Abstract
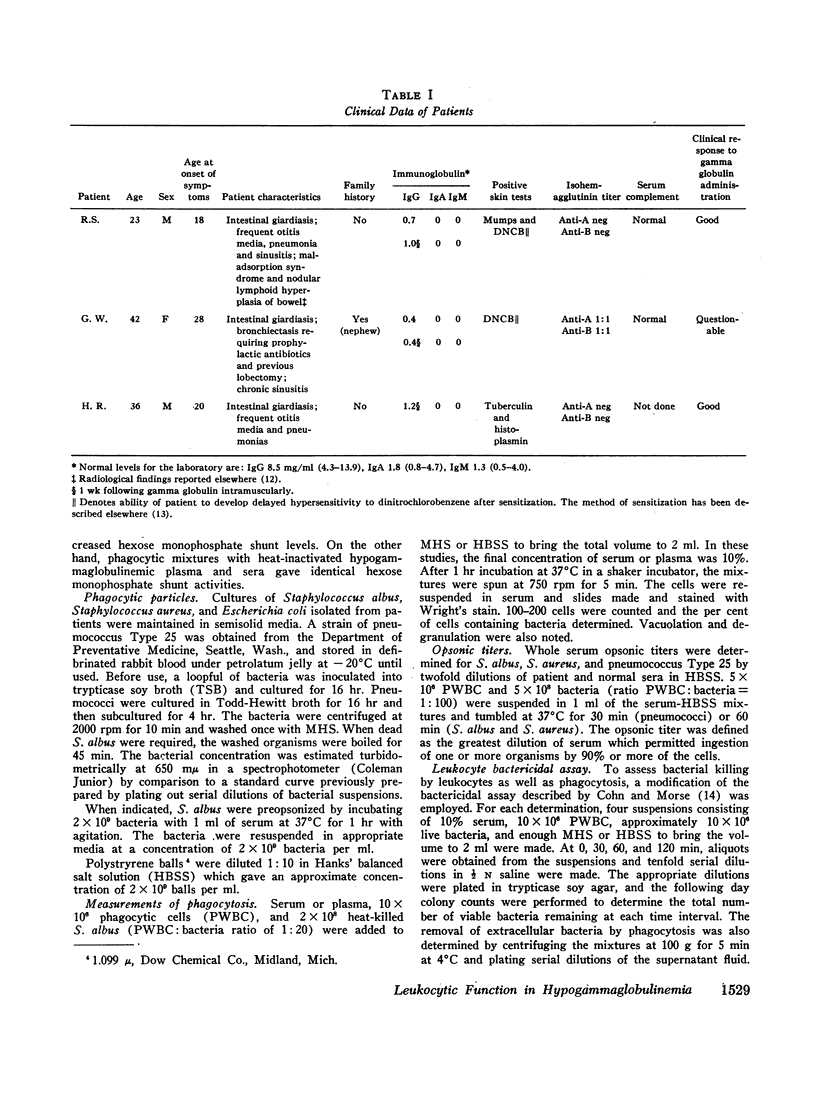
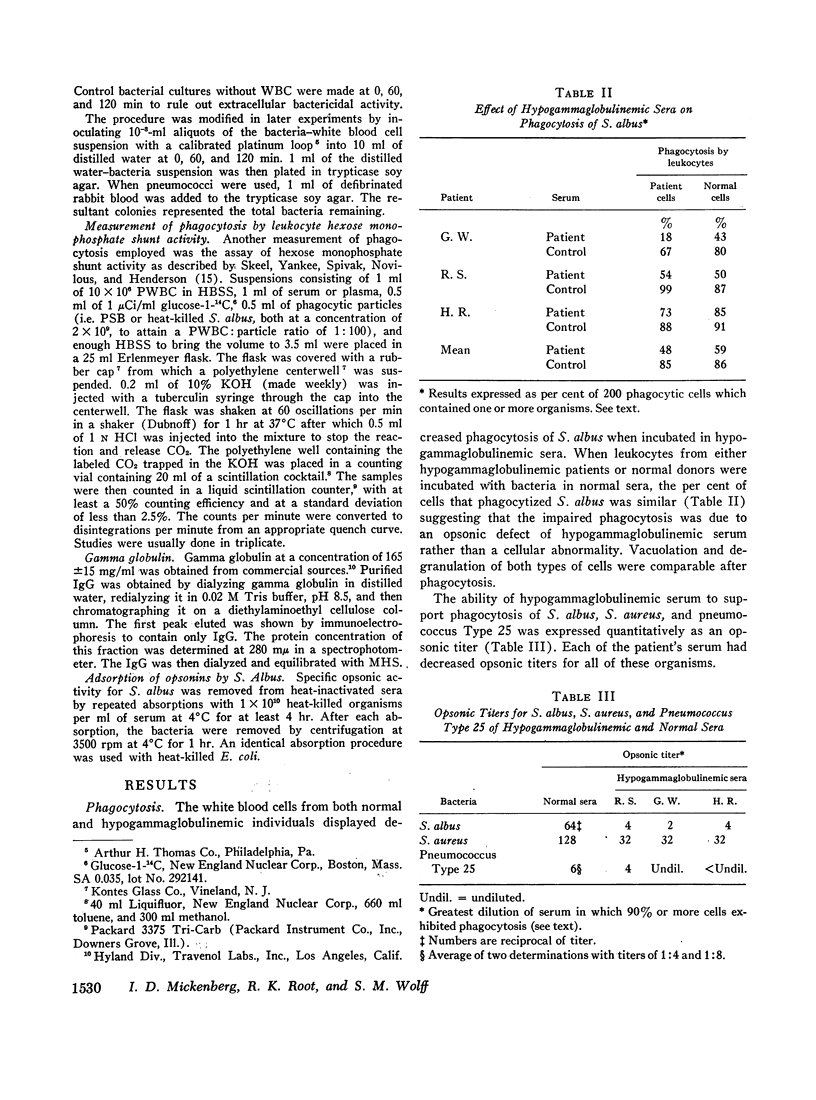
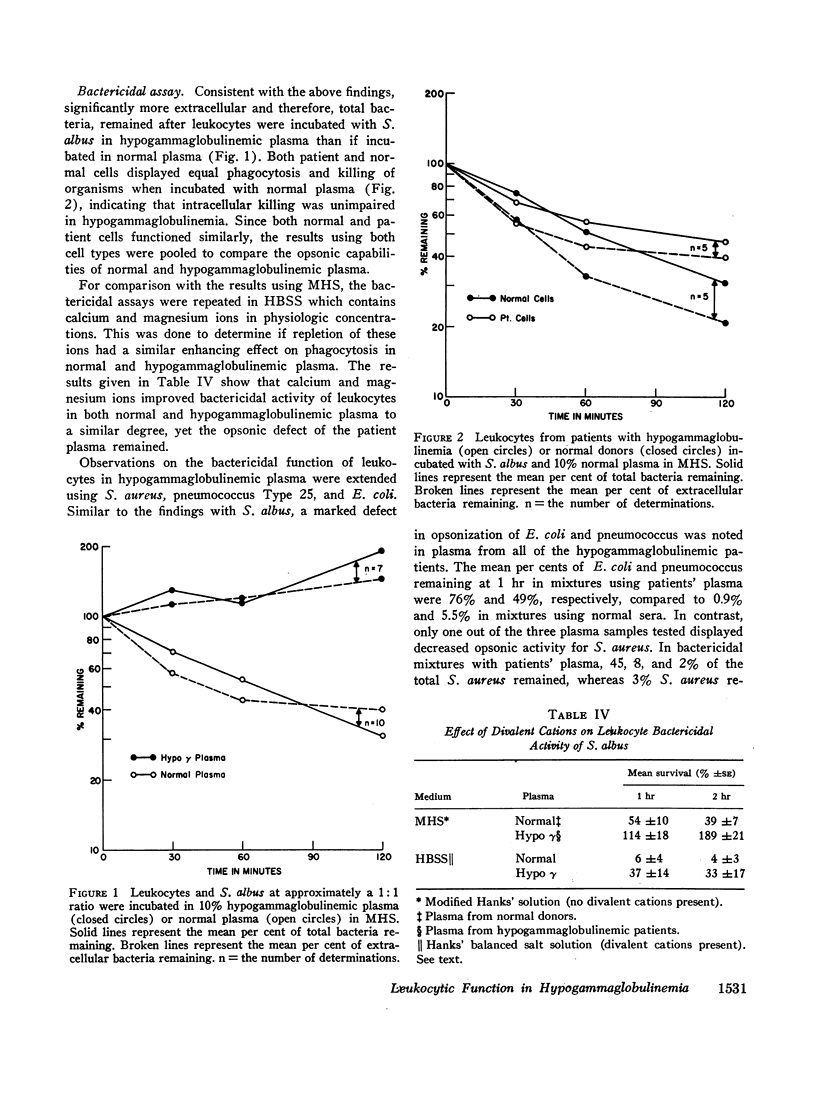
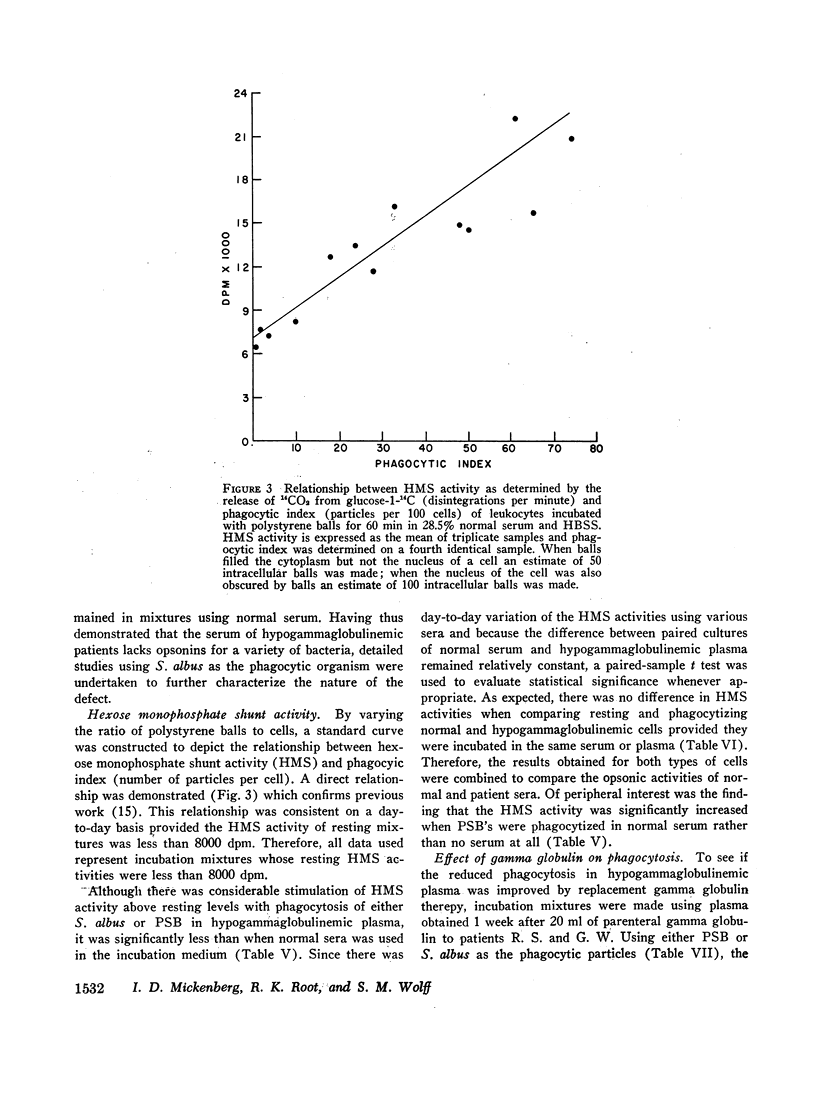
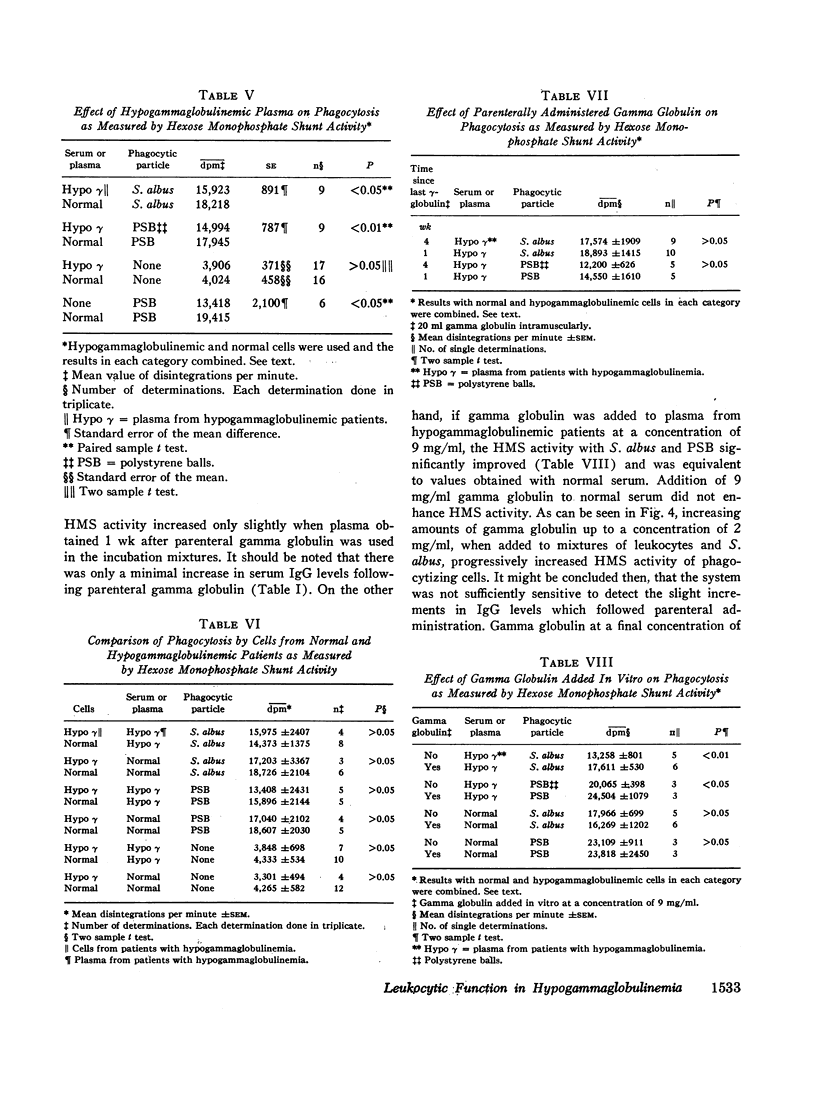
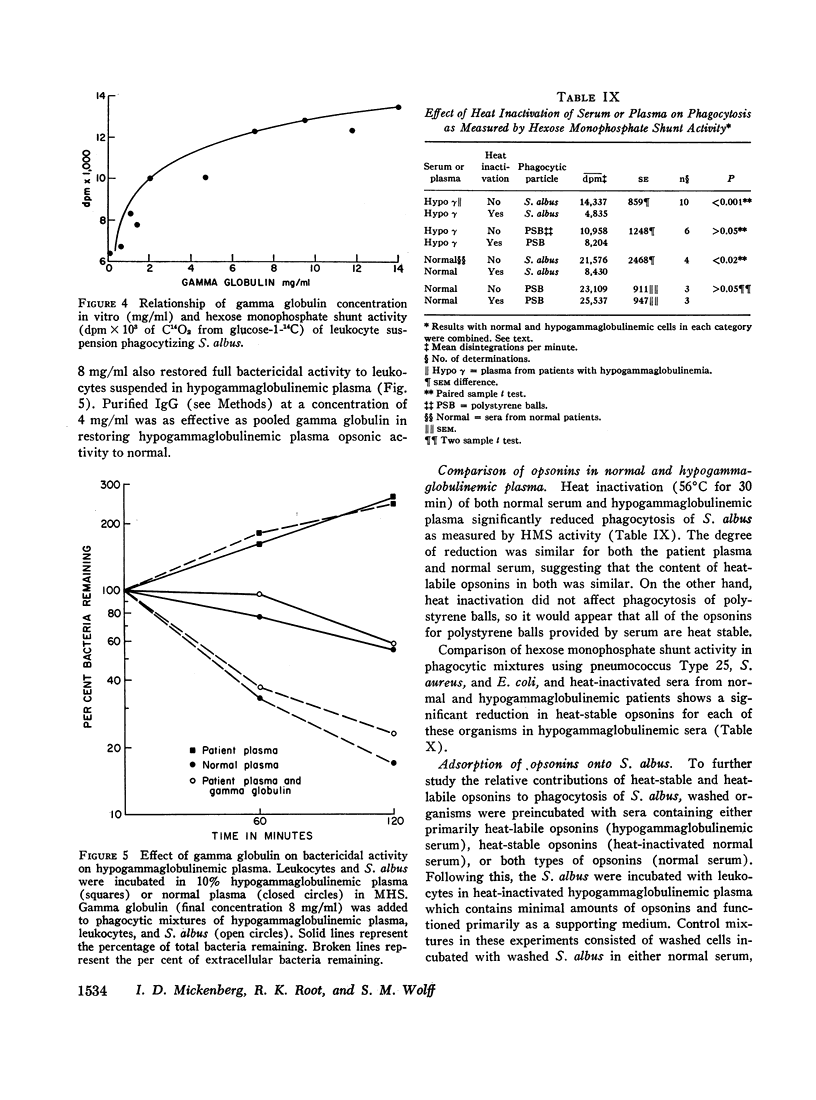
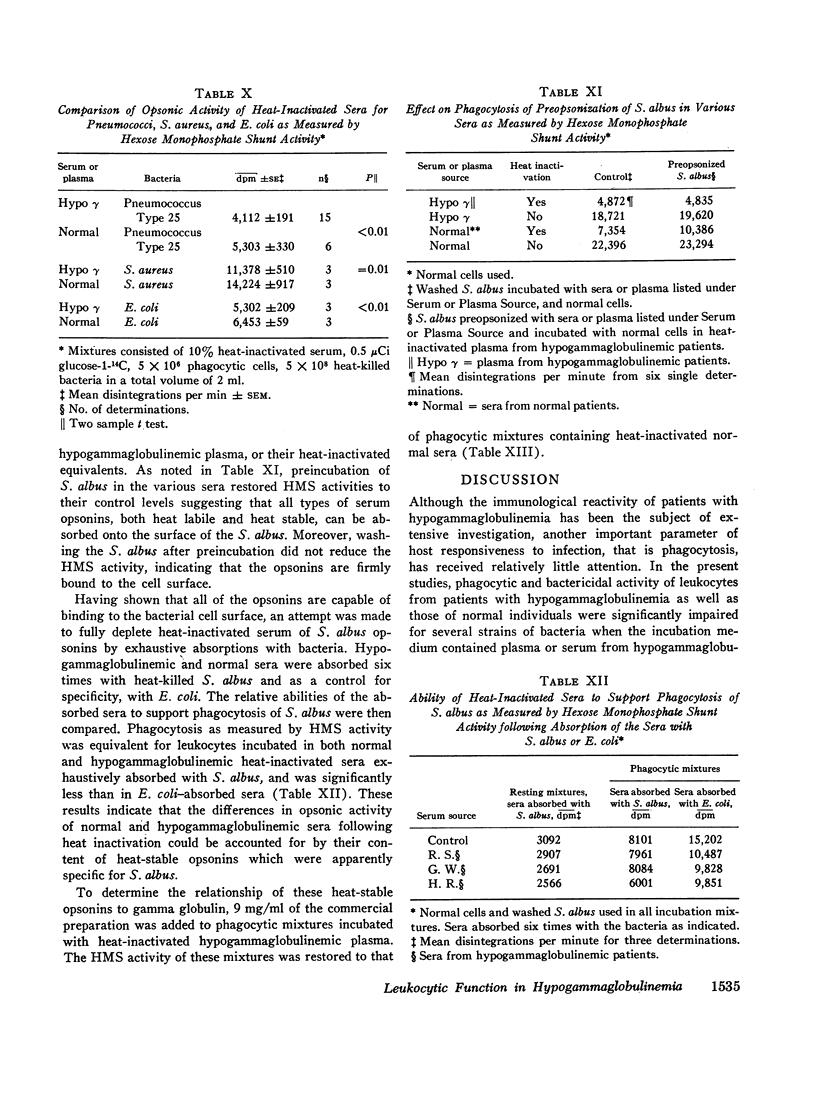
The phagocytic, bactericidal, and metabolic capabilities of circulating blood leukocytes from three adults (two males, one female) with hypogammaglobulinemia and recurrent pneumonia, chronic sinusitis, and intestinal giardiasis were studied. These functions were found to be normal when leukocytes from the patients were incubated in media containing normal human