Abstract
Introduction
Severe acute pancreatitis (SAP) requiring admission to a critical care unit is associated with high mortality and long lengths of stay. We describe the case mix, outcome, and activity of admissions with SAP who were identified from a high-quality clinical database.
Methods
We conducted a secondary analysis of the ICNARC (Intensive Care National Audit & Research Centre) Case Mix Programme Database of 219,468 admissions to 159 adult, general critical care units in England, Wales, and Northern Ireland for the period of 1995 to 2003 to identify admissions with SAP. The ability of the modified Glasgow criteria to discriminate hospital survivors from non-survivors was compared to that of the Acute Physiology and Chronic Health Evaluation (APACHE) II score and a number of individual physiological parameters.
Results
A total of 2,677 admissions with SAP were identified (1.2% of all admissions). Mortality for these admissions was 31% in the critical care unit and 42% in hospital. The median length of stay in the critical care unit was 3.8 days and was similar for survivors and non-survivors. Increasing numbers of modified Glasgow criteria were associated with increasing hospital mortality, but better discrimination was provided by the APACHE II score and by several physiological parameters.
Conclusion
SAP requiring critical care is associated with high mortality and long length of stay. The modified Glasgow criteria represent a simple measure of severity but were not designed to predict hospital mortality. It may be possible to develop a specific model for risk prediction in patients with SAP requiring critical care.
Introduction
Acute pancreatitis affects approximately 10 to 20 per million of the UK population per year [1] and approximately 25% of these require some form of critical care [2]. Severe acute pancreatitis (SAP) requiring admission to a critical care unit is associated with high mortality [1,3]. Management of SAP on the critical care unit is resource-intensive [4], and admissions have long stays in critical care [5]. However, data on outcomes and activity are sparse and predominantly from single specialist centres with limited numbers of patients.
A number of severity scoring approaches exist for acute pancreatitis, the most commonly used being the criteria of Ranson and colleagues [6], the Glasgow (Imrie) criteria [7], and the modified Glasgow criteria [8]. These scoring systems were devised predominantly for identifying SAP, and it is not clear how useful they are as prognostic tools in a critical care setting.
We interrogated a large national critical care database to ascertain the frequency of admissions with SAP, demographics of the patients, their first 24-hour illness severity, and subsequent outcome and activity. We applied the definitions of the modified Glasgow criteria and compared the discrimination of this score for predicting hospital mortality with that of the Acute Physiology and Chronic Health Evaluation (APACHE) II score and a number of physiological parameters.
Materials and methods
Case Mix Programme Database
Data were extracted for 219,468 admissions to 159 adult, general critical care units–intensive care units (ICUs) and combined intensive care/high-dependency units–from the Intensive Care National Audit & Research Centre (ICNARC) Case Mix Programme Database (CMPD) for the period of December 1995 to June 2003. Data were collected prospectively and abstracted locally by trained data collectors according to precise rules and definitions. The data underwent extensive validation before incorporation into the CMPD. The process of data validation and cleaning has been described in detail previously [9].
Selection of cases
The primary and secondary reasons for admission to the critical care unit are coded in the CMPD by means of a hierarchical coding system, the ICNARC Coding Method [10]. Admissions were identified as having SAP if their primary reason for admission was recorded as 'acute pancreatitis,' 'chronic pancreatitis,' or 'infective pancreatitis.' Admissions transferred in from another critical care unit were excluded. APACHE II exclusions (age less than 16 years, length of stay less than 8 hours) were applied when calculating APACHE II scores only [11]. Readmissions to the critical care unit within the same hospital stay were excluded from calculation of ultimate hospital mortality and length of stay in hospital.
Case mix
Age at admission and gender were extracted. Surgical status was defined by the source of admission to the critical care unit and (for admissions from theatre) by the National Confidential Enquiry into Patient Outcome and Death classification of surgery as elective (including scheduled) or emergency (including urgent). Three serious conditions from the past medical history were extracted using data collected as part of the chronic health evaluation section of the APACHE II score [11]. These were biopsy-proven cirrhosis documented at any time prior to or at admission to the critical care unit; hepatic encephalopathy (grade 1 or greater) occurring within the six months prior to admission; and steroid treatment, defined as more than or equal to 3 mg/kg prednisolone or equivalent daily for the six months prior to admission.
