Abstract
Direct current (DC) insulator-based dielectrophoretic (iDEP) microdevices have the potential to replace traditional alternating current (AC) dielectrophoretic devices for many cellular and biomolecular separation applications. The use of large DC fields suggest that electrode reactions and ion transport mechanisms can become important and impact ion distributions in the nanoliters of fluid in iDEP microchannels. This work tracked natural pH gradient formation in a 100 μm wide, 1 cm long microchannel under applicable iDEP protein manipulation conditions. Using fluorescence microscopy with the pH sensitive dye FITC Isomer I and the pH insensitive dye TRITC as a reference, pH was observed to drop drastically in the microchannels within 1 minute within a 3000 V/cm electric field; pH drops were observed in the range of 6 to 10 minutes within a 100 V/cm electric field and varied based on buffer conductivity. To address concerns of dye transport impacting intensity data, electrokinetic mobilities of FITC were carefully examined and found to be a) toward the anode and b) 1 to 2 orders of magnitude smaller than H+ transport which is responsible for pH drops from the anode toward the cathode. COMSOL simulations of ion transport showed qualitative agreement with experimental results. Results indicate that pH changes are severe enough and rapid enough to influence the net charge of a protein or cause aggregation during iDEP experiments. Results also elucidate reasonable time periods over which the phosphate buffering capacity can counter increases in H+ and OH− for unperturbed iDEP manipulations.
Keywords: COMSOL, Electroosmotic flow, FITC fluorescence, Insulator-based Dielectrophoresis, Natural pH gradients
1. Introduction
Dielectrophoresis (DEP) is the motion of polarizable particles in electric-field gradients first described by Pohl in 1951 [1]. In DEP, the polarization behavior of the particles governs their migrational motion giving rise to two cases of DEP behavior. In positive DEP, particles are attracted to areas of high electric fields. The contrary is the case for particles exhibiting negative DEP. Such particles are repulsed from regions of high electric field density. For the integration of DEP into microfluidic systems, two methods can be employed. In the classical method, microelectrodes are patterned onto channel walls. This unfavorably requires metal deposition steps in the fabrication process and can only provide electric field gradients in close vicinity to the microelectrodes.
A relatively new method of integrating DEP into microfluidic systems is insulator-based dielectrophoresis (iDEP). This technique uses insulating structures (posts) within an insulative channel as `obstacles'. When an electric field is applied at electrodes in reservoirs at the microchannel ends, spatially non-uniform electric fields are provoked around the posts in the microchannel. These gradients are rather uniform through the depth of the microchannel. Microfabrication requires only basic soft photolithography steps.
In iDEP, two regimes can be found: streaming and trapping. When the DEP force is strong enough to overcome diffusion and electrokinesis, trapping of particles can be achieved. However, if DEP cannot overcome electrokinesis, streaming behavior of particles is observed. This usually results in a focusing effect of particles in streamlines flowing through a microfluidic obstacle array [2]. Depending on their dielectrophoretic polarizability, particles migrate or focus at different rates in iDEP, opening the way for novel separation or fractionation techniques [3,4].
Proven applications of this technology are in cell trapping or cell streaming between or around arrays of insulating posts. More recently, researchers have been able to demonstrate manipulation with biomolecules, such as DNA [3,5–6] or proteins [7–9]. Interestingly, in the case of large (>10kbp) DNA, DEP trapping forces in the fN range have been reported [3,6]. Most relevant to this study, the DEP forces acting on proteins in solution can be estimated under consideration of the electric field gradients generated in insulator post arrays. In previous studies of protein iDEP [9, 10], the trapping forces were determined considering an overall prolate elliptic shape of proteins in solution. For example, using the typical dimension of an immunoglobuline G (IgG) molecule and experimental conditions as reported in reference [9], the maximum force generated by iDEP results in ~ 0.5 fN. Hence, the resulting trapping forces acting on proteins are only one magnitude lower than previously reported for DNA. As a result, streaming iDEP of proteins could indeed be demonstrated both numerically as well as experimentally for IgG and bovine serum albumin [9].
Despite the recent demonstrations of protein DEP [7–9], the mechanisms of protein polarization are little understood. Furthermore, the additional forces competing with iDEP are complex, but govern the often non-intuitive migration or trapping behavior. Those include electroosmosis, electrophoresis and diffusion. Another important factor that has been little investigated in iDEP applications is the influence of pH. Electrode reactions occur at electrode surfaces located in the channel-end reservoirs because high potentials are usually applied for iDEP applications. DC insulator DEP microfluidic devices are examples of electrochemical cells and water electrolysis reactions are known to take place on the electrode surfaces in contact with an aqueous medium [11], thus altering the electrode morphology, composition [12], and the surrounding solution [13]. The reactions produce H+ and OH− ions at the anode and cathode, respectively, via the following hydrolysis reactions:
| (1) |
[14]
| (2) |
[14] In microfluidic devices, the length and volume scales are small enough that the mass transport of H+ and OH− ions can result in the formation of a pH gradient across the microfluidic channel. Such gradients are known as “natural pH gradients,” which have been exploited to conduct isoelectric focusing (IEF) without the use of carrier ampholytes [15,16]. Changes in pH can affect the behavior of analytes. This is especially true for proteins, whose net charge, and therefore behavior in an electric field, is dependent on pH [17]. Therefore, it is important to discern if natural pH gradients form in iDEP devices and if so, the time scales at which this occurs. Cells in an iDEP device with a natural pH gradient may experience stress, and proteins may be transported or focused into undesired regions or potentially denature at pH extremes. Such events may be detrimental to the purpose of the iDEP process and alter analyte properties during iDEP device operation. In addition, local pH changes can influence the electroosmotic flow (EOF) behavior in a microfluidic channel by changing channel wall surface properties [13]. Use of Ag/AgCl electrodes or organic electrode materials such as PEDOT:PSS have been suggested to stop the formation of natural pH gradients [18,19]. However, it would be beneficial to map out the viable operating conditions for iDEP microchannels using traditional metal electrodes.
In this paper, we present a quantification of the fluorescent emission ratio of the pH sensitive dye FITC Isomer I and pH insensitive dye TRITC in a triangular post iDEP microdevice. The quantification is based on referencing the pH dependent FITC fluorescence intensity by the pH independent TRITC fluorescence intensity. Experiments are conducted with electric fields of 100 V/cm to 3000 V/cm using phosphate buffer solutions containing 0.3 mM CHAPS with conductivities from 0.01 to 0.10 S/m. These conditions mimic those in an iDEP microdevice able to successfully concentrate IgG between triangular or elliptical posts [9]. The formation of natural pH gradients are tracked over time by the FITC/TRITC fluorescent intensity profile along the centerline of the channel.
