Abstract
Sepsis is associated with cardiovascular changes that may lead to development of tissue hypoperfusion. Early recognition of sepsis and tissue hypoperfusion is critical to implement appropriate hemodynamic support and prevent irreversible organ damage. End points for resuscitation need to be defined and invasive hemodynamic monitoring is usually required. Targets for hemodynamic optimization should include intravascular volume, blood pressure, and cardiac output. Therapeutic interventions aimed at optimizing hemodynamics in patients with sepsis include aggressive fluid resuscitation, the use of vasopressor agents, inotropic agents and in selected cases transfusions of blood products. This review will cover the most important aspects of hemodynamic optimization for treatment of sepsis induced tissue-hypoperfusion.
Introduction
Severe sepsis and septic shock are among the most important causes of morbidity and mortality in patients admitted to the intensive care unit. It is estimated that approximately 200,000 patients die from severe sepsis in the USA every year and more than 150,000 in Europe [1]. Sepsis is associated with a spectrum of cardiovascular derangements that may lead to development of tissue hypoperfusion [2]. Tissue hypoperfusion is an important factor in the development of multiple organ failure. Therefore, recognition of sepsis-induced tissue hypoperfusion and timely clinical intervention to prevent and correct this phenomenon are fundamental aspects of managing critically ill patients with severe sepsis. This review focuses on the pathophysiology, recognition, and management of sepsis-induced tissue hypoperfusion. For a review of other aspects of sepsis management such as antimicrobial therapy, immunomodulatory therapy, corticosteroids, and other supportive therapies, the reader is referred to other recent articles [2,3].
Sepsis-induced tissue hypoperfusion
Our understanding of the pathophysiology underlying the development of sepsis, severe sepsis, and septic shock is continuously evolving [4,5]. It is important to examine the cardiovascular abnormalities that are present with sepsis and the clinical implications of these abnormalities in treating sepsis-induced tissue hypoperfusion.
The hemodynamic profile of severe sepsis and septic shock is initially characterized by components of hypovolemic, cardiogenic, and distributive shock [6]. In the early phases of sepsis, increased capillary leak and increased venous capacitance will result in a decrease in venous return to the heart. Cytokines released as a result of the host response to sepsis may also cause direct myocardial depression. The end result of these changes is a decrease in stroke volume and ejection fraction, leading to a compensatory tachycardia, increased ventricular compliance, and a decrease in arteriolar resistance. Fluid therapy will modify this hemodynamic profile. Fluid administration can increase venous return, compensating for the increased capillary leak and increased venous capacitance. Compensatory changes for the decreased ejection fraction include tachycardia, increased ventricular compliance, and decreased arteriolar resistance. In the early stages of sepsis, prior to fluid therapy, patients may present with a decreased cardiac output. Fluid therapy will usually result in a hyperdynamic state with a high normal or elevated cardiac output. After adequate restoration of left ventricular preload, hypotension – if present – is dependent on the degree of decreased systemic vascular resistance and on impairment of contractility.
Even with restoration of adequate blood pressure and normal or supranormal cardiac output, signs of tissue hypoperfusion may persist. This is often called 'distributive shock' and may be related to maldistribution of blood flow at the regional (splanchnic, mesenteric, and renal) or microvascular level and/or a cellular inability to utilize oxygen despite adequate oxygen delivery (cytotoxic hypoxia). Whether these abnormalities are present at the onset of sepsis or represent a progression of events is poorly understood. However, it is believed that early intervention with aggressive hemodynamic support can limit the damage of sepsis-induced tissue hypoperfusion and limit or prevent the development of endothelial injury. Support for this hypothesis is offered by the results of the early goal-directed therapy (EGDT) study conducted by Rivers and coworkers [7].
Septic shock defined as sepsis with refractory hypotension (systolic blood pressure <90 mmHg, mean arterial blood pressure <60 mmHg or a drop of ≥40 mmHg from baseline pressures), despite fluid administration, has traditionally been utilized to conceptualize the clinical syndrome of persistent sepsis-induced tissue hypoperfusion. Blood pressure alone is unlikely to be sufficient in identifying the presence or absence of tissue hypoperfusion in patients with sepsis; patients with sepsis-induced hypoperfusion can present with normal blood pressures. It is therefore important to recognize other signs that are indicative of tissue hypoperfusion. Markers of tissue hypoperfusion can be classified into two groups: indices of global hypoperfusion and indices of regional hypoperfusion (Table 1). A recent International Sepsis Definitions Conference recommended expanding the diagnostic criteria for sepsis [8]. Many of these criteria, including altered mental status, organ dysfunction parameters, acute oliguria, hyperlactatemia (>3 mmol/l), and decreased capillary refill or motling, suggest the presence of tissue hypoperfusion. It is clinically important that tissue hypoperfusion be recognized, despite what may appear to be 'normal' blood pressures, and should trigger timely and aggressive hemodynamic support interventions.