Mechanical ventilation during the first 24 hours following admission to the unit was identified by the recording of a ventilated respiratory rate. The following physiological parameters, selected a priori, were extracted from the data collected during the first 24 hours in the ICU: lowest arterial oxygen tension (PaO2), lowest PaO2/FIO2(PaO2/fractional inspired oxygen) gradient, lowest arterial pH, highest serum urea, highest serum creatinine, highest serum glucose, highest total serum bilirubin, lowest total serum calcium, lowest ionised serum calcium, lowest serum albumin, highest white blood cell count, and lowest platelet count. Overall acute severity of illness was summarised with the APACHE II Acute Physiology Score (APS) and the APACHE II score.
Seven of the eight modified Glasgow criteria for assessing severity of pancreatitis [8] were matched based on data available from the first 24 hours following admission to the unit. These were age older than 55 years, white blood cell count greater than 15 × 109/l, serum glucose greater than 10 mmol/l, serum urea greater than 16 mmol/l, PaO2 less than 8 kPa, serum albumin less than 32 g/l, and total serum calcium less than 2 mmol/l. The remaining criterion that could not be matched was lactate dehydrogenase more than 600 IU/l (lactate dehydrogenase is not recorded in the CMPD). According to the original definitions, these parameters should be assessed during a 48-hour period. However, data were available for only 24 hours.
Outcome
Mortality data were collected at discharge from the admitting critical care unit (critical care unit mortality) and at ultimate discharge from an acute hospital (ultimate hospital mortality).
Activity
Length of stay in the critical care unit was calculated (in fraction of days) from the date/time of admission to and date/time of discharge from or death in the critical care unit. For critical care unit survivors, post-discharge length of stay in hospital was calculated (in days) from the date of discharge from the critical care unit and date of ultimate discharge from an acute hospital or death in hospital. Transfers out to another ICU were identified as critical care unit survivors whose destination following discharge from the unit was an ICU in the same or another hospital. Readmissions to the critical care unit in the same hospital stay were identified from the postcode, date of birth, and gender and were confirmed by the participating units.
Analyses
The case mix, outcome, and activity of admissions with SAP were described using the variables defined above with numbers and percentages (for categorical variables) or medians and interquartile ranges (for continuous variables).
Ultimate hospital mortality was also reported by the number of modified Glasgow criteria and the APACHE II score. The ability of the modified Glasgow criteria to discriminate survivors from non-survivors was explored with receiver operating characteristic (ROC) curves. The ROC curve for the number of modified Glasgow criteria was compared to those for the APACHE II score and for the 12 individual physiological parameters listed above.
The effect of case mix on length of stay in the critical care unit was explored by comparing median lengths of stay by quartiles of APACHE II score and survival status. All analyses were performed using Stata 8.2 (StataCorp LP, College Station, TX, USA).
Results
Selection of cases
In total, 2,677 admissions were identified as SAP, representing 1.2% of all admissions in the CMPD. Of these, 2,376 (88.8%) were coded as acute pancreatitis, 159 (5.9%) as infective pancreatitis, and 142 (5.3%) as chronic pancreatitis. All were considered to represent SAP because their pancreatitis was recorded as the primary reason for admission to the critical care unit. Admissions coded with chronic pancreatitis were assumed to have a severe acute exacerbation of chronic pancreatitis.
Case mix
The case mix of admissions with SAP is summarised in Table 1. The age and gender distributions were comparable to those of all admissions in the CMPD [9]; however, as one would expect, the majority of admissions (84%) were non-surgical. Serious conditions in the past medical history were rare, and only 8, 13, and 27 admissions met the definitions of hepatic encephalopathy, biopsy-proven cirrhosis, and steroid treatment, respectively. The median APACHE II APS (for eligible admissions) was 13, and the median APACHE II score was 17. Between 14% and 72% of admissions met each of the seven modified Glasgow criteria.
Table 1.
