Abstract
The rise in noncommunicable chronic diseases associated with changing diet and lifestyles throughout the world is a major challenge for society. It is possible that certain dietary components within plants have roles both in reducing the incidence and progression of these diseases. We critically review the types of evidence used to support the health promoting activities of certain phytochemicals and plant-based foods and summarize the major contributions but also the limitations of epidemiological and observational studies and research with the use of cell and animal models. We stress the need for human intervention studies to provide high-quality evidence for health benefits of dietary components derived from plants.
INTRODUCTION
While, remarkably, the world still faces the scourge of severe malnutrition of which a major component is micronutrient deficiencies, changes in diet and lifestyle, increased urbanization, and reductions in physical activity have led to an astonishing rise in noncommunicable diseases, such as type 2 diabetes, cardiovascular disease (CVD), some cancers, and a range of inflammatory-associated conditions. While the increase in these diet-associated chronic diseases is well documented in Western countries, there is a rapid increase in many other countries within which the health and economic consequences are largely unknown. For example, India has more people with type 2 diabetes than any other country, with an 8% higher age-adjusted incidence than most European countries. Moreover, its onset occurs one or two decades earlier and at a lower obesity threshold than that typically observed in the West, and it is more strongly associated with coronary heart disease (Diamond, 2011). Enhancing the nutritional quality of plant-based foods through genetic manipulation and molecular breeding is an attractive and potentially useful contribution to tackling these twin global health burdens of micronutrient deficiencies and diet-related noncommunicable diseases (Martin et al., 2011). Plant-based approaches to alleviating micronutrient deficiencies have been widely advocated by international agencies, such as the Harvest Plus initiative, and well-defined targets in addressing iron, zinc, and vitamin A deficiencies have been agreed upon (Hotz and McClafferty, 2007). Furthermore, there is evidence that the introduction of orange-flesh sweet potatoes that are rich sources of the vitamin A precursor β-carotene has led to significant health benefits for children in parts of Africa (Low et al., 2007). By contrast, identifying plant metabolites whose manipulation in fresh and processed foods might have a significant effect in reducing the burden of chronic disease is more challenging, and it is this challenge that is the focus of this review.
In this article, we briefly review three major sources of evidence for the role of dietary plant secondary metabolites in contributing to the prevention of chronic disease: epidemiological studies, the use of animal and cell models, and human intervention studies. The large amounts of data from epidemiological and cell- and animal-based studies contrasts with the dearth of data from human intervention studies with well-characterized plant-based foods, and yet it is the latter that provides the most reliable evidence for health benefits and is the only source of data upon which health claims for novel foods or functional food products can be substantiated. Two of the major limitations in the design and execution of human intervention studies are the lack of validated biomarkers for health status and disease risk and the lack of well-characterized and contrasting plant foods required to test hypotheses for the health-promoting activity of specific plant metabolites. In this review, we describe the context within which nutrition scientists seek to gain evidence for health promoting benefits of specific foods and their chemical components and provide a critical review of existing approaches to acquire evidence for health benefits of specific plant compounds.
EPIDEMIOLOGICAL STUDIES
Evidence from epidemiological studies that correlate dietary intake of fruit and vegetables or specific food components with health is frequently quoted to justify the manipulation of dietary metabolites to enhance the nutritional quality of foods. There are two major types of epidemiological studies, both of which have important contributions to make: retrospective case-control studies and prospective cohort studies. Sometimes, a case-control study is nested within a larger cohort study and may be referred to as a case-cohort study. Both of these types of studies depend upon dietary assessment through, for example, food frequency questionnaires that depend upon accurate recall of diet, which is itself challenging. A few studies have sought to use biomarkers of intake, such as urinary isothiocyanates as a measure of cruciferous vegetable consumption (London et al., 2000), total urinary polyphenols (Medina-Remón et al., 2011), and serum carotenoids (Karppi et al., 2009; Beydoun et al., 2011). However, while the use of biomarkers may be more quantitative, they are likely to provide only a snapshot of diet as opposed to habitual intake.
The majority of studies undertaken from 1970 to 2000 were retrospective case-control studies, and many of these reported inverse associations between diets rich in fruit and vegetables in general, or particular classes of foods and secondary metabolites that occur in these foods, and the risk of cancer and CVD. Typically, case-control studies consider the retrospective analyses of diet through the use of food frequency questionnaires of several hundred people who have a disease, such as cancer, with a similar number of healthy matched controls. It is these studies that provided the evidence for the widespread dietary advice to consume several portions of fruit and vegetables per day to promote health. Case-control studies, however, suffer from two major problems. First, they can only provide an estimate of relative, as opposed to absolute risk, and thus can give a misleading impression of health benefits (Pearce, 1993; Deeks, 1998). Second, selection of the control group is complex and challenging (Lubin and Gail, 1984), particularly as diets rich in fruit and vegetables are often highly correlated with other lifestyle attributes and socioeconomic status. Furthermore, there may have been a historical bias in the reporting of studies that have a positive outcome, as opposed to those that find no association between diet and health, although many journals are now more prepared to publish reports of negative outcomes. However, compared with prospective cohort studies, case-control studies are relatively inexpensive and can be completed in a shorter time.
More recently, outputs from several much larger prospective cohort studies have been reported. These typically involve several thousands, or hundreds of thousands, of individuals whose diet and other lifestyle factors are monitored over a period of decades. Notable examples are the U.S.-based Nurses’ Health study, initially established in 1976 (Belanger et al., 1978), but with recruitment of additional cohorts in 1989 and 2010, the Health Professional’s Follow-Up Study established in 1986 (Grobbee et al., 1990), and the European Prospective Investigation in Cancer and Nutrition (EPIC), which has recruited over 520,000 people in 10 European Countries (Riboli and Kaaks, 1997). Within these studies, the incidence of disease has been correlated with diet and other lifestyle factors. In general, many of these large cohort studies have provided less support for an inverse relationship between fruit and vegetable consumption and overall cancer risk (Boffetta et al., 2010), although there is continuing support for relatively small protective effects of certain dietary components against cancer at specific sites (Gonzalez and Riboli, 2010). Meta-analyses of cohort studies, however, continue to support the inverse association between fruit and vegetable consumption and risk of CVD (Dauchet et al., 2006; He et al., 2006).
The large number of both case-control and cohort epidemiological studies facilitates the ability to cherry-pick studies that provide evidence of the importance of a particular food or food component in the diet, and it is often challenging for the nonspecialist to evaluate the quality of a particular study and how much weight should be given to its findings. Differences in outcomes of apparently similar studies are not restricted to the smaller case-control studies, as conflicting results have also been reported in large cohort studies. For example, fiber in the diet has been advocated as a preventive agent for colon cancer, and one may expect that this matter could be resolved through epidemiological evidence. However, while analyses of the EPIC cohort study in Europe concluded that a doubling of intake of dietary fiber in populations with low average intake of dietary fiber could reduce risk of colon cancer by 40% (Bingham et al., 2003), similar studies in the United States, Finland, and Sweden concluded that high dietary fiber was not associated with reduced risk of colon cancer (Fuchs et al., 1999; Pietinen et al., 1999; Terry et al., 2001). This difference may be due to contrasting consideration of other confounding factors that may influence colon cancer, such as smoking and the consumption of red meat, folate, and alcohol within the studies (Bingham, 2006), illustrating the complexity of interpreting epidemiological data.
A further example of the difficulties in the interpretation of epidemiological studies concerns the role of tomato and tomato-based products in the reduction of risk of prostate cancer and the possible involvement of lycopene. Following petitions for health claims on tomato and lycopene, the U.S. Food and Drug Administration (FDA) reviewed evidence from 18 epidemiological studies that sought to correlate consumption of tomato or tomato-based products with risk of prostate cancer (Kavanaugh et al., 2007). Two of these studies were disregarded due to lack of validation of the food frequency questionnaire or the clinical endpoint, while three were disregarded as they presented a reanalysis of previously published data. Of the remaining 13 studies, nine were case-control studies or case-cohort studies, of which six found no association between tomato consumption and three found an association. Two were ecological studies that sought to correlate country-wide incidence of prostate cancer with tomato consumption, neither of which found an association. The remaining two were large prospective cohort studies, both of which suggested that there was an inverse correlation between relatively high tomato consumption and risk of prostate cancer. The FDA subsequently allowed a health claim stating that “Very limited and preliminary scientific research suggests that eating one-half to one cup of tomatoes and/or tomato sauce a week reduces the risk of prostate cancer. FDA concludes that there is little scientific evidence supporting this claim.” The FDA also concluded that there was no evidence that lycopene reduced the risk of prostate cancer. Subsequent epidemiological studies have sought to correlate either lycopene supplement use or serum lycopene levels with prostate cancer incidence in prospective trials and have found no relationship (Karppi et al., 2009; Beilby et al., 2010; Kristal et al., 2010). Thus, while there is limited epidemiological evidence for tomato consumption in reducing risk of colon cancer, the bioactive components responsible for this remain unknown.
The majority of the larger case-control and cohort studies that seek to assess the role of diet in reduction of chronic disease frequently will analyze not only total fruit and vegetable consumption, but different categories of fruit and vegetables, and through integration with food compositional databases can also investigate individual chemical components. In this manner, more specific targets can be identified for plant scientists to focus upon. For example, there is much interest in how diet may influence the occurrence of type 2 diabetes, and diets rich in fruit and vegetables have been advocated as one component of lifestyle interventions to reduce risk. A recent study undertook a meta-analysis of all prospective cohorts that measured fruit and vegetable consumption and type 2 diabetes incidence and concluded that while there was no evidence for a preventive effect of fruit and vegetables in general, there was a statistically significant inverse effect of consumption of green leafy vegetables and diabetes (Carter et al., 2010). One problem with interpreting these data is that the various studies included in the meta-analysis used somewhat different definitions of leafy green vegetables and that from a botanical perspective there was no consistency with respect to plant families that may provide insight into potential bioactive components. This increases the challenge of identifying the bioactive components of these vegetables, which might include, among others, β-carotene or α-linolenic acid. A somewhat similar example is a prospective cohort study that sought to investigate the relationship between fruit and vegetable intake and the risk of prostate cancer. While total fruit and vegetable consumption was not related to prostate cancer risk, there was an association between increasing vegetable consumption and aggressive forms of prostate cancer (Kirsh et al., 2007). This association was mainly explained by intake of cruciferous vegetables, which contain glucosinolates and their bioactive derivatives.
