Abstract
Cell migration is a fundamental process in a wide array of biological and pathological responses. It is regulated by complex signal transduction pathways in response to external cues that couple to growth factor and chemokine receptors. In recent years, the target of rapamycin (TOR) kinase, as part of either TOR complex 1 (TORC1) or TOR complex 2 (TORC2), has been shown to be an important signaling component linking external signals to the cytoskeletal machinery in a variety of cell types and organisms. Thus, these complexes have emerged as key regulators of cell migration and chemotaxis.
Introduction
The ability of cells to migrate is a fundamental process in all living organisms. In vertebrates, cell migration is required for a wide array of biological processes that include embryogenesis, angiogenesis, epithelial wound healing, and immune responses. It is also involved in pathological conditions, such as arthritis, vascular disease, and neoplastic invasion (Ridley et al., 2003; Weijer, 2009). Cell migration has been well characterized in Drosophila melanogaster and Caenorhabditis elegans, where it is indispensable for their development (Marston and Goldstein, 2006; Montell, 2006). In addition, in the social amoeba Dictyostelium discoideum, directed cell migration or chemotaxis is essential for cells to aggregate into a mound that will later differentiate into a multicellular organism (Bagorda and Parent, 2008). In order for cells to move, they must first acquire a polarized morphology where F-actin is primarily enriched at the front and myosin II is assembled on the sides and at the back (Bagorda et al., 2006). Then, the polarized cells undergo a highly coordinated cycle of protrusions and retractions that are coupled with traction provided by the formation and release of adhesive contacts with the substrate (Le Clainche and Carlier, 2008). Not surprisingly, to accommodate the wide array of biological processes that depend on cell migration, the protrusion/retraction cycle is specifically regulated in different cell types. For example, slow-moving mesenchymal cells like fibroblasts exhibit strong cell–substrate adhesion forces that develop into mature contacts and give rise to slow migration speeds and almost no cell deformability (Friedl and Wolf, 2010; Parsons et al., 2010). On the other hand, fast-moving amoeboid cells like leukocytes and Dictyostelium discoideum exhibit weak and sparse adhesion to substrates, and, as a result, migrate orders of magnitude faster and show remarkable plasticity (Swaney et al., 2010). Regardless of the mode of migration used, during directed cell migration, cells must be able to determine where and when protrusions, retractions, and adhesions have to occur to migrate to the correct location. This is established by extracellular cues that act through receptor tyrosine kinase (RTK) and G protein–coupled receptor (GPCR) signal transduction pathways, which provide spatio-temporal information to direct the distribution of cytoskeletal elements and establish cell polarity (Citri and Yarden, 2006; Bagorda and Parent, 2008). Although Rho family GTP-binding proteins are important for regulating actin assembly to form protrusions, such as lamellipodia and filopodia, as well as force traction through actomyosin contractility, it is the upstream RTK and GPCR effectors that ultimately regulate the activity of Rho GTP-binding proteins (Jaffe and Hall, 2005; Heasman and Ridley, 2008; Berzat and Hall, 2010). In the past few years, our understanding of the signal transduction pathways that link receptors to Rho GTP-binding proteins has broadened to include products of phosphoinositide 3-kinase (PI3K), phospholipase A2 (PLA2), phospholipase C (PLC), adenylyl cyclase, and guanylyl cyclase (Bagorda and Parent, 2008; Stephens et al., 2008; King and Insall, 2009; Wang, 2009; Swaney et al., 2010). More recently, another highly conserved signaling component, the Ser/Thr protein kinase TOR (target of rapamycin), has also been shown to transduce migration signals to cytoskeletal elements. In this review, we highlight data linking TOR to the regulation of cell migration and chemotaxis.
TORC1 and TORC2: evolutionarily conserved signaling complexes
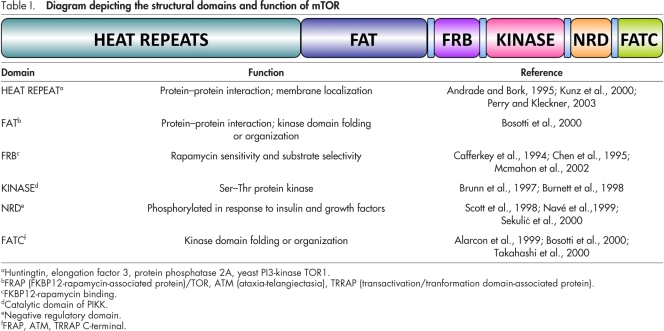
TOR, initially identified in Saccharomyces cerevisiae (Heitman et al., 1991; Cafferkey et al., 1994), is a member of the phosphatidylinositol kinase–related kinase (PIKK) family, which includes ATM (ataxia-telangiectasia mutated), ATR (ATM and Rad3-related), DNA-dependent protein kinase (DNA-PK), and hSMG1 (suppressor with morphological effect on genitalia) (Hoekstra, 1997; Abraham, 2001). These kinases possess Ser/Thr protein kinase activity and do not display lipid kinase activity (Brunn et al., 1997; Burnett et al., 1998). TOR is a large (290 kD) multi-domain protein (Table I) that is structurally and functionally conserved from yeast to mammals. Its name arises from the fact that TOR binds the bacterial macrolide rapamycin when it is complexed with FKBP12—a peptidyl prolyl isomerase (Heitman et al., 1991; Koltin et al., 1991). FKBP12–rapamycin binds to the FKBP12–rapamycin-binding domain of TOR (Table I), which inhibits TOR activity. Single amino acid substitutions in this domain block binding of FKBP12–rapamycin and generate a rapamycin-resistant form of TOR (Heitman et al., 1991; Chen et al., 1995; McMahon et al., 2002).