Case mix for patients with severe acute pancreatitis who were admitted to adult, general critical care units
| Case mix | N | Median (IQR) or n (%) | |
|---|---|---|---|
| Age in yearsa | 2,677 | 63 | (48–74) |
| Genderb | 2,677 | ||
| Male | 1,500 | (56.0) | |
| Female | 1,177 | (44.0) | |
| Surgical statusc | 2,675 | ||
| Non-surgical | 2,243 | (83.9) | |
| Elective/scheduled | 147 | (5.5) | |
| Emergency/urgent | 285 | (10.7) | |
| Past medical historyc | 2,633 | ||
| Biopsy-proven cirrhosis | 13 | (0.5) | |
| Hepatic encephalopathy | 8 | (0.3) | |
| Steroid treatment | 27 | (1.0) | |
| Mechanical ventilationc | 2,671 | 1,242 | (46.5) |
| Physiologya | |||
| Lowest PaO2, kPa | 2,470 | 9.8 | (8.6–11.3) |
| Lowest PaO2/FIO2, kPa | 2,467 | 20.4 | (13.7–29.8) |
| Lowest arterial pH | 2,467 | 7.30 | (7.20–7.36) |
| Highest serum urea, mmol/l | 2,307 | 9.3 | (5.3–16.0) |
| Highest serum creatinine, μmol/l | 2,559 | 112 | (78–212) |
| Highest serum glucose, mmol/l | 2,243 | 8.8 | (6.9–11.5) |
| Highest total serum bilirubin, μmol/l | 1,969 | 20 | (12–37) |
| Lowest total serum calcium, mmol/l | 1,791 | 1.86 | (1.62–2.08) |
| Lowest ionised serum calcium, mmol/l | 548 | 1.1 | (0.97–1.84) |
| Lowest serum albumin, g/l | 2,118 | 22 | (17–27) |
| Highest white blood cell count, × 109/l | 2,556 | 14.2 | (10.3–19.4) |
| Lowest platelet count, × 109/l | 2,320 | 174 | (122–240) |
| APACHE IIa | 2,434b | ||
| Acute Physiology Score | 13 | (9–17) | |
| APACHE II score | 17 | (13–22) | |
| Modified Glasgow criteriac | 2,677 | ||
| Serum albumin <32 g/l | 1,926 | (71.9) | |
| Age >55 years | 1,729 | (64.6) | |
| Total serum calcium <2 mmol/l | 1,179 | (44.0) | |
| White blood cell count >15 × 109/l | 1,163 | (43.4) | |
| Serum glucose >10 mmol/l | 826 | (30.9) | |
| Serum urea >16 mmol/l | 573 | (21.4) | |
| PaO2 <8 kPa | 375 | (14.0) | |
aValues expressed as median (IQR). bExclusion criteria: age less than 16 years, length of stay less than 8 hours. cValues expressed as number (percentage). APACHE, Acute Physiology and Chronic Health Evaluation; FIO2, fractional inspired oxygen; IQR, interquartile range; N, number of non-missing observations; PaO2, arterial oxygen tension.
Outcome
Of all admissions with SAP, 31% died in the admitting critical care unit (Table 2). Of all patients with SAP admitted to a critical care unit (excluding readmissions of the same patient within the same hospital stay), 42% died before ultimate discharge from an acute hospital (Table 2). Increasing numbers of modified Glasgow criteria were associated with increasing mortality, rising from 13% for admissions meeting no modified Glasgow criteria to 100% for the five admissions meeting all seven modified Glasgow criteria (Figure 1). Ultimate hospital mortality also increased with increasing APACHE II score, rising from 6% for admissions with an APACHE II score below 10 to 100% for the 13 admissions with an APACHE II score of 40 or higher (Figure 2).
Table 2.
