Abstract
Polymorphonuclear neutrophils (PMN) from patients with chronic granulomatous disease of childhood have impaired bactericidal activity and are deficient in diphosphopyridine nucleotide, reduced form of, (NADH) oxidase. Since hydrocortisone had been shown to inhibit NADH oxidation, experiments were undertaken to determine the effect of hydrocortisone on several parameters of human PMN function.
The phagocytic and bactericidal capacity of PMN with or without hydrocortisone (2.1 mM) was determined by quantitation of cell-free, cell-associated, and total bacteria. Phagocytosis of Staphylococcus aureus and several gram-negative rods was unimpaired by the presence of hydrocortisone in the media. In contrast, killing of bacteria was markedly impaired by hydrocortisone. After 30 min of incubation, there were 20-400 times as many bacteria surviving in hydrocortisone-treated PMN as in simultaneously run controls without hydrocortisone.
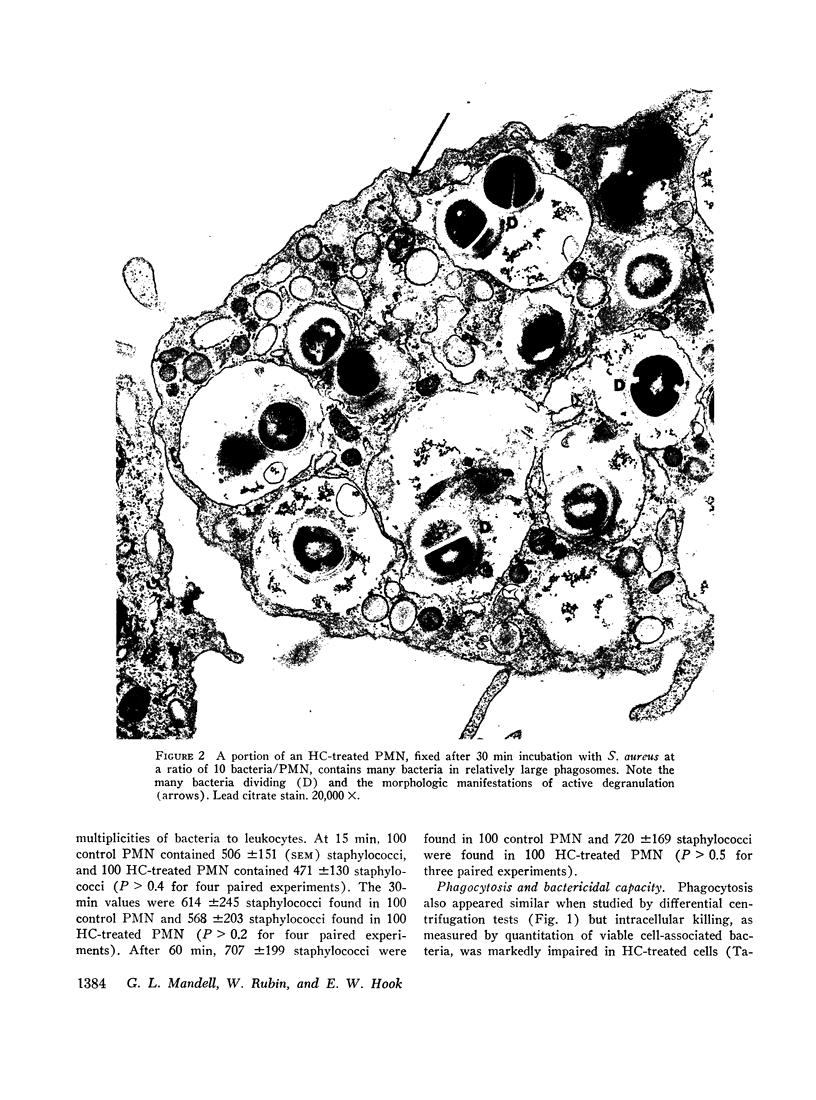
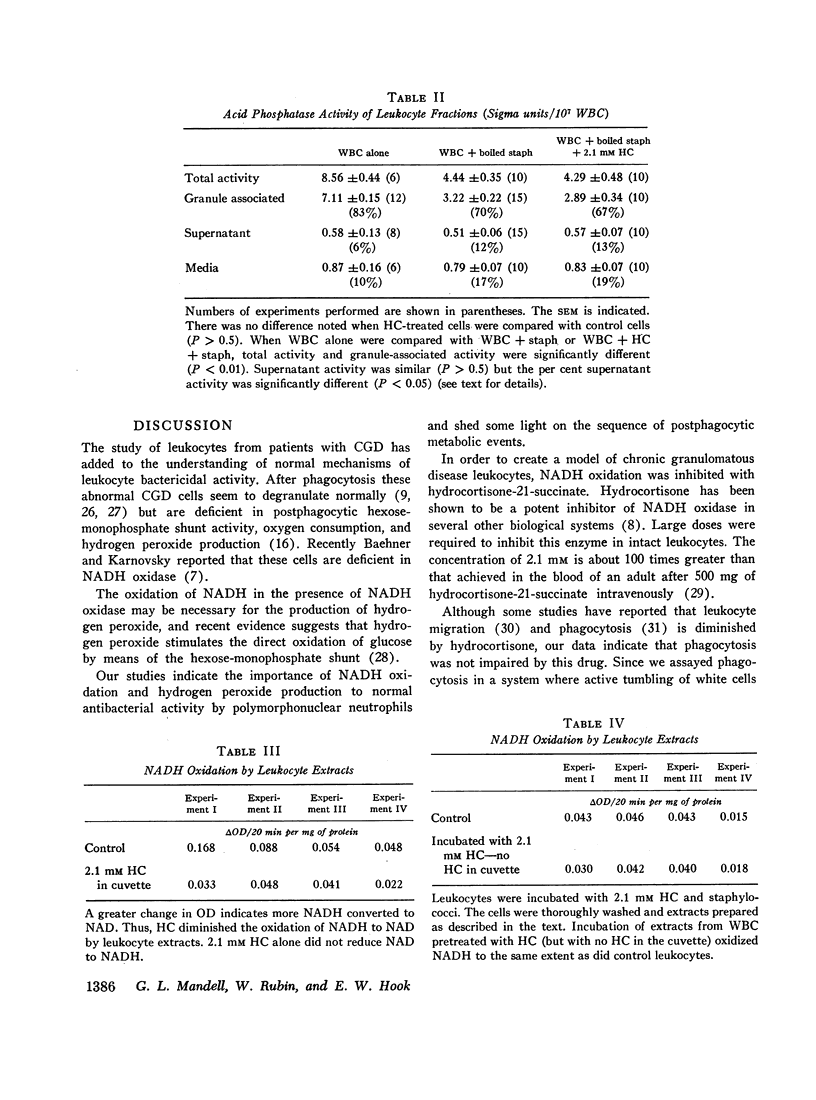
The defect of intracellular killing noted in the presence of hydrocortisone was not related to impaired degranulation. Quantitative kinetic studies of degranulation revealed no difference in the release of granule associated acid phosphatase in hydrocortisone-treated and control PMN after phagocytosis. Electron microscopy of PMN also indicated that the presence of hydrocortisone had no effect on the extent of degranulation after phagocytosis. These observations were confirmed by studies using histochemical techniques to detect lysosomal enzymes.
