Abstract
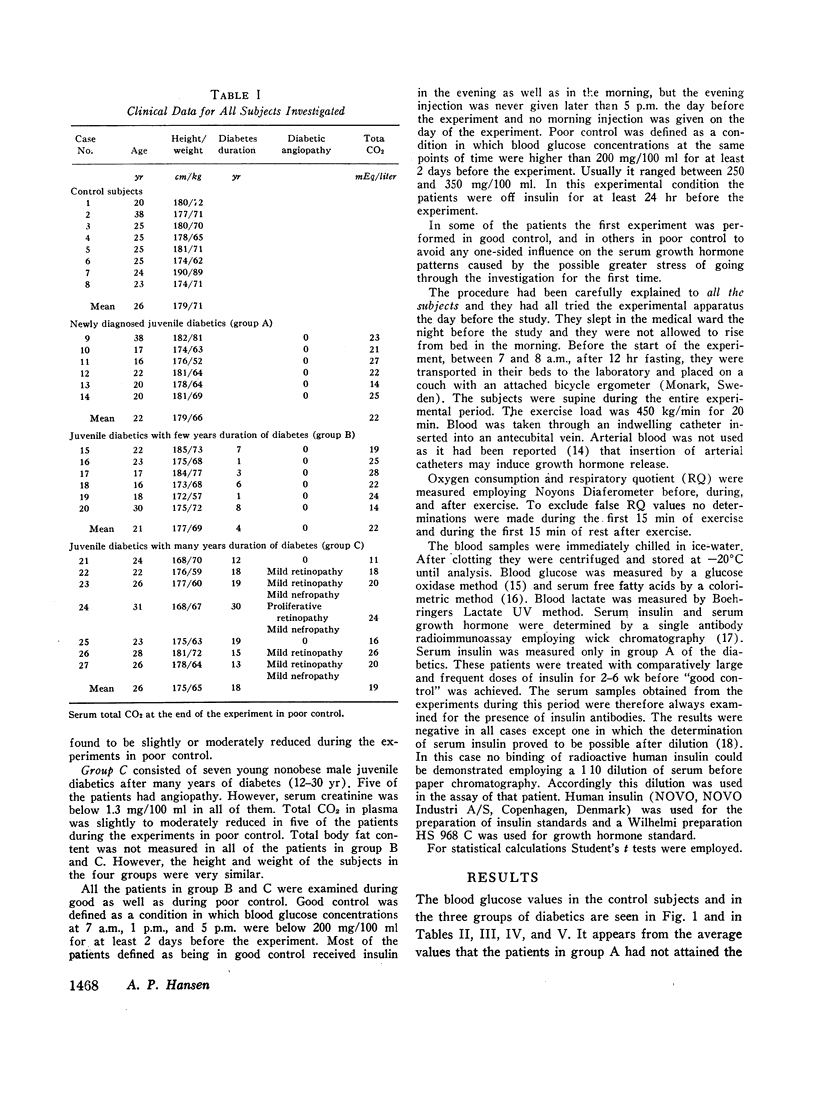
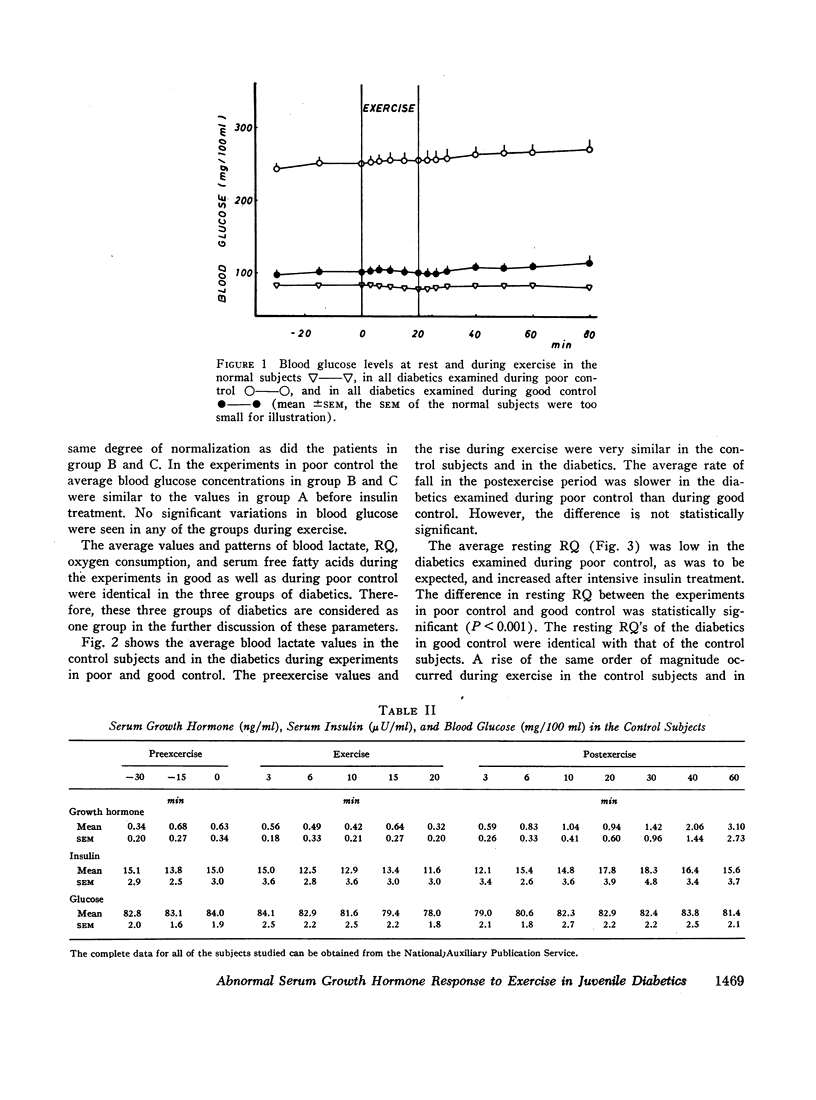
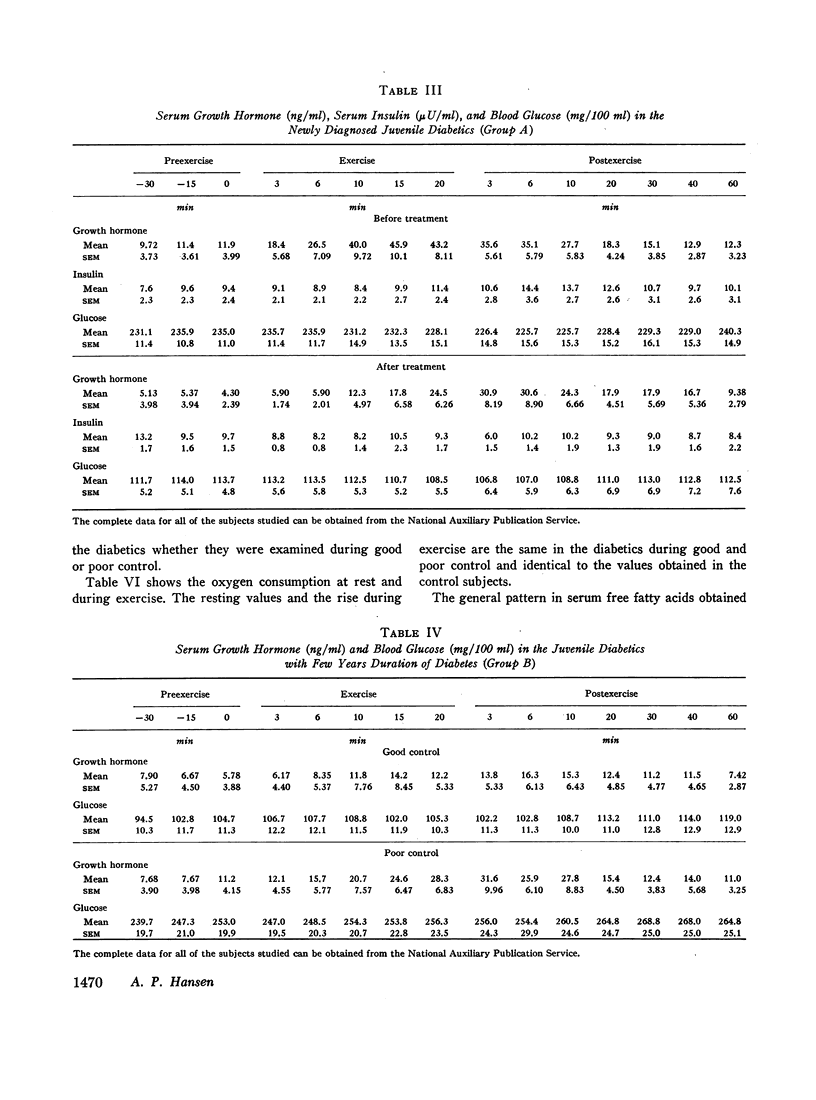
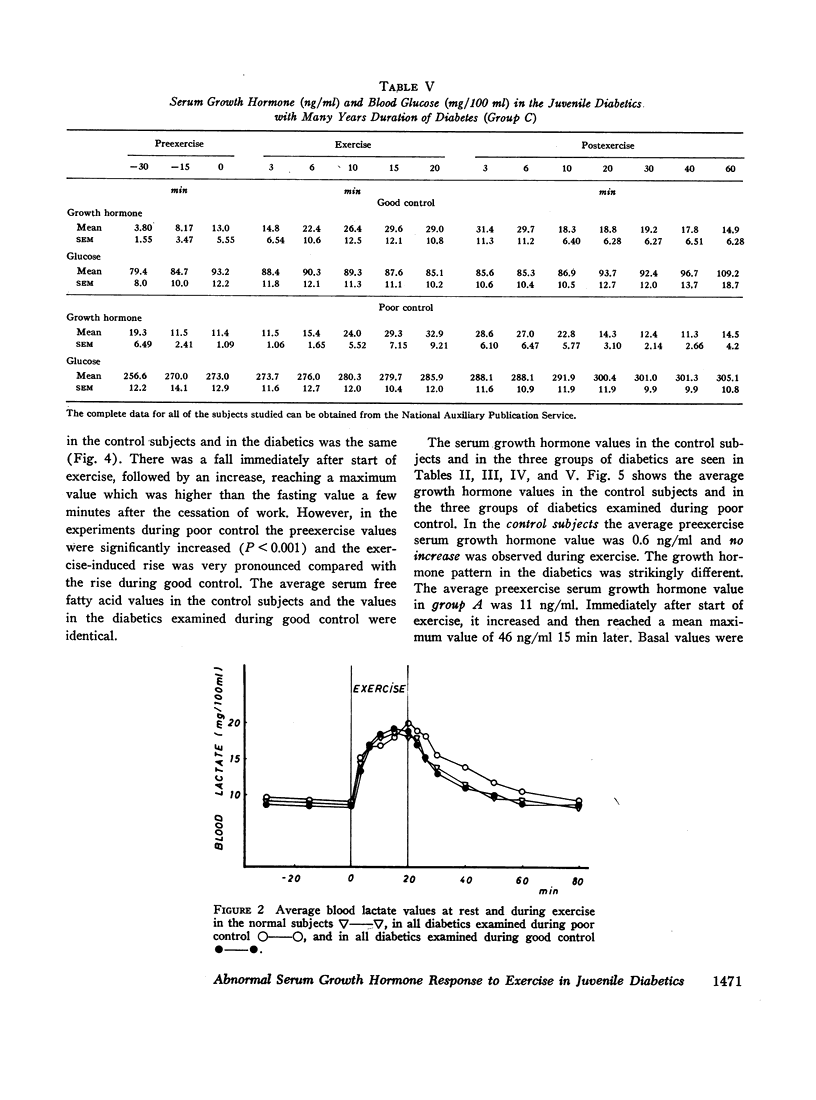
Groups of male nonobese juvenile diabetics with recent onset, short term (1-8 yr), and long-term (12-30 yr) diabetes as well as comparable nondiabetic controls were studied during exercise experiments. The chosen exercise load, 450 kg/min for 20 min never induced changes in serum growth hormone in our nondiabetic control subjects.