Unit and hospital outcome and activity for admissions with severe acute pancreatitis
| Outcome | N | Deaths | Percentage | |
|---|---|---|---|---|
| Critical care unit mortality | 2,677 | 818 | 30.6 | |
| Ultimate hospital mortalitya | 2,473 | 1,035 | 41.9 | |
| Activity | N | Median | IQR | |
| Length of stay in days | ||||
| Critical care unit | Survivors | 1,854 | 3.7 | 1.6–10.1 |
| Non-survivors | 814 | 3.8 | 1.1–13.4 | |
| All | 2,668 | 3.8 | 1.4–11.0 | |
| Post-dischargea,b | Hospital survivors | 1,427 | 15 | 9–30 |
| Non-survivors | 220 | 14 | 5–38 | |
| All | 1,647 | 15 | 8–30 | |
| Activity | N | n | Percentage | |
| Transferred out to another intensive care unit | 2,677 | 135 | 5.0 | |
| Readmission within the same hospital stay | 2,677 | 131 | 4.9 | |
aExcluding readmissions within the same hospital stay. bCritical care unit survivors only. IQR, interquartile range; N, number of non-missing observations.
Figure 1.
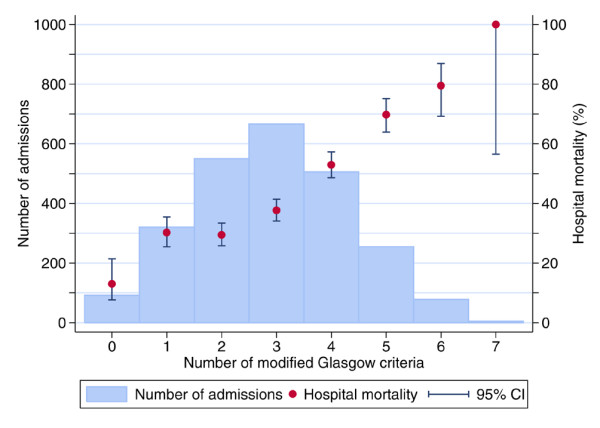
Ultimate hospital mortality by total number of modified Glasgow criteria. CI, confidence interval.
Figure 2.
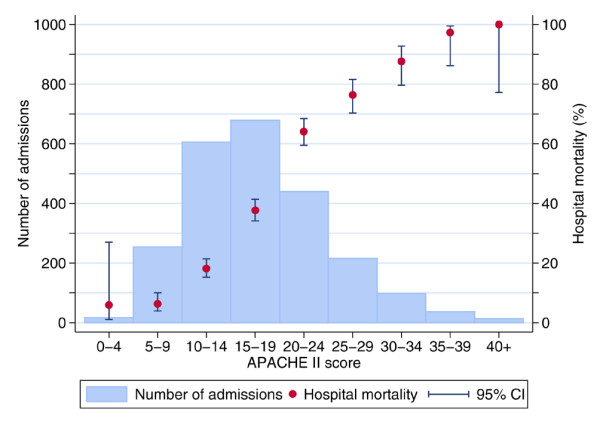
Ultimate hospital mortality by Acute Physiology and Chronic Health Evaluation (APACHE) II score. CI, confidence interval.
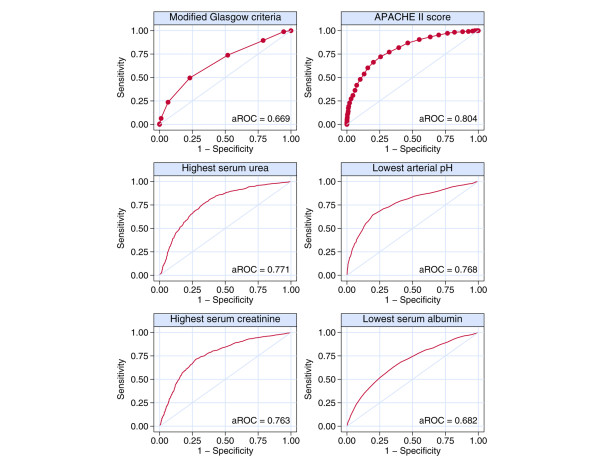
Areas under the ROC curves (aROCs) for the number of modified Glasgow criteria, APACHE II score, and 12 physiological parameters are shown in Table 3. The number of modified Glasgow criteria showed moderate ability to discriminate between hospital survivors and non-survivors (aROC = 0.669), but this was exceeded by the APACHE II score (aROC = 0.804) and four of the individual physiological parameters: highest serum urea, lowest arterial pH, highest serum creatinine, and lowest serum albumin. The ROC curves for the number of modified Glasgow criteria, the APACHE II score, and these four physiological parameters are shown in Figure 3.
