Abstract
A-kinase anchoring proteins (AKAPs) create compartmentalized environment inside the cell to bring various signaling molecules to their targets. In the heart, a slowly activating potassium channel (IKs) important for cardiac repolarization is tightly regulated by the sympathetic nervous system in an AKAP-dependent manner. IKs channel forms a macromolecular complex with AKAP9 and other enzymes, such as PKA, phosphatase, adenylyl cyclase and phosphodiesterase, all of which are responsible to control the phosphorylation state of the channel. Such a complex thus ensures the IKs channel to be regulated properly to maintain the normal cardiac rhythm. Disruptions of various elements of the complex have been found to cause severe pathological consequences, including the Long QT Syndrome.
A-kinase anchoring proteins (AKAPs) are scaffolding proteins that provide spatiotemporal control of enzymatic activities by serving as the structural and functional links between the associating enzyme molecules and their end targets, such as ion channels1. In the heart, where the excitation and contraction of cardiac muscles need to be tightly regulated to provide adequate and continuous blood supply to body organs, a growing list of AKAPs that includes mAKAP, AKAP15/18, Gravin and AKAP9 etc, has been shown to play critical roles in cardiac physiology and pathology2-4. In this review, we focus on AKAP9, also known as Yotiao, which was initially found to bind NMDA receptor subunit NR15, 6 and belongs to a multispliced AKAP family that includes the longer transcript AKAP350/AKAP450/CG-NAP 7-9 localized in the golgi and centrosomal regions. We will discuss the role of AKAP9 in the regulation of a slowly activating cardiac potassium channel (IKs) as well as its relevance to cardiac arrhythmic disorders, such as the Long QT Syndromes (LQTs).
I. Role of AKAP9 in IKs channel regulation and LQTs
The IKs channels are comprised of the pore-forming α- subunit KCNQ1 that conducts ionic current, as well as the auxiliary β-subunit KCNE1 that renders the channel its characteristic biophysical properties, including the slow kinetics 10, 11. The voltage-gated IKs channels open in response to depolarization to repolarize the cardiac cells. Mutations in both KCNQ1 and KCNE1 disrupt channel function, prolong action potential duration (APD) and cause LQT1 12 and 513, respectively. IKs channels are regulated by the sympathetic nervous system (SNS) via the β-adrenergic receptor/Gs/cAMP/PKA pathway. Walsh and Kass first demonstrated in the Guinea pig ventricular cells that cAMP analogues caused a large increase in the IKs amplitude as well as a slowing in the current decay during deactivation 14. Later, Kurokawa et al reconstituted such effects in a recombinant expression system by coexpressing AKAP9 (Yotiao) and demonstrated that AKAP9 forms a macromolecular complex with KCNQ1, Type II regulatory subunit of PKA (RII), and protein phosphatase 1 (PP1) and brings PKA to the vicinity of the channel to phosphorylate a serine residue (S27) on KCNQ1 amino terminus15. AKAP9 binds KCNQ1 carboxy terminus via a leucine zipper (LZ) motif. A previously known LQT mutation (G589D) near the LZ motif disrupted the interaction between Yotiao and KCNQ1 as well as the functional regulation of IKs by PKA phosphorylation 15. These results suggest for the first time that (1) AKAP9 plays an indispensable role in IKs channel regulation by SNS; (2) Dysfunctional SNS regulation of IKs channel may lead to pathological consequences such as LQT.
The role of AKAP9 in IKs channel regulation and LQTs was further demonstrated in a subsequent paper 16. AKAP9 was shown to possess two binding sites for KCNQ1, one located on its N-terminus (residues 29-46), the other on its C-terminus (residues 1574-1643). By performing sequence analysis targeted at these two regions in a large LQT cohort with negative genotype, Chen et al identified a putative AKAP9 mutation (S1570L) in a LQT patient and her family. The mutation is close to the C-terminal binding site on AKAP9 and was shown to reduce KCNQ1 binding and phosphorylation. More importantly it diminished cAMP-induced enhancement of IKs channel activities and was predicted by computer simulation to prolong APD, especially when cells were stimulated by isoproterenol. These results provided direct evidence that a defective AKAP9 may cause LQT.