2. Materials and Methods
2.1. Reagents
Phosphate buffer solution was made from potassium hydrogen phosphate (Mallinckrodt Baker, Inc., Phillipsburg, NJ), potassium dihydrogen phosphate (VWR International, West Chester, PA) and e-pure water (Millipore, Billerica, MA). Phosphate buffered saline (PBS) was made from 0.140 M NaCl (Mallinckrodt Baker, Inc., Phillipsburg, NJ), 0.025 M KH2PO4, 0.009 M K2HPO4 and e-pure water (Millipore, Billerica, MA). 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (Pluronic F108) were purchased from Sigma-Aldrich (Sigma-Aldrich, St Louis, MO) and used as received. Fluorescein-5-isothicyanate (FITC Isomer I) was purchased from Invitrogen (Invitrogen, Eugene, OR), dissolved into 1 mM aqueous solutions and pipetted into 100 μL aliquots. TRITC was purchased from Sigma-Aldrich, dissolved into 0.1 mM aqueous solutions, and pipetted into 100 μL aliquots. These aliquots were mixed together at a FITC:TRITC ratio of 2:1 (v/v) and the mixture carefully pipetted into phosphate solutions in order to ensure consistent relative concentrations between different runs. Sylgard 184 was purchased from KR Anderson (Phoenix, AZ). Alexa Fluor 488 chicken anti-rabbit IgG conjugate was obtained from Invitrogen (Carlsbad, CA, USA).
2.2. Microfabrication
Microchannels for iDEP and pH experiments were fabricated with PDMS from Sylgard 184. This procedure has been described in detail elsewhere [20]. Briefly, a master relief of SU-8 photoresist was patterned via photolithography on a silicon wafer. From this master wafer, a PDMS mold was formed resulting in channel cross sections of 10 × 100 μm2. Reservoir holes were punched with a diameter of 2 mm at corresponding positions in the PDMS mold. Finally, the microstructured PDMS mold was O2-plasma activated under vacuum (PDC-001 Harrick Plasma, Harrick, Ithaca, NY). Simultaneously, a cleaned class slide was treated with O2-plasma under similar conditions and the PDMS slab and glass slide were assembled directly after plasma activation. All channels were ~ 1 cm in length and filled with water immediately after assembly for storage prior to experiments. After experiments, devices were stored in buffer solution. However, prior to each experiment, the channels were incubated in 1 mM F108 solution in the corresponding buffer. Surface coating was necessary in order to prevent non-specific protein adsorption during iDEP experiments.
2.3. Protein iDEP experiments
Channels and reservoirs were filled with phosphate buffer (pH = 8) with a conductivity of 0.04 S/m. This solution also contained 0.3 mM in CHAPS. Prior to iDEP manipulation, one reservoir was filled with 1.2 nM IgG in the same buffer. A low voltage of 100 V/cm was applied in order to electrokinetically fill the channel with the protein solution. Once filled, the electric field was increased to 2400 V/cm within 3 min to manipulate IgG by DEP. This was observed by fluorescence microscopy on an inverted fluorescence microscope (IX 71, Olympus, Center Valley, PA) with filter sets for Alexa-Fluor 488 and a 40× objective (LUCPlan FL N, Olympus, Center Valley, PA). Images of fluorescence intensity were recorded with a CCD-Camera (Quantum 512SC, Photometrics, Tucson, AZ) at 0.2 frames/second. Image J software (version 1.43) was used for data analysis.
2.4. EOF Measurements
Linear channels without insulating posts were used for electroosmotic flow mobility measurements according to the Huang-method [21]. PDMS molds were assembled on glass slides under similar conditions as iDEP trapping experiments. For pH dependent measurements, 30 mM and 15 mM solutions were prepared at the corresponding pH (3, 6, 7, 8). Channels were filled with 30 mM concentration first. After a calibration phase of ~ 10 min at 300 V/cm (applied with a high voltage power supply from LabSmith model HVS448 6000D), the current decay was recorded after replacing an equal amount of 15 mM solution in the reservoir. The decay was recorded until a stable baseline was apparent again. The time to reach this baseline was obtained from the intersection of two linear fits of the decay and the stable baseline. Measurements were repeated for each pH accordingly in the same microchannel and repeated twice with three independent PDMS channels (n=9 for each pH).
2.5. Device Preparation for pH Measurements
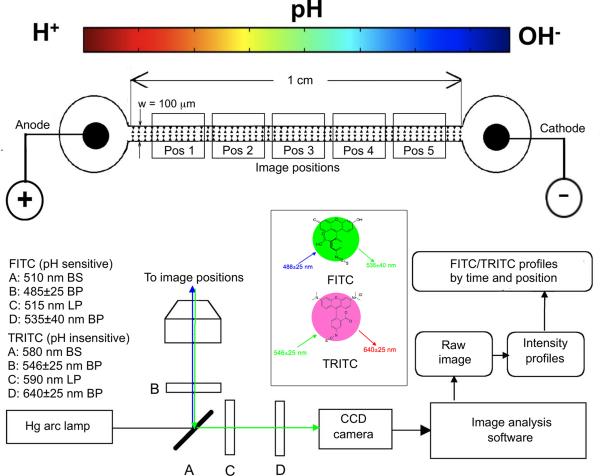
The same iDEP devices utilized for protein trapping were adapted for natural pH gradient experiments. Inlet and outlet ports were positioned on either side of a 1 cm section of the microchannel. The electrodes were 100 μm diameter Pt wires coiled into 2 mm flat contacts in each port. Electrodes were connected via soldered posts to leads from a HVS448 3000V High Voltage Sequencer (LabSmith, Inc. Livermore, CA). Fig. 1 shows a cartoon representation of the channel with the image capture positions and the optics system.
Figure 1.
iDEP microdevice schematic containing triangular posts within the 100 ⎧m wide channel spanning 1 cm between the anode/cathode wells. A fully motorized stage with position control in the x, y, and z directions was used to capture images at the specified positions 1 though 5 at specific times. Beamsplitters (BS), excitation filters, and longpass (LP) emission filters for FITC/TRITC were in a motorized turret and they are shown at A, B and C, respectively. Bandpass (BP) emissions filters were in a motorized filter wheel at D. Images were captured with a 1388×1040 resolution CCD camera, intensity profiles were obtained from unmodified images, and ratiometric analysis performed as outlined in Figure 2.