Table 3.
Area under the receiver operating characteristic curve for ultimate hospital mortality
| Variable | Na | aROC | 95% CI |
|---|---|---|---|
| Number of modified Glasgow criteria | 2,473 | 0.669 | 0.648–0.691 |
| APACHE II score | 2,369 | 0.804 | 0.786–0.821 |
| Highest serum urea, mmol/l | 2,140 | 0.771 | 0.751–0.791 |
| Lowest arterial pH | 2,288 | 0.768 | 0.748–0.788 |
| Highest serum creatinine, μmol/l | 2,370 | 0.763 | 0.744–0.783 |
| Lowest serum albumin, g/l | 1,972 | 0.682 | 0.658–0.706 |
| Lowest PaO2/FIO2, kPa | 2,291 | 0.660 | 0.638–0.683 |
| Lowest total serum calcium, mmol/l | 1,663 | 0.617 | 0.589–0.645 |
| Lowest platelet count, × 109/l | 2,152 | 0.599 | 0.574–0.623 |
| Lowest PaO2, kPa | 2,291 | 0.565 | 0.542–0.589 |
| Highest serum glucose, mmol/l | 2,078 | 0.560 | 0.534–0.585 |
| Highest total serum bilirubin, μmol/l | 1,826 | 0.545 | 0.518–0.572 |
| Lowest ionised serum calcium, mmol/l | 515 | 0.527 | 0.476–0.578 |
| Highest white blood cell count, × 109/l | 2,368 | 0.524 | 0.500–0.549 |
aExcluding readmissions within the same hospital stay and admissions missing ultimate hospital outcome. APACHE, Acute Physiology and Chronic Health Evaluation; aROC, area under the receiver operating characteristic curve; CI, confidence interval; FIO2, fractional inspired oxygen; N, number of non-missing observations; PaO2, arterial oxygen tension.
Figure 3.
Receiver operating characteristic curves for predicting ultimate hospital mortality by number of modified Glasgow criteria, Acute Physiology and Chronic Health Evaluation (APACHE) II score, and four physiological variables. aROC, area under the receiver operating characteristic curve.
Activity
Overall median length of stay in the critical care unit was similar for survivors and non-survivors (3.7 versus 3.8 days) (Table 2). However, median length of stay increased with increasing severity of illness for survivors and decreased with increasing severity of illness for non-survivors (Figure 4). Length of stay in hospital after discharge from the critical care unit was also similar for hospital survivors and non-survivors (median 15 versus 14 days) (Table 2), although the lengths of stay of non-survivors were more variable (interquartile range 5 to 38 days compared to 9 to 30 days for survivors). Five percent of all admissions with SAP were transferred out to another ICU, and 5% of admissions represented a readmission of a patient previously admitted during the same hospital stay (Table 2).
Figure 4.
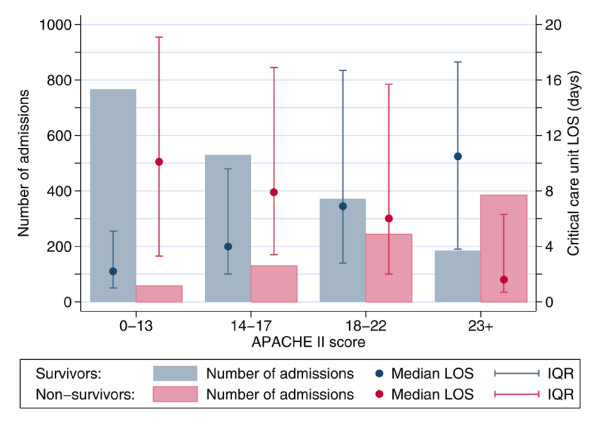
Length of stay (LOS) in the critical care unit by Acute Physiology and Chronic Health Evaluation (APACHE) II score and survival status. IQR, interquartile range.