Table 1.
Indices of sepsis-induced tissue hypoperfusion
| Measure | Details |
|---|---|
| Indices of global hypoperfusion | Hypotension |
| Tachycarda | |
| Oliguria | |
| Delayed capillary refill | |
| Clouded sensorium | |
| Elevated blood lactate | |
| Low mixed venous O2 saturation | |
| Indices of regional hypoperfusion | Markers of organ function |
| Cardiac: myocardial ischemia | |
| Renal: decreased urine output, increased blood urea nitrogen and creatinine | |
| Hepatic: increased transaminases, increased lactate dehydrogenase, increased bilirubin | |
| Splanchnic: stress ulceration, ileus, malabsorbtion | |
| Direct assessment | |
| Tonometry: increased gastric mucosal CO2 tension | |
| Sublingual capnometry: increased sublingual CO2 tension | |
| Near infrared spectroscopy: decreased tissue O2 saturation | |
| Orthogonal polarization spectral imaging: low flow velocity score |
Hemodynamic monitoring
Patients with evidence of sepsis-induced tissue hypoperfusion should be treated in a monitored area, preferably an intensive care unit. Noninvasive monitoring with continuous electrocardiography and pulse oxymetry should be initiated. Additional invasive monitoring is often utilized and can aid in determining the adequacy of hemodynamic support interventions. Arterial pressure monitoring with an indwelling arterial catheter can provide accurate and continuous blood pressure measurements. This is especially useful in patients with very low blood pressures, in whom noninvasive blood pressure measurements may be inaccurate, or in patients on vasopressors, in whom sudden changes in blood pressure may occur. The radial artery is preferred, although the femoral artery is also commonly utilized.
Central venous pressure (CVP) has been used for many years as a monitor of central venous blood volume. Normal CVP is approximately 2–8 mmHg. CVP is measured through a pressure transducer connected to a central venous catheter in the thoracic central veins (internal jugular or subclavian). The validity of CVP measurements in patients with sepsis is widely debated. It is commonly accepted that a very low CVP is indicative of low intravascular volumes and supports the administration of fluids (crystalloids or colloids) for volume expansion and improvement in tissue hypoperfusion. However, an elevated CVP does not always correlate with adequate intravascular volume. Despite these limitations, CVP measurement in conjunction with other measurements is often utilized to assess and guide resuscitation in patients with sepsis.
Despite the ongoing debate surrounding use of the pulmonary artery catheter (PAC), it is still utilized and when used appropriately it can provide important information to assist in choosing hemodynamic interventions in patients with sepsis. The PAC allows measurements of intracardiac pressures, determination of cardiac output (CO; through thermodilution), and mixed venous oxygen saturation (SvO2). Information obtained from the PAC can be useful in diagnosing different causes of shock as well as monitoring disease progression and response to therapeutic interventions. The pulmonary artery occlusion pressure (PAOP), as a reflection of left ventricular end-diastolic pressure, is presumed to correlate with left ventricular end-diastolic volume. Although clinicians assume that a high PAOP represents a high intravascular volume, many patients with elevated PAOP may still require higher intravascular volumes to ensure that there is adequate CO (e.g. in a patient with decreased ventricular compliance). Changes in PAOP in relation to intravascular volume are strongly influenced by myocardial compliance. It is important to recognize this relationship and appreciate that myocardial compliance may be different from patient to patient and may also change in an individual patient through the course of critical illness. It is useful to utilize the information provided by the PAC in a dynamic way, assessing changes in PAOP and their impact on CO as hemodynamic interventions are instituted. Current PACs offer the capability to monitor continuous CO and continuous SvO2.