Table I.
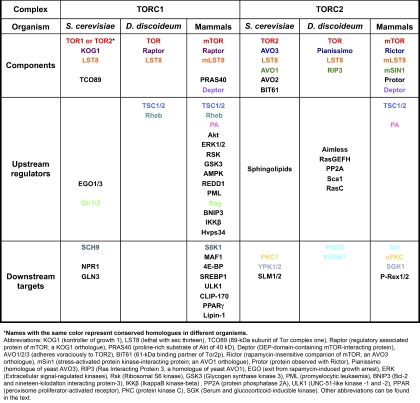
TOR exists in two functionally distinct multiprotein complexes named TOR complex 1 (TORC1) and TORC2. Each complex is highly conserved from yeast to mammals and is composed of specific core components and interactors (see Box 1 and recent reviews on the topic; Jacinto and Lorberg, 2008; Zoncu et al., 2011). The precise role of each component of TORC1 and TORC2 has yet to be fully understood. In mTORC1, LST8 has been proposed to act as a signal receiver (Kim et al., 2003), whereas Raptor functions as a scaffold for recruiting mTORC1 substrates, and PRAS40 and Deptor appear to be negative regulators (Fonseca et al., 2007; Wang et al., 2007; Peterson et al., 2009). In mTORC2, LST8 is required for the full catalytic kinase activity of mTOR and to a lesser extent, for structural stability of the complex (Guertin et al., 2006). Rictor and mSin1 interact with each other and also appear to be important for the structural integrity of mTORC2 (Wullschleger et al., 2005; Jacinto et al., 2006; Yang et al., 2006a). As in mTORC1, Deptor negatively regulates mTORC2 activity (Peterson et al., 2009). The function of Protor, a Rictor-binding component that lacks obvious functional domains, remains to be determined (Pearce et al., 2007; Woo et al., 2007).
Box 1.
Conserved core components and interactors of TORC1 and TORC2 in S. cerevisiae, D. discoideum, and mammals
Signaling upstream of TOR complexes
TORC1 regulates cell growth and metabolism (Laplante and Sabatini, 2009) and the pathways regulating the activity of mTORC1, which mostly focus on the GTPase-activating protein (GAP) tuberin, have been extensively studied. Tuberin is part of the hamartin/tuberin (TSC1/TSC2) complex and acts as a suppressor of mTORC1 activity (Inoki et al., 2002; Huang and Manning, 2008). It inhibits the GTP-binding protein Rheb (Ras homologue enriched in brain), which directly binds to the mTOR kinase domain and activates mTORC1 by an unknown mechanism (Garami et al., 2003; Inoki et al., 2003a; Tee et al., 2003; Bai et al., 2007). The activity of tuberin is regulated by inputs from growth factors, nutrients, and hypoxia, and involves Akt (Inoki et al., 2002), ERK1/2 (Ma et al., 2005), RSK (Roux et al., 2004), GSK3 (Inoki et al., 2006), AMP-dependent kinase, and HIF-1/REDD1 (Inoki et al., 2003b, 2006; Sofer et al., 2005; Box 1).
In contrast to mTORC1, the signaling pathways that lead to mTORC2 activation are not well characterized. The TSC1/TSC2 complex physically associates with mTORC2 and positively regulates its activity independently of the Rheb-GAP activity of TSC1/TSC2 (Huang and Manning, 2008). However, in Drosophila melanogaster, Rheb has been reported to negatively regulate dTORC2 through a dTORC1 and dS6K-dependent negative feedback loop (Yang et al., 2006b). In addition and similarly to mTORC1, phospholipase D and its metabolite phosphatidic acid appear to be critical for the formation of mTORC2 (Toschi et al., 2009). Also, Rac1 was shown to directly interact with mTOR and regulate both mTORC1 and mTORC2 activity (Saci et al., 2011), and PIP3 (phosphatidylinositol 3,4,5-trisphosphate), the product of PI3K, has been shown to directly stimulate mTORC2 kinase activity (Gan et al., 2011). However, reports are few and far between and no clear signaling pathway leading to the activation of mTORC2 has emerged. On the other hand, an extensive body of work in Dictyostelium discoideum has revealed how chemotactic signals mediated through GPCRs specifically regulate TORC2 activity through G proteins and Ras signals, independently of PI3K (Lee et al., 2005; Kamimura et al., 2008; Cai et al., 2010). In this organism, a Ras signaling complex, composed of two Ras GEFs (guanine exchange factor; Aimless and RasGEFH), a protein phosphatase (PP2A), and a scaffold designated Sca1, regulates the activation of RasC, which controls the chemoattractant-induced activation of TORC2 at the leading edge of chemotaxing cells (Kamimura et al., 2008; Charest et al., 2010). In addition, the membrane localization of the Sca1–RasGEF–PP2A complex is regulated through the Akt(PKB)-dependent phosphorylation of Sca1, which provides negative feedback to RasC and TORC2 (Charest et al., 2010). In this system, therefore, a clear path can be traced from a GPCR to the spatio-temporal activation of TORC2, which is important in regulating chemotaxis.
