Abstract
The concentrations of plasma glucose, free fatty acids, insulin, growth hormone, and placental prolactin in subhuman primate fetal and maternal plasma were examined following intravascular administration of insulin and glucagon to the fetus and mother. The neonatal plasma responses to these same stimuli were also examined.
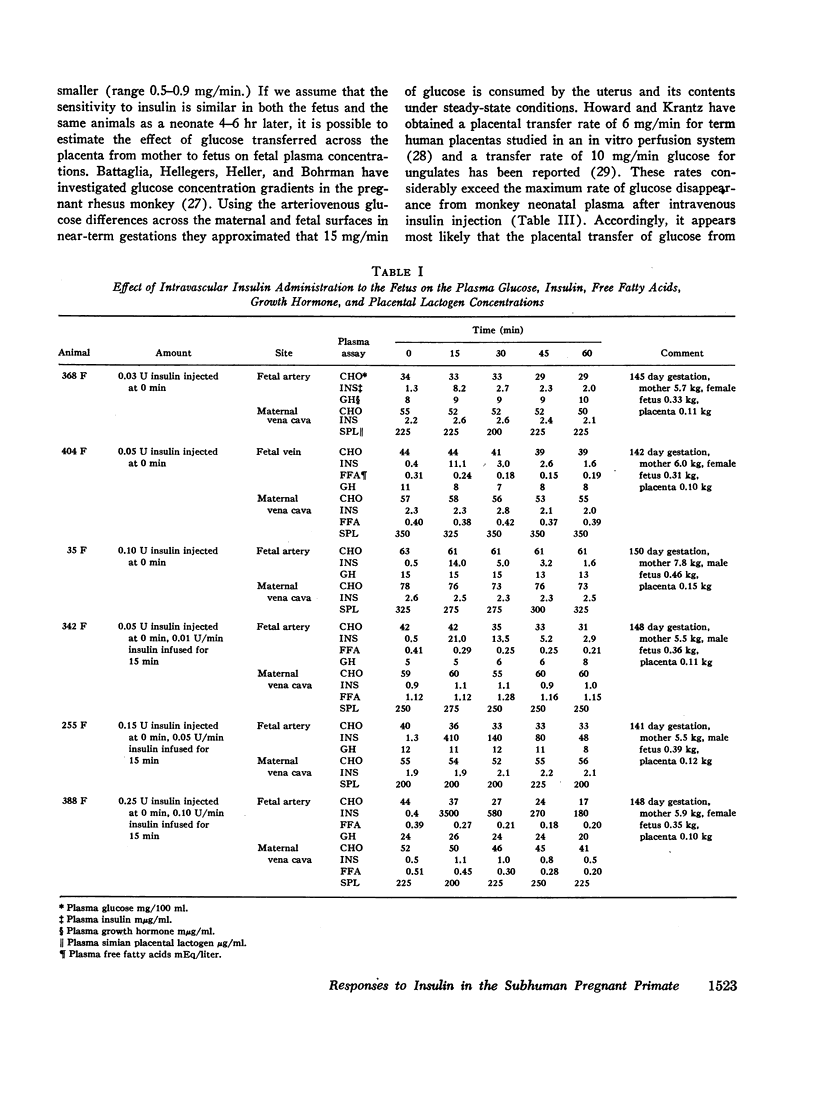
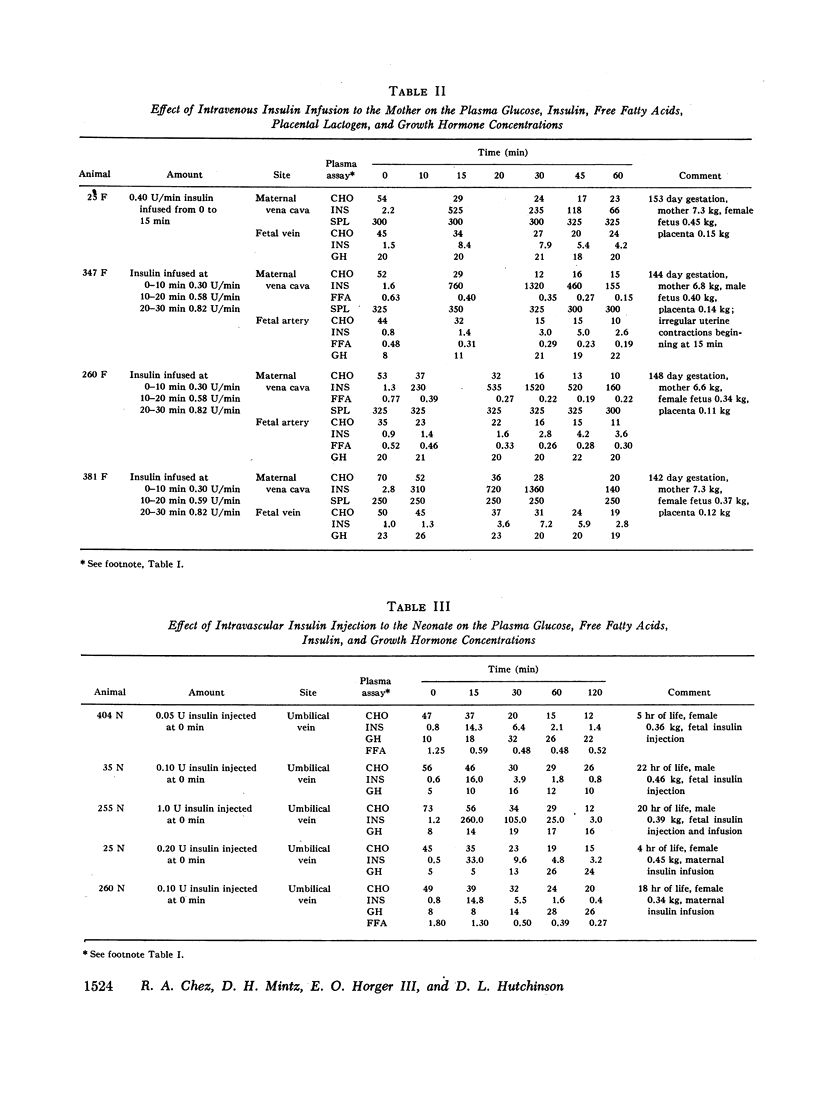
Fetal plasma glucose concentrations were minimally altered by direct fetal insulin injections, whereas neonatal glucose levels declined with similar injections. In both instances, however, plasma free fatty acid levels declined following insulin. When the amount of insulin given the fetus was increased, fetal plasma glucose concentrations did decline. Combined intravascular insulin injections and infusions in the mother were associated with a disappearance of the initial maternal to fetal plasma glucose concentration gradient and a nearly parallel fall in both maternal and fetal plasma glucose levels. It was concluded that insulin was biologically active in the fetus. Obtunded fetal plasma glucose responses to direct fetal insulin administration may be a function of placental transfer of glucose from the maternal pool.
Maternal plasma placental prolactin and fetal plasma growth hormone levels were unchanged in the presence of sustained maternal and fetal hypoglycemia. However, neonatal plasma growht hormone levels did increase in response to hypoglycemia. The observed bidirectional placental barrier to transfer of radioisotopically labeled growth hormone indicated that fetal plasma growth hormone was solely of fetal origin. These data suggested further that a change in the growth hormone-releasing mechanism may occur from fetal to neonatal life.
