Abstract
Central to our ability to hear and sense gravity is a cellular process known as mechanotransduction, which is initiated by the opening of mechanosensitive cation channels located near the tips of the stereocilia of auditory and vestibular inner ear hair cells. The molecular identity of the mechanotransduction channels has eluded researchers despite intensive investigations over the years. In this issue of the JCI, Kawashima et al. report their results obtained using mice with targeted deletion of both transmembrane channel–like 1 (Tmc1) and Tmc2. The use of inner ear hair cells isolated from these mice provided a nearly perfect system for testing the mechanotransduction channels without disrupting functions of other accessory proteins needed in the complicated molecular apparatus, and it allowed the authors to show that the proteins encoded by these genes are integral components of the mechanotransduction complex.
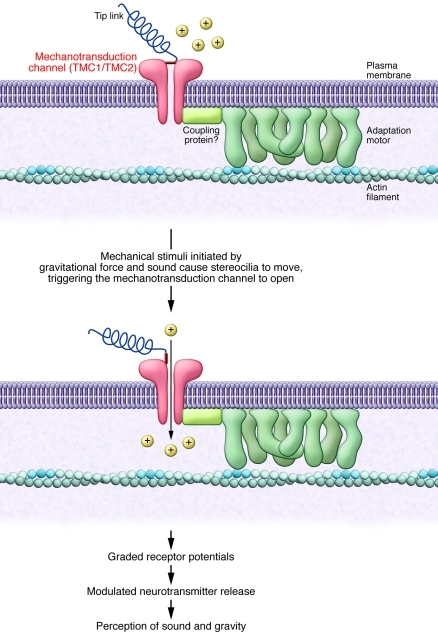
Auditory and vestibular inner ear hair cells are central to our ability to perceive sound and gravity, respectively. These cells convert mechanical stimuli initiated by sounds and gravitational force into electrical signals, a process known as mechanotransduction. Mechanotransduction is initiated when the mechanical stimuli cause stereocilia at the apical surface of the hair cells to move, triggering the opening of mechanosensitive cation channels located at the tip. Ionic currents that flow through the open channels initiate graded receptor potentials (refs. 1, 2, and Figure 1). This results in rapid changes in the membrane potential of the hair cells and subsequently modulates neurotransmitter release at the hair cell base. Auditory and vestibular inner ear hair cells synapse with auditory and vestibular nerves, respectively. Thus, changes in neurotransmitter release by the hair cells modulate postsynaptic action potentials to convey the mechanical information to the brain. These neuronal activities underlie our perception of sound and are necessary for maintaining balance and coordination of eye and head movements.
Figure 1. The mechanotransduction process in mammalian hair cells.
Displacement of the stereocilia creates tension in tip links that open the mechanotransduction channels located near the tips of stereocilia. The mechanotransduction channel is coupled to an adaptation motor so tension of the tip link can be properly adjusted.
Molecular identification of the mechanotransduction channels in the stereocilia of auditory and vestibular hair cells is fundamental for enhancing our understanding of mammalian auditory and vestibular systems, and has been a major focus of research in this field for many years. However, whereas the molecular mechanisms of transduction in the visual system are well understood (3), the molecular identity of the components of the auditory and vestibular mechanotransduction apparatus in mammals is largely unknown. Many research groups around the world have been searching for answers, and in this issue of the JCI, Kawashima and colleagues present an intriguing possibility (4). Specifically, they uncover key roles for transmembrane channel–like 1 (TMC1) and TMC2 in hair cell mechanotransduction in mice that indicate that these proteins are likely to be integral molecular components of the auditory and vestibular mechanotransduction apparatus.
Clues to the identity of the mechanotransduction channels
Over the years, several proteins have been proposed as candidate mechanotransduction channels, with most suggestions being based on studies in lower animals. At one time, a mammalian equivalent of bacterial large-conductance mechanosensitive channels such as MscL was considered a reasonable candidate (5). Cyclic nucleotide–gated (CNG) channels in hair cells (6) have also been prime contenders. However, the rapid dynamic changes required for auditory transduction are in the sub-millisecond range for high-frequency sensing, making such a proposal unlikely to be true (3). Members of the degenerin/epithelial Na+ channel (DEG/ENaC) family of ion channels have also been candidates, as they have been shown to be the major mechanoelectrical transduction channels in a Caenorhabditis elegans nociceptor (7). However, data obtained from transgenic mice suggest that these types of channels are unlikely to be the mammalian auditory and vestibular mechanotransduction channels (8). Work in zebrafish has identified members of the transient receptor potential (TRP) family of ion channels as additional candidates for the mechanotransduction channel of vertebrate auditory and vestibular hair cells (9). Later work conducted with targeted deletion of the TRPA1 channel in mice, however, showed that TRP channels are not essential for hearing and vestibular functions (10).