SvO2 can be measured in patients using a PAC. The determinants of SvO2 include CO, oxygen demand, hemoglobin, and arterial oxygen saturation. Normal SvO2 is 70–75%. In sepsis SvO2 may be elevated secondary to maldistribution of flow (blood returning to the venous circulation without opportunity for oxygen transfer). However, patients with sepsis may also present with a low or normal SvO2. Values of SvO2 must be interpreted within the overall context of the hemodynamic profile. Following SvO2 in patients with sepsis is useful because a low SvO2 is often associated with inadequate CO. Although a normal or high SvO2 does not always indicate adequate resuscitation, a low SvO2 should trigger aggressive interventions to increase oxygen delivery to the tissues and minimize sepsis-induced tissue hypoperfusion.
Recently, continuous measurement of central venous oxygen saturation (ScvO2) using a central venous catheter has garnered increasing attention. Rivers and coworkers [7] randomized patients with sepsis-induced hypoperfusion to receive either standard resuscitation or an early-goal directed protocol during the first 6 hours of admission to the emergency department. The EGDT protocol included a ScvO2 of 70% or more as one of the predefined end-points of resuscitation. In that study there was a significant improvement in mortality in the EGDT group compared with the standard resuscitation group (30.5% versus 46.5%; P = 0.009).
Monitoring techniques do not directly affect outcome. It is the proper use of this information to guide clinical interventions that potentially has an impact on patient outcomes. Current recommendations for hemodynamic management of septic shock include arterial canulation for monitoring of blood pressure and assessment of cardiac filling pressures (central venous catheterization, pulmonary artery catheterization, or echocardiography) [9].
Goals of hemodynamic support
Sepsis-induced tissue hypoperfusion leads to inadequate delivery of oxygen and nutrients to tissues. The principal goals of hemodynamic support in patients with sepsis are restoration of effective tissue perfusion and normalization of cellular metabolism. In order to facilitate hemodynamic optimization targets for treatment include intravascular volume, blood pressure, and CO.
Restoration of adequate intravascular volume should be the first goal in resuscitation. Adequate intravascular volume should be determined by achieving filling pressures associated with maximal increases in CO. In patients who are not undergoing invasive monitoring, establishing goals for clinical markers of perfusion such as heart rate (<100 beats/min), mean arterial pressure (>65 mmHg), and urine output (>0.5–1 cc/kg per hour) is appropriate. However, in many patients these markers may be unreliable as sepsis-induced tissue hypoperfusion may occur even with normal values. Variations in arterial pressure during positive pressure ventilation have been studied as a measure of responsiveness to increasing preload with volume [10,11]. Changes caused by positive pressure ventilation in systolic arterial pressure or pulse arterial pressure can predict which patients will respond to fluid loading (increased preload) with an increase in their CO. The degree of change observed in systolic arterial pressure or pulse arterial pressure during the respiratory cycle correlates directly with the response in terms of CO augmentation that a patient will have to a predetermined fluid challenge. When invasive monitoring is available to assess filling pressures, a CVP of 8–12 mmHg (higher in patients on mechanical ventilation or patients with poor compliance) or a PAOP of 12–15 mmHg are recommended as targets [9].
Blood flow to vital organs is usually preserved at a relatively constant rate by autoregulatory mechanisms when mean arterial pressure (MAP) is maintained between 60 and 120 mmHg. When MAP drops below 60 mmHg hypoperfusion can occur. It is important to appreciate that in patients with chronic hypertension the relationship between MAP and organ perfusion might be shifted to the right. This means that patients with chronic hypertension might need a higher MAP to ensure that there is adequate organ flow to vital organs. There is a paucity of data to indicate what MAP threshold should be used in patients with sepsis-induced hypoperfusion. One study demonstrated that raising the MAP from 65 to 75 to 85 mmHg was not associated with significant differences in measurements of oxygen delivery, or global or regional perfusion [12]. Current guidelines recommend the maintenance of a MAP of 65 mmHg or greater as an end-point for resuscitation [9,13]. It is important to supplement this end-point with other markers of perfusion as well as clinical considerations that may apply to individual patients.