serum. Phagocytosis of Staphylococcus albus and polystyrene balls by both patient and normal leukocytes was diminished when the cells were incubated in hypogammaglobulinemic plasma. A similar defect in opsonization by patient plasma was also noted for pneumococci, Escherichia coli and variably with Staphylococcus aureus. Both patient and normal sera had equivalent levels of heat-labile S. albus opsonins; normal serum, however, contained heat-stable S. albus-specific absorbable opsonins in significantly greater quantities to account for its superior opsonic capacity. The addition of commercial gamma globulin or purified IgG to hypogammaglobulinemic sera restored full S. albus opsonic activity. The relevancy of these observations to the impaired host defenses in these patients will be discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird D. C., Jacobs J. B., Silbiger M., Wolff S. M. Hypogammaglobulinemia with nodular lymphoid hyperplasia of the intestine. Report of a case with rectosigmoid involvement. Radiology. 1969 Jun;92(7):1535–1536. doi: 10.1148/92.7.1535. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssman O. Phagocytizing capacity of the neutrophil leucocyte in primary "acquired" hypogammaglobulinaemia. Acta Pathol Microbiol Scand. 1965;65(3):366–370. doi: 10.1111/apm.1965.65.3.366. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., ZAK S. J., CONDIE R. M., BRIDGES R. A. Clinical investigation of patients with agammaglobulinemia and hypogammaglobulinemia. Pediatr Clin North Am. 1960 May;7:397–433. doi: 10.1016/s0031-3955(16)30945-2. [DOI] [PubMed] [Google Scholar]
- Gewurz H., Pickering R. J., Christian C. L., Snyderman R., Mergenhagen S. E., Good R. A. Decreased C'-1q protein concentration and agglutinating activity in agammaglobulinaemia syndromes: an inborn error reflected in the complement system. Clin Exp Immunol. 1968 Jun;3(5):437–445. [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Jacobs J. C., de Capoa A., McGilvray