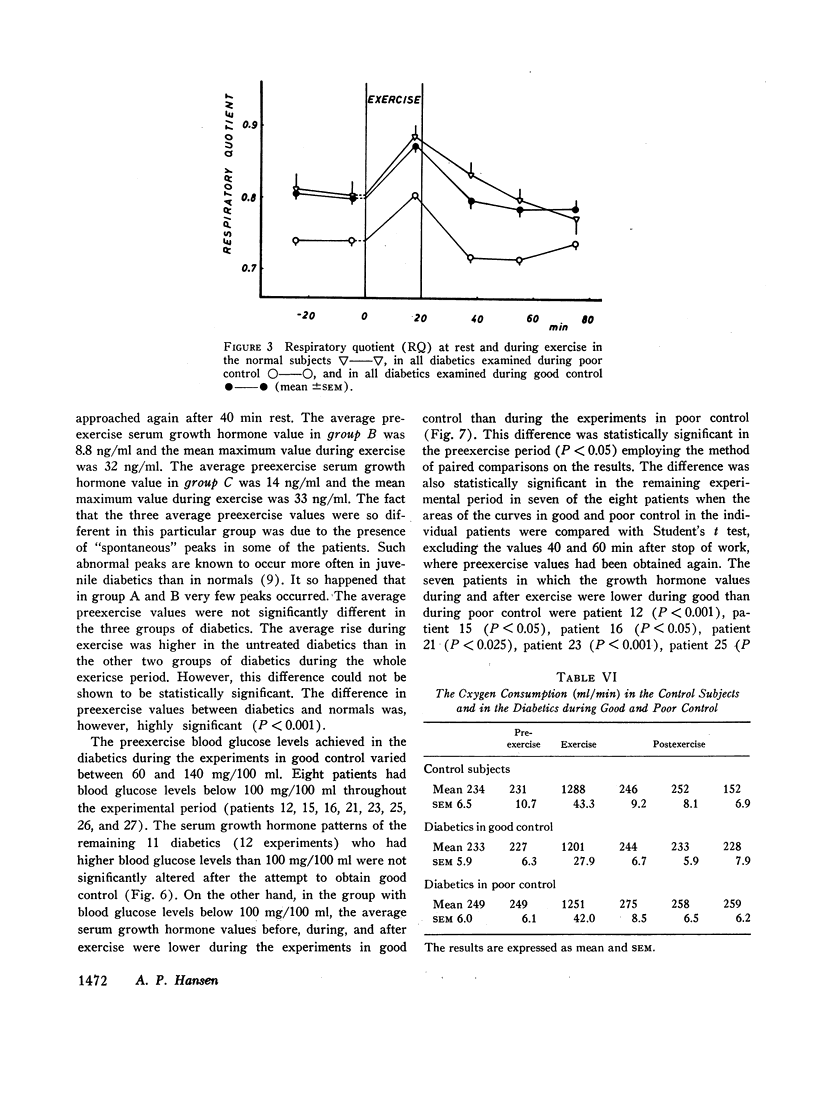
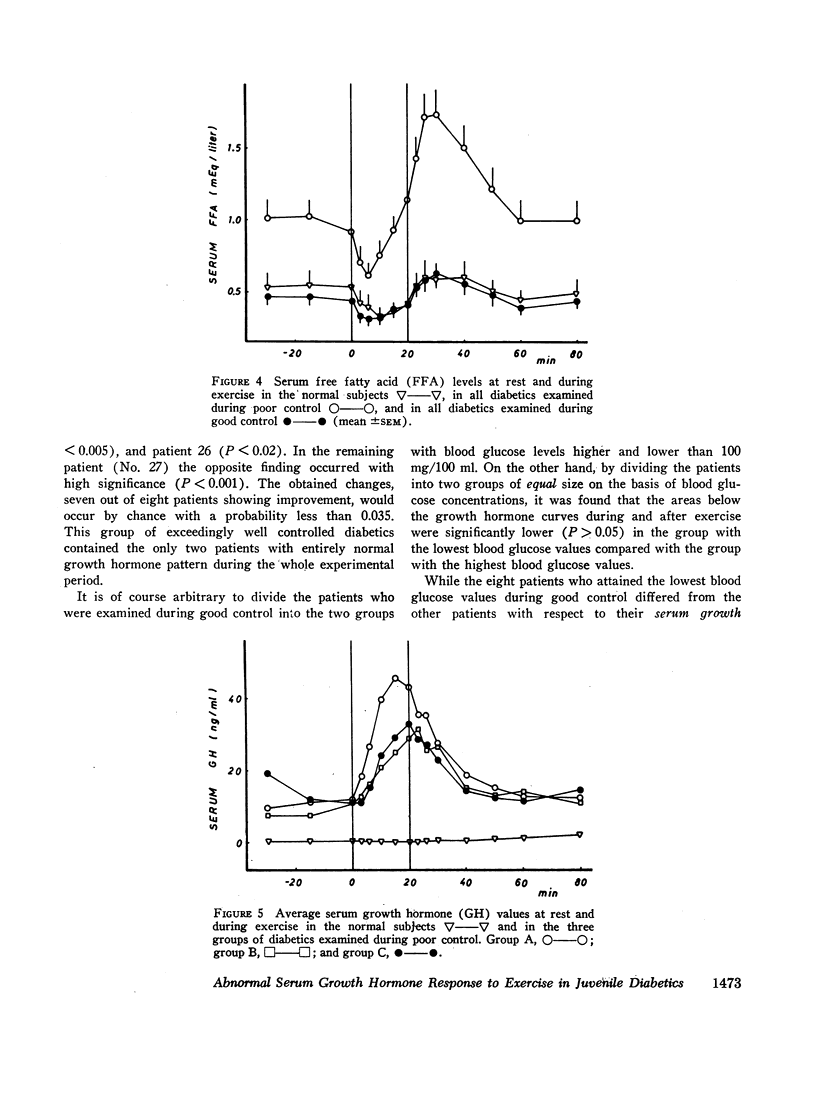
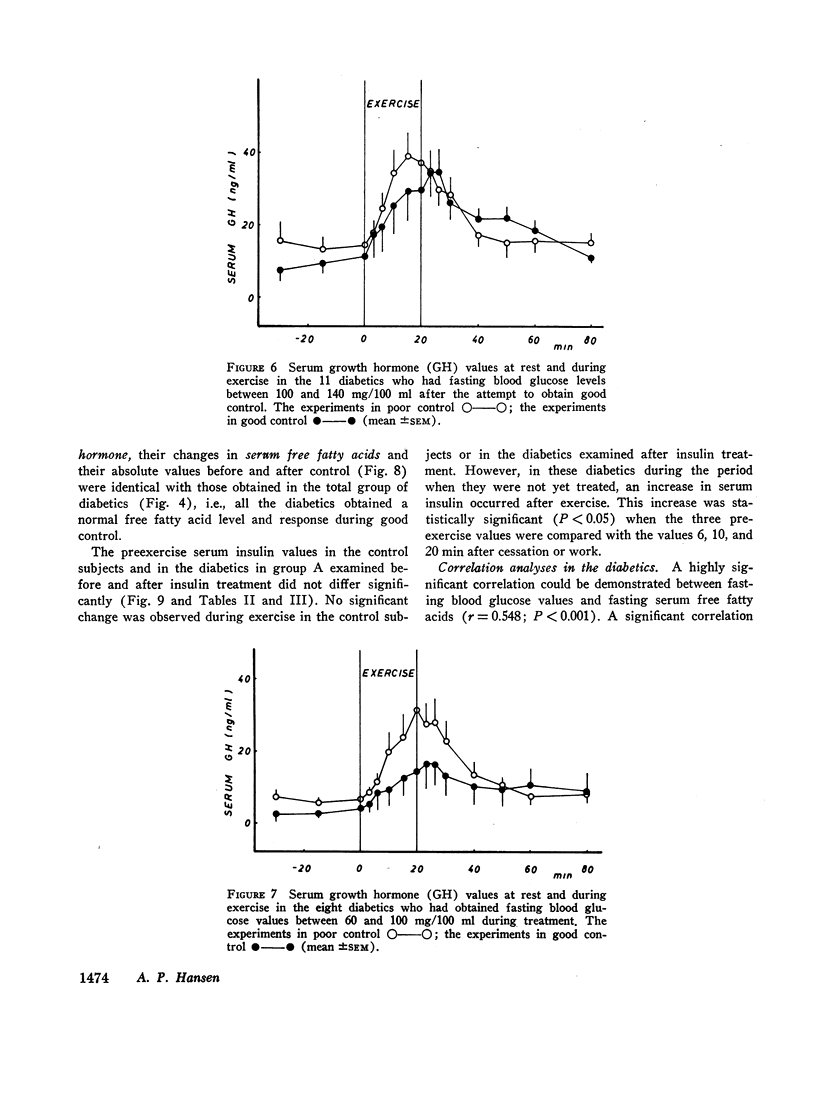
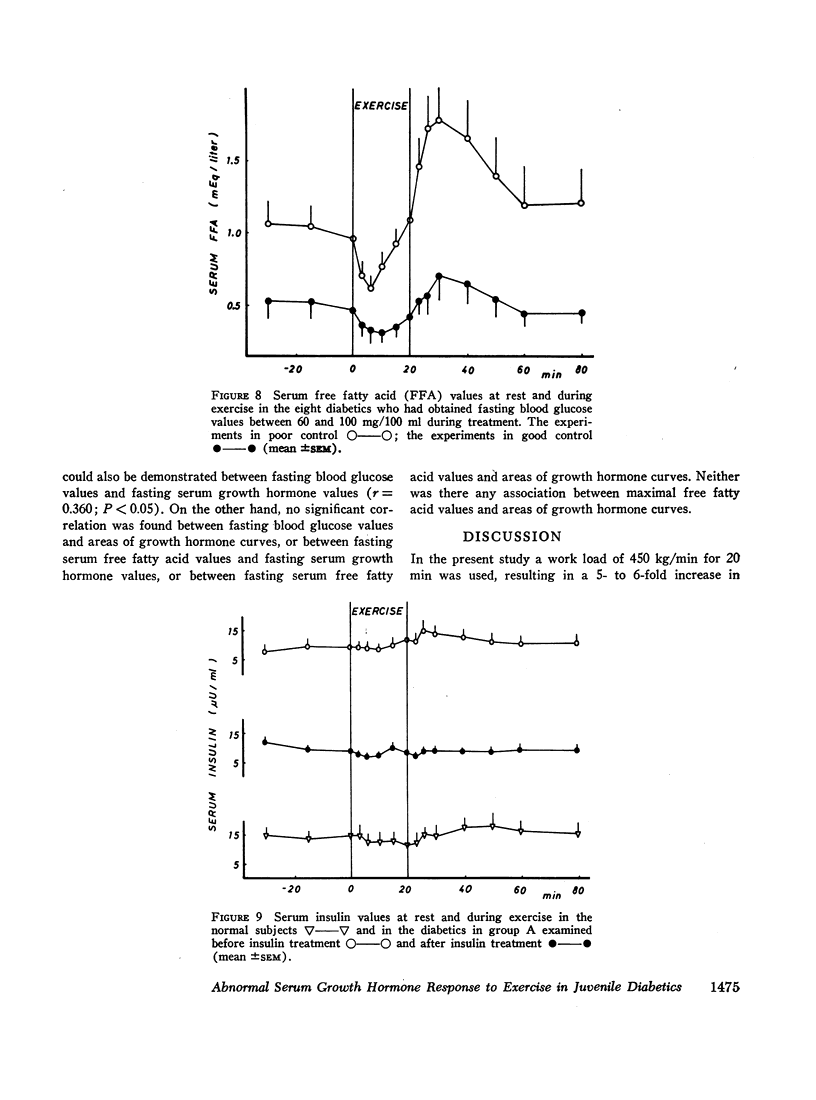
The principal results of the study were as follows: (a) an immediate high rise in serum growth hormone followed the commencement of exercise in all diabetics. The increase and pattern of serum growth hormone was not related to the duration of the diabetes. (b) The abnormal growth hormone response to exercise in diabetics was observed when the patients were in poor control as well as when they were in clinically excellent control (fasting blood glucose level between 100 and 140 mg/100 ml). (c) However, the abnormal serum growth hormone response was significantly diminished when exceedingly strict control was achieved (fasting blood glucose level between 60 and 100 mg/100 ml). In two of these experiments an entirely normal growth hormone pattern was obtained. (d) The change in serum growth hormone pattern during regulation was totally unrelated to the changes in serum free fatty acid patterns. A normal free fatty acid level and exercise pattern was obtained much earlier during the improved control. (e) Fasting serum growth hormone levels were also significantly raised in the juvenile diabetics irrespective of the diabetes duration. (f) Fasting