Most patients with sepsis will have adequate CO after aggressive fluid resuscitation. However, in early unresuscitated sepsis and in a subgroup of patients later in the disease process and after adequate resuscitation, there is evidence of low or inadequate CO. The CO can be measured using various techniques; those most commonly utilized in critically ill patients include thermodilution measurements with PACs and echocardiographically derived measurements. In addition, the measurement of SvO2 or ScvO2 can be utilized as a surrogate for adequate CO. An adequate CO is an important end-point for resuscitation in patients with sepsis-induced tissue hypoperfusion. As a general rule one should aim for a cardiac index (CO [l/min]/body surface area [m2]) of 3.0 l/min per m2 or greater. A SvO2 of at least 65% or a ScvO2 of at least 70% can be used as targets of adequate oxygen delivery. The importance of implementing early goal-directed hemodynamic support in patients with sepsis-induced hypoperfusion (defined as low blood pressure despite fluid resuscitation or a lactate >4 mmol/l) has been identified by the results of the study by Rivers and coworkers [7]. The Surviving Sepsis Campaign has recommended that the following end-points of resuscitation be targeted [14]: CVP 8–12 mmHg (higher in mechanically ventilated patients); MAP at least 65 mmHg; urine output at least 0.5 ml/kg per hour; and ScvO2 or SvO2 of at least 70%. These end-points have been incorporated into the Surviving Sepsis Campaign sepsis resuscitation bundle (Table 2), and should be achieved as a group within first 6 hours of treatment [15]. At our institution we have adopted a protocol (based on the work of Rivers and coworkers [7]) that is implemented in patients with sepsis-induced hypoperfusion as soon as they are identified in the emergency department or general ward, and is then continued as a guide for resuscitation once the patient is transferred to the intensive care unit (Figure 1).
Table 2.
Surviving Sepsis Campaign: sepsis resuscitation bundle
| Step | Details |
|---|---|
| 1 | Serum lactate measured |
| 2 | Blood cultures obtained before antibiotic administration |
| 3 | From the time of presentation, broad-spectrum antibiotics administered within 3 hours for ED admissions and 1 hour for non-ED intensive care unit admissions |
| 4 | In the event of hypotension and/or lactate >4 mmol/l (36 mg/dl): |
| Deliver an initial minimum of 20 ml/kg of crystalloid (or colloid equivalent) | |
| Apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure >65 mmHg | |
| 5 | In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate >4 mmol/l (36 mg/dl): |
| Achieve central venous pressure >8 mmHg | |
| Achieve central venous oxygen saturation >70% |
ED, emergency department.
Figure 1.
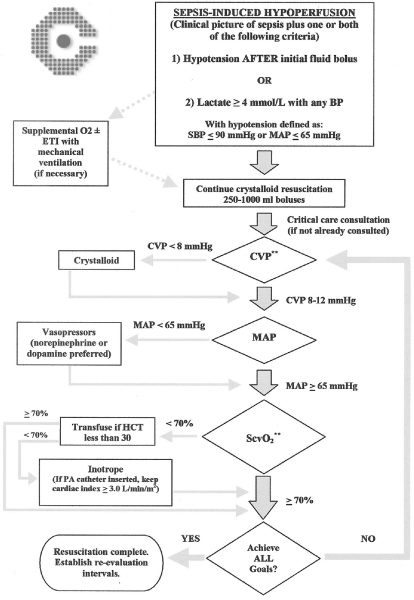
Goal-directed resuscitation protocol for severe sepsis (employed at authors' institution). BP, blood pressure; CVP, central venous pressure; ETI, endothracheal intubation; HCT, hematocrit; MAP, mean arterial pressure; PA, pulmonary artery; SBP, systolic blood pressure; ScvO2, central venous oxygen saturation. Adapted with permission from Rivers and coworkers [7].
Therapy
Fluid resuscitation
The initial step in optimizing hemodynamics and treating sepsis-induced hypoperfusion is aggressive fluid resuscitation. Although experts agree on the value and importance of early and aggressive fluid resuscitation, controversy over the optimal type of fluid continues. The debate has centered on the use of crystalloids (saline, Ringers' lactate) versus the use of colloids (albumin, hydroxyethyl starches). There are no prospective randomized trials in patients with sepsis and septic shock that clearly address this issue. Meta-analyses of clinical studies performed in general critical care patient populations, mostly surgical and nonseptic, have demonstrated no difference in clinical outcomes between patients fluid resuscitated with crystalloids and those receiving colloids [16-18]. Extrapolation of these results to patients with sepsis-induced tissue hypoperfusion is difficult. In patients with septic shock targets of resuscitation can be achieved with both types of fluids, although studies have demonstrated that less fluid is required to achieve these goals when colloids are utilized [19].
