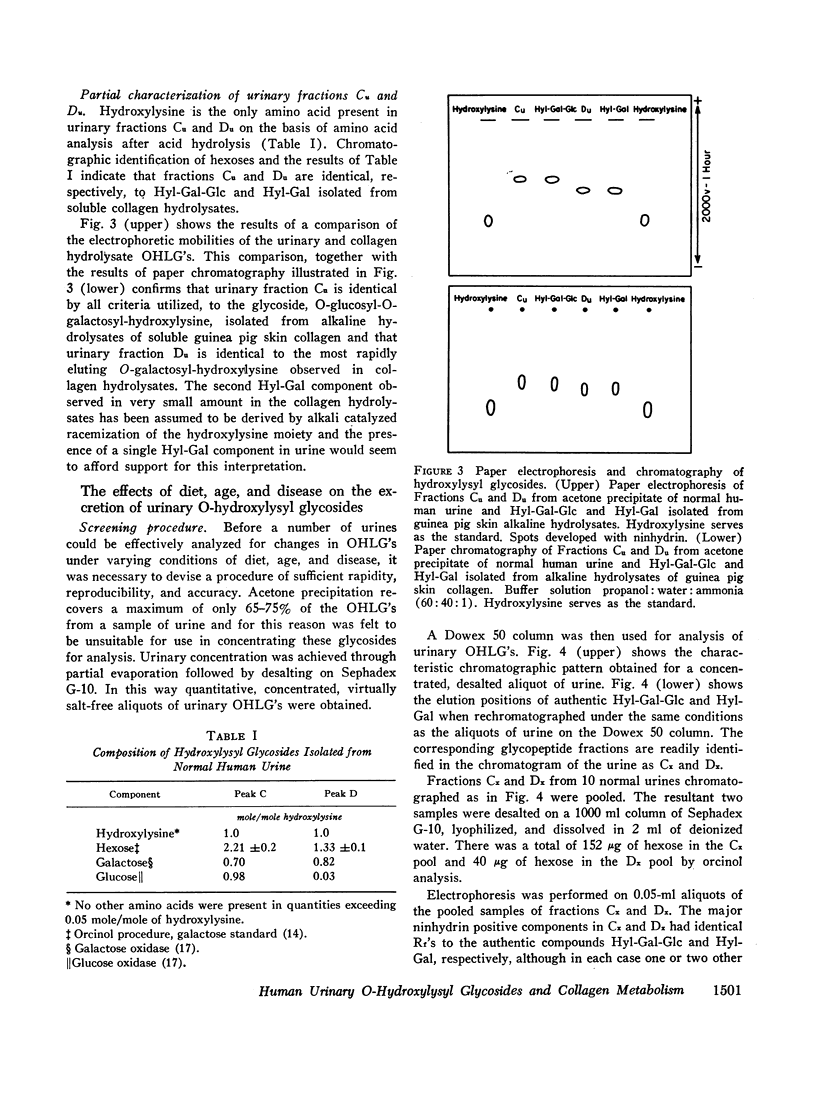
Abstract
Two O-hydroxylysyl glycosides, Hyl-Gal-Glc and Hyl-Gal, have been isolated from normal human urine and shown to be identical to two glycosides isolated from alkaline hydrolysates of collagen. A relatively sample and reproducible analytical procedure has been devised to measure the levels of these glycosides in human urine. By the use of this procedure it was shown that a normal diet has only a small effect on 24-hr urinary excretion levels of these glycosides indicating an endogenous origin. Urinary glycoside levels appear to be highest in children, roughly paralleling collagen turnover as indicated by urinary hydroxyproline levels. Collagen turnover equivalents calculated from urinary hydroxylysyl glycoside levels were found to be significantly larger than collagen turnover equivalents calculated from urinary hydroxyproline levels. This suggests that urinary glycosides are more quantitative indicators of collagen metabolism than urinary hydroxyproline.