Signaling downstream of TOR complexes
The best-characterized TOR substrates include a subgroup of related AGC (cAMP-dependent, cGMP-dependent, and protein kinase C) family kinases (Jacinto and Lorberg, 2008; Pearce et al., 2010). AGC kinases are activated by phosphorylation of a conserved Ser/Thr residue in their activation loop (also called T-loop), which can occur via autophosphorylation or through other protein kinases such as PDK1 (3-phosphoinositide–dependent protein kinase 1) (Mora et al., 2004). In addition to phosphorylation at the T-loop motif, several AGC kinases are also phosphorylated at Ser or Thr residues within their hydrophobic motif in the C terminus (Jacinto and Lorberg, 2008; Alessi et al., 2009). From yeast to mammals, TOR complexes have been shown to phosphorylate a subset of AGC kinases at a conserved noncatalytic residue within their C-terminal hydrophobic motif, which consists of Phe-X-X-Phe-Ser/Thr-Tyr (Jacinto and Lorberg, 2008; Alessi et al., 2009). mTORC1 phosphorylates the hydrophobic motif of S6K1, whereas mTORC2 phosphorylates the hydrophobic motif of SGK1, Akt, and PKC (Pearson et al., 1995; Sarbassov et al., 2004, 2005; García-Martínez and Alessi, 2008). Similarly, in Dictyostelium discoideum, TORC2 phosphorylates the Akt homologues PKBA and PKBR1 (Kamimura et al., 2008).
A wide array of non-AGC kinases, transcription factors, and other regulators act as effectors of TOR in yeast and mammals (Box 1). Notably, mTORC1 controls the phosphorylation state of 4E-BP1, an important translation initiation machinery component (Haghighat et al., 1995; Beretta et al., 1996; Kim et al., 2002). Phosphorylation of 4E-BP1 induces its dissociation from eIF4E and promotes the initiation of protein translation (Haghighat et al., 1995)—a main effect of mTORC1 activation. In addition, mTORC1 directly phosphorylates and inactivates MAF1 (a key repressor or RNA polymerase II transcription) and contributes to RNA polymerase III–dependent transcription (Kantidakis et al., 2010; Michels et al., 2010; Shor et al., 2010). Interestingly, mTORC1 also regulates microtubule dynamics by physically interacting with CLIP-170, the human homologue of yeast Bik1p, which belongs to a family of conserved microtubule-associated proteins (Choi et al., 2000; Jiang and Yeung, 2006). Phosphorylation of CLIP-170 by mTORC1 positively regulates the association of CLIP-170 with microtubules, which enhances their assembly, elongation, and stability. Finally, a few reports have suggested the existence of cross talk between mTORC1 and mTORC2, as S6K1 has been reported to phosphorylate Rictor and positively regulate mTORC2. Although this phosphorylation event does not affect mTORC2 integrity or in vitro kinase activity, it causes an increase in 14-3-3 binding to Rictor and mTORC2-dependent phosphorylation of Akt on S473 (Dibble et al., 2009; Julien et al., 2010; Treins et al., 2010). As Akt can positively regulate mTORC1 (see previous section), these findings underscore a potential cross talk between mTORC1 and mTORC2.
TORC1 and cell migration
Studies using rapamycin have implicated mTORC1 as a regulator of mammalian cell migration under normal conditions as well as in the context of tumor metastasis (Fig. 1). In aortic smooth muscle cells, rapamycin inhibits fibronectin-induced activation of mTORC1 and S6K1 and markedly diminishes chemotaxis of smooth muscle cells toward fibronectin (Poon et al., 1996; Sakakibara et al., 2005), thereby implicating mTORC1 in matrix protein–induced cell migration. Further, mTORC1 regulates growth factor–induced cell migration, as rapamycin treatment inhibits growth factor–induced cell migration of a wide array of cell lines (Attoub et al., 2000; Berven et al., 2004; Wong et al., 2004; Wan et al., 2005; Liu et al., 2006, 2008, 2010b; Zhou and Wong, 2006). Interestingly, the rapamycin-mediated inhibition of IGF-I–stimulated motility of Rh30 (rhabdomyosarcoma) cells can be prevented by either the expression of a rapamycin-resistant mutant of mTOR (mTORrr), a constitutively active version of S6K1, or the down-regulation of 4E-BP1 (Liu et al., 2006). Therefore, mTORC1 can regulate cell motility via both S6K1 and 4E-BP1 pathways.
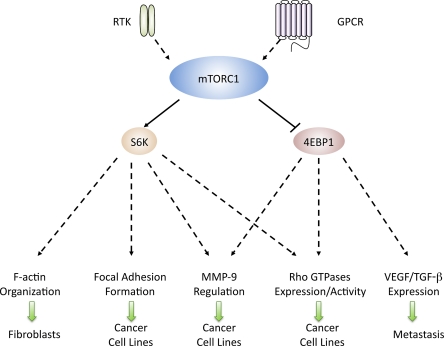
Figure 1.
mTORC1 and cell migration. S6K1 and 4E-BP1 control cell motility by regulating (1) F-actin reorganization, (2) focal adhesion formation, (3) MMP-9 up-regulation (4) Rho expression and activity, and (5) VEGF and TGF-β expression in various cell types.