II. Molecular components of the AKAP9/IKs complex
AKAP9 provides a structural entity to integrate various signaling molecules and the targeted IKs channel. Two pairs of enzymes have been shown to associate with AKAP9. The first pair involves PKA and PP1, which phosphorylates or dephosphorylates the channel, respectively. The second pair involves adenylyl cyclase (AC) and phosphodiesterase (PDE), which increases or decreases cAMP inside the cell, respectively, to control PKA activities. Together the two pairs of opposing enzymes act to balance and fine-tune the phosphorlation state of IKs channel (Figure 1).
Figure 1.
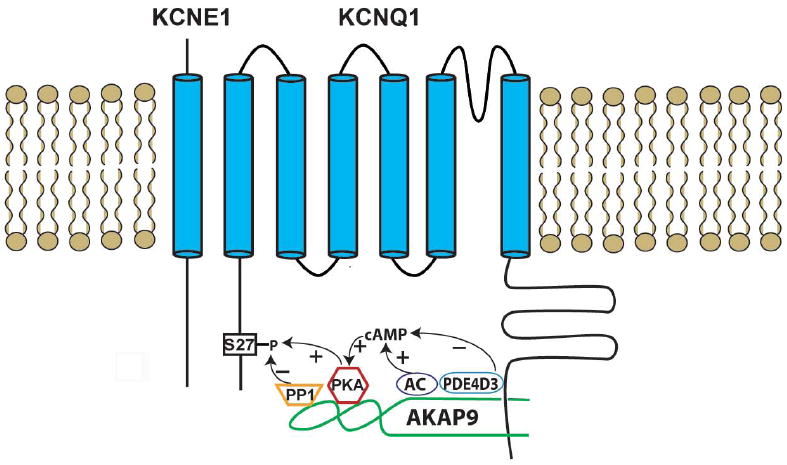
A schematic diagram of the IKs /AKAP9 (Yotiao) macromolecular complex. IKs channels are comprised of α-subunits (KCNQ1) and β-subunits (KCNE1). S27 is a PKA phosphorylation site on KCNQ1 N-terminus. AKAP9 interacts with KCNQ1 C-terminus and harbors two pairs of enzymes that control the phosphorylation state of the channel. PKA phosphorylates IKs channel, while PP1 dephosphorylate it. AC and PDE control cAMP level, which in turn activates PKA.
PKA. PKA holoenzyme consists of two regulatory subunits and two catalytic subunits. The regulatory subunits associate with their catalytic counterparts under low cAMP level. Upon receptor stimulation, cAMP concentration rises. The catalytic subunits are released from the regulatory subunits. One of the essential functions of AKAPs is to harbor the RII subunits. All AKAPs have a signature RII binding motif, which features an amphipathic helix of 14-18 residues that are able to dock to RII with high affinity17. Early works identified a region on AKAP9 (residues 1440-1457: LEEEVAKVIVSMSIAFAQ) as the primary binding site for PKA RII 6, 18. Recently crystal structures of AKAP binding motif bound to RII have been solved. The structures showed that the RII binding motif of AKAP binds to a hydrophobic interface formed by the two N-termini (D/D domain) of the RII dimer19, 20. It remains to be seen whether the RII binding site on AKAP9 for RII follows the same pattern. The details in the crystal structures will allow us to design peptides to disrupt interaction between AKAP and RII, thus providing valuable tools to study AKAP function21, 22. The primary function of the AKAP9-bound PKA is to phosphorylate the S27 residue on KCNQ1. But it is also noteworthy that AKAP9 itself is a PKA substrate and that phosphorylation of AKAP9 participates in the process of IKs channel regulation 23, 24. Whether AKAP9 is self-phosphorylated by the associating PKA is an interesting possibility that remains to be tested.
PP1. PP1 is a serine/threonine phosphatase that dephosphorylates its substrate. Scaffolding proteins such as AKAPs provide the non-specific PP1 a structural platform to act on a specific group of substrates 25-27. The role of PP1 in the IKs regulation is evidenced not only by its detection in the IKs/AKAP9 macromolecular complex, but also by the functional experimental data. Addition of okadaic acid, a PP1 inhibitor, enhanced the effect of cAMP-dependent IKs regulation 15. Previous works have shown that PP1 bound to residues 1171-1229 of AKAP9. A hallmark PP1 binding motif (KVxF) is present in AKAP9, but was found to be not essential for PP1 interaction 6. Lack of finer mapping for PP1 binding site hinders further studies of the physiological role of PP1 in IKs regulation.