The recently published SAFE (Saline versus Albumin Fluid Evaluation) study [20] prospectively randomized 7000 critically ill patients to receive 4% albumin or 0.9% saline for fluid resuscitation. There were no significant differences between the two groups in the following outcome measures: mortality, days spent in the intensive care unit, days spent in the hospital, days of mechanical ventilation, or days of renal replacement therapy. A subgroup analysis conducted in patients with sepsis revealed a trend (nearly statistically significant if this had been the primary analysis) toward improved mortality in the group of patients treated with albumin. Unless further studies with clear answers emerge, clinicians will need to include cost considerations and specific clinical scenarios in their decision making process to select the appropriate type of fluid for resuscitating patients with sepsis.
Patients with sepsis-induced tissue hypoperfusion often present with significant intravascular volume deficits. It is important to initiate aggressive resuscitation as soon as possible. The first step should be an adequate volume challenge with at least 20 cc/kg of crystalloids or an equivalent amount of colloids. Resuscitation should continue until end-points of CVP, MAP, and CO are met with the administration of fluid challenges (500–1000 cc crystalloids or 300–500 cc colloids). Once end-points are met it is important to re-evaluate tissue perfusion continuously to determine the need for further volume expansion.
Blood transfusions
Blood transfusions have been associated with immunosuppression, and there are concerns regarding the ultimate ability of stored red blood cells to carry and deliver oxygen [21,22]. Studies evaluating outcomes based on different hemoglobin thresholds for transfusions in a general population of critically ill patients have suggested that keeping hemoglobin level above 10 g/dl offered no benefits when compared with a more conservative target of 7 g/dl [23]. However, these studies did not include patients with sepsis-induced tissue hypoperfusion. In contrast, transfusion of packed red blood cells as part of the EGDT protocol in patients with a hematocrit below 30% and a ScvO2 below 70%, despite achieving a CVP above 8 mmHg and a MAP above 65 mmHg, was associated with significant improvement in mortality in the study conducted by Rivers and coworkers [7]. It is probably more appropriate to target hemoglobin of 8–10 g/dl in patients with sepsis, recognizing that some patients with altered oxygen transport will likely benefit from blood transfusions targeted at achieving a ScvO2 or 70% or more.
Vasopressors
Some patients with sepsis-induced hypoperfusion may remain hypotensive despite adequate fluid replacement. In these patients vasopressor agents to increase MAP should be utilized. Catecholamines such as dopamine, epinephrine (adrenaline), norepinephrine (noradrenaline), and phenylephrine have traditionally been used to raise blood pressure in patients with septic shock (Table 3). The ideal vasopressor has been a subject of unresolved discussion, mostly resulting from the lack of convincing data supporting improved outcomes with a particular agent. Other important factors to consider in choosing a vasopressor include other hemodynamic effects, individual patient characteristics, and potential effects of vasopressors on regional vascular beds (splanchnic circulation and renal circulation). Dopamine and epinephrine are more likely to cause or exacerbate tachycardia than norepinephrine and phenylephrine. Dopamine, epinephrine, and norepinephrine will increase CO through β receptor stimulation. This effect is much more pronounced with dopamine and epinephrine than with norepinephrine. Phenylephrine is a pure α agonist and is anticipated to decrease CO.
Table 3.
Vasoactive drugs utilized in treating sepsis-induced hypoperfusion
| Drug | Dosage | Comments |
|---|---|---|
| Dobutamine | 1–40 μg/kg per min | Strong inotropic effect may produce vasodilation; utilized as pure inotrope agent. |
| Causes tachycardia | ||
| Dopamine | 1–20 μg/kg per min | Effects vary with dose. Predominantly vasoconstrictor with positive inotropy. |
| Causes tachycardia. Effects on renal vasculature are not protective against renal failure | ||
| Epinephrine | 1–20 μg/min | Strong inotropic, chronotropic, and vasoconstrictor. |
| Concerns about ischemia and splanchnic circulation | ||
| Norepinephrine | 0.03–1.5 μg/kg per min | Strong vasoconstrictor with modest effect on contractility. Does not produce tachycardia |
| Phenylephrine | 0.5–8 μg/kg per min | Pure vasoconstrictor. No effect on contractility or heart rate |
| Vasopressin | 0.01–0.04 U/min | Not recommended as first-line agent. Increases blood pressure; may cause splanchnic and cardiac ischemia |
Several studies have evaluated the effects of vasopressors on the splanchnic circulation [24-26]. The body of literature available appears to indicate that epinephrine is associated with the least favorable regional perfusion profile, with dopamine and norepinephrine demonstrating more favorable effects on regional splanchnic perfusion. Dopamine stimulates D1(dopaminergic) receptors in the renal regional circulation, producing vasodilation and increased blood flow. This is one of the reasons why for many years clinicians have utilized low doses of dopamine to protect kidney function. However, a well designed randomized clinical trial [27] and a recent meta-analysis [28] demonstrated no benefit in outcomes (mortality, need for renal replacement therapy, and peak serum creatinine) with the use of renal dose dopamine. Current recommendations strongly emphasize that dopamine at so called renal doses (low doses) should not be utilized in critically ill patients to protect renal function [9,13]. For many years it was proposed that norepinephrine was associated with significant vasoconstriction, resulting in decreased renal blood flow. However, animal and human studies have demonstrated beneficial effects on renal perfusion when norepinephrine is utilized to increase blood pressure in septic shock [29,30]. There are few published studies evaluating vasopressor agents against each other in terms of outcome benefits. One randomized clinical study [31] found that norepinephrine was more effective than dopamine in correcting sepsis-induced hypotension. No benefit in mortality was demonstrated, although the size of the study (n = 32) is a limiting factor in making outcome conclusions. Another cohort study [32] identified the use of norepinephrine as an independent factor associated with decreased mortality in patients with septic shock.