Discussion
This paper presents case mix, outcome, and activity data for a large cohort of patients with SAP who were identified from a high-quality clinical database. The modified Glasgow criteria were found to provide a simple score that summarised the severity of acute pancreatitis, with an increasing number of modified Glasgow criteria associated with increasing mortality and providing moderate discrimination between hospital survivors and non-survivors. However, better discrimination was provided by the APACHE II score and by several individual physiological measurements.
The main strength of this study is the large cohort, which is representative of all patients with SAP who were admitted to adult, general critical care units in the UK. Previous studies of a comparable size have been based on hospital episode statistics [12-15], which do not allow the analysis of detailed physiological measurements which was possible in this study. Previous studies with detailed clinical data have been much smaller and have tended to concentrate on admissions from single or small numbers of specialist centres. Even the studies used to develop scoring systems have been based on between 100 [6] and 405 [8] patients with pancreatitis.
The main weakness of this study is the reliance on data collected for a different purpose. Of the severity scoring systems for acute pancreatitis, we opted to use the modified Glasgow criteria as these had the greatest overlap with the data included in the CMPD. Even so, data were not available for one of the eight criteria (lactate dehydrogenase), and it was necessary to score the criteria during a 24-hour period rather than the prescribed 48 hours. This may reduce the utility of the modified Glasgow criteria for predicting outcome as some studies have noted that severity of pancreatitis may worsen after the first 24 hours [16,17]. Furthermore, the modified Glasgow criteria were not designed for predicting outcomes in patients with SAP, but rather to identify SAP.
Consensus criteria exist for the definition of SAP [18], but it was not possible to match these criteria in the CMPD. We feel that a diagnosis of pancreatitis as the primary reason for admission to a critical care unit can be considered to be a reasonable pragmatic definition of SAP. The lead author of the consensus criteria has also noted that in the more than 10 years since their conception, they remain poorly validated [19].
A consensus conference on the management of critically ill patients with SAP in 2004 [20] led to the first international recommendations regarding the question of when patients should be admitted to a critical care unit. The consensus group concluded that the use of disease-specific and global severity scores may assist in the identification of high-risk patients but should not replace close clinical observation. The levels of discrimination observed in this study support this recommendation.
Conclusion
SAP requiring admission to a critical care unit is associated with high mortality and long length of stay; this review identified 2,677 admissions to 159 critical care units with hospital mortality of 42% and a median length of stay in critical care of four days. Although the modified Glasgow criteria provide a simple tool for summarising the severity of acute pancreatitis, they were not designed for predicting hospital mortality in patients with SAP requiring critical care. Good discrimination between survivors and non-survivors was obtained by using the APACHE II score. Good discrimination was also found for four individual physiological parameters–highest serum urea, lowest arterial pH, highest serum creatinine, and lowest serum albumin–suggesting that it may be possible to produce a new score to provide a more accurate outcome prediction for this population.
Key messages
• SAP requiring admission to a critical care unit is associated with high mortality and long length of stay.
• Increasing numbers of modified Glasgow criteria were associated with increasing hospital mortality, although the criteria were not designed for this purpose and only seven of the eight criteria could be identified in these data.
• Good discrimination was obtained with the APACHE II score and with four individual physiological parameters: highest serum urea, lowest arterial pH, highest serum creatinine, and lowest serum albumin.
Abbreviations
APACHE = Acute Physiology and Chronic Health Evaluation; APS = Acute Physiology Score; aROC = area under the receiver operating characteristic curve; CMPD = Case Mix Programme Database; FIO2 = fractional inspired oxygen; ICNARC = Intensive Care National Audit & Research Centre; ICU = intensive care unit; PaO2 = arterial oxygen tension; ROC = receiver operating characteristic; SAP = severe acute pancreatitis.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MS conceived the study. DAH performed the analyses and drafted the manuscript. GD'A performed the referencing. All authors participated in the interpretation of the study and critical revision of the manuscript and have read and approved the final manuscript.
Acknowledgements
This study was supported by ICNARC. The authors wish to thank everyone in the ICUs participating in the Case Mix Programme [21]. These results have been presented as a poster at the 17th European Society of Intensive Care Medicine (ESICM) Annual Congress, Berlin, Germany, 10 to 13 October 2004.