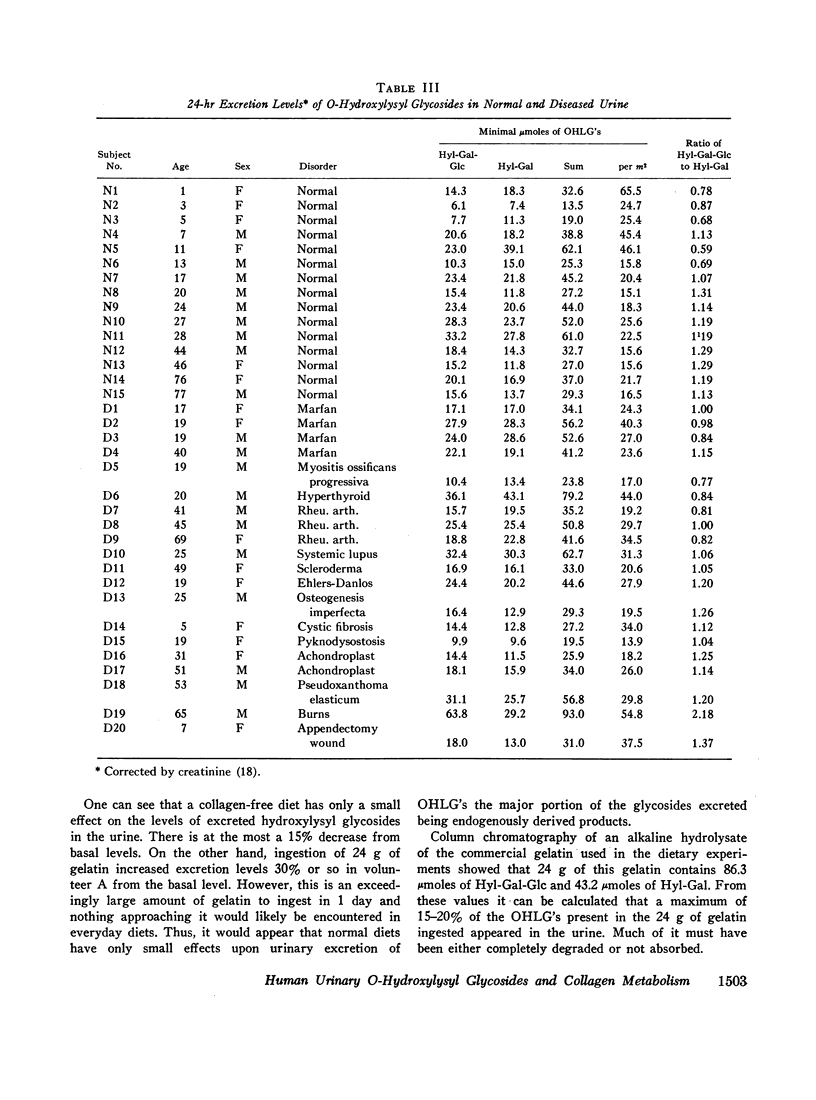
The ratio of Hyl-Gal-Glc to Hyl-Gal was measured in urines of diseased as well as normal individuals and a bimodal distribution was found. Alkaline hydrolysates of different human connective tissue collagens showed that only bone collagen, of the collagens examined, had a low ratio of Hyl-Gal-Glc to Hyl-Gal compared to human urine. Other collagens examined had higher ratios than found in human urine. On the basis of these results it is postulated that the bimodal distribution of glycoside ratios represents two populations of collagen turnover, the lower ratio population having a high bone collagen turnover, the lower ratio population having a high bone collagen turnover relative to the second population. Examination of the types of subjects making up the two populations supports this hypothesis. These data suggest that urinary O-hydroxylysyl glycoside excretion, in addition to providing a more quantitative estimate of collagen turnover than urinary hydroxyproline, may prove to be of value as a specific means of studying the metabolism of bone collagen.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON A. J., MACLAGAN N. F. The isolation and estimation of urinary mucoproteins. Biochem J. 1955 Apr;59(4):638–644. doi: 10.1042/bj0590638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENOIT F. L., THEIL G. B., WATTEN R. H. HYDROXYPROLINE EXCRETION IN ENDOCRINE DISEASE. Metabolism. 1963 Dec;12:1072–1082. [PubMed] [Google Scholar]
- Bosmann H. B., Eylar E. H. Attachment of carbohydrate to collagen. Isolation, purification and properties of the glucosyl transferase. Biochem Biophys Res Commun. 1968 Jan 11;30(1):89–94. doi: 10.1016/0006-291x(68)90717-1. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Cunningham L. W. Evidence for the linkage of a disaccharide to hydroxylysine in tropocollagen. J Biol Chem. 1966 Sep 10;241(17):3882–3888. [PubMed] [Google Scholar]
- CRABBE P., ISSELBACHER K. J. URINARY HYDROXYPROLINE EXCRETION IN MALABSORPTION STATES. Gastroenterology. 1965 Mar;48:307–311. [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D. A comparison of glycopeptides derived from soluble and insoluble collagens. J Biol Chem. 1968 May 10;243(9):2390–2398. [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D., Segrest J. P. The isolation of identical hydroxylysyl glycosides from hydrolysates of soluble collagen and from human urine. J Biol Chem. 1967 May 25;242(10):2570–2571. [PubMed] [Google Scholar]