Diets rich in fruit and vegetables have been associated in several epidemiological studies with reduction in CVD, and flavonoids that are present in significant amounts in many fruit and vegetables have been implicated. A recent study sought to associate specific subclasses of flavonoids, as opposed to types of foods, with the incidence of hypertension within three U.S.-based cohort studies through the use of databases of flavonoid content of foods maintained by the USDA. Anthocyanins and some flavone and flavan-3-ol compounds were associated with a modest reduction in hypertension, and it was suggested that this may result from similarities in the chemical structure of these compounds (Cassidy et al., 2011). Moreover, it has been shown that the total levels of polyphenols in the diet assessed through urinary analysis rather than a dietary assessment is inversely associated with blood pressure and hypertension (Medina-Remón et al., 2011).
While many factors complicate the interpretation of epidemiological studies, there is also a limitation to the extent of resolution that this type of evidence can provide. Hence, to provide convincing evidence of the health-promoting activity of particular foods and their chemical components, epidemiological data are often complemented with that obtained from experimental studies with cell and animal models. However, such studies present many challenges in interpretation and extrapolation to humans.
CELL AND ANIMAL MODELS FOR HEALTH-PROMOTING AND DISEASE-PREVENTING ACTIVITY OF PHYTOCHEMICALS
Studies to determine biological efficacy of specific phytochemicals and plant-based foods that have been implicated in epidemiological studies involve experimentation with in vitro and in vivo systems. These aim to determine the bioactivity of the pure compound, a plant extract or plant-based food, and may be used to test their potential effects within cell and animal models of specific human diseases. The types of models used and the progressive approach from cells to animal models as a prerequisite to human studies is largely based upon the approach adopted within the pharmaceutical industry. However, there are several important differences in assessing the activity of a candidate drug as opposed to the activity of dietary phytochemicals, the most notable of which is the need within the former to establish toxicity as a prerequisite for human studies.
Advantages of Cell Models
Cell models offer an appealing and ethical alternative to animal experimentation for initial efficacy and mechanistic studies. They can provide an indication of biological activity for the phytochemical in question, which can be used to design animal experiments with the least number of animals necessary to test hypotheses of bioactivity. The main advantage of the use of in vitro cell models is practical convenience, such as ease of culturing, their relatively low cost, and moderate throughput capabilities. Moreover, it is relatively easy to alter gene expression to test specific hypotheses concerning mechanisms of action of phytochemicals. Culture of homogeneous cell lines isolated from various tissues offers simplicity and the possibility to observe effects of dietary factors in well-defined and well-controlled environments. This allows for better reproducibility of responses within an experiment and replication between experiments. There is a vast selection of cell lines that can be used for efficacy experiments, available mainly from two biorepositories, the American Type Culture Collection, which holds over 3500 cell lines, and the European Collection of Cell Cultures, which holds more than 1100 cell lines. Cell models are relatively easy for many biochemical and molecular scientists to use, with few ethical and regulatory issues compared with the use of animal and human studies.
Cell line experiments are particularly relevant for initial studies on understanding mechanism of disease prevention by plant products. For example, the mechanistic basis for the bioactivity of sulforaphane, a secondary plant product derived from broccoli, has been extensively studied in cell models since its identification in the early 1990s (Zhang et al., 1992). Twenty years on, through the use of cells from different cancerous tissues, macrophages, and endothelial tissue, multiple mechanisms appear to be induced by sulforaphane, including suppression of phase 1 enzymes (i.e., mainly cytochrome P450 enzymes that activate a xenobiotic through the addition of a reactive group such as a hydroxyl radical), induction of phase 2 detoxification enzymes (i.e., enzymes that catalyze the conjugation of the activated xenobiotic with such compounds as glutathione, glucuronic acid, or sulfate, thereby reducing potential toxicity), induction of apoptosis, cell cycle arrest, inhibition of histone deacetylation, anti-inflammatory properties, binding to proteins, and interaction with extracellular signaling molecules (Juge et al., 2007; Mi et al., 2007; Shan et al., 2010; Youn et al., 2010). Some of these mechanisms have also been shown to occur in animal models (Juge et al., 2007). Whether these mechanisms also occur in vivo at physiological concentrations largely remains to be investigated. Similar studies have been undertaken with many other potentially bioactive dietary compounds.
While cell lines from a variety of tissues have been used to explore the biological activity of plant-derived metabolites, hepatic and intestinal cell lines have also been used to explore human metabolism of plant compounds and transport across the gut epithelium. For example, the Caco-2 cell line was isolated from human colon adenocarcinoma tissue in the late 1970s and since then it has been a valuable tool in studies on absorption of drugs and phytochemicals (Fogh et al., 1977). Culture of Caco-2 cells under conventional conditions makes these cells a model of colon cancer that has been used extensively in determining potential cancer preventive properties of various phytochemicals and plant-derived products (Zhu et al., 2002; Traka et al., 2005, 2009; McDougall et al., 2008; Savini et al., 2009). However, it is the property of these cells to differentiate into functional small intestinal cells that has made them a useful model in predicting intestinal absorption. When Caco-2 cells are grown as a monolayer for more than 21 d under confluent conditions, they become polarized and adopt morphological and biochemical characteristics of normal enterocytes, with characteristic dome formations and expression of intestinal specific enzymes, such as sucrase isomaltase and alkaline phosphatase (Pinto et al., 1983; Grasset et al., 1984; Hidalgo et al., 1989; Hara et al., 1993). For this reason, Caco-2 cells have been used extensively to model absorption of phytochemicals (Whitley et al., 2003; O’Sullivan et al., 2010; Richelle et al., 2010) as well as their interaction with drug absorption (Reboul et al., 2005; Chen et al., 2009; Soler-Rivas et al., 2010).
Limitations of Cell Models
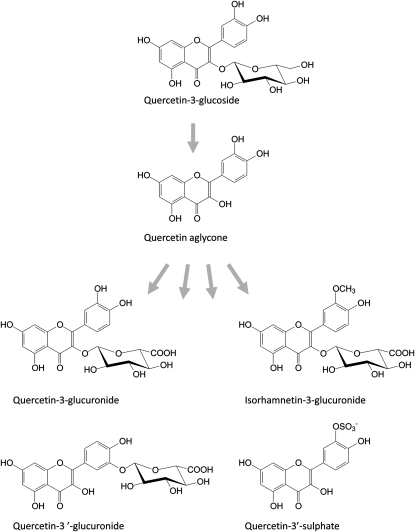
Despite the benefits of using cell lines, there are important limitations that must be considered before interpreting an in vitro cell experiment designed to prove efficacy of plant-derived products. One of the most important issues is the types of metabolites to which the cells are exposed. Within plants, many secondary plant metabolites are glycosylated. Following consumption, they are frequently deglycosylated within the lumen of the gut or the gut epithelium prior to further phase 2 metabolism either within the enterocyte or the liver, resulting in a range of metabolites within the systemic circulation to which tissues are directly exposed. For example, following degylcosylation, the flavonoid quercetin is metabolized to quercetin sulfate and quercetin glucuronides, which may also become methylated (Figure 1). The quercetin aglycone itself is not present in the plasma, and yet this is the compound to which the majority of cells are exposed. Several studies have shown that the bioactivity of quercetin aglycone is distinct from its various metabolites (Lodi et al., 2009; Winterbone et al., 2009). This type of exposure of mammalian cells to plant metabolites that they would never encounter in vivo is a major problem with assessing biological activity and defining plant targets for manipulation. It is also noteworthy that the antioxidant activity of many phytochemicals, notably flavonoids, is based largely upon the in vitro activity of the aglycone. Metabolites that are actually found within the systemic circulation commonly have much lower levels of antioxidant activity (Janisch et al., 2004; Rimbach et al., 2004; Shirai et al., 2006).
Figure 1.
Human Metabolism of Quercetin Glycoside.
Deglycosylation occurs either within the lumen of the gastrointestinal tract or an enterocyte. Within the systemic circulation, only glucuronide and sulfate metabolites are found.
In a somewhat analogous manner, many other phytochemicals are extensively metabolized by the gut microbiota prior to absorption, so again the compounds in the systemic circulation to which cells are exposed are different from those obtained directly from the plant and to which cell models are frequently exposed in vitro. These include such flavonoid compounds as anthocyanins and flavan-3-ols for which there is increasingly good epidemiological evidence of health benefits, as described above. In addition to biological difficulties with cell models, there are also many practical issues, such as maintaining appropriate environmental conditions and modeling in vitro exposure to lipophillic compounds, such as carotenoids. A further issue is the tendency for experimental scientists to expose in vitro cell cultures to concentrations of plant-derived metabolites far in excess of the levels that are present in the systemic circulation. This can result in the description of a range of biological activities of dietary compounds that may be of value within a pharmaceutical context but is of little relevance to normal dietary intake. Indeed, one of the major challenges in this field of research is to elucidate how transient exposure, typically for a few hours, of human tissues after a meal to low levels of dietary phytochemicals within the systemic circulation can be of biological significance.
Inherent characteristics of cells in culture can also prove problematic in understanding bioactivity. There are two main types of animal cell lines, primary cells and immortalized cells. With the exception of some cells isolated from tumors, primary cells isolated directly from healthy tissue have a finite doubling potential, called the Hayflick limit, when cells undergo senescence and stop dividing (Hayflick, 1965). Immortalized cells, on the other hand, have acquired the ability to proliferate indefinitely either through naturally occurring oncogenic mutations, as in cells isolated from tumors, or deliberate modification, such as induction of an oncogene or loss of a tumor suppressor gene. The most common modification is through simian virus 40–mediated induction of the large T-antigen (Colby and Shenk, 1982). However, such transformed cells typically exhibit significant alterations in physiological and biological properties. Most notably, these cells are associated with aneuploidy, epigenetic changes, spontaneous hypermutability, loss of contact inhibition (the characteristic of healthy cells to arrest growth in response to contact with other cells), and alterations in biochemical functions related to cell cycle checkpoints, such as Rb, p53, and pp2A proteins (Huschtscha and Holliday, 1983; Röscheisen et al., 1994; Velicescu et al., 2003; Ahuja et al., 2005).
Additionally, cell culture of homogeneous cell types is far removed from the in vivo situation, where the original tissue exists in the presence of multiple other neighboring cell types and interacts with them through paracrine signaling. For example, in prostate tissue the cytokine transforming growth factor-β, produced by stromal cells, supports tumor growth in adjacent epithelial cells, but tumor growth is not sustained in the absence of transforming growth factor-β signaling (Verona et al., 2007). Features of the cell culture technique may also affect how representative the response of cells will be to the in vivo situation. This is because cultured cells are not maintained under hypoxic conditions as would happen in vivo, and furthermore, they are cultured in the absence of the original blood circulation, which supports growth but also circulates important factors, such as signaling molecules and hormones.