Although the mechanisms by which mTORC1 regulates cell migration remain to be determined, a few reports provide evidence that S6K1 controls cell migration by regulating: (a) F-actin reorganization, (b) focal adhesion formation, (c) tissue remodeling through the proteolytical digestion of extracellular matrix via up-regulation of MMP-9 (matrix metalloproteinase 9; Vaillant et al., 2003; Khandoga et al., 2006), and (d) Rho expression and activity (Fig. 1). Activated mTOR and S6K1 along with PI3K, Akt1, and PDK1 are enriched in actin arcs, a caveolin-enriched cytoskeletal structure located at the leading edge of migrating Swiss 3T3 cells, and mTOR and p70S6K activation is required for actin arc formation (Berven et al., 2004). On the other hand, in Rh30 cells, down-regulation of Raptor or S6K1 suppresses IGF-I–stimulated tyrosine phosphorylation of FAK and paxillin (Liu et al., 2008). Thus, the kinase activity of S6K1 seems essential for IGF-I–stimulated focal adhesion formation. In SKOV-3 and CaOV-3 ovarian cancer cells, the expression of a constitutively active form of S6K1 induces MMP-9 expression and enhances its activity, which is independent of de novo protein synthesis as it is not affected by cycloheximide treatment (Zhou and Wong, 2006). In contrast, the effects of 4E-BP1 on cell migration appear to be mediated through changes in mRNA translation and protein synthesis. In activated CD4+ T cells, the chemokine CCL5-induced mTOR-dependent phosphorylation of 4E-BP1 ultimately leads to its release from eIF4E. eIF4E associates with the scaffold proteins eIF4G and eIF4A and forms the eIF4F heterotrimeric initiation complex, which initiates mRNA translation and protein synthesis of a wide array of targets, including cyclinD1 and MMP-9 (Murooka et al., 2008). Pretreatment with rapamycin or cycloheximide abolishes CCL5-induced up-regulation of cyclin D1 and MMP-9 while also significantly reducing CCL5-mediated T cell chemotaxis (Murooka et al., 2008). Thus, S6K1 and 4E-BP1 seem to independently regulate the expression and activity of MMP-9 during migration. Finally, recent studies suggest that rapamycin inhibits the expression and activity of the small GTP-binding proteins RhoA, Cdc42, and Rac1 in a panel of tumor cells including Rh30, HeLa (cervical cancer), PC-3 (prostate cancer), Rh1 (Ewing sarcoma), and U373 (glioblastoma) cells (Liu et al., 2010b). Similar effects were observed by the expression of constitutively active 4EBP1-5A or the down-regulation of S6K1. Notably, overexpression of constitutively active RhoA, but not Rac1 and Cdc42, prevented the rapamycin-mediated inhibition of lamellipodia formation and cell migration (Liu et al., 2010b). Thus, in these cell lines, mTORC1-mediated regulation of cell motility depends on RhoA in a 4E-BP1– and S6K1-dependent fashion.
mTORC1 has also been implicated as a regulator of tumor cell metastasis and angiogenesis in a variety of cancers (Guba et al., 2002; Luan et al., 2003; Boffa et al., 2004; Wan et al., 2005; Kobayashi et al., 2007). The effects of rapamycin on metastasis have been linked to changes in VEGF, a key regulator of both metastasis and angiogenesis (Ferrara, 2002; Turner et al., 2003). Rapamycin treatment significantly inhibits the secretion of VEGF both in cultured mouse colon adenocarcinoma cells as well as adenocarcinoma tumor-bearing mice (Guba et al., 2002). In B13LM cells (a lymphatic metastasis-prone pancreatic tumor cell line), dose-dependent reductions of VEGF-A and VEGF-C expression were observed after rapamycin treatment (Kobayashi et al., 2007). Further, in renal and nonsmall lung cancer cells, after treatment with rapamycin, a reduction in both VEGF-A and TGF-β expression were observed at both the mRNA and protein levels (Luan et al., 2003; Boffa et al., 2004). Moreover, treatment of tumor-bearing mice with rapamycin gives rise to a significant reduction in the circulating levels of TGF-β (Boffa et al., 2004). These mechanistic studies suggest that mTORC1-regulated tumor metastasis and angiogenesis are associated with, at least in part, the production of VEGF and TGF-β.
TORC2 and cell migration
TORC2 regulates cytoskeleton organization.
Accumulating evidence indicates that TORC2 is a key regulator of the actin cytoskeleton (Fig. 2). In Saccharomyces cerevisiae, TORC2 is required for the cell cycle–dependent polarization of the actin cytoskeleton (Schmidt et al., 1996). In Dictyostelium discoideum, knockout of individual TORC2 components leads to the loss of cell polarity and the random extension of pseudopods from multiple points of the cells (Chen et al., 1997; Lee et al., 1999, 2005). In human neutrophils, inhibition of mTORC2 function by Rictor knockdown also leads to cell polarity defects and uniform cortical F-actin accumulation (Liu et al., 2010a). Although no obvious alterations in the actin cytoskeleton is observed in embryonic fibroblasts derived from Rictor knockout mice (Guertin et al., 2006; Shiota et al., 2006), siRNA-mediated knockdown of mTOR, Rictor, or mLST8 prevents actin polymerization and cell spreading in NIH3T3 fibroblasts (Jacinto et al., 2004). In contrast, in HeLa cells, lentivirus shRNA-mediated Rictor and mTOR knockdown leads to increased stress fiber and cytoplasmic paxillin patch formation (Sarbassov et al., 2004). Together, these studies highlight the possibility that mTORC2 may have distinct effects on the actin cytoskeleton in different cell types.
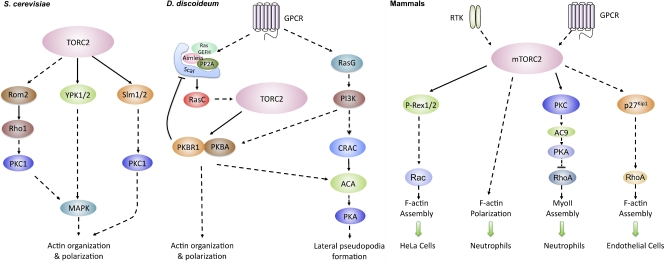
Figure 2.