2.6. Fluorescence detection and image capture
The triangular post microdevices were filled with 0.01, 0.05, or 0.10 S/m phosphate buffers (pH=8) containing 0.3 mM CHAPS, 50 μM FITC and 10 μM TRITC, and then mounted on a Zeiss Axiovert 200M fluorescence microscope (Carl Zeiss Microimaging, Thornwood, NY). In controls for FITC transport, only one port was filled with FITC containing buffer, while the channel and other port were filled with FITC-free buffer. Both ports and the channel were filled with FITC and TRITC containing buffer for the pH change experiments. FITC was excited with a 485±25 nm bandpass filter, and emissions filtered with a 515 nm longpass filter followed by a 535±40 nm bandpass filter. Five adjacent microchannel positions were monitored between the ports (see Fig. 1). TRITC was excited with a 546±25 nm bandpass filter, and emissions filtered with a 590 nm longpass filter, followed by a 640±25 nm bandpass filter. A 510 nm beamsplitter was used with FITC and a 580 nm beamsplitter was used for TRITC (see Fig. 1). For the experiments, electric fields of 100 V/cm or 3000 V/cm were applied across the 1 cm microchannel, and an x, y, z motorized stage obtained 1.0 sec exposure images at specified times at each position. Images were recorded with a 1388×1040 resolution (pixel size: 6.45 μm × 6.45 μm) B&W CCD camera (Zeiss, AxioCam MRm).
For both calibration measurements and pH change experiments, Axiovision 4.8 software (Carl Zeiss Microimaging, Thornwood, NY) was used to obtain emission intensity profiles for both dyes along the centerline of the channel as shown in Fig. 2. Greyscale pixel intensity values ranged between 0 and 4095, indicating black (no fluorescence) and white (strong fluorescence), respectively. FITC/TRITC fluorescence intensity ratio profiles were calculated on a pixel by pixel basis as shown in Eq. 3.
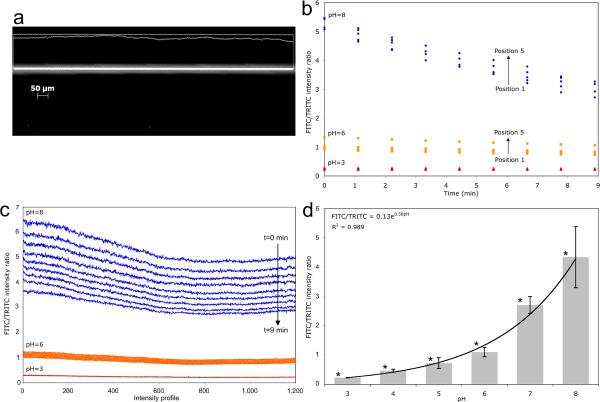
Figure 2.
Calibration controls for pH dependent FITC/TRITC emissions ratios at 535±40 nm and 640±25 nm, respectively. a) 535±40 nm (FITC filtered) microscope image of a 20 ⎧m diameter capillary. Intensity values were measured along the capillary centerline (intensity profile is projected above). Separate FITC and TRITC images were taken at each position every ~1.1 minutes for 9 minutes. Three independent experiments were conducted (n=3). b) FITC/TRITC intensity ratios were calculated according to Eq (3) on a pixel by pixel basis. FITC/TRITC profiles for each position were averaged and are shown for pH 3, pH 6, and pH 8. c) To ascertain time dependence, FITC/TRITC profiles from the same position were averaged. The compiled profiles are shown at each time from 1 min to 9 min at pH 3, 6 and 8. d) Intensity profiles were averaged over pixels, time and position, then used to calculate a FITC/TRITC ratio for pH values 3 through 8. 95% confidence intervals with asterisks indicate no overlap between adjacent pH controls.
| (3) |
Where Rijk is the FITC/TRITC intensity ratio, PI is pixel intensity, and i is the pixel location within a single profile. j and k correspond to channel position (see Fig. 1) and time point, respectively. To correlate Rijk to pH, PBS solutions with fixed pH from 3 to 8 containing 50 μM FITC and 10 μM TRITC were loaded into 20 μm ID fused silica capillaries, and 1.0 sec exposure images recorded at five positions; this sequence was computer automated and repeated every ~1.1 min for 9 minutes. FITC/TRITC ratios, Rijk, averaged over pixel location, i, and pH values of 3, 6 and 8 are shown in Fig. 2b. FITC/TRITC ratios, Rijk, were averaged over channel position, and intensity vs. location profiles for each time are shown in Fig. 2c for pH values of 3, 6 and 8. While FITC intensity decreased over the first10 minutes, it then remained constant (Data not shown). The system was therefore allowed to equilibrate for 10 minutes before field application and the start of the experiment (excluding section 2.7 controls). Further, an average was calculated simultaneously over position and over time. This calibration was repeated three times with 95% confidence intervals calculated from the combined population of position, time, and pixel location as shown in Fig. 2d. All pH readings in the 3 to 8 range were discernible at a 95% confidence level. Further, the calibration data are fit to an exponential with an R2 value of 0.9888. This demonstrates the data is reproducible and reliable at discerning order of magnitude changes in [H+] within these channels.
2.7. Controls for FITC transport through the microchannels
FITC transport controls were conducted to experimentally ascertain a) diffusion timescales of FITC, b) electrophoretic mobility of FITC, and c) make comparisons with literature/calculated values. For the diffusion control experiments, one port and the microchannel were filled with a 0.10 S/m phosphate buffer, 0.3 mM CHAPS, and no FITC. The other port was filled with the same buffer solution but with 50 μM FITC. The purpose was to observe FITC diffusion into the microchannel due to the concentration difference, but in the absence of other transport mechanisms. Intensities were measured at five positions along the channel and the time between FITC observations was used to estimate a diffusion coefficient.
For the electrophoretic FITC controls, the experiment was set up exactly the same as the diffusion controls with FITC loaded into only 1 port, but the electric field was turned on and images captured. Four control experiments with FITC solution in either the cathode or the anode port, and either 100 V/cm or 3000 V/cm electric field strength were conducted.
2.8. Simulations in COMSOL Multiphysics 4.1
AutoCAD drawings of the iDEP channels were imported into COMSOL 4.1 (COMSOL, Inc., Burlington, MA). Electric currents and laminar flow modules were used to model electroosmotic flow (EOF), and transport of dilute species module was used to model mass transport of ions due to diffusion, electrophoretic migration and EOF, as well as the water association reaction. Parameters are provided in Table SI1 in supporting information.
3. Results and Discussion
This section is organized by information on iDEP manipulation, results of pH measurements, control results for the pH measurements, and simulations of the transport phenomena in the channel.