One way to avoid the problems caused by the absence of a representative microenvironment is to culture whole tissues ex vivo through organ/tissue culture. Organ culture maintains the biologically relevant structural and functional features of in vivo tissues, which overcomes the limitations of single homogeneous cell culture. However, tissues exhibit very short lifespan and are therefore not suited for longer experiments. Availability of tissue is also an issue that prevents their use in medium- to high-throughput analyses of biological efficacy. Nevertheless, they can be valuable in understanding biochemical processes in response to plant secondary metabolites (Mehta and Lansky, 2004; Papini et al., 2004; Moronvalle-Halley et al., 2005; Konsue and Ioannides, 2010).
Advantages of Animal Models
Animal models are widely used to explore the biological activity of dietary phytochemicals, with the expectation that the protective effects and mechanistic insights gained might be extrapolated to humans. For this purpose, a plethora of animal models is used that attempt to recapitulate human disease. One of the main advantages of the use of animals, especially mice, to model responses in nutritional studies is the high degree of orthology between mice and humans; the proportion of mouse genes with a single identifiable ortholog in the human genome is ~80% (Waterston et al., 2002). This makes the mouse an ideal model to investigate environmental and genetic manipulation rarely feasible in humans. The small size and short gestation and life span of rodents make them amenable to rapid breeding of a large number of animals and, consequently, the feasibility of many studies in a relatively short period. Preclinical experiments with animal models can thus be performed in relative short time periods, enabling the chronic study of disease response to nutritional manipulation and providing invaluable pharmacological, toxicological, and biological data that may predict the clinical efficacy of dietary compounds. Genetic homogeneity between animals and the lack of influence of variable environmental factors during these experiments also facilitates these studies. Additionally, it is possible to perform a comprehensive analysis of the disease response through proteomics, transcriptomics, and metabolomics analyses, crucially both pre- and postmortem. These analyses make animals particularly suited for studies that attempt to establish mechanisms of action for a specific phytochemical and have been an invaluable tool in deciphering the molecular pathways involved in the response to phytochemicals.
Limitations of Animal Models
Despite the extensive use of animals in nutrition research, it is important to recognize the limitations of these models. A recent systematic review of 76 highly cited animal studies that assessed preventive or therapeutic interventions published in seven leading scientific journals of high impact factor found that only a minority were replicated in human randomized trials (Hackam and Redelmeier, 2006). The main limitation is that despite the fact that animal models in preventative studies are selected because they present similar characteristics to the disease under investigation, they never exhibit identical morphological, physiological, or biochemical characteristics. For example, the PTEN knockout mouse prostate model (Wang et al., 2003), described in more detail below, exhibits disease progression similar to humans but at vastly accelerated rates, equivalent to men getting prostate cancer in late adolescence as opposed to typically late in life (Luchman et al., 2008), whereas the TRAMP mouse prostate cancer model progresses at a slower rate but the cancer is of neuroendocrine origin compared with epithelial origin seen in humans (Greenberg et al., 1995). The APCmin mouse model of colon cancer, used widely in cancer prevention studies, differs from humans as tumors are frequently found in the small intestine, which is rare in humans, as opposed to the colon itself (Fodde et al., 1994). Additionally, differences in immune system development, activation, and response to challenge between rodents and humans (Mestas and Hughes, 2004) will inevitably affect translation of responses to humans.
Another major limitation of animal studies particularly relevant for nutritional research is the difference between humans and rodents in the absorption and metabolism of dietary phytochemicals. Following ingestion, small animals exhibit more rapid gastric emptying, which affects absorption (e.g., 10 min in rodents compared with 1 h in humans in the fasting state) (Campbell, 1996). Binding of compounds to components in blood, such as glutathione, also tends to be more efficient in humans compared with other species, meaning there are lower circulating levels of free active unbound phytochemicals able to reach different tissues. Metabolic degradation also varies between humans and animals. In the liver, where the majority of detoxification occurs, phase 1 cytochrome P450 enzymes (CYPs) are responsible for catalyzing the oxidation of organic substances, including ingested food products and drugs, thus facilitating their metabolism and excretion through the subsequent activity of phase 2 detoxification enzymes. We now know that there are important differences, not only in the amount for CYPs present in different species (the CYP2C family is larger and more complex in mice than in humans, whereas CYP3A is the most important of all human CYPs) but also in the substrate specificities of some CYPs, such as CYP2A, CYP2B, and CYP2C (Martignoni et al., 2006). Metabolic rates between species are also different; for example, small animals clear drugs and chemicals faster than humans, primarily because of faster blood flow and proportionally larger organs (Campbell, 1996), which makes identification of the human equivalent dose from an animal study also challenging.
Importantly, most animal experiments involve the use of highly inbred strains of mice, which, due to their genetic homogeneity, are expected to respond similarly when exposed to phytochemicals. This is a double-edged sword; although mouse models remove the real genetic variation in natural populations, which can confound the results and conclusions of experimental measurements, at the same time they are not representative of the genetic variation exhibited in humans. Also, rodents will express alleles that are not necessarily the most dominant human equivalents or the exact human functional orthologs.
Types of Animal Models
Appropriate models used for nutrition studies include (1) genetically modified mice, through either a disease-driven directed approach, where a known human disease-causing gene is identified and the corresponding gene in the mouse is targeted for genetic modification, or a mutagenesis-driven nondirected approach, where genetic modification in mice leads to a phenotype that resembles human disease (Semsarian, 2009); (2) syngeneic models, where disease is induced mainly by external stimuli such as chemical or surgical intervention; and (3) xenogeneic models, mainly used in cancer prevention studies, where xenografts of human origin are injected into the host either subcutaneously (unnatural site) or orthotopically (primary tumor source site) (de Jong and Maina, 2010). A few examples of animal models used to study the health benefits of phytochemicals are presented below.
Animal Models for Studies on Cancer Prevention
Cancer prevention is a favorite target for claims related to the bioactivity of plant-derived products, not least due to the appealing potential that cancer might be prevented by a simple modification of diet without the use of drugs. An example of mice genetically engineered by a directed approach used for studies on bioactivity of phytochemicals is the prostate-specific PTEN-null mouse model. PTEN deletion in an epithelial stem cell can be an early initiating event leading to prostatic intraepithelial neoplasia, a precursor of the disease, and subsequently to cancer in humans (Wang et al., 2006, 2009). Excision of the PTEN gene in mice is achieved in a spatio-temporal manner by the use of the Cre/loxP system, where transcription of Cre4 recombinase is driven by a prostate-specific promoter and loxP sites flank the PTEN gene (Wang et al., 2003). Recently, the dietary isothiocyanate sulforaphane derived from broccoli was shown to suppress transcriptional changes induced by the PTEN deletion in this model as well as inducing additional changes in gene expression in PTEN null tissue, which could partly explain the epidemiological evidence for a reduced risk of prostate cancer in people with high consumption of cruciferous vegetables (Traka et al., 2010). Although no histological differences were seen compared with mice on control diet lacking sulforaphane, this could potentially be due to the aggressive nature of disease progression in this model as discussed above. In TRAMP mice, another well-documented model of prostate disease in which expression of the oncogene SV40 is driven by a prostate-specific promoter, both tomato (Pannellini et al., 2010) and lycopene (Konijeti et al., 2010), a putative bioactive ingredient of tomatoes, were shown to reduce significantly the incidence of prostate cancer, although certain tomato preparations were shown to have no effect (Konijeti et al., 2010).
An example of nondirected approach to generate genetically engineered mice used to establish health benefits of phytochemicals is the adenomatous polyposis coli (APC)min mouse model. Mice highly susceptible to spontaneous intestinal adenoma formation carrying the APCmin mutation were identified in the progeny of a C57BL/6J male mutagenized by ethylnitrosourea. When the diet of APCmin mice was supplemented with an anthocyanin-rich red grape extract, the overall adenoma burden was halved and this was associated with reduced Akt expression, an important cell signaling molecule driving cell proliferation (Cai et al., 2010). Similarly, green tea polyphenols and epigallocatechin-3-gallate decreased tumor incidence and multiplicity in this model significantly and induced molecular events that contributed to cancer prevention (Hao et al., 2007).
Syngeneic rodent models used extensively for studies on prevention of cancer include 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary tumors in rats, shown to be reduced by the isoflavones genistein and daidzein (Mishra et al., 2009), DMBA-induced skin tumors in mice, alleviated by topical application of sulforaphane (Xu et al., 2006) or resveratrol (Yusuf et al., 2009), and lung tumors in mice induced by combined administration of the tobacco smoke carcinogens benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, shown to be reduced by phenethyl isothiocyanate, sulforaphane (Conaway et al., 2005), and indole-3-carbinol (Kassie et al., 2007).
Xenogeneic models are also used routinely to exhibit growth suppression of human cancer cells in immunodeficient nude mice. Examples include growth suppression of human non-small cell lung cancer cells by grape seed proanthocyanidins (Akhtar et al., 2009) and pancreatic tumor xenografts inhibited by curcumin combined with omega-3 polyunsaturated fatty acids (Swamy et al., 2008).
Models for Studies on Prevention of CVD
Animal models of CVD have focused on genetically engineered rodents because diets supplemented with cholesterol as high as 10% w/w are not usually sufficient to create significant hypercholesterolemia and subsequent atherosclerosis in healthy rodents, unlike in humans. This resistance to atherogenesis is likely due to the difference in lipid metabolism of normal rats and mice, which carry the majority of cholesterol in high-density lipoprotein rather than low-density lipoprotein, as do humans (Bergen and Mersmann, 2005; Russell and Proctor, 2006).
Studies in nutritional research using genetically engineered mouse models of CVD to determine efficacy of phytochemicals in prevention of atherosclerosis, mainly are based on knockout ApoE−/− mice. ApoE−/− mice have high total plasma cholesterol concentrations and exhibit atherosclerosis largely confined to the aortic root area or more widely distributed atherosclerosis when they are on a high-cholesterol diet, which makes them an appropriate model of human CVD (Lutgens et al., 1999). ApoE−/− mice fed an atherogenic diet supplemented with an anti-inflammatory dietary mixture containing resveratrol, lycopene, catechin, vitamins E and C, and fish oil showed a substantial reduction in the development of atherosclerosis (Verschuren et al., 2011). Similar results have been obtained with red wine polyphenols (Waddington et al., 2004) and pomegranate juice (Aviram et al., 2000).
Another model of human CVD is the JCR:LA-cp rat, which develops an extreme obese/insulin resistant and atherosclerosis-prone phenotype with spontaneously arising ischemic lesions of the heart that resemble the atherosclerosis commonly seen in human coronary arteries (Russell et al., 1998). Diet supplemented with prebiotic fiber lowered serum cholesterol levels in these rats (Parnell and Reimer, 2010).