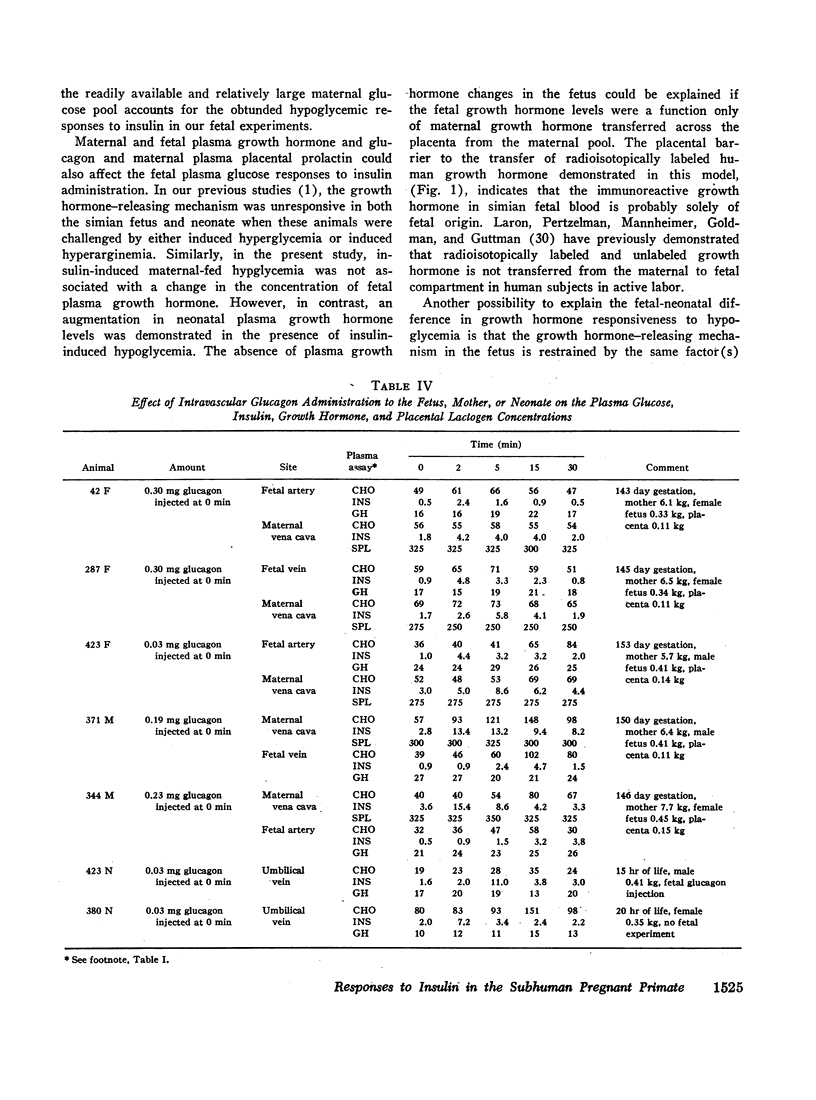
Direct maternal intravascular glucagon administration led to augmentation in both maternal and fetal plasma insulin and glucose levels. Direct fetal glucagon injections enhanced both maternal and fetal plasma insulin levels. These simultaneous changes in both plasma pools were consistent with the demonstration of a bidirectional placental transfer of radioisotopically labeled glucagon. The role of endogenously produced glucagon in these studies remains to be clarified.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER D. P., HUGGETT A. S., NIXON D. A., WIDDAS W. F. The placental transfer of sugars in the sheep: the influence of concentration gradient upon the rates of hexose formation as shown in umbilical perfusion of the placenta. J Physiol. 1955 Aug 29;129(2):367–383. doi: 10.1113/jphysiol.1955.sp005360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATTAGLIA F. C., HELLEGERS A. E., HELLER C. J., BEHRMAN R. GLUCOSE CONCENTRATION GRADIENTS ACROSS THE MATERNAL SURFACE, THE PLACENTA, AND THE AMNION OF THE RHESUS MONKEY (MACACA MULATTA). Am J Obstet Gynecol. 1964 Jan 1;88:32–37. doi: 10.1016/0002-9378(64)90226-1. [DOI] [PubMed] [Google Scholar]
- BERSON S. A., YALOW R. S., BAUMAN A., ROTHSCHILD M. A., NEWERLY K. Insulin-I131 metabolism in human subjects: demonstration of insulin binding globulin in the circulation of insulin treated subjects. J Clin Invest. 1956 Feb;35(2):170–190. doi: 10.1172/JCI103262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocek R. M., Beatty C. H. Effect of insulin on the carbohydrate metabolism of fetal rhesus monkey muscle. Endocrinology. 1969 Sep;85(3):615–618. doi: 10.1210/endo-85-3-615. [DOI] [PubMed] [Google Scholar]
- CHINARD F. P., DANESINO V., HARTMANN W. L., HUGGETT A. S., PAUL W., REYNOLDS S. R. The transmission of hexoses across the placenta in the human and the rhesus monkey (Macaca mulatta). J Physiol. 1956 May 28;132(2):289–303. doi: 10.1113/jphysiol.1956.sp005525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. M., Jr, Cahill G. F., Jr, Soeldner J. S. Effects of exogenous insulin on the rate of fatty acid synthesis and glucose C-14 utilization in the twenty-day rat fetus. Diabetes. 1968 Jun;17(6):362–368. doi: 10.2337/diab.17.6.362. [DOI] [PubMed] [Google Scholar]
- Coltart T. M., Beard R. W., Turner R. C., Oakley N. W. Blood glucose and insulin relationships in the human mother and fetus before onset of labour. Br Med J. 1969 Oct 4;4(5674):17–19. doi: 10.1136/bmj.4.5674.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltart T. M., Beard R. W., Turner R. C., Oakley N. W. Blood glucose and insulin relationships in the human mother and fetus before onset of labour. Br Med J. 1969 Oct 4;4(5674):17–19. doi: 10.1136/bmj.4.5674.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drash A., Field J. B., Garces L. Y., Kenny F. M., Mintz D., Vazquez A. M. Endogenous insulin and growth hormone response in children with newly diagnosed diabetes mellitus. Pediatr Res. 1968 Mar;2(2):94–102. doi: 10.1203/00006450-196803000-00004. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Odell W. D. Acute release of thyrotropin in the newborn. J Clin Invest. 1969 Sep;48(9):1670–1677. doi: 10.1172/JCI106132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIDOTTI G., KANAMEISHI D., FOA P. P. Chick embryo heart as a tool for studying cell permeability and insulin action. Am J Physiol. 1961 Nov;201:863–868. doi: 10.1152/ajplegacy.1961.201.5.863. [DOI] [PubMed] [Google Scholar]
- Grumbach M. M., Kaplan S. L., Sciarra J. J., Burr I. M. Chorionic growth hormone-prolactin (CGP): secretion, disposition, biologic activity in man, and postulated function as the "growth hormone" of the 2d half of pregnancy. Ann N Y Acad Sci. 1968 Feb 5;148(2):501–531. doi: 10.1111/j.1749-6632.1968.tb20372.x. [DOI] [PubMed] [Google Scholar]
- HOLMBERG N. G., KAPLAN B., KARVONEN M. J., LIND J., MALM M. Permeability of human placenta to glucose, fructose, and xylose. Acta Physiol Scand. 1956 May 31;36(4):291–299. doi: 10.1111/j.1748-1716.1956.tb01326.x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hazzard W. R., Crockford P. M., Buchanan K. D., Vance J. E., Chen R., Williams R. H. A double antibody immunoassay for glucagon. Diabetes. 1968 Apr;17(4):179–186. doi: 10.2337/diab.17.4.179. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hinkley C. M., Stenger V. G., Blechner J. N. Glucose concentration gradients across the uterus and placenta of the pregnant primate. Am J Obstet Gynecol. 1969 Jul 15;104(6):893–897. doi: 10.1016/0002-9378(69)90643-7. [DOI] [PubMed] [Google Scholar]
- Howard J. M., Krantz K. E. Transfer and use of glucose in the human placenta during in vitro perfusion and the associated effects of oxytocin and papaverine. Am J Obstet Gynecol. 1967 Jun 15;98(4):445–458. doi: 10.1016/0002-9378(67)90094-4. [DOI] [PubMed] [Google Scholar]
- Katz H. P., Grumbach M. M., Kaplan S. L. Diminished growth hormone response to arginine in the puerperium. J Clin Endocrinol Metab. 1969 Nov;29(11):1414–1419. doi: 10.1210/jcem-29-11-1414. [DOI] [PubMed] [Google Scholar]
- Lambert A. E., Jeanrenaud B., Renold A. E. Enhancement by caffeine of glucagon-induced and tolbutamide-induced insulin release from isolated foetal pancreatic tissue. Lancet. 1967 Apr 15;1(7494):819–820. doi: 10.1016/s0140-6736(67)92782-1. [DOI] [PubMed] [Google Scholar]
- Laron Z., Pertzelan A., Mannheimer S., Goldman J., Guttmann S. Lack of placental transfer of human growth hormone. Acta Endocrinol (Copenh) 1966 Dec;53(4):687–692. doi: 10.1530/acta.0.0530687. [DOI] [PubMed] [Google Scholar]
- Lumley J. M., Wood C. Influence of hypoxia on glucose transport across the human placenta. Nature. 1967 Oct 28;216(5113):403–404. doi: 10.1038/216403a0. [DOI] [PubMed] [Google Scholar]