More recently, vasopressin has been proposed as a potential vasopressor in patients with sepsis. Vasopressin – an endogenous hormone with vasoconstrictor effects – plays an important role in the physiologic response to hypotension [33]. Serum vasopressin levels in septic shock are decreased in comparison with other shock states (e.g. cardiogenic shock), resulting in a 'relative deficiency' of vasopressin [34,35]. This deficiency appears to develop over the first 24 hours of septic shock after an initial increase in vasopressin serum levels [36]. These findings led to several small studies that demonstrated the ability of low-dose vasopressin infusions (0.01–0.04 U/min) to increase blood pressure and decrease the amount of catecholamines utilized in patients with vasodilatory shock [34,37-39]. Initial enthusiasm has been followed by caution following studies suggesting potential detrimental effects of vasopressin on splanchnic circulation and cardiac performance [40,41]. In addition, a recently published study [42] demonstrated that administration of a nonselective nitric oxide inhibitor (NG-methyl-L-arginine) in septic shock produced significant increases in mean arterial blood pressure and mortality. This study suggests that increasing the blood pressure with certain drugs, despite its intuitive appeal as something beneficial, can be associated with worse outcomes.
Vasopressors should be utilized in patients who remain hypotensive after volume expansion or during volume resuscitation in the presence of life-threatening hypotension. Vasopressor therapy should be targeted to maintain a MAP of 65 mmHg or greater. Norepinephrine or dopamine should be used as first-line agents to correct hypotension in patients with sepsis [9,13]. Epinephrine and phenylephrine are recommended in patients who do not respond to initial vasopressors [9,13]. Finally, vasopressin is not recommended as a first-line agent. It can be used as hormone replacement, given at low doses (0.01–0.04 U/min) 24 hours after the onset of shock, in cases refractory to other vasopressors [9,13].
Inotropes
Cardiac function is impaired in most patients with sepsis-induced hypotension after fluid resuscitation. Myocardial dysfunction in sepsis is complex and usually characterized by ventricular dilation, decreased ejection fraction, and impaired contractile response to fluid resuscitation [43,44]. In patients with low CO despite fluid administration, it is recommended that inotropic agents be utilized to increase CO. The agent of choice is dobutamine, a catecholamine with selective β1 adrenergic effect. Studies have shown that dobutamine, at doses ranging from 2 to 28 μg/kg per min, results in increased cardiac index, stroke volume, and heart rate in patients with sepsis [45-47]. In patients with hypotension dobutamine should be used in conjunction with a vasopressor agent. Dopexamine, a dopamine analog with β and dopamine agonist effects, has also been proposed as a useful inotrope agent for patients with sepsis. Currently not approved for use in the USA, it has been evaluated in small studies in Europe [48,49]. The use of inotropes to achieve supranormal or 'supraphysiologic' CO in sepsis is not recommended. Although, early studies conducted in high-risk surgical patients suggested improved outcomes with an approach that increased oxygen delivery to 'supraphysiologic' levels, two prospective randomized clinical trials [50,51] failed to demonstrate outcome benefits with supranormal oxygen delivery. Current recommendations strongly emphasize that strategies to increase oxygen delivery beyond normal values should not be implemented in patients with sepsis [9,14].