Finally, surgically induced myocardial infarction in rats has been used as a syngeneic model for the prevention of CVD. In this model, curcumin (Morimoto et al., 2008), a broccoli diet (Mukherjee et al., 2010), and sulforaphane administration (Piao et al., 2010) were found to inhibit myocardial hypertrophy, improve systolic function, and reduce myocardial infarction.
Models for Studies on Prevention on Metabolic Syndrome and Type 2 Diabetes
Models for metabolic syndrome are increasingly sought after, as this complex cluster of abnormalities that include insulin resistance, visceral adiposity, atherogenic dyslipidemia, and endothelial dysfunction, all of which are associated with obesity, is also a risk factor for type 2 diabetes and CVD (Huang, 2009), but possibly more amenable to amelioration through dietary change than these more advanced disease states. There are several animal models of metabolic syndrome relating both to the pathophysiology of metabolic syndrome and its associated diseases (Kennedy et al., 2010; Varga et al., 2010). The most commonly used in nutritional studies include diet-induced obesity in rodents, leptin-deficient (Lepob/ob), and leptin receptor-deficient (LepRdb/db) mice.
Diet-induced obesity is achieved when otherwise healthy rats and mice are allowed ad libitum access to a high-fat diet, thus developing obesity, hyperinsulinemia, hyperglycemia, and hypertension (Collins et al., 2004). This pathology has been successfully reduced by simultaneous addition in the diet of plant-derived preparations, such as pomegranate seed oil (Vroegrijk et al., 2011), grape skin extract (Hogan et al., 2011), blood orange extract (Titta et al., 2010), barley β-glucan (Choi et al., 2010), anthocyanins from cherries (Jayaprakasam et al., 2006), and green tea (−)-epigallocatechin-3-gallate (Lee et al., 2009).
Leptin is a protein that functions as a hormone regulating feed intake and energy expenditure and is expressed predominantly in adipose tissue. Both Lepob/ob and LepRdb/db mice have nearly identical metabolic profiles, demonstrating obesity, hyperinsulinemia, hyperglycemia, and elevated total cholesterol levels (Kennedy et al., 2010). Green tea (Richard et al., 2009) and curcumin (Weisberg et al., 2008) are among some of the dietary interventions shown to reduce the severity of diseased phenotype in these models.
Thus, despite many limitations, animal models have been widely used to provide evidence for the health-promoting and disease-preventing activity of a wide range of dietary phytochemicals. However, there is considerable skepticism within the nutrition community of the ability to extrapolate from animal studies to humans, although we consider that they serve an important role to gain insight into underlying mechanisms. For example, the European Food Safety Authority, which regulates health and function claims associated with either food components or specific foods, does not consider health claims unless they contain evidence from human intervention studies.
HUMAN INTERVENTION STUDIES
Complementing both epidemiological data and the use of animal and cell models, there are data from human intervention studies. These can be loosely grouped into two major classes. First, there have been a relatively small number of large scale prospective trials with dietary supplements, mainly based upon the antioxidant hypothesis of bioactivity, with primary endpoints of predominantly lung and prostate cancer, and secondary endpoints of cancer at other sites and CVD. Second, there has been a larger number of shorter term studies that have focused on dietary interventions with supplements, specific foods and diets, and mainly biomarkers of cardiovascular risk as opposed to clinical endpoints. Design of intervention studies with specific foods or dietary manipulation is complex and challenging. It is not possible to adopt the classic double-blinded randomized control trial design typically used to evaluate pharmaceuticals, and many ethical issues arise if one seeks to modify diet for several weeks. Indeed, one of the important contributions that plant scientists could make to this field of study is to develop plant genotypes that have contrasting levels of bioactive compounds but are otherwise identical for the use in dietary intervention studies, as reviewed recently by Martin et al. (2011).
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study (The ATBC Cancer Prevention Study Group, 1994) and the Beta-Carotene and Retinol Efficacy study (Omenn et al., 1996b) assessed the combinations of vitamin E and β-carotene, and β-carotene and retinyl palmiate on incidence of lung cancer in studies involving 29,133 and 18,314 smokers (or those with other enhanced risk factors). Contrary to expectations, both studies reported a higher incidence of lung cancer (28 and 17% respectively) for those taking β-carotene supplements (Albanes et al., 1996; Omenn et al., 1996a). However, as a secondary endpoint, the ATBC study indicated a reduction in risk of prostate cancer with vitamin E. Two other studies of similar design, the Physicians Health Study and the Skin Cancer Prevention Study, involving 22,071 and 1805 volunteers, respectively, did not report any positive or negative effects on lung and skin cancer, although the volunteers in these studies were not of enhanced risk (Greenberg et al., 1996; Hennekens et al., 1996). By contrast, an intervention trial in China provided evidence for a 21% reduction in stomach cancer and a 13% reduction in total cancer mortality following 5 years of supplementation with a combination of vitamin E, β-carotene, and selenium (Blot et al., 1993). Based upon this study and the secondary results of the ATBC study, a randomized placebo controlled trial of selenium and vitamin E for prostate cancer prevention (SELECT) was undertaken involving 35,533 men. After more than 5 years, there was no evidence of any reduction in risk of cancer with the supplements, while the potential risk of type 2 diabetes appeared to be increased (Lippman et al., 2009). Thus, several large intervention studies have not provided evidence that dietary antioxidants can reduce incidence of cancer. This may be due to the precise dose and delivery system used for the compound of interest or the nature of the study population, or, maybe, these compounds lack the expected biological activity.
For smaller scale intervention studies, clinical endpoints, particularly with respect to cancer, are very challenging or probably not possible. The majority of studies are focused on assessing the effect of dietary interventions on biomarkers associated with CVD. These would include both biochemical markers, such as blood lipid profiles, pro- and anti-inflammatory cytokines, and physiological biomarkers, such as blood pressure and flow-mediated dilation of blood vessels. A combination of biomarkers can be integrated into overall estimates of cardiovascular risk with the use of widely accepted algorithms (British Cardiac Society; British Hypertension Society; Diabetes UK; HEART UK; Primary Care Cardiovascular Society; Stroke Association, 2005). While there have been studies that have considered a wide range of foods and their bioactive components, maybe the greatest body of work is concerned with dietary flavonoids. The diverse range of activity that these compounds exhibit in cell and animal models, combined with epidemiological studies, provide the background evidence to support such studies. A recent meta-analysis of 133 trials with flavonoids and flavonoid-rich foods suggested that foods such as chocolate and green tea and a limited number of soy products, which contain relatively high levels of different flavonoid subclasses, could have a beneficial effect on some CVD biomarkers. However, this review highlighted the methodological weaknesses that exist in trials reported up to now, such as small size of the majority of studies and poorly reported data from randomized controlled trials, although this should not be interpreted as lack of effectiveness (Hooper et al., 2008). In addition to studies with flavonoid-rich foods, similar intervention studies have been undertaken that focus on other classes of natural products. Foods rich in β-carotene have been used in several studies to investigate how they could be used to alleviate vitamin A deficiency (van Jaarsveld et al., 2005; Low et al., 2007) and other carotenoids, such as lycopene, lutein, and zeaxanthin, to investigate effects on biomarkers of cardiovascular risk, DNA damage, inflammation, and macular degeneration (Chopra et al., 2000; Astley et al., 2004; Kopsell et al., 2006; Paterson et al., 2006; Beck et al., 2010). There have also been several human intervention studies with cruciferous vegetables to attempt to obtain experimental evidence to support epidemiological data for the health promoting effects of these vegetables (Kensler et al., 2005; Al Janobi et al., 2006; Gasper et al., 2007; Traka et al., 2008; Li et al., 2009; Navarro et al., 2009a, 2009b; Yanaka et al., 2009; Brauer et al., 2011). Systematic reviews of human intervention studies that have involved both carotenoid-rich and glucosinolate-rich foods, as have been done for flavonoid-rich foods (Hooper et al., 2008), would be timely.
Human studies also cast doubt on the antioxidant theory of biological activity of dietary phytochemicals. As commented above, human metabolism of phytochemicals can reduce greatly their antioxidant capacity as shown within in vitro cell studies. Several studies have also attempted to demonstrate that a polyphenol-rich diet results in an increase in plasma antioxidant capacity, but few, if any, have demonstrated a significant increase. This is due to two main factors: First, the endogenous phenolic, α-tocopherol, and ascorbate concentrations in the plasma range between 169 and 380 μM (Clifford, 2004). The additional concentrations that can be obtained from dietary sources is relatively low, probably <1% from average diets and up to 5% for diets that are particularly rich in polyphenols (Clifford, 2004). Moreover, these additional marginal increases are transient, and it is difficult to envisage how these changes might have a significant impact upon health. However, it is conceivable that in certain elderly populations in which the plasma ascorbate levels can become depleted, heavy consumption of tea and coffee may have a significant effect on plasma antioxidant activity. A more detailed critique of the biological relevance of direct antioxidant effects of polyphenols has recently been published (Hollman et al., 2011). The biological activity of carotenoids is frequently attributed to their lipophilic antioxidant effects observed in cell and animal systems. Whether this is their main mode of action following normal dietary consumption within humans still remains to be elucidated. It now seems more likely that phytochemicals may have health-promoting effects through more complex changes on cell signaling pathways leading to, for example, changes in expression of pro- and anti-inflammatory cytokines that can influence the risk of chronic disease, as is evident through research with model systems (Youn et al., 2006, 2010) and human intervention studies (Traka et al., 2008).
While there are both biochemical and physiological biomarkers associated with risk of CVD that represent useful endpoints for dietary intervention studies, the same is not true for other forms of chronic disease, and this is a major limitation in the design of human intervention studies. It is inconceivable that it is possible to obtain data on the effects of a dietary intervention on primary cancer risk, as opposed to the use of supplements as described above, due to the required size of the studies to obtain appropriate power, their duration, and ethical issues concerned with altering diet, such as the complete removal of a certain food group from the control arm of the study. It is possible that dietary intervention studies could be adopted to assess the risk of reoccurrence of some cancers, such as bladder cancer and some forms of breast cancer that have relatively high reoccurrence rates. A similar strategy could be used to look at incidence of reoccurrence of other chronic diseases, such as inflammatory bowel disease after periods of remission.
While various biomarkers associated with CVD are accepted by the clinical community, there has been very little progress in identifying robust biomarkers for risk of other forms of chronic disease, such as cancer and cognitive decline. Biomarker development, either in the form of single biochemical traits or, for example, more complex metabolomic fingerprints of plasma and urine (Legido-Quigley et al., 2010), is a major target of the biomedical community.