serum growth hormone levels were also significantly decreased during regulation. Furthermore, a significant correlation between blood glucose and fasting serum growth hormone concentration was established. (g) In the juvenile diabetics a significant increase in serum insulin was observed at the point of time when exercise was concluded.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIERMAN E. L., DOLE V. P., ROBERTS T. N. An abnormality of nonesterified fatty acid metabolism in diabetes mellitus. Diabetes. 1957 Nov-Dec;6(6):475–479. doi: 10.2337/diab.6.6.475. [DOI] [PubMed] [Google Scholar]
- Baker L., Root A. W., Haque N., Kaye R. Metabolic homeostasis in juvenile diabetes mellitus. I. Role of growth hormone. Metabolism. 1969 Feb;18(2):110–114. doi: 10.1016/0026-0495(69)90103-6. [DOI] [PubMed] [Google Scholar]
- Boden G., Soeldner J. S., Gleason R. E., Marble A. Elevated serum human growth hormone and decreased serum insulin in prediabetic males after intravenous tolbutamide and glucose. J Clin Invest. 1968 Apr;47(4):729–739. doi: 10.1172/JCI105768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlström S. Studies on fatty acid metabolism in diabetics during exercise. I. Plasma free fatty acid concentration in juvenile, newly diagnosed diabetics during exercise. Acta Med Scand. 