TORC2 regulates cytoskeleton organization and cell migration in yeast, amoebae, and mammalian cells. (A) In Saccharomyces cerevisiae, TOR2 regulates actin organization and polarization through PKC1 and MAPK pathways. (B) In Dictyostelium discoideum, GPCRs specifically regulate TORC2 through a Ras signaling complex, which controls actin assembly and polarization as well as the synthesis of cAMP and the activation of PKA. (C) In mammalian cells, mTORC2 also regulates Rac and PKC and plays a key role in neutrophil chemotaxis by independently regulating F-actin polarization and myosin II phosphorylation. In endothelial cells, mTORC2 decreases p27Kip1 levels, which results in high RhoA activity and increased chemotaxis.
The molecular mechanism by which TORC2 mediates actin reorganization has been extensively studied in Saccharomyces cerevisiae where the protein kinases PKC1, YPK2 (yeast protein kinase 2), and SLM (synthetic lethal with Mss4) are involved (Fig. 2). In these cells, TOR2 activates the GTP-binding protein Rho1 through the GTP exchange factor Rom2, which in turn triggers the activation of PKC1. Active PKC1 controls the polarization of the actin cytoskeleton via the MAP kinase cascade (Kamada et al., 1995, 1996; Schmidt et al., 1997; Loewith et al., 2002), and up-regulation of Rho1, PKC1, or PKC1-controlled MAP kinase suppresses the actin defect of tor2 mutant (Helliwell et al., 1998). YPK2 is an AGC kinase that is directly activated by TORC2 via phosphorylation on Ser641 and Thr659 (Kamada et al., 2005). YPK2 activity is greatly reduced in tor2 mutants and overexpression of a constitutively active mutant of YPK2 restores MAP kinase activation and suppresses the actin cytoskeleton organization defects of tor2 mutants (Kamada et al., 2005). SLM1 and SLM2, homologous pleckstrin homology (PH) domain–containing proteins that bind to phosphatidylinositol-4,5-bisphosphate (PIP2), physically interact with AVO2 and BIT61 and mediate TORC2 signaling to the actin cytoskeleton (Fadri et al., 2005). Overexpression of PKC1, but not activated forms of the MAP kinase components can restore proper actin filament assembly and actin polarization in slm1/slm2-null cells (Fadri et al., 2005). Thus, SLM signaling likely involves a MAP kinase–independent PKC1 signaling branch or may act in a pathway that has an overlapping function with PKC1.
Consistent with studies from yeast, mTORC2 also regulates the activation of PKC-α and Rac (Fig. 2). In HeLa cells, PKC-α activity is reduced in Rictor and mTOR knockdown cells and the morphology of the actin cytoskeleton in PKC-α knockdown cells is similar to that of Rictor knockdown cells (Sarbassov et al., 2004). In NIH3T3 cells, knockdown of mTOR, LST8, or Rictor results in a 20–30% decrease Rac1 activity. In addition, the expression of an active form of Rac1 or RhoA restores the formation of membrane ruffles, lamellipodia, and stress fibers in mTOR, mLST8, or Rictor knockdown cells (Jacinto et al., 2004). The mechanism by which this occurs could involve P-Rex1 and P-Rex2 (PIP3-dependent Rac exchange factor), Rac GEFs linking GPCRs, Gβγ, and PI3K signaling to Rac activation. In HeLa cells, exogenously expressed P-Rex1 and P-Rex2 interact with mTOR through their tandem DEP (disheveled, EGL-10, and pleckstrin) domain (Hernández-Negrete et al., 2007). Moreover, P-Rex1 appears to link mTOR signaling to Rac activation, as cells expressing dominant-negative constructs or shRNA-mediated knockdown of P-Rex1 specifically decrease mTORC2-dependent leucine-induced activation of Rac (Hernández-Negrete et al., 2007). As the yeast Rom2 also harbors a DEP domain, P-Rex may represent the mammalian orthologue of Rom2.
TORC2 regulates cell migration.
The ability of mTORC2 to regulate actin networks suggests that it may be involved in regulating cell migration. Although several studies on glioblastoma cells lines, Rh30, HeLa, and endothelial cells have implicated mTORC2 as a positive regulator of cell motility (Liu et al., 2006; Hernández-Negrete et al., 2007; Masri et al., 2007; Dada et al., 2008), in-depth mechanistic insight has come from studies in Dictyostelium discoideum (Fig. 2). In this system, the binding of the chemoattractant cAMP to specific GPCRs leads to the activation of signal transduction pathways that regulate gene expression, the production and degradation of cAMP, and chemotaxis (Bagorda et al., 2006). Null mutations of TORC2 components give rise to cells with severe cell polarity defects, reduced chemotactic speeds and directionality, and the inability to activate adenylyl cyclase (Chen et al., 1997; Lee et al., 1999, 2005). Through a Ras signaling complex that activates RasC, chemoattractant addition stimulates TORC2 specifically at the leading edge of chemotaxing cells (Cai et al., 2010; Charest et al., 2010). TORC2 phosphorylates PKBA as well as PKBR1. Unlike PKBA, which harbors a PH domain, PKBR1 lacks a PH domain and is constitutively anchored to the plasma membrane (independently of PI3K activity) via a Myr site at its N terminus (Meili et al., 2000; Kamimura et al., 2008). Interestingly, PKBR1 appears to be the major effector of TORC2 during chemotaxis toward cAMP, as cells lacking PKBA show mild chemotaxis defects and retain a normal phosphorylation pattern of PKB substrates in aggregating cells. On the other hand, cells lacking PKBR1, or components of the Ras signaling complex, have a similar phenotype as TORC2 mutants, exhibiting both chemotaxis and adenylyl cyclase activity defects during aggregation (Kamimura et al., 2008; Cai et al., 2010). Once activated, PKBR1 phosphorylates several substrates, including Sca1, Talin, two Ras GEFs, and a Rho GAP (Kamimura et al., 2008; Charest et al., 2010). Although P-Sca1 has been shown to negatively regulate RasC activity, the precise role of the phosphorylation of other PKBR1 substrates during chemotaxis remains to be determined. Together, these studies establish a pathway arising from GPCRs, through G proteins and a Ras signaling complex, which activates TORC2 to regulate cell polarity, actin assembly, and adenylyl cyclase activity.