While many dietary phytochemicals may have positive effects upon health, there may also be negative effects. The ability of dietary phytochemicals to induce and/or suppress phase 1 and phase 2 metabolism may raise concerns that these compounds will interfere with drug metabolism, and certain examples of such activity are known. For example, the furancoumarin begamottin that is found in grapefruit is an inhibitor of certain cytochrome P450s, including CYP34A, which, in turn prevents the metabolism of statins that subsequently accumulate in the bloodstream and have detrimental effects (Girennavar et al., 2006). Dietary polyphenols may also have negative effects on absorption of micronutrients. For example, phenolic compounds in the diet may complex with non-haem iron within the intestinal lumen making it unavailable for absorption. Thus, care should be taken when advocating a polyphenol-rich diet to certain sectors of the population, such as young women, who are frequently deficient in iron (Hurrell et al., 1999).
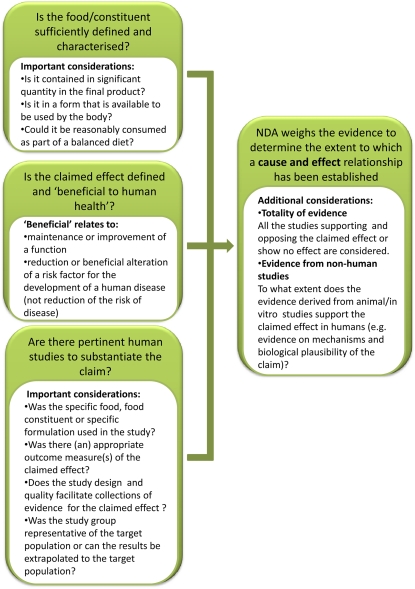
Health claims for foods that may have modified levels of phytochemicals are regulated in the U.S. by the FDA and in Europe by the European Food Safety Authority (EFSA). The FDA’s Code of Federal Regulations Title 21 section 101.14 of 2003 provides for four levels of claims based upon the extent of evidence, ranging from “significant scientific agreement for the claim” to “very limited and preliminary scientific for the claim.” In Europe, the criteria to assess health claims arose from the trans-European project PASSCLAIM (Process for the Assessment of Scientific Support for Claims on Foods) that led to EU regulations in 2006. Health claims for specific food components or proprietary products are submitted by the national government to EFSA who uses set criteria to assess the scientific validity of claims. Evidence of health benefits has to be derived from human intervention studies, while data from animal studies are used to provide evidence of the underlying mechanisms (Figure 2).
Figure 2.
Main Issues Addressed by the EFSA Panel on Dietetic Products, Nutrition, and Allergies in the Evaluation of Health Claims.
[See online article for color version of this figure.]
OPPORTUNITIES FOR THE PLANT SCIENTIST
What then should be the targets for the plant science community? One of the most valuable contributions will be the development of near isogenic genotypes of common foods that vary in specific phytochemicals that can be used within human intervention studies to ask specific questions concerning biological activity of different phytochemicals when provided not as supplements but within a common chemical and physical food matrix. The most notable approach to this is the manipulation of the phenylpropanoid and/or flavonoid pathways in tomato to develop fruits that have contrasting flavonoid compositions, including potentially all of the major subclasses of dietary flavonoids (Butelli et al., 2008; Luo et al., 2008; Martin et al., 2011). This system has the advantages of targeting phytochemicals for which there is considerable circumstantial evidence for health benefits within a tractable system that is appropriate for human studies in contrast with the use of other plant model systems. While there has been some experimentation of these types of materials with animal models, the use of these in short- and long-term intervention trials with humans will potentially make a major contribution to our understanding of the dietary role of these compounds in a manner that is not feasible through further epidemiological and animal studies.
The ability to manipulate carotenoid levels, exemplified through the development of ‘golden rice’ (Ye et al., 2000), has had greatest focus on expression of β-carotene to help alleviate vitamin A deficiency, but similar approaches could be used to modify levels of a range of carotenoids, such as phytoene, lycopene, zeaxanthin, and lutein, to assess their effect on biomarkers associated with, for example, CVD in a range of fruit and vegetable crops to investigate their potential health-promoting activity (Namitha and Negi, 2010). Again, as with tomatoes and flavonoids, the development of a range of genotypes of a single species with contrasting carotenoid profiles would be of immense value to the nutrition research community, although the technological challenges in manipulating carotenoid biosynthesis may be considerable (Fraser et al., 2009).
Sulfur-containing secondary metabolites are also appealing targets for manipulation. Evidence for the cancer-protecting effects of cruciferous vegetables remains strong (Kirsh et al., 2007; Lam et al., 2009), and recent studies suggest that diets rich in these vegetable may also reduce the risk of CVD (Zhang et al., 2011). To what extent this effect is mediated by glucosinolates and their degradation products remains to be resolved. The biochemical genetics of glucosinolate biosynthesis and accumulation has been thoroughly explored in Arabidopsis (Sønderby et al., 2010), and it has recently been shown it is possible to express these compounds, albeit at very low levels, in non-Brassicales species (Geu-Flores et al., 2009). Although marker-assisted selection approaches have been used to develop broccoli varieties with contrasting levels of glucosinolates (Mithen et al., 2003), and these have been used in human intervention studies (Gasper et al., 2005; Traka et al., 2008), there are conceivably many opportunities both to enhance and decrease their levels in cruciferous vegetables and salad plants via genetic modification approaches to test specific hypotheses concerning the glucosinolates themselves, as well as their degradation products. Likewise, sulfur compounds in Allium crops, such as onions and garlic, have been associated with health benefits (Griffiths et al., 2002; Ried et al., 2008). The main focus of these compounds among breeding companies has been to develop cultivars with milder flavors partly through reducing the levels of certain volatile compounds, but similar approaches could be adapted specifically for exploring in a rigorous manner the health promoting activity of certain secondary metabolites which may not necessarily contribute to flavor.
References
- Ahuja D., Sáenz-Robles M.T., Pipas J.M. (2005). SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24: 7729–7745 [DOI] [PubMed] [Google Scholar]
- Akhtar S., Meeran S.M., Katiyar N., Katiyar S.K. (2009). Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting insulin-like growth factor binding protein-3, tumor cell proliferation, and angiogenic factors. Clin. Cancer Res. 15: 821–831 [DOI] [PubMed] [Google Scholar]
- Albanes D., et al. (1996). Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: Effects of base-line characteristics and study compliance. J. Natl. Cancer Inst. 88: 1560–1570 [DOI] [PubMed] [Google Scholar]
- Al Janobi A.A., Mithen R.F., Gasper A.V., Shaw P.N., Middleton R.J., Ortori C.A., Barrett D.A. (2006). Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ionisation mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 844: 223–234 [DOI] [PubMed] [Google Scholar]
- Astley S.B., Elliott R.M., Archer D.B., Southon S. (2004). Evidence that dietary supplementation with carotenoids and carotenoid-rich foods modulates the DNA damage: Repair balance in human lymphocytes. Br. J. Nutr. 91: 63–72 [DOI] [PubMed] [Google Scholar]
- Aviram M., Dornfeld L., Rosenblat M., Volkova N., Kaplan M., Coleman R., Hayek T., Presser D., Fuhrman B. (2000). Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am. J. Clin. Nutr. 71: 1062–1076 [DOI] [PubMed] [Google Scholar]
- Beck K., Conlon C., Kruger R., Coad J., Stonehouse W. (2010). The effect of gold kiwifruit consumed with an iron fortified breakfast cereal meal on iron status in women with low iron stores: A 16 week randomised controlled intervention study. BMC Public Health 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilby J., Ambrosini G.L., Rossi E., de Klerk N.H., Musk A.W. (2010). Serum levels of folate, lycopene, β-carotene, retinol and vitamin E and prostate cancer risk. Eur. J. Clin. Nutr. 64: 1235–1238 [DOI] [PubMed] [Google Scholar]
- Belanger C.F., Hennekens C.H., Rosner B., Speizer F.E. (1978). The nurses’ health study. Am. J. Nurs. 78: 1039–1040 [PubMed] [Google Scholar]
- Bergen W.G., Mersmann H.J. (2005). Comparative aspects of lipid metabolism: Impact on contemporary research and use of animal models. J. Nutr. 135: 2499–2502 [DOI] [PubMed] [Google Scholar]
- Beydoun M.A., Shroff M.R., Chen X., Beydoun H.A., Wang Y., Zonderman A.B. (2011). Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J. Nutr. 141: 903–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham S. (2006). The fibre-folate debate in colo-rectal cancer. Proc. Nutr. Soc. 65: 19–23 [DOI] [PubMed] [Google Scholar]
- Bingham S.A., et al. ; European Prospective Investigation into Cancer and Nutrition (2003). Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): An observational study. Lancet 361: 1496–1501 [DOI] [PubMed] [Google Scholar]
- Blot W.J., et al. (1993). Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 85: 1483–1492 [DOI] [PubMed] [Google Scholar]
- Boffetta P., et al. (2010). Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 102: 529–537 [DOI] [PubMed] [Google Scholar]
- Brauer H.A., Libby T.E., Mitchell B.L., Li L., Chen C., Randolph T.W., Yasui Y.Y., Lampe J.W., Lampe P.D. (2011). Cruciferous vegetable supplementation in a controlled diet study alters the serum peptidome in a GSTM1-genotype dependent manner. Nutr. J. 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Cardiac Society; British Hypertension Society; Diabetes UK; HEART UK; Primary Care Cardiovascular Society; Stroke Association (2005). JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart 91(suppl. 5): v1–v52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelli E., Titta L., Giorgio M., Mock H.P., Matros A., Peterek S., Schijlen E.G., Hall R.D., Bovy A.G., Luo J., Martin C. (2008). Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 26: 1301–1308 [DOI] [PubMed] [Google Scholar]