3.1. Protein iDEP
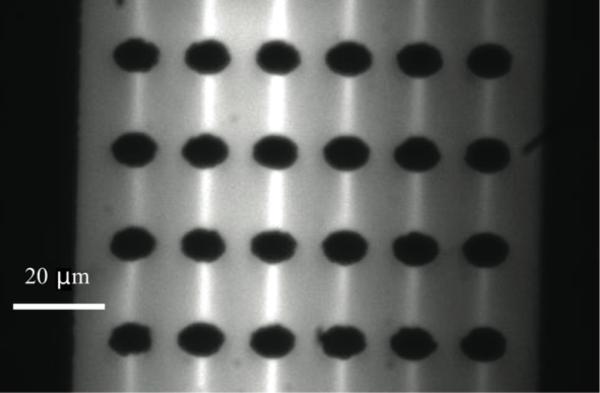
Bioanalytically important IgG iDEP manipulation was carried out in a PDMS microfluidic channel exhibiting insulating posts with an elliptic base. IgG was fluorescently labeled to enable visualization of iDEP focusing via fluorescent video microscopy. As demonstrated in Fig. 3, we observe streamline concentration of IgG at a buffer conductivity of 0.04 S/m and an applied field of 2400 V/cm. This finding is in good agreement with our studies in triangular post devices and for bovine serum albumin in elliptic post arrays [9] employing buffers with low conductivity and the zwitterionic detergent CHAPS. As demonstrated in a comparative study with numerical simulations, this streamline behavior corresponds to positive iDEP, when iDEP forces on proteins cannot overcome electrokinetic forces [9]. Furthermore, we observe such streaming behavior as demonstrated in Figure 3 for time scales of ~20 min under applied electric field. After this time, the streamline behavior vanishes and aggregation governs the migration and trapping processes. It is thus important to investigate the influence of high electric fields on the solution conditions in iDEP.
Figure 3.
Fluorescence microscopy image obtained during iDEP manipulation of IgG in an array of elliptic posts. The light areas correspond to regions where the concentration of IgG was increased due to positive DEP. The applied electric field strength was 2400 V/cm. The IgG concentration was 1.7 nM, buffer pH was 8 and conductivity was 0.04 S/m.
3.2. Fluorescent measurements of pH gradient formation
The approach to measure pH as a function of position in the iDEP microdevices is based on the pH- dependent fluorescence behavior of FITC Isomer I with increased emissions at higher pH, as well as the pH independent fluorescence behavior of TRITC. FITC is highly sensitive from pH 6 to 8, but discernable differences occur for the 3–10 pH range [22]. Our calibration curve correlating pH with FITC/TRITC intensity ratios, R, is shown in Fig. 2d and is in good agreement with the literature [23,24]. An exponential fit was obtained with a coefficient of determination of 0.99 as follows:
| (4) |
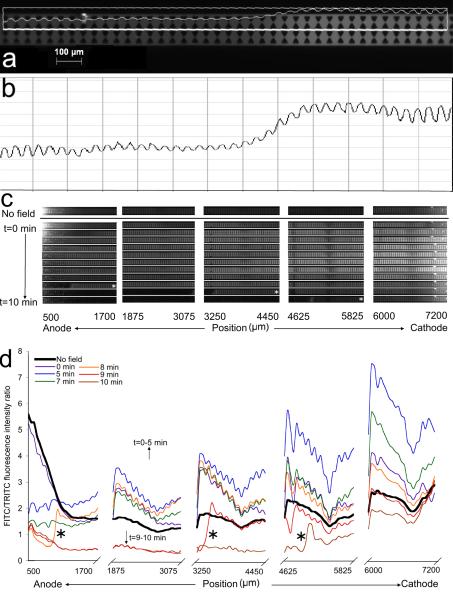
Fig. 4 shows the results of a single experiment (σ=0.01 S/m, E=100 V/cm). Fig. 4a and c show FITC microscopy images taken with 488±25 nm excitation and 535±40 nm emission filters. A corresponding TRITC image (not shown) was taken with 546±25 nm excitation, and combined 590 nm longpass and 680±nm bandpass emission filters. FITC and TRITC intensity profiles were generated along the channel centerline as shown in Fig 4a. As seen in Fig. 4a and 4b, the proximity of the insulating posts cause oscillations in the intensity profile. To reduce this noise, the FITC/TRITC profiles shown in Fig. 4d were binned by performing a running average over 50 pixels. Fig. 4c shows raw FITC images from a typical experiment. The top row shows pH 8 solution in the channel with the electric field off at the end of the 10 minute waiting period described in Section 2.6. The remaining rows show the change in FITC intensity by position from anode to cathode (left to right) and with time from 0 to 10 min (top to bottom). The profiles are depicted in Fig. 4d for a subset of the times. An increase in fluorescence intensity can be observed at the cathode end from 3 minutes to 6 minutes. It is possible that this increase is due to electrokinetic transport of FITC dianion or OH− transport causing a pH rise. Approximately 8 min into the experiment, FITC intensity drastically drops at the anode end, and a sharp low pH front is observed moving from the anode to the cathode. The FITC/TRITC ratio drops to 0.5, indicating a pH value of 4. A velocity of 22 μm/s was estimated from this front between t=8 min and t=10 min over a distance of 3400 μm (See Fig 4c), taking into account time between images at different positions; this rate is the same order of magnitude as EOF velocities estimated from the EOF mobilities reported in Table 1. This behavior was reliably reproduced in five 100 V/cm experiments with 0.01 S/m buffer and yielded ending pH values from 2.7–4.0. Fewer clean pH fronts were observed in 0.05 S/m buffer solutions and no significant pH change was observed in 0.10 S/m buffer solutions in a 100 V/cm electric field. This has been attributed to the greater buffering capacity of the 0.05 and 0.10 S/m conductivity buffer solutions. The differences observed in the 0.05 S/m runs were attributed to aging effects [12] and differences in active surface area of the wire electrodes and are discussed in greater detail later. In Table 2, 100 V/cm and 3000 V/cm results are tabulated with the first time point where a pH front was observed, and the estimated front velocity.
Figure 4.
Analysis of pH sensitive FITC/TRITC fluorescent emission results in an iDEP channel. a) 535±40 nm (FITC filtered) microscope image with overlaid intensity profile measured along the channel centerline. b) Unbinned intensity profile. c) FITC fluorescence images taken during the application of a 100 V/cm electric field to a microchannel and device filled with 0.01 S/m buffer solution, 50 μM FITC and 10 μM TRITC. d) The FITC/TRITC fluorescence intensity ratio along the length of the microchannel at different time points. The transition between two different pH values at t=8 min, t=9 min and t=10 min at the first, third and fourth channel positions from the anode are marked by asterisks and correspond to the similarly marked images in Fig 4c.