mTORC2 also plays a key role during neutrophil chemotaxis by independently regulating F-actin polarization and myosin II (MyoII) phosphorylation. Rictor knockdown or prolonged rapamycin treatment strongly inhibits neutrophil polarity and chemotaxis to the GPCR ligands fMLP (N-formyl-methionine-leucine-phenylalanine) and LTB4 (leukotriene B4), as well as cAMP production (Liu et al., 2010a). However, in contrast to Dictyostelium discoideum, the effects of mTORC2 are not mediated through Akt (PKB). Instead, and in accordance with findings in yeast and mammalian systems, it appears that PKC is mediating part of mTORC2’s effects by regulating adenylyl cyclase activity and cAMP production. Cyclic AMP then regulates MyoII assembly through a RhoA/ROCK-dependent pathway. Interestingly, although mTORC1 is required for GM-CSF (granulocyte macrophage colony-stimulating factor)–induced neutrophil migration (Gomez-Cambronero, 2003; Liu et al., 2010a), it is dispensable for fMLP- and LTB4-mediated chemotaxis (Liu et al., 2010a). Thus, mTORC2 appears to specifically regulate neutrophil chemotaxis toward GPCR ligands. Similarly to neutrophils, in mouse bone marrow–derived mast cells, the GPCR-mediated chemotaxis via prostaglandin E2 (PGE2) receptors is specifically dependent on mTORC2 (Kuehn et al., 2011). Rictor-targeted shRNA results in a significant attenuation in PGE2-mediated chemotaxis, yet the selective inhibition of mTORC1 by rapamycin treatment or by Raptor knockdown fails to decrease PGE2-mediated chemotaxis in these cells.
Rictor levels are elevated in a wide array of glioma cell lines and primary glioma tumor cells (Masri et al., 2007), as well as in invasive breast ductal carcinomas (Zhang et al., 2010). Furthermore, overexpression or knockdown of Rictor in glioma cell lines results in either increased or decreased cell migration, (Masri et al., 2007), although a separate group reported that mTORC2 negatively regulates invasion in two glioma cell lines (Das et al., 2011). Intriguingly, Rictor could mediate its effects on cell migration in an mTORC2-independent fashion. Indeed, Zhang et al. (2010) present evidence suggesting that the inhibition of migration observed in MDA-MB-231 breast cancer cells with reduced Rictor levels is mediated independently of mTORC2 through a direct interaction with PKC-ζ. They show that mSin1 knockdown does not alter chemotaxis of MDA-MB-231 cells, nor does it affect Rictor–PKC-ζ interaction (Zhang et al., 2010). In this context, Rictor can form a complex with the integrin-linked kinase and regulate Akt phosphorylation in an mTORC2-independent fashion (McDonald et al., 2008). It will be interesting to see if these mTORC2-independent effects of Rictor impact mTORC2 or even mTORC1 signals.
Although rapamycin FKBP12 cannot bind to mTORC2, prolonged rapamycin treatment inhibits mTORC2 function by sequestering mTOR and interfering with mTORC2 assembly in some cell lines (Sarbassov et al., 2006). Surprisingly, prolonged but not short-term rapamycin treatment inhibits endothelial cell (Sun et al., 2001) as well as mesangial cell migration (Daniel et al., 2004) through the cyclin-dependent kinase inhibitor (p27Kip1). A recent study revealed that siRNA knockdown of Rictor increases p27Kip1 levels, resulting in the inhibition of RhoA activity and inhibition of VEGF-mediated endothelial chemotaxis (Moss et al., 2010). These findings provide novel mechanistic insight into the role of cyclin-dependent kinase proteins outside the nucleus (Denicourt and Dowdy, 2004).