- Cai H., Marczylo T.H., Teller N., Brown K., Steward W.P., Marko D., Gescher A.J. (2010). Anthocyanin-rich red grape extract impedes adenoma development in the Apc(Min) mouse: Pharmacodynamic changes and anthocyanin levels in the murine biophase. Eur. J. Cancer 46: 811–817 [DOI] [PubMed] [Google Scholar]
- Campbell D.B. (1996). Extrapolation from animals to man. The integration of pharmacokinetics and pharmacodynamics. Ann. N. Y. Acad. Sci. 801: 116–135 [DOI] [PubMed] [Google Scholar]
- Carter P., Gray L.J., Troughton J., Khunti K., Davies M.J. (2010). Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 341: c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy A., O’Reilly E.J., Kay C., Sampson L., Franz M., Forman J.P., Curhan G., Rimm E.B. (2011). Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 93: 338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Lu Z., Viljoen A., Hamman J. (2009). Intestinal drug transport enhancement by Aloe vera. Planta Med. 75: 587–595 [DOI] [PubMed] [Google Scholar]
- Choi J.S., Kim H., Jung M.H., Hong S., Song J. (2010). Consumption of barley beta-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 54: 1004–1013 [DOI] [PubMed] [Google Scholar]
- Chopra M., O’Neill M.E., Keogh N., Wortley G., Southon S., Thurnham D.I. (2000). Influence of increased fruit and vegetable intake on plasma and lipoprotein carotenoids and LDL oxidation in smokers and nonsmokers. Clin. Chem. 46: 1818–1829 [PubMed] [Google Scholar]
- Clifford M.N. (2004). Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 70: 1103–1114 [DOI] [PubMed] [Google Scholar]
- Colby W.W., Shenk T. (1982). Fragments of the simian virus 40 transforming gene facilitate transformation of rat embryo cells. Proc. Natl. Acad. Sci. USA 79: 5189–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S., Martin T.L., Surwit R.S., Robidoux J. (2004). Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: Physiological and molecular characteristics. Physiol. Behav. 81: 243–248 [DOI] [PubMed] [Google Scholar]
- Conaway C.C., Wang C.X., Pittman B., Yang Y.M., Schwartz J.E., Tian D., McIntee E.J., Hecht S.S., Chung F.L. (2005). Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 65: 8548–8557 [DOI] [PubMed] [Google Scholar]
- Dauchet L., Amouyel P., Hercberg S., Dallongeville J. (2006). Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 136: 2588–2593 [DOI] [PubMed] [Google Scholar]
- de Jong M., Maina T. (2010). Of mice and humans: Are they the same?—Implications in cancer translational research. J. Nucl. Med. 51: 501–504 [DOI] [PubMed] [Google Scholar]
- Deeks J. (1998). When can odds ratios mislead? Odds ratios should be used only in case-control studies and logistic regression analyses. BMJ 317: 1155–1156, author reply 1156–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. (2011). Medicine: Diabetes in India. Nature 469: 478–479 [DOI] [PubMed] [Google Scholar]
- Fodde R., et al. (1994). A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc. Natl. Acad. Sci. USA 91: 8969–8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Wright W.C., Loveless J.D. (1977). Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J. Natl. Cancer Inst. 58: 209–214 [DOI] [PubMed] [Google Scholar]
- Fraser P.D., Enfissi E.M., Bramley P.M. (2009). Genetic engineering of carotenoid formation in tomato fruit and the potential application of systems and synthetic biology approaches. Arch. Biochem. Biophys. 483: 196–204 [DOI] [PubMed] [Google Scholar]
- Fuchs C.S., Giovannucci E.L., Colditz G.A., Hunter D.J., Stampfer M.J., Rosner B., Speizer F.E., Willett W.C. (1999). Dietary fiber and the risk of colorectal cancer and adenoma in women. N. Engl. J. Med. 340: 169–176 [DOI] [PubMed] [Google Scholar]
- Gasper A.V., Al-Janobi A., Smith J.A., Bacon J.R., Fortun P., Atherton C., Taylor M.A., Hawkey C.J., Barrett D.A., Mithen R.F. (2005). Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am. J. Clin. Nutr. 82: 1283–1291 [DOI] [PubMed] [Google Scholar]
- Gasper A.V., Traka M., Bacon J.R., Smith J.A., Taylor M.A., Hawkey C.J., Barrett D.A., Mithen R.F. (2007). Consuming broccoli does not induce genes associated with xenobiotic metabolism and cell cycle control in human gastric mucosa. J. Nutr. 137: 1718–1724 [DOI] [PubMed] [Google Scholar]
- Geu-Flores F., Nielsen M.T., Nafisi M., Møldrup M.E., Olsen C.E., Motawia M.S., Halkier B.A. (2009). Glucosinolate engineering identifies a gamma-glutamyl peptidase. Nat. Chem. Biol. 5: 575–577 [DOI] [PubMed] [Google Scholar]
- Girennavar B., Poulose S.M., Jayaprakasha G.K., Bhat N.G., Patil B.S. (2006). Furocoumarins from grapefruit juice and their effect on human CYP 3A4 and CYP 1B1 isoenzymes. Bioorg. Med. Chem. 14: 2606–2612 [DOI] [PubMed] [Google Scholar]
- Gonzalez C.A., Riboli E. (2010). Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Cancer 46: 2555–2562 [DOI] [PubMed] [Google Scholar]
- Grasset E., Pinto M., Dussaulx E., Zweibaum A., Desjeux J.F. (1984). Epithelial properties of human colonic carcinoma cell line Caco-2: Electrical parameters. Am. J. Physiol. 247: C260–C267 [DOI] [PubMed] [Google Scholar]
- Greenberg E.R., Baron J.A., Karagas M.R., Stukel T.A., Nierenberg D.W., Stevens M.M., Mandel J.S., Haile R.W. (1996). Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 275: 699–703 [DOI] [PubMed] [Google Scholar]
- Greenberg N.M., DeMayo F., Finegold M.J., Medina D., Tilley W.D., Aspinall J.O., Cunha G.R., Donjacour A.A., Matusik R.J., Rosen J.M. (1995). Prostate cancer in a transgenic mouse. Proc. Natl. Acad. Sci. USA 92: 3439–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Trueman L., Crowther T., Thomas B., Smith B. (2002). Onions—A global benefit to health. Phytother. Res. 16: 603–615 [DOI] [PubMed] [Google Scholar]
- Grobbee D.E., Rimm E.B., Giovannucci E., Colditz G., Stampfer M., Willett W. (1990). Coffee, caffeine, and cardiovascular disease in men. N. Engl. J. Med. 323: 1026–1032 [DOI] [PubMed] [Google Scholar]
- Hackam D.G., Redelmeier D.A. (2006). Translation of research evidence from animals to humans. JAMA 296: 1731–1732 [DOI] [PubMed] [Google Scholar]
- Hao X., Sun Y., Yang C.S., Bose M., Lambert J.D., Ju J., Lu G., Lee M.J., Park S., Husain A., Wang S. (2007). Inhibition of intestinal tumorigenesis in Apc(min/+) mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr. Cancer 59: 62–69 [DOI] [PubMed] [Google Scholar]
- Hara A., Hibi T., Yoshioka M., Toda K., Watanabe N., Hayashi A., Iwao Y., Saito H., Watanabe T., Tsuchiya M. (1993). Changes of proliferative activity and phenotypes in spontaneous differentiation of a colon cancer cell line. Jpn. J. Cancer Res. 84: 625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. (1965). The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37: 614–636 [DOI] [PubMed] [Google Scholar]
- He F.J., Nowson C.A., MacGregor G.A. (2006). Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet 367: 320–326 [DOI] [PubMed] [Google Scholar]
- Hennekens C.H., Buring J.E., Manson J.E., Stampfer M., Rosner B., Cook N.R., Belanger C., LaMotte F., Gaziano J.M., Ridker P.M., Willett W., Peto R. (1996). Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 334: 1145–1149 [DOI] [PubMed] [Google Scholar]
- Hidalgo I.J., Raub T.J., Borchardt R.T. (1989). Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96: 736–749 [PubMed] [Google Scholar]
- Hogan S., Canning C., Sun S., Sun X., Kadouh H., Zhou K. (2011). Dietary supplementation of grape skin extract improves glycemia and inflammation in diet-induced obese mice fed a Western high fat diet. J. Agric. Food Chem. 59: 3035–3041 [DOI] [PubMed] [Google Scholar]
- Hollman P.C., Cassidy A., Comte B., Heinonen M., Richelle M., Richling E., Serafini M., Scalbert A., Sies H., Vidry S. (2011). The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 141: 989S–1009S [DOI] [PubMed] [Google Scholar]
- Hooper L., Kroon P.A., Rimm E.B., Cohn J.S., Harvey I., Le Cornu K.A., Ryder J.J., Hall W.L., Cassidy A. (2008). Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 88: 38–50 [DOI] [PubMed] [Google Scholar]
- Hotz C., McClafferty B. (2007). From harvest to health: challenges for developing biofortified staple foods and determining their impact on micronutrient status. Food Nutr. Bull. 28(2, suppl.): S271–S279 [DOI] [PubMed] [Google Scholar]
- Huang P.L. (2009). A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2: 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurrell R.F., Reddy M., Cook J.D. (1999). Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 81: 289–295 [PubMed] [Google Scholar]
- Huschtscha L.I., Holliday R. (1983). Limited and unlimited growth of SV40-transformed cells from human diploid MRC-5 fibroblasts. J. Cell Sci. 63: 77–99 [DOI] [PubMed] [Google Scholar]
- Janisch K.M., Williamson G., Needs P., Plumb G.W. (2004). Properties of quercetin conjugates: Modulation of LDL oxidation and binding to human serum albumin. Free Radic. Res. 38: 877–884 [DOI] [PubMed] [Google Scholar]
- Jayaprakasam B., Olson L.K., Schutzki R.E., Tai M.H., Nair M.G. (2006). Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 54: 243–248 [DOI] [PubMed] [Google Scholar]
- Juge N., Mithen R.F., Traka M. (2007). Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 64: 1105–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karppi J., Kurl S., Nurmi T., Rissanen T.H., Pukkala E., Nyyssönen K. (2009). Serum lycopene and the risk of cancer: The Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. Ann. Epidemiol. 19: 512–518 [DOI] [PubMed] [Google Scholar]
- Kassie F., Anderson L.B., Scherber R., Yu N., Lahti D., Upadhyaya P., Hecht S.S. (2007). Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 67: 6502–6511 [DOI] [PubMed] [Google Scholar]
- Kavanaugh C.J., Trumbo P.R., Ellwood K.C. (2007). The U.S. Food and Drug Administration’s evidence-based review for qualified health claims: tomatoes, lycopene, and cancer. J. Natl. Cancer Inst. 99: 1074–1085 [DOI] [PubMed] [Google Scholar]
- Kennedy A.J., Ellacott K.L., King V.L., Hasty A.H. (2010). Mouse models of the metabolic syndrome. Dis. Model. Mech. 3: 156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler T.W., et al. (2005). Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomarkers Prev. 14: 2605–2613 [DOI] [PubMed] [Google Scholar]