Table 1.
Average electroosmotic mobilities and standard deviations in PDMS channels without post arrays. Values were averaged over three measurements in each channel and three independent channels for each pH except for pH 3, where representing repetitive data in one channel only.
| pH | μEOF×10−8[m2/Vs] |
|---|---|
| 3 | 0.48 ± 0.07 |
| 6 | 2.45 ± 0.80 |
| 7 | 2.89 ± 1.00 |
| 8 | 2.43 ± 0.70 |
Table 2.
Onset times when a significant pH change was first detected in individual experiments, along with the estimated propagation rate of the observed pH front and measured average currents. The propagation rate estimates were made by comparing the positions of the front in consecutive time points. Currents recorded at a period of 62.5 ms by the High Voltage Sequencer were averaged over time and their 95% confidence intervals over 10 minutes were calculated.
| σ=0.01 S/m | σ=0.05 S/m | σ=0.10 S/m | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset time | Est. propagation rate (μm/s) | Avg. current (μA) | Onset time | Est. propagation rate (μm/s) | Avg. current (μA) | Onset time | Est. propagation rate (μm/s) | Avg. current (μA) | |
| 100 V/cm | t = 6 min | 31 | 2.5±0.01 | t = 4 min | 29 | 1.3±0.03 | N/O | N/A | 1.9±0.01 |
| t = 8 min | 22 | 2.3±0.01 | t = 9 min | 33 | 2.7±0.01 | N/O | N/A | 0.39±0.01 | |
| t = 4 min | 110 | 1.7±0.01 | t = 9 min | 26 | 3.0±0.01 | N/O | N/A | 1.3±0.01 | |
| t = 3 min | 31 | 2.5±0.01 | N/O | N/A | 1.8±0.01 | N/O | N/A | 3.9±0.01 | |
| t = 9 min | 19 | 2.0±0.01 | N/O | N/A | 3.1±0.01 | ||||
| N/O | N/A | 3.0±0.01 | |||||||
|
| |||||||||
| 3000 V/cm | t = 0 min | 115 | 27.2±0.45 | t = 0 min | 115 | 31.5±0.37 | |||
| t = 0 min | 115 | 36.6±0.42 | N/C | t = 0 min | 115 | 42.7±0.72 | |||
| t = 0 min | 115 | 21.6±0.18 | t = 0 min | >115 | 27.2±+0.14 | ||||
N/O is not observed, N/C is not conducted, and N/A is not applicable.
3000 V/cm electric fields were applied to iDEP microchannels filled with 0.01 and 0.10 S/m buffer solutions. In all cases, FITC/TRITC ratios dropped throughout the channel rapidly from the anode to the cathode to final intensity ratios between 0.2–0.4 (pH=2.7–3.9) with 0.01 S/m buffer solution and to 0.2–0.3 (pH=2.7–3.4) with 0.10 S/m buffer solution. The very similar behaviors of the 0.01 and 0.10 S/m buffer solutions under a 3000 V/cm electric field suggest that the electrode reactions overpower any buffering capabilities of the solution. This is a concern for iDEP protein focusing experiments which are typically conducted at these higher fields.
Current was recorded by the High Voltage Sequencer during experiments and concurrently used as an indicator of electrode reaction rates where higher currents suggest greater H+ and OH− generation rates. Average current and total charge passed through the channel over 10 minutes are also given in Table 2. It was observed that when Pt electrodes age by generating 100 V/cm electric fields for more than one hour, no pH changes were observed over 10 minutes with 0.01, 0.05 or 0.10 S/m buffer solutions; three experiments were carried out with each buffer solution, with identical results (data not shown). It is known that Pt surfaces undergo chemical and morphological changes over their lifetime [12]. It is possible that the water electrolysis reactions become slower or stop as the Pt surfaces become passivated or adsorb material. Interestingly, when a 3000 V/cm electric field was applied, rapid changes in intensity were again observed suggesting larger fields can overcome surface passivation. In summary, drops in pH were observed moving from the anode to the cathode for all 3000 V/cm experiments regardless of electrode age. Drops in pH were also observed with 100 V/cm at 0.01 S/m, observed at 0.05 S/m with unused electrode surfaces, but rarely observed at 0.10 S/m irrespective of electrode age. The observed pH front was consistent with the solution's buffering capacity reaching a threshold and velocities are consistent with predicted H+ mobility rates.
Since changes in FITC concentration can affect the fluorescence intensity and thus reliability of pH estimates, control experiments were run to determine EOF as a function of pH, electrophoretic mobility of the FITC molecule, and diffusion of the FITC molecule, as discussed in the following sections.
3.3. EOF measurements
The pH dependence of EOF was investigated in PDMS channels without post arrays. Table 1 shows that the EOF remains almost constant in the pH range 6–8, however is decreased by ~ 80 % to 0.48*10−8 m2/Vs at pH 3. This is in accordance with the acidic pK of ~3.5 [25] of silanol-groups responsible for negative surface charges on PDMS surfaces. We can thus conclude, that the EOF is reduced to a great extent at low pH, which will have consequences for iDEP manipulation and natural pH gradient formation. In the case of iDEP, electroosmosis is reduced, favoring the formation of iDEP streamlines and thus concentration enhancement of proteins. With regards to pH gradients, EOF toward the cathode is greatly reduced and would slow the total electrokinetic migration of H+ ions also toward the cathode. Despite this, pH 3 fronts are observed moving from the anode to the cathode.
3.4. Electrophoretic mobility of FITC
FITC has several prototropic forms that are cationic, neutral, anionic or dianionic [26]. The dianionic form is the most strongly fluorescing form, followed by the anionic forms. The neutral and cationic forms fluoresce as a result of deprotonation in the excited state, but less intensely. In neutral and slightly acidic conditions, the anionic and dianionic forms are present, while the neutral forms are dominant around pH ~3 [26]. Thus, in the experimental conditions, FITC molecules are expected to have a charge of 0, −1, or −2. Unfortunately, the coexistence of FITC molecules with different charges and quantum yields mean that smaller changes in fluorescence intensity may not be due solely to a pH change but may be influenced by a concentration change due to electrokinetic transport of FITC. This is explored theoretically and experimentally and summarized in Table SI2 in the supporting information. One source reported the radius of fluorescein sodium as 0.75 nm [27]. Since FITC and fluorescein sodium are close in structure, the radius of 0.75 nm was assumed to be a good approximation for FITC Isomer I. Based on these estimated properties, the electrophoretic mobility of FITC toward the anode was calculated with the following mobility equation [28] to be −1.13 × 10−8 m2/Vs for the anionic form, and −2.27 × 10−8 m2/Vs for the dianionic form; the neutral form does not experience electrophoretic transport.