1967 May;181(5):609–621. doi: 10.1111/j.0954-6820.1967.tb07284.x. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Notes on the glucose oxidase method. Scand J Clin Lab Invest. 1967;19(4):379–384. doi: 10.3109/00365516709090653. [DOI] [PubMed] [Google Scholar]
- Copinschi G., Hartog M., Earll J. M., Havel R. J. Effect of various blood sampling procedures on serum levels of immunoreactive human growth hormone. Metabolism. 1967 May;16(5):402–409. doi: 10.1016/0026-0495(67)90130-8. [DOI] [PubMed] [Google Scholar]
- Drash A., Field J. B., Garces L. Y., Kenny F. M., Mintz D., Vazquez A. M. Endogenous insulin and growth hormone response in children with newly diagnosed diabetes mellitus. Pediatr Res. 1968 Mar;2(2):94–102. doi: 10.1203/00006450-196803000-00004. [DOI] [PubMed] [Google Scholar]
- FRIEDBERG S. J., HARLAN W. R., Jr, TROUT D. L., ESTES E. H., Jr The effect of exercise on the concentration and turnover of plasma nonesterified fatty acids. J Clin Invest. 1960 Jan;39:215–220. doi: 10.1172/JCI104021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatourechi V., Molnar G. D., Service F. J., Ackerman E., Rosevear J. W., Moxness K. E., Taylor W. F. Growth hormone and glucose interrelationships in diabetes: studies with insulin infusion during continuous blood glucose analysis. J Clin Endocrinol Metab. 1969 Mar;29(3):319–327. doi: 10.1210/jcem-29-3-319. [DOI] [PubMed] [Google Scholar]