- Kirsh V.A., Peters U., Mayne S.T., Subar A.F., Chatterjee N., Johnson C.C., Hayes R.B.; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (2007). Prospective study of fruit and vegetable intake and risk of prostate cancer. J. Natl. Cancer Inst. 99: 1200–1209 [DOI] [PubMed] [Google Scholar]
- Konijeti R., Henning S., Moro A., Sheikh A., Elashoff D., Shapiro A., Ku M., Said J.W., Heber D., Cohen P., Aronson W.J. (2010). Chemoprevention of prostate cancer with lycopene in the TRAMP model. Prostate 70: 1547–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsue N., Ioannides C. (2010). Differential response of four human livers to modulation of phase II enzyme systems by the chemopreventive phytochemical phenethyl isothiocyanate. Mol. Nutr. Food Res. 54: 1477–1485 [DOI] [PubMed] [Google Scholar]
- Kopsell D.A., Lefsrud M.G., Kopsell D.E., Wenzel A.J., Gerweck C., Curran-Celentano J. (2006). Spinach cultigen variation for tissue carotenoid concentrations influences human serum carotenoid levels and macular pigment optical density following a 12-week dietary intervention. J. Agric. Food Chem. 54: 7998–8005 [DOI] [PubMed] [Google Scholar]
- Kristal A.R., Arnold K.B., Neuhouser M.L., Goodman P., Platz E.A., Albanes D., Thompson I.M. (2010). Diet, supplement use, and prostate cancer risk: Results from the prostate cancer prevention trial. Am. J. Epidemiol. 172: 566–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.K., Gallicchio L., Lindsley K., Shiels M., Hammond E., Tao X.G., Chen L., Robinson K.A., Caulfield L.E., Herman J.G., Guallar E., Alberg A.J. (2009). Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol. Biomarkers Prev. 18: 184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.S., Kim C.T., Kim Y. (2009). Green tea (-)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann. Nutr. Metab. 54: 151–157 [DOI] [PubMed] [Google Scholar]
- Legido-Quigley C., Stella C., Perez-Jimenez F., Lopez-Miranda J., Ordovas J., Powell J., van-der-Ouderaa F., Ware L., Lindon J.C., Nicholson J.K., Holmes E. (2010). Liquid chromatography-mass spectrometry methods for urinary biomarker detection in metabonomic studies with application to nutritional studies. Biomed. Chromatogr. 24: 737–743 [DOI] [PubMed] [Google Scholar]
- Li F., Hullar M.A., Schwarz Y., Lampe J.W. (2009). Human gut bacterial communities are altered by addition of cruciferous vegetables to a controlled fruit- and vegetable-free diet. J. Nutr. 139: 1685–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman S.M., et al. (2009). Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi F., Jimenez R., Moreno L., Kroon P.A., Needs P.W., Hughes D.A., Santos-Buelga C., Gonzalez-Paramas A., Cogolludo A., Lopez-Sepulveda R., Duarte J., Perez-Vizcaino F. (2009). Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis 204: 34–39 [DOI] [PubMed] [Google Scholar]
- London S.J., Yuan J.M., Chung F.L., Gao Y.T., Coetzee G.A., Ross R.K., Yu M.C. (2000). Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: A prospective study of men in Shanghai, China. Lancet 356: 724–729 [DOI] [PubMed] [Google Scholar]
- Low J.W., Arimond M., Osman N., Cunguara B., Zano F., Tschirley D. (2007). A food-based approach introducing orange-fleshed sweet potatoes increased vitamin A intake and serum retinol concentrations in young children in rural Mozambique. J. Nutr. 137: 1320–1327 [DOI] [PubMed] [Google Scholar]
- Lubin J.H., Gail M.H. (1984). Biased selection of controls for case-control analyses of cohort studies. Biometrics 40: 63–75 [PubMed] [Google Scholar]
- Luchman H.A., Benediktsson H., Villemaire M.L., Peterson A.C., Jirik F.R. (2008). The pace of prostatic intraepithelial neoplasia development is determined by the timing of Pten tumor suppressor gene excision. PLoS ONE 3: e3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Butelli E., Hill L., Parr A., Niggeweg R., Bailey P., Weisshaar B., Martin C. (2008). AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. 56: 316–326 [DOI] [PubMed] [Google Scholar]
- Lutgens E., Daemen M., Kockx M., Doevendans P., Hofker M., Havekes L., Wellens H., de Muinck E.D. (1999). Atherosclerosis in APOE*3-Leiden transgenic mice: From proliferative to atheromatous stage. Circulation 99: 276–283 [DOI] [PubMed] [Google Scholar]
- Martignoni M., Groothuis G.M., de Kanter R. (2006). Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2: 875–894 [DOI] [PubMed] [Google Scholar]
- Martin C., Butelli E., Petroni K., Tonelli C. (2011). How can research on plants contribute to promoting human health? Plant Cell 23: 1685–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G.J., Ross H.A., Ikeji M., Stewart D. (2008). Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J. Agric. Food Chem. 56: 3016–3023 [DOI] [PubMed] [Google Scholar]
- Medina-Remón A., et al. ; PREDIMED Study Investigators (2011). Total polyphenol excretion and blood pressure in subjects at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 21: 323–331 [DOI] [PubMed] [Google Scholar]
- Mehta R., Lansky E.P. (2004). Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur. J. Cancer Prev. 13: 345–348 [DOI] [PubMed] [Google Scholar]
- Mestas J., Hughes C.C. (2004). Of mice and not men: Differences between mouse and human immunology. J. Immunol. 172: 2731–2738 [DOI] [PubMed] [Google Scholar]
- Mi L., Wang X., Govind S., Hood B.L., Veenstra T.D., Conrads T.P., Saha D.T., Goldman R., Chung F.L. (2007). The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 67: 6409–6416 [DOI] [PubMed] [Google Scholar]
- Mishra P., Kar A., Kale R.K. (2009). Prevention of chemically induced mammary tumorigenesis by daidzein in pre-pubertal rats: The role of peroxidative damage and antioxidative enzymes. Mol. Cell. Biochem. 325: 149–157 [DOI] [PubMed] [Google Scholar]
- Mithen R., Faulkner K., Magrath R., Rose P., Williamson G., Marquez J. (2003). Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor. Appl. Genet. 106: 727–734 [DOI] [PubMed] [Google Scholar]
- Morimoto T., Sunagawa Y., Kawamura T., Takaya T., Wada H., Nagasawa A., Komeda M., Fujita M., Shimatsu A., Kita T., Hasegawa K. (2008). The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Invest. 118: 868–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moronvalle-Halley V., Sacré-Salem B., Sallez V., Labbe G., Gautier J.C. (2005). Evaluation of cultured, precision-cut rat liver slices as a model to study drug-induced liver apoptosis. Toxicology 207: 203–214 [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Lekli I., Ray D., Gangopadhyay H., Raychaudhuri U., Das D.K. (2010). Comparison of the protective effects of steamed and cooked broccolis on ischaemia-reperfusion-induced cardiac injury. Br. J. Nutr. 103: 815–823 [DOI] [PubMed] [Google Scholar]
- Namitha K.K., Negi P.S. (2010). Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 50: 728–760 [DOI] [PubMed] [Google Scholar]
- Navarro S.L., Chang J.L., Peterson S., Chen C., King I.B., Schwarz Y., Li S.S., Li L., Potter J.D., Lampe J.W. (2009a). Modulation of human serum glutathione S-transferase A1/2 concentration by cruciferous vegetables in a controlled feeding study is influenced by GSTM1 and GSTT1 genotypes. Cancer Epidemiol. Biomarkers Prev. 18: 2974–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro S.L., Peterson S., Chen C., Makar K.W., Schwarz Y., King I.B., Li S.S., Li L., Kestin M., Lampe J.W. (2009b). Cruciferous vegetable feeding alters UGT1A1 activity: Diet- and genotype-dependent changes in serum bilirubin in a controlled feeding trial. Cancer Prev. Res. (Phila.) 2: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenn G.S., Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Glass A., Keogh J.P., Meyskens F.L., Valanis B., Williams J.H., Barnhart S., Hammar S. (1996a). Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 334: 1150–1155 [DOI] [PubMed] [Google Scholar]
- Omenn G.S., et al. (1996b). Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Cancer Inst. 88: 1550–1559 [DOI] [PubMed] [Google Scholar]
- O’Sullivan L., Jiwan M.A., Daly T., O’Brien N.M., Aherne S.A. (2010). Bioaccessibility, uptake, and transport of carotenoids from peppers (Capsicum spp.) using the coupled in vitro digestion and human intestinal Caco-2 cell model. J. Agric. Food Chem. 58: 5374–5379 [DOI] [PubMed] [Google Scholar]
- Pannellini T., Iezzi M., Liberatore M., Sabatini F., Iacobelli S., Rossi C., Alberti S., Di Ilio C., Vitaglione P., Fogliano V., Piantelli M. (2010). A dietary tomato supplement prevents prostate cancer in TRAMP mice. Cancer Prev. Res. (Phila.) 3: 1284–1291 [DOI] [PubMed] [Google Scholar]
- Papini S., Rosellini A., Campani D., DeMatteis A., Selli C., Revoltella R.P. (2004). Selective growth of epithelial basal cells from human prostate in a three-dimensional organ culture. Prostate 59: 383–392 [DOI] [PubMed] [Google Scholar]
- Parnell J.A., Reimer R.A. (2010). Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: A dose-response study in JCR:LA-cp rats. Br. J. Nutr. 103: 1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson E., Gordon M.H., Niwat C., George T.W., Parr L., Waroonphan S., Lovegrove J.A. (2006). Supplementation with fruit and vegetable soups and beverages increases plasma carotenoid concentrations but does not alter markers of oxidative stress or cardiovascular risk factors. J. Nutr. 136: 2849–2855 [DOI] [PubMed] [Google Scholar]
- Pearce N. (1993). What does the odds ratio estimate in a case-control study? Int. J. Epidemiol. 22: 1189–1192 [DOI] [PubMed] [Google Scholar]
- Piao C.S., Gao S., Lee G.H., Kim S., Park B.H., Chae S.W., Chae H.J., Kim S.H. (2010). Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial K(ATP) channels. Pharmacol. Res. 61: 342–348 [DOI] [PubMed] [Google Scholar]
- Pietinen P., Malila N., Virtanen M., Hartman T.J., Tangrea J.A., Albanes D., Virtamo J. (1999). Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 10: 387–396 [DOI] [PubMed] [Google Scholar]
- Pinto M., Robineleon S., Appay M.D., Kedinger M., Triadou N., Dussaulx E., Lacroix B., Simonassmann P., Haffen K., Fogh J., Zweibaum A. (1983). Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 47: 323–330 [Google Scholar]