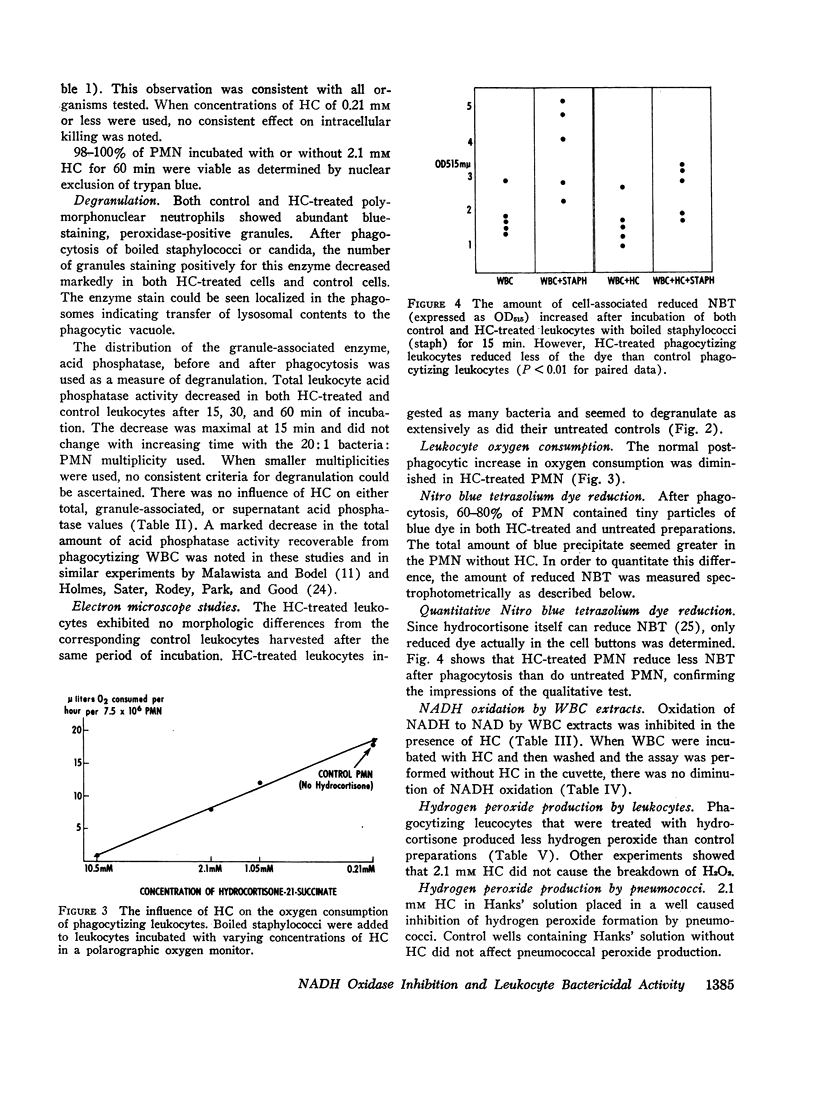
After phagocytosis, hydrocortisone-treated PMN demonstrated less NADH oxidase activity, oxygen consumption, and hydrogen peroxide production than postphagocytic control PMN. In addition, Nitro blue tetrazolium dye reduction was diminished in hydrocortisone-treated PMN.
