS, Nishikura K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci U S A. 1989;86:2647–2651. doi: 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AH, Quigley GJ, Kolpak FJ, Crawford JL, van Boom JH, van der Marel G, Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979;282:680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wong SK, Sato S, Lazinski DW. Substrate recognition by ADAR1 and ADAR2. RNA. 2001;7:846–858. doi: 10.1017/s135583820101007x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TC, Ayata M, Ueda S, Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991;65:2191–2199. doi: 10.1128/jvi.65.5.2191-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Henras A, Chanfreau G, Feigon J. Structural basis for recognition of the AGNN tetraloop RNA fold by the double-stranded RNA-binding domain of Rnt1p RNase III. Proc Natl Acad Sci U S A. 2004;101:8307–8312. doi: 10.1073/pnas.0402627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff B, Nishikura K. Substitutional A-to-I RNA editing. WIREs RNA. 2010;1:90–101. doi: 10.1002/wrna.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff BE, Sakurai M, Nishikura K. Elucidating the inosinome: global approaches to adenosine-to-inosine RNA editing. Nat Rev Genet. 2011;12:81–85. doi: 10.1038/nrg2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wells KS, Emeson RB. Substrate-dependent contribution of double-stranded RNA-binding motifs to ADAR2 function. Mol Biol Cell. 2006;17:3211–3220. doi: 10.1091/mbc.E06-02-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Carmichael GG. The fate of dsRNA in the nucleus: a p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell. 2001;106:465–475. doi: 10.1016/s0092-8674(01)00466-4. [DOI] [PubMed] [Google Scholar]