New clues provided by human genetic studies
Despite the numerous candidates proposed up to now, the identity of the mammalian auditory and vestibular mechanotransduction channels remains elusive. A major difficulty in identifying these channels arises from the fact that there are many accessory molecules required for the mechanotransduction apparatus and they have to be properly assembled (Figure 1). Proper assembly of the mechanotransduction apparatus is a condition hard to meet in the reconstituted systems commonly used for functional verification of candidate molecules, but it is essential if functional assessments are to be made.
Clues as to the identity of the mammalian auditory and vestibular mechanotransduction channels are also provided by the extensive data obtained from human studies of genes associated with deafness (11). Among the genes identified in such studies is TMC1. Mutations in TMC1 are associated with profound human prelingual non-syndromic deafness of both dominant (DFNA36) and recessive (DFNB7/B11) forms (12). In Iranian (13) and Turkish populations (14), TMC1 is one of the genes most commonly associated with deafness. Brownstein et al. (15) found a mutation in TMC1 to be a founder allele in the Moroccan Jewish population. For the population of Israelis of Moroccan Jewish ancestry who are deaf, recessive mutations in TMC1 were detected in more than a third of them. Consistent with these human studies linking TMC1 to an essential role in hearing, mice with Tmc1 mutations (e.g., Beethoven [Bth] and deafness [dn] mutant mice) generated in a large-scale N-ethyl-N-nitrosourea (ENU) mutagenesis program (16) are also deaf (17).
TMC1 and TMC2 are predicted to encode membrane proteins with six transmembrane domains. Although the specific functions of the proteins encoded by these genes are unknown, bioinformatic analysis (18) and data obtained from in vitro heterologous systems (19) suggest that they function as membrane channels or transporters (18–20). Consistent with the genetic data linking TMC1 and TMC2 to a role in hearing, it has been shown that Tmc1 and Tmc2 mRNA is expressed in the inner ear (12, 17). Moreover, it has been suggested that the two genes may functionally compensate for each other in inner ear functions (21).
Double knockout of Tmc1 and Tmc2 reveals their roles
In this issue of the JCI, Kawashima et al. report their novel results showing that TMC1 and TMC2 encode functionally redundant stereocilia components that are necessary for hair cell mechanotransduction in mice (4). Key to this conclusion was the observation that mice with targeted deletion of both Tmc1 and Tmc2 (Tmc1ΔTmc2Δ mice) showed profound deafness and vestibular dysfunction. Interestingly, mice with targeted deletion of only Tmc1 showed severe hearing loss without vestibular dysfunction, similar to what is observed in human patients with TMC1 mutation (12, 15). These results suggest that there might be functional compensation between Tmc1 and Tmc2. A molecular explanation for the observation was provided by quantitative RT-PCR, which showed that Tmc2 expression persisted in vestibular hair cells into adult life but was markedly reduced in cochlear hair cells after the early postnatal stage. Therefore, TMC1 was the only TMC channel expressed in the adult cochlea.
Using GFP-tagged TMC proteins to find the location of these proteins in the hair cells enabled Kawashima et al. to solve another key piece of the puzzle (4). This approach pinpointed TMC proteins to the tips of stereocilia at the apical surface of the hair cells, which is where mechanotransduction channels should be localized. High-resolution scanning electron microscopy indicated that stereocilia in hair cells from Tmc1ΔTmc2Δ mice were structurally normal, with intact tip links. However, isolated hair cells tested in vitro completely lacked mechanotransduction activity. Tmc1 and Tmc2 were then shown to be sufficient for mechanotransduction individually, since exogenous expression of either of the two genes rescued mechanotransduction activity in auditory and vestibular hair cells obtained from the Tmc1ΔTmc2Δ mice. Importantly, exogenous expression of either Tmc1 or Tmc2 rescued not only the mechanotransduction currents in the hair cells, but also uptake of fluorescent FM1-43 and gentamicin dye, which are both known to enter hair cells via the mechanotransduction channels. These data support the conclusion that either Tmc1 or Tmc2 is sufficient for carrying out mechanotransduction in hair cells in mice.
Tmc1ΔTmc2Δ mice are valuable tools for studying deafness
Preserving the integrity of the mammalian mechanotransduction apparatus (Figure 1) while selectively removing one piece of the jigsaw (the mechanotransduction channel) has been an insurmountable task for auditory physiologists for many years. Finally, the use of hair cells isolated from the Tmc1ΔTmc2Δ mice generated by Kawashima et al. (4) seems to provide a nearly perfect system in which the mechanotransduction channels may be the only missing piece in this complicated device (Figure 1). How the other accessory proteins (e.g., the tip links and motor proteins) interact with the mechanotransduction channels can now be tested experimentally. Where does the mechanical gate of the tip link anchor with the channels encoded by Tmc1 and Tmc2? how does stereocilia turnover (22) affect the function of mechanotransduction channels? and how do the whole mechanotransduction apparatus regenerate after chemical or noise insults? — these are just a few of the questions it is now possible to tackle with the Tmc1ΔTmc2Δ mice generated by Kawashima et al. (4). Although many aspects about the structure and functions of TMC channels still need to be answered, the work of Kawashima et al. (4) appears to have firmly established that both TMC1 and TMC2 are integral components of the mechanotransduction complex. This advances our understanding of the molecular mechanisms underlying the pathogenesis of human DFNA36, DFNB7/B11, and other types of deafness.