References
- Beckingham IJ, Bornman PC. Acute pancreatitis. BMJ. 2001;322:595–598. doi: 10.1136/bmj.322.7286.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998;42:886–891. doi: 10.1136/gut.42.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint R, Windsor J, Bonham M. Trends in the management of severe acute pancreatitis: interventions and outcome. ANZ J Surg. 2004;74:335–342. doi: 10.1111/j.1445-1433.2004.02940.x. [DOI] [PubMed] [Google Scholar]
- Sigurdsson GH. Intensive care management of acute pancreatitis. Dig Surg. 1995;11:231–241. [Google Scholar]
- Toh SK, Phillips S, Johnson CD. A prospective audit against national standards of the presentation and management of acute pancreatitis in the South of England. Gut. 2000;46:239–243. doi: 10.1136/gut.46.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson JHC, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69–81. [PubMed] [Google Scholar]
- Imrie CW, Benjamin IS, Ferguson JC, McKay AJ, Mackenzie I, O'Neill J, Blumgart LH. A single-centre double-blind trial of Trasylol therapy in primary acute pancreatitis. Br J Surg. 1978;65:337–341. doi: 10.1002/bjs.1800650514. [DOI] [PubMed] [Google Scholar]
- Blamey SL, Imrie CW, O'Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut. 1984;25:1340–1346. doi: 10.1136/gut.25.12.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult general critical care units in England, Wales and Northern Ireland: the Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care. 2004;8:R99–111. doi: 10.1186/cc2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Goldfrad C, Rowan K. Development and testing of a hierarchical method to code the reason for admission to intensive care units: the ICNARC Coding Method. Br J Anaesth. 2001;87:543–548. doi: 10.1093/bja/87.4.543. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–828. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- Wilson C, Imrie CW. Changing patterns of incidence and mortality from acute pancreatitis in Scotland, 1961–1985. Br J Surg. 1990;77:731–734. doi: 10.1002/bjs.1800770705. [DOI] [PubMed] [Google Scholar]
- McKay CJ, Evans S, Sinclair M, Carter CR, Imrie CW. High early mortality rate from acute pancreatitis in Scotland, 1984–1995. Br J Surg. 1999;86:1302–1305. doi: 10.1046/j.1365-2168.1999.01246.x. [DOI] [PubMed] [Google Scholar]
- Tinto A, Lloyd DA, Kang JY, Majeed A, Ellis C, Williamson RC, Maxwell JD. Acute and chronic pancreatitis–diseases on the rise: a study of hospital admissions in England 1989/90–1999/2000. Aliment Pharmacol Ther. 2002;16:2097–2105. doi: 10.1046/j.1365-2036.2002.01367.x. [DOI] [PubMed] [Google Scholar]
- Goldacre MJ, Roberts SE. Hospital admission for acute pancreatitis in an English population, 1963–98: database study of incidence and mortality. BMJ. 2004;328:1466–1469. doi: 10.1136/bmj.328.7454.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Heath DI, Imrie CW. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br J Surg. 1990;77:1260–1264. doi: 10.1002/bjs.1800771120. [DOI] [PubMed] [Google Scholar]
- Khan AA, Parekh D, Cho Y, Ruiz R, Selby RR, Jabbour N, Genyk YS, Mateo R. Improved prediction of outcome in patients with severe acute pancreatitis by the APACHE II score at 48 hours after hospital admission compared with the APACHE II score at admission. Arch Surg. 2002;137:1136–1140. doi: 10.1001/archsurg.137.10.1136. [DOI] [PubMed] [Google Scholar]
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- Bradley EL 3rd. Atlanta redux. Pancreas. 2003;26:105–106. doi: 10.1097/00006676-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Nathans AB, Curtis JR, Beale RJ, Cook DJ, Moreno RP, Romand JA, Skerrett SJ, Stapleton RD, Ware LB, Waldmann CS. Management of the critically ill patient with severe acute pancreatitis. Crit Care Med. 2004;32:2524–2536. doi: 10.1097/01.CCM.0000148222.09869.92. [DOI] [PubMed] [Google Scholar]
- Participating units. ICNARC: Intensive Care National Audit & Research Centre Web site. http://www.icnarc.org/audit/cmp/participating-units/