AC. Membrane bound ACs are activated by Gs-coupled receptors, including the β-adrenergic receptors, and are responsible for cAMP synthesis, a prelude to PKA activation. Recent studies showed that ACs associated with various AKAPs, including AKAP79/150, mAKAP and AKAP9 28-31. AKAP9 associates with AC1, 2, 3, and 9, but not 4, 5, and 6. Residues 808-957 of AKAP9 bind directly to AC2 N-terminus. Interestingly, expression of AKAP9 inhibited the activity of AC2 and 3, but not AC1 or 9 32. Disrupting the interaction between AKAP9 and AC enhanced AC activities. However, it is unclear which AC subtype is present in the AKAP9/IKs complex and what role it may play in the regulation of IKs activity.
PDE. PDEs hydrolize cyclic nucleotides in the cells. Some isoforms of PDE specifically degrade cAMP, a key second messenger, thus providing negative controls over the downstream effectors, including PKA 33. More importantly, PDEs create localized cAMP gradients/compartments to fine-tune various cellular functions 34, 35. PDEs have been shown to associate with AKAPs. For example, PDE4D3 was shown to interact with mAKAP 36 and AKAP450 37. Terrnoire et al demonstrated that PDE4D3, but not PDE4D5, is a member of the IKs/AKAP9 complex and interacts with AKAP9 38. Addition of PDE4D3 to the expression system diminished the cAMP-induced IKs channel regulation. These results point to an interesting possibility: if PDE4D3 activity were to be down-regulated in disease, or if a patient were to carry a mutation that renders IKs channel unable to bind PDE4D3, these would create a situation where IKs channel gains in function. Gain in function of IKs channel is known to associate with atrial fibrillation39. This intriguing hypothesis remains to be tested.
In summary, the AKAP9/IKs macromolecular complex integrates various elements in the Gs/cAMP/PKA pathway. AKAP9 is pivotal in creating a compartmentalized environment that ensures IKs channels to function properly. Many questions remain to be answered. For example, it is unclear how AKAP9 coordinates various signaling molecules, which have seemingly opposing functions, to act in concert to control the phosphorylation state of IKs channels. It is of great interest to test or to visualize the changes in the local cAMP gradient as a result of the altered enzyme activities. Also intriguing is the question that how phosphorylation of a single serine residue (S27 in KCNQ1) may cause such a significant change in channel biophysical properties. With newly developed experimental techniques and tools, these questions will be addressed to provide insights into the molecular mechanism of how IKs channels are regulated by the SNS.
Abbreviations footnote
- AKAP
A kinase-anchoring protein
- PKA
protein kinase A
- AC
adenylyl cyclase
- PP1
protein phosphatase 1
- PDE
phosphodiesterase
- IKs
the slow outward potassium current
- SNS
sympathetic nervous system
- LZ
leucine zipper
References
- 1.Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life. 2009;61:394–406. doi: 10.1002/iub.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauban JR, O’Donnell M, Warrier S, et al. AKAP-scaffolding proteins and regulation of cardiac physiology. Physiology (Bethesda) 2009;24:78–87. doi: 10.1152/physiol.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McConnachie G, Langeberg LK, Scott JD. AKAP signaling complexes: getting to the heart of the matter. Trends Mol Med. 2006;12:317–323. doi: 10.1016/j.molmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Dodge-Kafka KL, Langeberg L, Scott JD. Compartmentation of cyclic nucleotide signaling in the heart: the role of A-kinase anchoring proteins. Circ Res. 2006;98:993–1001. doi: 10.1161/01.RES.0000218273.91741.30. [DOI] [PubMed] [Google Scholar]
- 5.Lin JW, Wyszynski M, Madhavan R, et al. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J Neurosci. 1998;18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westphal RS, Tavalin SJ, Lin JW, et al. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt PH, Dransfield DT, Claudio JO, et al. AKAP350, a multiply spliced protein kinase A-anchoring protein associated with centrosomes. J Biol Chem. 1999;274:3055–3066. doi: 10.1074/jbc.274.5.3055. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi M, Shibata H, Shimakawa M, et al. Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the golgi apparatus. J Biol Chem. 1999;274:17267–17274. doi: 10.1074/jbc.274.24.17267. [DOI] [PubMed] [Google Scholar]