Perspectives
Although it is becoming clear that both TOR complex 1 and 2 regulate cytoskeletal networks and cell migration, we are far from understanding the mechanism by which this takes place. This is primarily due to the complexity of the TOR signaling cascades and their impact on a wide array of effectors, as well as to the distinct migratory behaviors exhibited by different cells and the chemotactic input (i.e., RTK vs. GPCR). In this context, it will be very interesting to see if the TORC2-mediated effects on chemotaxis of amoeboid-like cells extend to other cell types with distinct cellular architecture and motility behaviors. Indeed, in contrast to mesenchymal cells such as fibroblasts, where microtubules primarily extend to the front (Kupfer et al., 1982; Gundersen and Bulinski, 1988), amoeboid-like cells such Dictyostelium discoideum, neutrophils, and migrating lymphocytes have an extensive microtubule network at their back and the microtubule organizing center is positioned behind the nucleus (Ratner et al., 1997; Eddy et al., 2002; Kriebel et al., 2008). This distinct cellular architecture underscores the dramatically different mechanisms regulating the motility machinery in these two cell types. Indeed, in contrast to neutrophils where RhoA activity is restricted to the back (Wong et al., 2007), high RhoA activity has been reported at the leading edge of HeLa and MDCK cells (Kurokawa and Matsuda, 2005) and Rho signaling has been shown to stabilize microtubules at the leading edge of fibroblast cells (Palazzo et al., 2004). It is therefore foreseeable that distinct TOR signals mediate distinct effects on cell types with very different cytoskeletal organizations and motility machineries. This could have interesting consequences during metastasis, where dynamic epithelial-to-mesenchymal as well as mesenchymal-to-amoeboid transitions dramatically alter the motility behavior of cells (Friedl and Wolf, 2010). Importantly, as it has been observed with the PI3K pathway (Ferguson et al., 2007), the effects of TOR signaling may also be dependent on the microenvironment, which adds another layer of complexity to the picture. In any case, to move the field forward, it is important to use approaches that precisely target TORC1 or TORC2. Although rapamycin certainly represents a powerful tool in the TOR field, in an increasing number of cells, long-term treatment with rapamycin also inhibits TORC2 (Sarbassov et al., 2006; Liu et al., 2010a; Moss et al., 2010). We have yet to fully appreciate the extent by which TOR complexes regulate a wide array of processes, including cell migration, and the future will undoubtedly continue to bring unexpected insight.
Acknowledgments
We thank Drs. Philippe Afonzo and Michael Weiger for their critical input.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Abbreviations used in this paper:
- GPCR
- G protein–coupled receptor
- MMP
- matrix metalloproteinase
- PH
- pleckstrin homology
- PI3K
- phosphoinositide 3-kinase
- TOR
- target of rapamycin
- TORC
- TOR complex
References
- Abraham R.T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177–2196 10.1101/gad.914401 [DOI] [PubMed] [Google Scholar]
- Alarcon C.M., Heitman J., Cardenas M.E. 1999. Protein kinase activity and identification of a toxic effector domain of the target of rapamycin TOR proteins in yeast. Mol. Biol. Cell. 10:2531–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi D.R., Pearce L.R., García-Martínez J.M. 2009. New insights into mTOR signaling: mTORC2 and beyond. Sci. Signal. 2:pe27 10.1126/scisignal.267pe27 [DOI] [PubMed] [Google Scholar]
- Andrade M.A., Bork P. 1995. HEAT repeats in the Huntington’s disease protein. Nat. Genet. 11:115–116 10.1038/ng1095-115 [DOI] [PubMed] [Google Scholar]
- Attoub S., Noe V., Pirola L., Bruyneel E., Chastre E., Mareel M., Wymann M.P., Gespach C. 2000. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J. 14:2329–2338 10.1096/fj.00-0162 [DOI] [PubMed] [Google Scholar]
- Bagorda A., Parent C.A. 2008. Eukaryotic chemotaxis at a glance. J. Cell Sci. 121:2621–2624 10.1242/jcs.018077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagorda A., Mihaylov V.A., Parent C.A. 2006. Chemotaxis: moving forward and holding on to the past. Thromb. Haemost. 95:12–21 [PubMed] [Google Scholar]
- Bai X., Ma D., Liu A., Shen X., Wang Q.J., Liu Y., Jiang Y. 2007. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 318:977–980 10.1126/science.1147379 [DOI] [PubMed] [Google Scholar]
- Beretta L., Gingras A.C., Svitkin Y.V., Hall M.N., Sonenberg N. 1996. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 15:658–664 [PMC free article] [PubMed] [Google Scholar]
- Berven L.A., Willard F.S., Crouch M.F. 2004. Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration. Exp. Cell Res. 296:183–195 10.1016/j.yexcr.2003.12.032 [DOI] [PubMed] [Google Scholar]
- Berzat A., Hall A. 2010. Cellular responses to extracellular guidance cues. EMBO J. 29:2734–2745 10.1038/emboj.2010.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffa D.J., Luan F., Thomas D., Yang H., Sharma V.K., Lagman M., Suthanthiran M. 2004. Rapamycin inhibits the growth and metastatic progression of non-small cell lung cancer. Clin. Cancer Res. 10:293–300 10.1158/1078-0432.CCR-0629-3 [DOI] [PubMed] [Google Scholar]
- Bosotti R., Isacchi A., Sonnhammer E.L. 2000. FAT: a novel domain in PIK-related kinases. Trends Biochem. Sci. 25:225–227 10.1016/S0968-0004(00)01563-2 [DOI] [PubMed] [Google Scholar]
- Brunn G.J., Hudson C.C., Sekulić A., Williams J.M., Hosoi H., Houghton P.J., Lawrence J.C., Jr, Abraham R.T. 1997. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 277:99–101 10.1126/science.277.5322.99 [DOI] [PubMed] [Google Scholar]