- MILNER R. D., HALES C. N. EFFECT OF INTRAVENOUS GLUCOSE ON CONCENTRATION OF INSULIN IN MATERNAL AND UMBILICAL-CORD PLASMA. Br Med J. 1965 Jan 30;1(5430):284–286. doi: 10.1136/bmj.1.5430.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer V., Knobil E. Growth hormone secretion in the unanesthetized rhesus monkey in response to noxious stimuli. Endocrinology. 1967 Jan;80(1):163–171. doi: 10.1210/endo-80-1-163. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Wright A. D. Plasma glucose, non-esterified fatty acid, insulin and growth hormone response to glucagon in the newborn. Clin Sci. 1967 Apr;32(2):249–255. [PubMed] [Google Scholar]
- Mintz D. H., Chez R. A., Horger E. O., 3rd Fetal insulin and growth hormone metabolism in the subhuman primate. J Clin Invest. 1969 Jan;48(1):176–186. doi: 10.1172/JCI105966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz D. H., Stock R., Finster J. L., Taylor A. L. The effect of normal and diabetic pregnancies on growth hormone responses to hypoglycemia. Metabolism. 1968 Jan;17(1):54–61. doi: 10.1016/s0026-0495(68)80007-1. [DOI] [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Paterson P., Page D., Taft P., Phillips L., Wood C. Study of fetal and maternal insulin levels during labour. J Obstet Gynaecol Br Commonw. 1968 Sep;75(9):917–921. doi: 10.1111/j.1471-0528.1968.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Paterson P., Phillips L., Wood C. Relationship between maternal and fetal blood glucose during labor. Am J Obstet Gynecol. 1967 Aug 1;98(7):938–945. doi: 10.1016/0002-9378(67)90080-4. [DOI] [PubMed] [Google Scholar]
- Portman O. W., Behrman R. E., Soltys P. Transfer of free fatty acids across the primate placenta. Am J Physiol. 1969 Jan;216(1):143–147. doi: 10.1152/ajplegacy.1969.216.1.143. [DOI] [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- SPELLACY W. N., GOETZ F. C., GREENBERG B. Z., ELLS J. THE HUMAN PLACENTAL GRADIENT FOR PLASMA INSULIN AND BLOOD GLUCOSE. Am J Obstet Gynecol. 1964 Nov 15;90:753–757. doi: 10.1016/0002-9378(64)90938-x. [DOI] [PubMed] [Google Scholar]
- Samaan N., Yen S. C., Friesen H., Pearson O. H. Serum placental lactogen levels during pregnancy and in trophoblastic disease. J Clin Endocrinol Metab. 1966 Dec;26(12):1303–1308. doi: 10.1210/jcem-26-12-1303. [DOI] [PubMed] [Google Scholar]
- Solomon S., Friesen H. G. Endocrine relations between mother and fetus. Annu Rev Med. 1968;19:399–430. doi: 10.1146/annurev.me.19.020168.002151. [DOI] [PubMed] [Google Scholar]
- Tobin J. D., Roux J. F., Soeldner J. S. Human fetal insulin response after acute maternal glucose administration during labor. Pediatrics. 1969 Nov;44(5):668–671. [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Measurements of endogenous glucagon in plasma and the influence of blood glucose concentration upon its secretion. J Clin Invest. 1962 Apr;41:682–689. doi: 10.1172/JCI104525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDAS W. F. Transport mechanisms in the foetus. Br Med Bull. 1961 May;17:107–111. doi: 10.1093/oxfordjournals.bmb.a069882. [DOI] [PubMed] [Google Scholar]