- 9.Witczak O, Skalhegg BS, Keryer G, et al. Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450. Embo J. 1999;18:1858–1868. doi: 10.1093/emboj/18.7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barhanin J, Lesage F, Guillemare E, et al. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 11.Sanguinetti MC, Curran ME, Zou A, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Curran ME, Splawski I, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 13.Splawski I, Tristani-Firouzi M, Lehmann MH, et al. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 14.Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242:67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- 15.Marx SO, Kurokawa J, Reiken S, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Marquardt ML, Tester DJ, et al. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007;104:20990–20995. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr DW, Hausken ZE, Fraser ID, et al. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. J Biol Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 18.Feliciello A, Cardone L, Garbi C, et al. Yotiao protein, a ligand for the NMDA receptor, binds and targets cAMP-dependent protein kinase II(1) FEBS Lett. 1999;464:174–178. doi: 10.1016/s0014-5793(99)01585-9. [DOI] [PubMed] [Google Scholar]
- 19.Kinderman FS, Kim C, von Daake S, et al. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol Cell. 2006;24:397–408. doi: 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold MG, Lygren B, Dokurno P, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–395. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Alto NM, Soderling SH, Hoshi N, et al. Bioinformatic design of A-kinase anchoring protein-in silico: a potent and selective peptide antagonist of type II protein kinase A anchoring. Proc Natl Acad Sci U S A. 2003;100:4445–4450. doi: 10.1073/pnas.0330734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hundsrucker C, Rosenthal W, Klussmann E. Peptides for disruption of PKA anchoring. Biochem Soc Trans. 2006;34:472–473. doi: 10.1042/BST0340472. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Kass RS. Dual roles of the A kinase-anchoring protein Yotiao in the modulation of a cardiac potassium channel: a passive adaptor versus an active regulator. Eur J Cell Biol. 2006;85:623–626. doi: 10.1016/j.ejcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Kurokawa J, Kass RS. Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J Biol Chem. 2005;280:31347–31352. doi: 10.1074/jbc.M505191200. [DOI] [PubMed] [Google Scholar]
- 25.Gold MG, Barford D, Komander D. Lining the pockets of kinases and phosphatases. Curr Opin Struct Biol. 2006;16:693–701. doi: 10.1016/j.sbi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Le AV, Tavalin SJ, Dodge-Kafka KL. Identification of AKAP79 as a Protein Phosphatase 1 Catalytic Binding Protein. Biochemistry. 2011;50:5279–5291. doi: 10.1021/bi200089z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redden JM, Dodge-Kafka KL. AKAP Phosphatase Complexes in The Heart. J Cardiovasc Pharmacol. 2011 doi: 10.1097/FJC.0b013e31821e5649. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauman AL, Soughayer J, Nguyen BT, et al. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dessauer CW. Adenylyl cyclase--A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol. 2009;76:935–941. doi: 10.1124/mol.109.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapiloff MS, Piggott LA, Sadana R, et al. An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem. 2009;284:23540–23546. doi: 10.1074/jbc.M109.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efendiev R, Samelson BK, Nguyen BT, et al. AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J Biol Chem. 2010;285:14450–14458. doi: 10.1074/jbc.M110.109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piggott LA, Bauman AL, Scott JD, et al. The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci U S A. 2008;105:13835–13840. doi: 10.1073/pnas.0712100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 34.Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100:950–966. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 35.Rochais F, Abi-Gerges A, Horner K, et al. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res. 2006;98:1081–1088. doi: 10.1161/01.RES.0000218493.09370.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodge KL, Khouangsathiene S, Kapiloff MS, et al. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. Embo J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tasken KA, Collas P, Kemmner WA, et al. Phosphodiesterase 4D and protein kinase a type II constitute a signaling unit in the centrosomal area. J Biol Chem. 2001;276:21999–22002. doi: 10.1074/jbc.C000911200. [DOI] [PubMed] [Google Scholar]
- 38.Terrenoire C, Houslay MD, Baillie GS, et al. The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J Biol Chem. 2009;284:9140–9146. doi: 10.1074/jbc.M805366200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]