Thus, impairment of NADH oxidase activity in normal human PMN by hydrocortisone results in reduced intracellular killing of bacteria, diminished postphagocytic oxygen consumption, decreased ability to reduce Nitro blue tetrazolium, and decreased hydrogen peroxide production. These abnormalities are similar to those seen in the PMN of patients with chronic granulomatous disease of childhood.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. L. Deficiency of reduced nicotinamide-adenine dinucleotide oxidase in chronic granulomatous disease. Science. 1968 Dec 13;162(3859):1277–1279. doi: 10.1126/science.162.3859.1277. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967 Feb 17;155(3764):835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- CAGAN R. H., KARNOVSKY M. L. ENZYMATIC BASIS OF THE RESPIRATORY STIMULATION DURING PHAGOCYTOSIS. Nature. 1964 Oct 17;204:255–257. doi: 10.1038/204255a0. [DOI] [PubMed] [Google Scholar]
- CREPEA S. B., MAGNIN G. E., SEASTONE C. V. Effect of ACTH and cortisone on phagocytosis. Proc Soc Exp Biol Med. 1951 Aug;77(4):704–706. doi: 10.3181/00379727-77-18899. [DOI] [PubMed] [Google Scholar]
- Evans H. M., Schulemann W. THE ACTION OF VITAL STAINS BELONGING TO THE BENZIDINE GROUP. Science. 1914 Mar 27;39(1004):443–454. doi: 10.1126/science.39.1004.443. [DOI] [PubMed] [Google Scholar]
- Good R. A., Quie P. G., Windhorst D. B., Page A. R., Rodey G. E., White J., Wolfson J. J., Holmes B. H. Fatal (chronic) granulomatous disease of childhood: a hereditary defect of leukocyte function. Semin Hematol. 1968 Jul;5(3):215–254. [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KETCHEL M. M., FAVOUR C. B., STURGIS S. H. The in vitro action of hydrocortisone on leucocyte migration. J Exp Med. 1958 Feb 1;107(2):211–218. doi: 10.1084/jem.107.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUS F. W., NICKERSON J. F., PERRY W. I., WALKER A. P. Peroxide and peroxidogenic bacteria in human saliva. J Bacteriol. 1957 Jun;73(6):727–735. doi: 10.1128/jb.73.6.727-735.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauder E., Kahle L. L., Moreno H., Partin J. C. Leukocyte degranulation and vacuole formation in patients with chronic granulomatous disease of childhood. J Clin Invest. 1968 Aug;47(8):1753–1762. doi: 10.1172/JCI105865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., White L. R. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N Engl J Med. 1969 Feb 27;280(9):460–466. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Hook E. W. Leukocyte bactericidal activity in chronic granulomatous disease: correlation of bacterial hydrogen peroxide production and susceptibility to intracellular killing. J Bacteriol. 1969 Oct;100(1):531–532. doi: 10.1128/jb.100.1.531-532.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Hook E. W. Leukocyte function in chronic granulomatous disease of childhood. Studies on a seventeen year old boy. Am J Med. 1969 Sep;47(3):473–486. doi: 10.1016/0002-9343(69)90231-9. [DOI] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON R. E., WYNGAARDEN J. B., GUERRA S. L., BRODIE B. B., BUNIM J. J. The physiological disposition and metabolic fate of hydrocortisone in man. J Clin Invest. 1955 Dec;34(12):1779–1794. doi: 10.1172/JCI103233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D. E., TOMPSETT R. The survival of staphylococci within human leukocytes. J Exp Med. 1952 Feb;95(2):209–230. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium. J Biophys Biochem Cytol. 1958 Nov 25;4(6):727–730. doi: 10.1083/jcb.4.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. Studies on lysosomes. II. The effect of cortisone on the release of acid hydrolases from a large granule fraction of rabbit liver induced by an excess of vitamin A. J Clin Invest. 1963 May;42:661–669. doi: 10.1172/JCI104757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhorst D. B., Holmes B., Good R. A. A newly defined X-linked trait in man with demonstration of the Lyon effect in carrier females. Lancet. 1967 Apr 8;1(7493):737–739. doi: 10.1016/s0140-6736(67)91360-8. [DOI] [PubMed] [Google Scholar]
- Yielding K. L., Tomkins G. M. INHIBITION OF THE ENZYMIC OXIDATION OF DPNH BY STER HORMONES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1730–1739. doi: 10.1073/pnas.45.12.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZATTI M., ROSSI F., MENEGHELLI V. METABOLIC AND MORPHOLOGICAL CHANGES OF POLYMORPHONUCLEAR LEUCOCYTES DURING PHAGOCYTOSIS. Br J Exp Pathol. 1965 Apr;46:227–233. [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Arginine-rich proteins of polymorphonuclear leukocyte lysosomes. Antimicrobial specificity and biochemical heterogeneity. J Exp Med. 1968 May 1;127(5):927–941. doi: 10.1084/jem.127.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]