- Hunter W. M., Fonseka C. C., Passmore R. The rôle of growth hormone in the mobilization of fuel for muscular exercise. Q J Exp Physiol Cogn Med Sci. 1965 Oct;50(4):406–416. doi: 10.1113/expphysiol.1965.sp001806. [DOI] [PubMed] [Google Scholar]
- Jacobs H. S., Nabarro J. D. Plasma 11-hydroxycorticosteroid and growth hormone levels in acute medical illnesses. Br Med J. 1969 Jun 7;2(5657):595–598. doi: 10.1136/bmj.2.5657.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL S. Plasma free fatty acids in diabetic acidosis and starvation. Scand J Clin Lab Invest. 1956;8(1):81–82. doi: 10.3109/00365515609049249. [DOI] [PubMed] [Google Scholar]
- Laurell S., Tibbling G. Colorimetric micro-determination of free fatty acids in plasma. Clin Chim Acta. 1967 Apr;16(1):57–62. doi: 10.1016/0009-8981(67)90269-0. [DOI] [PubMed] [Google Scholar]
- Molnar G. D., Ackerman E., Rosevear J. W., Gatewood L. C., Moxness K. E. Continuous blood glucose analysis in ambulatory fed subjects. I. General methodology. Mayo Clin Proc. 1968 Dec;43(12):833–851. [PubMed] [Google Scholar]
- Orskov H., Thomsen H. G., Yde H. Wick chromatography for rapid and reliable immunoassay of insulin, glucagon and growth hormone. Nature. 1968 Jul 13;219(5150):193–195. doi: 10.1038/219193b0. [DOI] [PubMed] [Google Scholar]