| (5) |
This suggests that electrophoretic transport of FITC is the same order of magnitude as its transport by EOF, but in the opposing direction. Electroosmotic flow is from the anode to the cathode when the PDMS walls are negatively charged and ranges in magnitude at different pHs as reported in Table 1, while electrophoretic migration of the negatively charged FITC is from the cathode towards the anode. By combining EOF measured at pH 7 and electrophoretic mobilities of FITC, a compound electrokinetic mobility (μEK) was calculated,
| (6) |
as equal to 1.76 × 10−8 m2/Vs for the anionic form, and 6.2 × 10−9 m2/Vs for the dianionic form, indicating net FITC transport from the anode to the cathode. μEK of the neutral form is equal to the EOF mobility, which is 0.48 × 10−8 m2/Vs for pH=3, which is where the neutral form is dominant. Electrophoretic mobilities for H+ and OH− were also obtained from the literature and are +3.6*10−7 m2/Vs [28] and −2.1*10−7 m2/Vs [28], respectively.
FITC transport controls were experimentally explored according to the procedure in section 2.7 with the FITC loaded only into the cathode port at 100 V/cm and at 3000 V/cm and then only in the anode port for 100 and 3000 V/cm. When FITC was loaded only into the cathode end and a 100 V/cm electric field was applied, FITC migrated toward the anode; the front reached the port in 6 minutes corresponding to an electrokinetic mobility of −2.5 × 10−9 m2/Vs. After 8 minutes running the experiment, the fluorescence began to decrease at the anode end consistent with formation of a natural pH gradient. Fluorescence was not observed for either high voltage, nor for FITC loaded into the anode port and a 100 V/cm field applied.
Electrokinetic mobility estimates summarizing and comparing these different theoretically and experimentally derived values are given in Table SI2 in the supporting information. Electroosmotic flow will transport the uniformly concentrated dyes and the spatially varying H+/OH− ions at the exact same rate. As pH drops, electroosmotic flow decreases (Table 1) and electrophoretic mobilities become more dominant. Note that while the dianionic form of FITC has higher quantum yields than the anionic form, both have electrophoretic mobilities towards the anode that are an order of magnitude smaller than the electrophoretic mobility of H+ toward the cathode. Further, as the pH drops, the combined FITC electrokinetic mobility switches from being weakly toward the cathode to being weakly toward the anode O(10−9) and eventually converts to the neutral form while H+ transport toward the cathode remains O(10−7). All of the data given in Table SI2 supports that the H+ electrokinetic mobilities are 1 to 2 orders of magnitude greater than FITC electrokinetic mobilities and support our conclusions that the intensity drops seen moving toward the cathode in Figure 4 experiments are due to the formation of a natural pH gradient. Our FITC/TRITC dye concentrations are uniform everywhere in solution, suggesting decreases in intensity are not explainable as electrophoretically created voids of dye, it can only be due to the transport of H+ into the channel quenching the dye's fluorescence. We observe decreases in intensity from the anode to the cathode at roughly the correct ion transport rate, which support our conclusions that substantial darkening of the channel is primarily due to increases in H+ concentrations. Lastly, the decreases in intensity are buffer conductivity dependent which is also consistent with H+ and OH− driven effects and not dye transport effects.
3.5. Diffusion Controls
Since experiments were designed to start with uniform 50 μM FITC solution, diffusion was expected to come into effect when another transport mechanism caused a local concentration change. EOF is a bulk fluid transport mechanism and thus would not alter local concentrations. Biased electrophoretic transport could cause concentration changes in an initially homogenous solution. The diffusion coefficients for fluorescein derivatives are reported in the 1.76–4.94 × 10−8 m2/s range [26]. From Eq. (4), the combined electrokinetic flux would be approximately 5 orders of magnitude greater than the diffusional flux in a 100 V/cm electric field, and approximately 6 orders of magnitude greater in a 3000 V/cm field, although this is not a direct comparison since electrokinetic transport depends on concentration while diffusion depends on concentration gradient. Therefore the induced diffusional flux would not have a significant impact on the observed electrokinetic transport.
A rate of 8.43×10−10 mol/m2s, was measured from the diffusional control experiments as described in section 2.7, which is an order of magnitude greater than literature for H+ at 9.31×10−9 mol/m2s [29] and two orders of magnitude greater than diffusion coefficients for fluorescein derivatives in the 1.76–4.94 × 10−8 m2/s range [30]. The apparent enhancement of diffusion coefficient may be due to a pressure head introduced during the dye port loading. It was concluded that the observed losses of fluorescence in the natural pH gradient experiments are a result of a pH drop originating at the anode port, which has a stronger effect on the observed fluorescence than any FITC transport within the microchannel.
3.6. Modeling of Ion Transport in Microfluidic Channels
Mass flux in the x-direction for a charged species i as a result of diffusion, electrophoretic migration and EOF mechanisms is [28],
| (7) |
The right hand side terms from left to right represent mass diffusion, electrophoretic migration and EOF, respectively [28]. Di is the diffusivity of ion i, and Ci is the concentration of ion i. Z is the valence number of the particle, e is the elementary charge, η is the dynamic viscosity, a is particle radius, E is the electric field in the x-direction, ε is the electric permittivity of the fluid, and ζ is the zeta potential of the wall surface. The constitutive equation for the transport of dilute species module is analogous to Eq. 4, and was used to model the system in conjunction with the electric currents and laminar flow modules over a 160 μm long section of the microchannel with triangular posts. 25 μm long extensions were added to either end of this section, to set the desired boundary conditions. Key points of the model are discussed here, and the model file is available as supporting information.