Conclusion
Herein we review the principles of hemodynamic optimization in patients with sepsis-induced hypoperfusion. Although we have much to learn regarding this very important aspect of sepsis, it is important to recognize tissue hypoperfusion as a medical emergency. As such it is essential to implement therapeutic interventions aimed at correcting tissue hypoperfusion as soon as possible. The initial intervention should be administration of volume (crystalloids or colloids). For patients who require vasopressors, norepinephrine or dopamine should be utilized as first-line agents. Some patients may require dobutamine to increase low CO. Implementation of hemodynamic interventions targeting predefined end-points is a time-sensitive therapy that has a significant impact on decreasing morbidity and mortality from sepsis.
Abbreviations
CO = cardiac output; CVP = central venous pressure; EGDT = early goal-directed therapy; MAP = mean arterial pressure; PAC = pulmonary artery catheter; PAOP = pulmonary artery occlusion pressure; ScvO2 = central venous oxygen saturation; SvO2 = mixed venous oxygen saturation.
Competing interests
The authors declare that they have no competing interests.
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med. 2003;31:946–955. doi: 10.1097/01.CCM.0000057403.73299.A6. [DOI] [PubMed] [Google Scholar]
- Sessler CN, Perry JC, Varney KL. Management of severe sepsis and septic shock. Curr Opin Crit Care. 2004;10:354–363. doi: 10.1097/01.ccx.0000139363.76068.7b. [DOI] [PubMed] [Google Scholar]
- Bochud PY, Calandra T. Pathogenesis of sepsis: new concepts and implications for future treatment. BMJ. 2003;326:262–266. doi: 10.1136/bmj.326.7383.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, Heard SO, Martin C, Napolitano LM, Susla GM. et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32:1928–1948. doi: 10.1097/01.CCM.0000139761.05492.D6. [DOI] [PubMed] [Google Scholar]
- Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28:2729–2732. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM. et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.CCM.0000117317.18092.E4. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Carlet JM, Gerlach H, Ramsey G, Levy M. The surviving sepsis guidelines: not another 'groundhog day'. Crit Care Med. 2004;32:1601–1602. doi: 10.1097/01.CCM.0000130996.14896.F6. [DOI] [PubMed] [Google Scholar]
- Institute of Healthcare Improvement homepage. http://www.IHI.org
- Choi PT, Yip G, Quinonez LG, Cook DJ. Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999;27:200–210. doi: 10.1097/00003246-199901000-00053. [DOI] [PubMed] [Google Scholar]
- Cook D, Guyatt G. Colloid use for fluid resuscitation: evidence and spin. Ann Intern Med. 2001;135:205–208. doi: 10.7326/0003-4819-135-3-200108070-00013. [DOI] [PubMed] [Google Scholar]
- Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998;316:961–964. doi: 10.1136/bmj.316.7136.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackow EC, Falk JL, Fein IA, Siegel JS, Packman MI, Haupt MT, Kaufman BS, Putnam D. Fluid resuscitation in circulatory shock: a comparison of the cardiorespiratory effects of albumin, hetastarch, and saline solutions in patients with hypovolemic and septic shock. Crit Care Med. 1983;11:839–850. doi: 10.1097/00003246-198311000-00001. [DOI] [PubMed] [Google Scholar]
- Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1703–1721. [PubMed] [Google Scholar]
- Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3029. doi: 10.1001/jama.269.23.3024. [DOI] [PubMed] [Google Scholar]
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31:1659–1667. doi: 10.1097/01.CCM.0000063045.77339.B6. [DOI] [PubMed] [Google Scholar]
- Marik PE, Mohedin M. The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA. 1994;272:1354–1357. doi: 10.1001/jama.272.17.1354. [DOI] [PubMed] [Google Scholar]