- Parker M. L., Pildes R. S., Chao K. L., Cornblath M., Kipnis D. M. Juvenile diabetes mellitus, a deficiency in insulin. Diabetes. 1968 Jan;17(1):27–32. doi: 10.2337/diab.17.1.27. [DOI] [PubMed] [Google Scholar]
- ROTH J., GLICK S. M., YALOW R. S., BERSON S. A. Secretion of human growth hormone: physiologic and experimental modification. Metabolism. 1963 Jul;12:577–579. [PubMed] [Google Scholar]
- Sabeh G., Mendelsohn L. V., Corredor D. G., Sunder J. H., Friedman L. M., Morgan C. R., Danowski T. S. Growth hormone in insulin-treated diabetes mellitus. Metabolism. 1969 Sep;18(9):748–753. doi: 10.1016/0026-0495(69)90003-1. [DOI] [PubMed] [Google Scholar]
- UNGER R. H. HIGH GROWTH-HORMONE LEVELS IN DIABETIC KETOACIDOSIS: A POSSIBLE CAUSE OF INSULIN RESISTANCE. JAMA. 1965 Mar 15;191:945–947. doi: 10.1001/jama.1965.03080110069026. [DOI] [PubMed] [Google Scholar]
- VAUGHAN B. E., BOLING E. A. Rapid assay procedures for tritium-labeled water in body fluids. J Lab Clin Med. 1961 Jan;57:159–164. [PubMed] [Google Scholar]
- Yde H. Abnormal growth hormone response to ingestion of glucose in juvenile diabetics. Acta Med Scand. 1969 Dec;186(6):499–504. doi: 10.1111/j.0954-6820.1969.tb01511.x. [DOI] [PubMed] [Google Scholar]