E., Morse J. H., Schullinger J. N., Blanc W. A., Heird W. C., Miller O. J., Rossen R. D., Walzer R. A. Complement deficiency and chromosomalbreaks in a case of Swiss-type agammaglobulinaemia. Lancet. 1968 Mar 9;1(7541):499–503. doi: 10.1016/s0140-6736(68)91467-0. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R., Sheagren J. N., Lipsett M. B., Wolff S. M. Alternate-day prednisone therapy. Evaluation of delayed hypersensitivity responses, control of disease and steroid side effects. N Engl J Med. 1969 Jun 26;280(26):1427–1431. doi: 10.1056/NEJM196906262802601. [DOI] [PubMed] [Google Scholar]
- Miller M. E., Hummeler K. Thymic dysplasia ("Swiss agammaglobulinemia"). II. Morphologic and functional observations. J Pediatr. 1967 May;70(5):737–744. doi: 10.1016/s0022-3476(67)80324-x. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Skeel R. T., Yankee R. A., Spivak W. A., Novikovs L., Henderson E. S. Leukocyte preservation. I. Phagocytic stimulation of the hexose monophosphate shunt as a measure of cell viability. J Lab Clin Med. 1969 Feb;73(2):327–337. [PubMed] [Google Scholar]
- Winkelstein J. A., Drachman R. H. Deficiency of pneumococcal serum opsonizing activity in sickle-cell disease. N Engl J Med. 1968 Aug 29;279(9):459–466. doi: 10.1056/NEJM196808292790904. [DOI] [PubMed] [Google Scholar]