Acknowledgments
Research done in the author’s laboratory is funded by grants from the National Institute on Deafness and Other Communication Disorders (NIDCD 4R33DC010476, R01DC010204, and R01DC006483).
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(12):4633–4636. doi:10.1172/JCI61167
See the related article beginning on page 4796.
References
- 1.Hudspeth AJ. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dallos P, Santos-Sacchi J, Flock A. Intracellular recordings from cochlear outer hair cells. Science. 1982;218(4572):582–584. doi: 10.1126/science.7123260. [DOI] [PubMed] [Google Scholar]
- 3. Hille B. Sensory transduction and excitable cells. In: Hille B, ed.Ion Channels Of Excitable Membranes . Sunderland, Massachusetts, USA: Sinauer Associates, Inc.; 2001:237–268. [Google Scholar]
- 4.Kawashima Y, et al. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel–like genes. J Clin Invest. 2011;121(12):4796–4809. doi: 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gullingsrud J, Kosztin D, Schulten K. Structural determinants of MscL gating studied by molecular dynamics simulations. Biophys J. 2001;80(5):2074–2081. doi: 10.1016/S0006-3495(01)76181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolesnikov SS, Rebrik TI, Zhainazarov AB, Tavartkiladze GA, Kalamkarov GR. A cyclic-AMP-gated conductance in cochlear hair cells. FEBS Lett. 1991;290(1–2):167–170. doi: 10.1016/0014-5793(91)81251-3. [DOI] [PubMed] [Google Scholar]
- 7.Geffeney SL, et al. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. . Neuron. 2011;71(5):845–857. doi: 10.1016/j.neuron.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng BG, Ahmad S, Chen S, Chen P, Price MP, Lin X. Acid-sensing ion channel 2 contributes a major component to acid-evoked excitatory responses in spiral ganglion neurons and plays a role in noise susceptibility of mice. J Neurosci. 2004;24(45):10167–10175. doi: 10.1523/JNEUROSCI.3196-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corey DP, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432(7018):723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 10.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 11.Dror AA, Avraham KB. Hearing loss: mechanisms revealed by genetics and cell biology. Annu Rev Genet. 2009;43:411–437. doi: 10.1146/annurev-genet-102108-134135. [DOI] [PubMed] [Google Scholar]
- 12.Kurima K, et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet. 2002;30(3):277–284. doi: 10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- 13.Hildebrand MS, et al. Mutations in TMC1 are a common cause of DFNB7/11 hearing loss in the Iranian population. Ann Otol Rhinol Laryngol. 2010;119(12):830–835. doi: 10.1177/000348941011901207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duman D, Sirmaci A, Cengiz FB, Ozdag H, Tekin M. Screening of 38 genes identifies mutations in 62% of families with nonsyndromic deafness in Turkey. Genet Test Mol Biomarkers. 2011;15(1–2):29–33. doi: 10.1089/gtmb.2010.0120. [DOI] [PubMed] [Google Scholar]
- 15.Brownstein Z, et al. Targeted genomic capture and massively parallel sequencing to identify genes for hereditary hearing loss in middle eastern families. Genome Biol. 2011;12(9):R89. doi: 10.1186/gb-2011-12-9-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrabe de Angelis MH, et al. Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nat Genet. 2000;25(4):444–447. doi: 10.1038/78146. [DOI] [PubMed] [Google Scholar]
- 17.Vreugde S, et al. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet. 2002;30(3):257–258. doi: 10.1038/ng848. [DOI] [PubMed] [Google Scholar]
- 18.Keresztes G, Mutai H, Heller S. TMC and EVER genes belong to a larger novel family, the TMC gene family encoding transmembrane proteins. BMC Genomics. 2003;4(1):24. doi: 10.1186/1471-2164-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labay V, Weichert RM, Makishima T, Griffith AJ. Topology of transmembrane channel-like gene 1 protein. Biochemistry. 2010;49(39):8592–8598. doi: 10.1021/bi1004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurima K, Yang Y, Sorber K, Griffith AJ. Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis. Genomics. 2003;82(3):300–308. doi: 10.1016/S0888-7543(03)00154-X. [DOI] [PubMed] [Google Scholar]
- 21.de Heer AM, et al. Progressive sensorineural hearing loss and normal vestibular function in a Dutch DFNB7/11 family with a novel mutation in TMC1. Audiol Neurootol. 2011;16(2):93–105. doi: 10.1159/000313282. [DOI] [PubMed] [Google Scholar]
- 22.Manor U, Kachar B. Dynamic length regulation of sensory stereocilia. Semin Cell Dev Biol. 2008;19(6):502–510. doi: 10.1016/j.semcdb.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]