- Meier-Hellmann A, Bredle DL, Specht M, Spies C, Hannemann L, Reinhart K. The effects of low-dose dopamine on splanchnic blood flow and oxygen uptake in patients with septic shock. Intensive Care Med. 1997;23:31–37. doi: 10.1007/s001340050287. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139–2143. doi: 10.1016/S0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Decker MJ. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29:1526–1531. doi: 10.1097/00003246-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Leone M, Garnier F, Bourgoin A, Antonini F, Martin C. Renal effects of norepinephrine in septic and nonseptic patients. Chest. 2004;126:534–539. doi: 10.1378/chest.126.2.534. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Kellum JA, Wisniewski SR, Pinsky MR. Effects of norepinephrine on the renal vasculature in normal and endotoxemic dogs. Am J Respir Crit Care Med. 1999;159:1186–1192. doi: 10.1164/ajrccm.159.4.9802055. [DOI] [PubMed] [Google Scholar]
- Martin C, Papazian L, Perrin G, Saux P, Gouin F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103:1826–1831. doi: 10.1378/chest.103.6.1826. [DOI] [PubMed] [Google Scholar]
- Martin C, Viviand X, Leone M, Thirion X. Effect of norepinephrine on the outcome of septic shock. Crit Care Med. 2000;28:2758–2765. doi: 10.1097/00003246-200008000-00012. [DOI] [PubMed] [Google Scholar]
- Holmes CL, Patel BM, Russell JA, Walley KR. Physiology of vasopressin relevant to management of septic shock. Chest. 2001;120:989–1002. doi: 10.1378/chest.120.3.989. [DOI] [PubMed] [Google Scholar]
- Landry DW, Levin HR, Gallant EM, Ashton RC Jr, Seo S, D'Alessandro D, Oz MC, Oliver JA. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122–1125. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345:588–595. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D. Circulating vasopressin levels in septic shock. Crit Care Med. 2003;31:1752–1758. doi: 10.1097/01.CCM.0000063046.82359.4A. [DOI] [PubMed] [Google Scholar]
- Tsuneyoshi I, Yamada H, Kakihana Y, Nakamura M, Nakano Y, Boyle WA III. Hemodynamic and metabolic effects of low-dose vasopressin infusions in vasodilatory septic shock. Crit Care Med. 2001;29:487–493. doi: 10.1097/00003246-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Malay MB, Ashton RC Jr, Landry DW, Townsend RN. Low-dose vasopressin in the treatment of vasodilatory septic shock. J Trauma. 1999;47:699–703. doi: 10.1097/00005373-199910000-00014. discussion 703–705. [DOI] [PubMed] [Google Scholar]
- Patel BM, Chittock DR, Russell JA, Walley KR. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology. 2002;96:576–582. doi: 10.1097/00000542-200203000-00011. [DOI] [PubMed] [Google Scholar]
- van Haren FM, Rozendaal FW, van der Hoeven JG. The effect of vasopressin on gastric perfusion in catecholamine-dependent patients in septic shock. Chest. 2003;124:2256–2260. doi: 10.1378/chest.124.6.2256. [DOI] [PubMed] [Google Scholar]
- Klinzing S, Simon M, Reinhart K, Bredle DL, Meier-Hellmann A. High-dose vasopressin is not superior to norepinephrine in septic shock. Crit Care Med. 2003;31:2646–2650. doi: 10.1097/01.CCM.0000094260.05266.F4. [DOI] [PubMed] [Google Scholar]
- Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D. et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Gris P, Coffernils M, Leon M, Pinsky M, Reuse C, Kahn RJ. Myocardial depression characterizes the fatal course of septic shock. Surgery. 1992;111:660–667. [PubMed] [Google Scholar]
- Jardin F, Sportiche M, Bazin M, Bourokba A, Margairaz A. Dobutamine: a hemodynamic evaluation in human septic shock. Crit Care Med. 1981;9:329–332. doi: 10.1097/00003246-198104000-00010. [DOI] [PubMed] [Google Scholar]
- Tell B, Majerus TC, Flancbaum L. Dobutamine in elderly septic shock patients refractory to dopamine. Intensive Care Med. 1987;13:14–18. doi: 10.1007/BF00263550. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Roman A, Kahn RJ. Dobutamine administration in septic shock: addition to a standard protocol. Crit Care Med. 1990;18:689–693. doi: 10.1097/00003246-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Colardyn FC, Vandenbogaerde JF, Vogelaers DP, Verbeke JH. Use of dopexamine hydrochloride in patients with septic shock. Crit Care Med. 1989;17:999–1003. doi: 10.1097/00003246-198910000-00007. [DOI] [PubMed] [Google Scholar]
- Hannemann L, Reinhart K, Meier-Hellmann A, Wallenfang G, Bredle DL. Dopexamine hydrochloride in septic shock. Chest. 1996;109:756–760. doi: 10.1378/chest.109.3.756. [DOI] [PubMed] [Google Scholar]
- Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]