- JASIN H. E., FINK C. W., WISE W., ZIFF M. Relationship between urinary hydroxyproline and growth. J Clin Invest. 1962 Oct;41:1928–1935. doi: 10.1172/JCI104650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIVIRIKKO K. I., KOIVUSALO M., LAITINEN O., LAMBERG B. A. HYDROXYPROLINE IN THE SERUM AND URINE OF PATIENTS WITH HYPERTHYROIDISM. J Clin Endocrinol Metab. 1964 Feb;24:222–223. doi: 10.1210/jcem-24-2-222. [DOI] [PubMed] [Google Scholar]
- KLEIN L., CURTISS P. H., Jr, DAVIS J. H. Collagen breakdown in thermal burns. Surg Forum. 1962;13:459–461. [PubMed] [Google Scholar]
- MAXFIELD M., STEFANYE D. The amino acid composition of normal human urinary mucoprotein. J Biol Chem. 1962 Aug;237:2522–2524. [PubMed] [Google Scholar]
- PLATT W. D., DOOLITTLE L. H., HARTSHORN J. W. URINARY HYDROXYPROLINE EXCRETION IN METASTATIC CANCER OF BONE. N Engl J Med. 1964 Aug;271:287–290. doi: 10.1056/NEJM196408062710604. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., KEISER H. R., SJOERDSMA A. Gastrointestinal absorption and renal excretion of hydroxyproline peptides. Lancet. 1962 Sep 15;2(7255):527–528. doi: 10.1016/s0140-6736(62)90400-2. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., SJOERDSMA A. Significance of urinary hydroxyproline in man. J Clin Invest. 1961 May;40:843–849. doi: 10.1172/JCI104318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- STEARNS G., NEWMAN K. J., McKINLEY J. B., JEANS P. C. The protein requirements of children from one to ten years of age. Ann N Y Acad Sci. 1958 Jan 10;69(5):857–868. doi: 10.1111/j.1749-6632.1958.tb49724.x. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J Biol Chem. 1969 Feb 25;244(4):602–612. [PubMed] [Google Scholar]
- Spiro R. G. The structure of the disaccharide unit of the renal glomerular basement membrane. J Biol Chem. 1967 Oct 25;242(20):4813–4823. [PubMed] [Google Scholar]
- WOESSNER J. F., Jr Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem J. 1962 May;83:304–314. doi: 10.1042/bj0830304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P. H., Klein L. The quantitative relationship of urinary peptide hydroxyproline excretion to collagen degradation. J Clin Invest. 1969 Jan;48(1):1–10. doi: 10.1172/JCI105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIFF M., KIBRICK A., DRESNER E., GRIBETZ H. J. Excretion of hydroxyproline in patients with rheumatic and non-rheumatic diseases. J Clin Invest. 1956 Jun;35(6):579–587. doi: 10.1172/JCI103311. [DOI] [PMC free article] [PubMed] [Google Scholar]