The electric field was modeled with the electric currents module. Potential differences corresponding to 100 and 3000 V/cm, and conductivities of 0.01 and 0.10 S/m were used in separate runs of the model. EOF was modeled with the laminar flow module using the electroosmotic slip boundary condition and an EOF mobility of 1.5×10−8 m2/Vs, according to experimentally reported conditions employing F108 coating agents [31]. Built in expressions for electrophoretic migration and convective mass transfer available in the transport of dilute species module in COMSOL 4.1 were used. The influx of H+ and OH− ions into the system were modeled by dividing the current density in the y-direction from the electric currents module by the Faraday's constant, and defined as flux discontinuity boundary conditions at either end of the 160 μm section. The water association reaction, shown below, was also modeled. The conditions specified in the model are given in a table SI1 in supporting information.
| (8) |
[29]
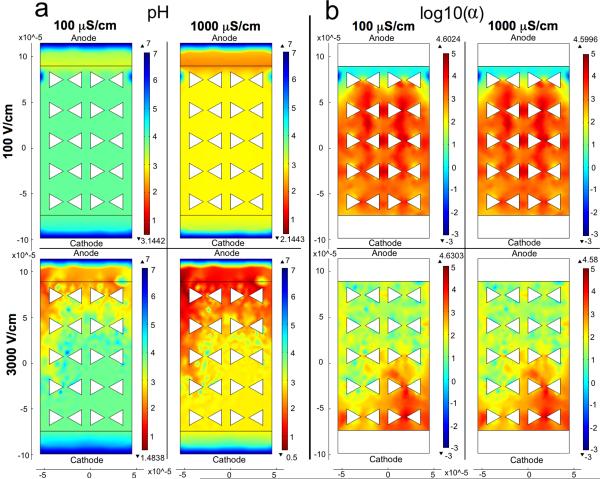
The steady-state solution of this model showed that the pH drop becomes greater both with increasing field strength, which was consistent with experimental results (See Fig. 5a). The simulation showed greater pH drop with increasing buffer conductivity, which is not consistent with the experimental results. However, this discrepancy can be easily explained. The simulation does not account for the buffering effect; thus, the increased conductivity only increases the current, which increases the water hydrolysis reaction rate. In reality, higher conductivity is due to higher concentration of phosphate ions and thus, higher buffering capacity. The solution also showed that electrophoretic flux is 3 to 5 orders of magnitude stronger than the diffusional flux for H+ and OH−, for the case of a 100 V/cm electric field, at both buffer conductivities (See Fig. 5b). The ratio of the electrophoretic flux to the diffusion flux for a species was defined as α,
| (9) |
Surface plots of pH and log10(α) for the four electric field and conductivity combinations are shown in Fig. 5b. By expressing α logarithmically, an order of magnitude comparison between electrophoretic and diffusional fluxes can be made. This result agrees with predictions from comparisons of electrophoretic mobilities and diffusion coefficients. For a higher potential difference (e.g., a 3000 V/cm electric field) it is expected that the calculated electrophoretic flux would be even more dominant over the diffusional flux.
Figure 5.
Comparison of pH and log10(α) values plotted for different electric field strengths and conductivities. α is the ratio of the electrophoretic flux to the diffusion flux as defined in Eq. 9. The anode is at the top of the plots. The flux terms representing the H+ and OH− carrying currents were defined at the interior boundaries, and the boundaries beyond those are set to concentrations of 10−7 M for both species. Simulation conditions are outlined in Table SI1. a) pH values plotted by COMSOL confirm experimentally observed trends of higher electric field strengths resulting in larger pH drops. However, COMSOL does not account for buffering strength and so conductivity dependence is not comparable between experiments and these COMSOL results. b) Order of magnitude differences between the electrophoretic and diffusional fluxes are shown with larger values indicating more dominant electrophoretic migration.
While this model demonstrates the phenomenon and is useful for comparing the relative importances of the transport mechanisms, it is not considered to be rigorous and quantitatively predictive. One reason for this is gradients around the posts that significantly diminish within the first five rows in the simulation, and are therefore considered to reflect more accurately the entrance of the channel. In addition, unnaturally steep gradients were observed in the 3000 V/cm cases, which suggest the onset of instabilities for those solutions.
Conclusions
Evidence has been presented herein that pH changes occur in less than 10 minutes within an iDEP device predominantly at higher electric fields and lower buffer conductivities. In less than 1 minute of 3000 V/cm field application, the pH within the microchannels dropped to pHs in the range of 2.7–3.4. In 100 V/cm electric fields and 0.01 S/m buffer solutions, the pH within the microchannels dropped to 2.7–4.0 over time spans of 6 to 10 minutes. Also in 100 V/cm fields, increasing the buffer conductivity decreased the propensity of the system to form a pH gradient. This is consistent with the solution exceeding its buffering capacity thus allowing pH shifts. Competing dye transport via electrokinetic mobilities and diffusion were carefully examined and it was determined that the transport rates were orders of magnitude too small to account for the observed decreases in intensity from the anode to the cathode. Thus, we conclude that substantial darkening of the channel is due to fluorescent dye quenching as a result of increases in H+ concentrations. The source of the H+ is electrochemical reactions at the anode; these ions are then transported electrophoretically and via electroosmotic flow into the channel during the course of 10-minute iDEP experiments. The decreases in intensity are buffer conductivity dependent which is also consistent with H+ and OH− driven effects and not dye transport effects. Lastly, Pt electrode aging effects were observed in the smaller electric fields and suggest electrode passivation may be a strategy to reduce electrode catalyzed reactions. However, larger applied potentials are able to overcome electrode passivation effects. In summary, drops in pH are observed moving from the anode to the cathode for all 3000 V/cm experiments regardless of electrode age; drops in pH were also observed for 100 V/cm at 0.01 S/m, less consistently at 0.05 S/m, but not at 0.10 S/m.
It is interesting to relate the pH gradient study to iDEP of proteins. Considering a change in pH in the iDEP experiments, it is likely that the isoelectric point of a protein is reached or the solution pH even falls below it. As a consequence, conditions favoring protein aggregation may arise. In our iDEP manipulations with IgG exhibiting an isoelectric point of ~5, fluorescent aggregates become apparent after ~20 min of application of the high electric fields investigated in this study. Interestingly, the addition of the zwitterionic agent CHAPS shifts the time at which this effect becomes dominant. In comparison, for both investigated field strengths, the pH changes to acidic conditions in a time scale of 10 min in the FITC experiments. Protein streamline iDEP is however still apparent after 10 min. It can further be noted that the pH changes towards the acidic range can reduce EOF. As a consequence, streamline iDEP of proteins is enhanced compared to electroosmotic flow. We would thus expect an increase in the concentration enhancement due to streamline iDEP. This was also evidenced in our previous study, which found a higher concentration factor for iDEP of IgG experimentally as was evidenced by numerical simulations [9]. The conductivity dependence is also a particularly important result for iDEP devices because lower conductivities yield greater polarizations and thus cleaner separations, yet these same conditions are more amenable to formation of pH gradients.
The general conclusion that can be drawn from this study is that the behavior of iDEP devices is influenced by the electrode reactions that take place during the application of an electric field. Utilizing ion sinks in the respective wells may be a way to overcome this issue and potentially increase the efficiency of iDEP devices. Knowledge of ion gradients in these devices and strategies for controlling the ion gradients are needed. Our study thus demonstrates that pH changes can indeed influence iDEP applications for proteins and gives future guidelines for the operation conditions of separation or fractionation devices based on iDEP. Despite these challenges, insulator-based DC dielectrophoretic microdevices have the potential to replace traditional AC dielectrophoretic devices for many cellular and biomolecular separation applications.