- Reboul E., Borel P., Mikail C., Abou L., Charbonnier M., Caris-Veyrat C., Goupy P., Portugal H., Lairon D., Amiot M.J. (2005). Enrichment of tomato paste with 6% tomato peel increases lycopene and beta-carotene bioavailability in men. J. Nutr. 135: 790–794 [DOI] [PubMed] [Google Scholar]
- Riboli E., Kaaks R. (1997). The EPIC Project: Rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 26(suppl. 1): S6–S14 [DOI] [PubMed] [Google Scholar]
- Richard D., Kefi K., Barbe U., Poli A., Bausero P., Visioli F. (2009). Weight and plasma lipid control by decaffeinated green tea. Pharmacol. Res. 59: 351–354 [DOI] [PubMed] [Google Scholar]
- Richelle M., Sanchez B., Tavazzi I., Lambelet P., Bortlik K., Williamson G. (2010). Lycopene isomerisation takes place within enterocytes during absorption in human subjects. Br. J. Nutr. 103: 1800–1807 [DOI] [PubMed] [Google Scholar]
- Ried K., Frank O.R., Stocks N.P., Fakler P., Sullivan T. (2008). Effect of garlic on blood pressure: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbach G., Weinberg P.D., de Pascual-Teresa S., Alonso M.G., Ewins B.A., Turner R., Minihane A.M., Botting N., Fairley B., Matsugo S., Uchida Y., Cassidy A. (2004). Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim. Biophys. Acta 1670: 229–237 [DOI] [PubMed] [Google Scholar]
- Röscheisen C., Haupter S., Zechner U., Speit G. (1994). Characterization of spontaneous and induced mutations in SV40-transformed normal and ataxia telangiectasia cell lines. Somat. Cell Mol. Genet. 20: 493–504 [DOI] [PubMed] [Google Scholar]
- Russell J.C., Graham S.E., Richardson M. (1998). Cardiovascular disease in the JCR:LA-cp rat. Mol. Cell. Biochem. 188: 113–126 [PubMed] [Google Scholar]
- Russell J.C., Proctor S.D. (2006). Small animal models of cardiovascular disease: Tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc. Pathol. 15: 318–330 [DOI] [PubMed] [Google Scholar]
- Savini I., Arnone R., Catani M.V., Avigliano L. (2009). Origanum vulgare induces apoptosis in human colon cancer caco2 cells. Nutr. Cancer 61: 381–389 [DOI] [PubMed] [Google Scholar]
- Semsarian C. (2009). Use of mouse models for the analysis of human disease. Curr. Protoc. Hum. Genet. 60: 15.2.1–15.2.10 [DOI] [PubMed] [Google Scholar]
- Shan Y., Zhao R., Geng W., Lin N., Wang X., Du X., Wang S. (2010). Protective effect of sulforaphane on human vascular endothelial cells against lipopolysaccharide-induced inflammatory damage. Cardiovasc. Toxicol. 10: 139–145 [DOI] [PubMed] [Google Scholar]
- Shirai M., Kawai Y., Yamanishi R., Kinoshita T., Chuman H., Terao J. (2006). Effect of a conjugated quercetin metabolite, quercetin 3-glucuronide, on lipid hydroperoxide-dependent formation of reactive oxygen species in differentiated PC-12 cells. Free Radic. Res. 40: 1047–1053 [DOI] [PubMed] [Google Scholar]
- Soler-Rivas C., Marín F.R., Santoyo S., García-Risco M.R., Señoráns F.J., Reglero G. (2010). Testing and enhancing the in vitro bioaccessibility and bioavailability of Rosmarinus officinalis extracts with a high level of antioxidant abietanes. J. Agric. Food Chem. 58: 1144–1152 [DOI] [PubMed] [Google Scholar]
- Sønderby I.E., Geu-Flores F., Halkier B.A. (2010). Biosynthesis of glucosinolates—Gene discovery and beyond. Trends Plant Sci. 15: 283–290 [DOI] [PubMed] [Google Scholar]
- Swamy M.V., Citineni B., Patlolla J.M., Mohammed A., Zhang Y., Rao C.V. (2008). Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr. Cancer 60(suppl. 1): 81–89 [DOI] [PubMed] [Google Scholar]
- Terry P., Giovannucci E., Michels K.B., Bergkvist L., Hansen H., Holmberg L., Wolk A. (2001). Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J. Natl. Cancer Inst. 93: 525–533 [DOI] [PubMed] [Google Scholar]
- The ATBC Cancer Prevention Study Group (1994). The alpha-tocopherol, beta-carotene lung cancer prevention study: Design, methods, participant characteristics, and compliance. Ann. Epidemiol. 4: 1–10 [DOI] [PubMed] [Google Scholar]
- Titta L., et al. (2010). Blood orange juice inhibits fat accumulation in mice. Int. J. Obes. (Lond.) 34: 578–588 [DOI] [PubMed] [Google Scholar]
- Traka M., et al. (2008). Broccoli consumption interacts with GSTM1 to perturb oncogenic signalling pathways in the prostate. PLoS ONE 3: e2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M., Gasper A.V., Smith J.A., Hawkey C.J., Bao Y., Mithen R.F. (2005). Transcriptome analysis of human colon Caco-2 cells exposed to sulforaphane. J. Nutr. 135: 1865–1872 [DOI] [PubMed] [Google Scholar]
- Traka M.H., Chambers K.F., Lund E.K., Goodlad R.A., Johnson I.T., Mithen R.F. (2009). Involvement of KLF4 in sulforaphane- and iberin-mediated induction of p21(waf1/cip1). Nutr. Cancer 61: 137–145 [DOI] [PubMed] [Google Scholar]
- Traka M.H., Spinks C.A., Doleman J.F., Melchini A., Ball R.Y., Mills R.D., Mithen R.F. (2010). The dietary isothiocyanate sulforaphane modulates gene expression and alternative gene splicing in a PTEN null preclinical murine model of prostate cancer. Mol. Cancer 9: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Jaarsveld P.J., Faber M., Tanumihardjo S.A., Nestel P., Lombard C.J., Benadé A.J. (2005). Beta-carotene-rich orange-fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified-relative-dose-response test. Am. J. Clin. Nutr. 81: 1080–1087 [DOI] [PubMed] [Google Scholar]
- Varga O., Harangi M., Olsson I.A., Hansen A.K. (2010). Contribution of animal models to the understanding of the metabolic syndrome: A systematic overview. Obes. Rev. 11: 792–807 [DOI] [PubMed] [Google Scholar]
- Velicescu M., Yu J.M., Herbert B.S., Shay J.W., Granada E., Dubeau L. (2003). Aneuploidy and telomere attrition are independent determinants of crisis in SV40-transformed epithelial cells. Cancer Res. 63: 5813–5820 [PubMed] [Google Scholar]
- Verona E.V., Elkahloun A.G., Yang J., Bandyopadhyay A., Yeh I.T., Sun L.Z. (2007). Transforming growth factor-beta signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res. 67: 5737–5746 [DOI] [PubMed] [Google Scholar]
- Verschuren L., Wielinga P.Y., van Duyvenvoorde W., Tijani S., Toet K., van Ommen B., Kooistra T., Kleemann R. (2011). A dietary mixture containing fish oil, resveratrol, lycopene, catechins, and vitamins E and C reduces atherosclerosis in transgenic mice. J. Nutr. 141: 863–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroegrijk I.O., van Diepen J.A., van den Berg S., Westbroek I., Keizer H., Gambelli L., Hontecillas R., Bassaganya-Riera J., Zondag G.C., Romijn J.A., Havekes L.M., Voshol P.J. (2011). Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem. Toxicol. 49: 1426–1430 [DOI] [PubMed] [Google Scholar]
- Waddington E., Puddey I.B., Croft K.D. (2004). Red wine polyphenolic compounds inhibit atherosclerosis in apolipoprotein E-deficient mice independently of effects on lipid peroxidation. Am. J. Clin. Nutr. 79: 54–61 [DOI] [PubMed] [Google Scholar]
- Wang S., Gao J., Lei Q., Rozengurt N., Pritchard C., Jiao J., Thomas G.V., Li G., Roy-Burman P., Nelson P.S., Liu X., Wu H. (2003). Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4: 209–221 [DOI] [PubMed] [Google Scholar]
- Wang S., Garcia A.J., Wu M., Lawson D.A., Witte O.N., Wu H. (2006). Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc. Natl. Acad. Sci. USA 103: 1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Kruithof-de Julio M., Economides K.D., Walker D., Yu H., Halili M.V., Hu Y.P., Price S.M., Abate-Shen C., Shen M.M. (2009). A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461: 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston R.H., et al. ; Mouse Genome Sequencing Consortium (2002). Initial sequencing and comparative analysis of the mouse genome. Nature 420: 520–562 [DOI] [PubMed] [Google Scholar]
- Weisberg S.P., Leibel R., Tortoriello D.V. (2008). Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 149: 3549–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley A.C., Stoner G.D., Darby M.V., Walle T. (2003). Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid—Extensive binding to protein and DNA. Biochem. Pharmacol. 66: 907–915 [DOI] [PubMed] [Google Scholar]
- Winterbone M.S., Tribolo S., Needs P.W., Kroon P.A., Hughes D.A. (2009). Physiologically relevant metabolites of quercetin have no effect on adhesion molecule or chemokine expression in human vascular smooth muscle cells. Atherosclerosis 202: 431–438 [DOI] [PubMed] [Google Scholar]
- Xu C., Huang M.T., Shen G., Yuan X., Lin W., Khor T.O., Conney A.H., Kong A.N. (2006). Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 66: 8293–8296 [DOI] [PubMed] [Google Scholar]
- Yanaka A., Fahey J.W., Fukumoto A., Nakayama M., Inoue S., Zhang S., Tauchi M., Suzuki H., Hyodo I., Yamamoto M. (2009). Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev. Res. (Phila.) 2: 353–360 [DOI] [PubMed] [Google Scholar]
- Ye X., Al-Babili S., Klöti A., Zhang J., Lucca P., Beyer P., Potrykus I. (2000). Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287: 303–305 [DOI] [PubMed] [Google Scholar]
- Youn H.S., Kim Y.S., Park Z.Y., Kim S.Y., Choi N.Y., Joung S.M., Seo J.A., Lim K.M., Kwak M.K., Hwang D.H., Lee J.Y. (2010). Sulforaphane suppresses oligomerization of TLR4 in a thiol-dependent manner. J. Immunol. 184: 411–419 [DOI] [PubMed] [Google Scholar]
- Youn H.S., Saitoh S.I., Miyake K., Hwang D.H. (2006). Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem. Pharmacol. 72: 62–69 [DOI] [PubMed] [Google Scholar]
- Yusuf N., Nasti T.H., Meleth S., Elmets C.A. (2009). Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol. Carcinog. 48: 713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Shu X.O., Xiang Y.B., Yang G., Li H., Gao J., Cai H., Gao Y.T., Zheng W. (2011). Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am. J. Clin. Nutr. 94: 240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Talalay P., Cho C.G., Posner G.H. (1992). A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 89: 2399–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Meisinger J., Van Thiel D.H., Zhang Y., Mobarhan S. (2002). Effects of soybean extract on morphology and survival of Caco-2, SW620, and HT-29 cells. Nutr. Cancer 42: 131–140 [DOI] [PubMed] [Google Scholar]