Supplementary Material
Acknowledgements
Initial seed funding for this research was provided by NSF CBET 0636254, “SGER: Exploration and Quantification of Ion Gradients in a Capillary Microdevice”. This study was also supported by National Institute of Health under grant 1R21RR025826-01A2.
List of abbreviations
- AC
Alternating current
- CF
Carboxyfluorescein
- DC
Direct current
- iDEP
Insulator-based dielectrophoresis
- NC
Normalized current
- NI
Normalized intensity
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- [1].Pohl HA. Dielectrophoresis. Cambridge University Press; Cambridge, UK: 1978. [Google Scholar]
- [2].Cummings EB, Singh AK. Anal. Chem. 2003;75:4724–4731. doi: 10.1021/ac0340612. [DOI] [PubMed] [Google Scholar]
- [3].Regtmeier J, Duong TT, Eichhorn R, Anselmetti D, Ros A. Anal. Chem. 2007;79:3925–3932. doi: 10.1021/ac062431r. [DOI] [PubMed] [Google Scholar]
- [4].Regtmeier J, Eichhorn R, Bogunovic L, Ros A, Anselmetti D. Anal. Chem. 2010;82:7141–7149. doi: 10.1021/ac1005475. [DOI] [PubMed] [Google Scholar]
- [5].Gallo-Villanueva RC, Rodriguez,-Lopez RC, Diaz-De-La-Garza RI. Electrophoresis. 2000;30:4195–4205. doi: 10.1002/elps.200900355. [DOI] [PubMed] [Google Scholar]
- [6].Chou CF, Tegenfeldt JO, Bakajin O, Chan SS, Cox EC, Darton N, Duke T, Austin RH. Biophys.J. 2002;83:2170–2179. doi: 10.1016/S0006-3495(02)73977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lapizco-Encinas BH, Ozuna-Chacon S, Rito-Palomares M. J. Chromatogr. A. 2008;1206:45–51. doi: 10.1016/j.chroma.2008.05.077. [DOI] [PubMed] [Google Scholar]
- [8].Clarke RW, White SS, Zhou D, Ying L, Klenerman D. Angew. Chem. Int. Ed. Engl. 2005;24:3747–3750. doi: 10.1002/anie.200500196. [DOI] [PubMed] [Google Scholar]
- [9].Nakano A, Chao T-C, Camacho-Alanis F, Ros A. Electrophoresis. 2011 doi: 10.1002/elps.201100037. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Clarke RW, Piper JD, Ying LM, Klenerman D. Phys.Rev.Lett. 2007;98:198102. doi: 10.1103/PhysRevLett.98.198102. [DOI] [PubMed] [Google Scholar]
- [11].Macounova K, Cabrera CR, Holl MR, Yager P. Anal. Chem. 2000;72:3745–3751. doi: 10.1021/ac000237d. [DOI] [PubMed] [Google Scholar]
- [12].Gencoglu A, Minerick A. Lab Chip. 2009;9:1866–1873. doi: 10.1039/b820126a. [DOI] [PubMed] [Google Scholar]
- [13].Minerick A, Zhou R, Takhistov P, Chang H-S. Electrophoresis. 2003;24:3703–3717. doi: 10.1002/elps.200305644. [DOI] [PubMed] [Google Scholar]
- [14].Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. John Wiley & Sons, Inc.; New York, NY: 2001. [Google Scholar]
- [15].Svensson H. Acta Chem. Scand. 1961;15:325–341. [Google Scholar]
- [16].Sommer GJ, Hatch AV. Electrophoresis. 2009;30:742–757. doi: 10.1002/elps.200800598. [DOI] [PubMed] [Google Scholar]
- [17].Ou J, Glawdel T, Samy R, Wang S, Liu Z, Ren CL, Pawliszyn J. Anal. Chem. 2008;80:7401–7407. doi: 10.1021/ac8010928. [DOI] [PubMed] [Google Scholar]
- [18].Guzman KAD, Karnik RN, Newman JS, Majumdar A. J. Microelectromech. S. 2006;15:237–245. [Google Scholar]
- [19].Erlandsson PG, Robinson ND. Electrophoresis. 2011;32:784–790. doi: 10.1002/elps.201000617. [DOI] [PubMed] [Google Scholar]
- [20].Duong T, Kim G, Ros R, Streek M, Schmid F, Brugger J, Ros A, Anselmetti D. Microelectronic Engineering. 2003;67-68:905–912. [Google Scholar]
- [21].Huang X, Gordon MJ, Zare RN. Anal.Chem. 1988;60:1837–1838. [Google Scholar]
- [22].Allard E, Larpent C. J. Polym. Sci. A Polym. Chem. 2008;46:6206–6213. [Google Scholar]
- [23].Burns A, Sengupta P, Zedayko T, Baird B, Wiesner U. Small. 2006;2:723–726. doi: 10.1002/smll.200600017. [DOI] [PubMed] [Google Scholar]
- [24].Yumura S, Fukui Y. J. Cell Sci. 1998;111:2097–2108. doi: 10.1242/jcs.111.15.2097. [DOI] [PubMed] [Google Scholar]
- [25].Lide DR. CRC Handbook of Chemistry and Physics. 84 ed. CRC Press LCC; Boca Raton: 2004. [Google Scholar]
- [26].Sjöback R, Nygren J, Kubista M. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995;51:L7–L21. [Google Scholar]
- [27].Fu BM, Adamson RH, Curry FE. Am. J. Physiol. Heart. Circ. Physiol. 1998;274:2062–2073. [Google Scholar]
- [28].Bruus H. Theoretical Microfluidics. Oxford University Press; Oxford, UK: 2008. [Google Scholar]
- [29].Millero FJ. Physical Chemistry of Natural Waters. Wiley-Interscience; New York, NY: 2001. [Google Scholar]
- [30].Tucker EB, Tucker JE. Protoplasma. 1993;174:36–44. [Google Scholar]
- [31].Hellmich W, Regtmeier J, Duong TT, Ros R, Anselmetti D, Ros A. Langmuir. 2005;21:7551–7557. doi: 10.1021/la0510432. [DOI] [PubMed] [Google Scholar]
- [32].Ren X, Bachman M, Sims C, Li GP, Allbritton N. J. Chromatogr. B. 2001;762:117–125. doi: 10.1016/s0378-4347(01)00327-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.