- Burnett P.E., Barrow R.K., Cohen N.A., Snyder S.H., Sabatini D.M. 1998. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. USA. 95:1432–1437 10.1073/pnas.95.4.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferkey R., McLaughlin M.M., Young P.R., Johnson R.K., Livi G.P. 1994. Yeast TOR (DRR) proteins: amino-acid sequence alignment and identification of structural motifs. Gene. 141:133–136 10.1016/0378-1119(94)90141-4 [DOI] [PubMed] [Google Scholar]
- Cai H., Das S., Kamimura Y., Long Y., Parent C.A., Devreotes P.N. 2010. Ras-mediated activation of the TORC2-PKB pathway is critical for chemotaxis. J. Cell Biol. 190:233–245 10.1083/jcb.201001129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest P.G., Shen Z., Lakoduk A., Sasaki A.T., Briggs S.P., Firtel R.A. 2010. A Ras signaling complex controls the RasC-TORC2 pathway and directed cell migration. Dev. Cell. 18:737–749 10.1016/j.devcel.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zheng X.F., Brown E.J., Schreiber S.L. 1995. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc. Natl. Acad. Sci. USA. 92:4947–4951 10.1073/pnas.92.11.4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.Y., Long Y., Devreotes P.N. 1997. A novel cytosolic regulator, Pianissimo, is required for chemoattractant receptor and G protein-mediated activation of the 12 transmembrane domain adenylyl cyclase in Dictyostelium. Genes Dev. 11:3218–3231 10.1101/gad.11.23.3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H., Adames N.R., Chan T.F., Zeng C., Cooper J.A., Zheng X.F. 2000. TOR signaling regulates microtubule structure and function. Curr. Biol. 10:861–864 10.1016/S0960-9822(00)00599-6 [DOI] [PubMed] [Google Scholar]
- Citri A., Yarden Y. 2006. EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 7:505–516 10.1038/nrm1962 [DOI] [PubMed] [Google Scholar]
- Dada S., Demartines N., Dormond O. 2008. mTORC2 regulates PGE2-mediated endothelial cell survival and migration. Biochem. Biophys. Res. Commun. 372:875–879 10.1016/j.bbrc.2008.05.154 [DOI] [PubMed] [Google Scholar]
- Daniel C., Pippin J., Shankland S.J., Hugo C. 2004. The rapamycin derivative RAD inhibits mesangial cell migration through the CDK-inhibitor p27KIP1. Lab. Invest. 84:588–596 10.1038/labinvest.3700078 [DOI] [PubMed] [Google Scholar]
- Das G., Shiras A., Shanmuganandam K., Shastry P. 2011. Rictor regulates MMP-9 activity and invasion through Raf-1-MEK-ERK signaling pathway in glioma cells. Mol. Carcinog. 50:412–423 10.1002/mc.20723 [DOI] [PubMed] [Google Scholar]
- Denicourt C., Dowdy S.F. 2004. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 18:851–855 10.1101/gad.1205304 [DOI] [PubMed] [Google Scholar]
- Dibble C.C., Asara J.M., Manning B.D. 2009. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol. Cell. Biol. 29:5657–5670 10.1128/MCB.00735-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy R.J., Pierini L.M., Maxfield F.R. 2002. Microtubule asymmetry during neutrophil polarization and migration. Mol. Biol. Cell. 13:4470–4483 10.1091/mbc.E02-04-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadri M., Daquinag A., Wang S., Xue T., Kunz J. 2005. The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2. Mol. Biol. Cell. 16:1883–1900 10.1091/mbc.E04-07-0564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson G.J., Milne L., Kulkarni S., Sasaki T., Walker S., Andrews S., Crabbe T., Finan P., Jones G., Jackson S., et al. 2007. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat. Cell Biol. 9:86–91 10.1038/ncb1517 [DOI] [PubMed] [Google Scholar]
- Ferrara N. 2002. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer. 2:795–803 10.1038/nrc909 [DOI] [PubMed] [Google Scholar]
- Fonseca B.D., Smith E.M., Lee V.H., MacKintosh C., Proud C.G. 2007. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J. Biol. Chem. 282:24514–24524 10.1074/jbc.M704406200 [DOI] [PubMed] [Google Scholar]
- Friedl P., Wolf K. 2010. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 188:11–19 10.1083/jcb.200909003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X., Wang J., Su B., Wu D. 2011. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 286:10998–11002 10.1074/jbc.M110.195016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garami A., Zwartkruis F.J., Nobukuni T., Joaquin M., Roccio M., Stocker H., Kozma S.C., Hafen E., Bos J.L., Thomas G. 2003. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell. 11:1457–1466 10.1016/S1097-2765(03)00220-X [DOI] [PubMed] [Google Scholar]
- García-Martínez J.M., Alessi D.R. 2008. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 416:375–385 10.1042/BJ20081668 [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J. 2003. Rapamycin inhibits GM-CSF-induced neutrophil migration. FEBS Lett. 550:94–100 10.1016/S0014-5793(03)00828-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guba M., von Breitenbuch P., Steinbauer M., Koehl G., Flegel S., Hornung M., Bruns C.J., Zuelke C., Farkas S., Anthuber M., et al. 2002. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat. Med. 8:128–135 10.1038/nm0202-128 [DOI] [PubMed] [Google Scholar]
- Guertin D.A., Stevens D.M., Thoreen C.C., Burds A.A., Kalaany N.Y., Moffat J., Brown M., Fitzgerald K.J., Sabatini D.M. 2006. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell. 11:859–871 10.1016/j.devcel.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Gundersen G.G., Bulinski J.C. 1988. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc. Natl. Acad. Sci. USA. 85:5946–5950 10.1073/pnas.85.16.5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighat A., Mader S., Pause A., Sonenberg N. 1995. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 14:5701–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman S.J., Ridley A.J. 2008. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9:690–701 10.1038/nrm2476 [DOI] [PubMed] [Google Scholar]
- Heitman J., Movva N.R., Hall M.N. 1991. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 253:905–909 10.1126/science.1715094 [DOI] [PubMed] [Google Scholar]
- Helliwell S.B., Schmidt A., Ohya Y., Hall M.N. 1998. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol. 8:1211–1214 10.1016/S0960-9822(07)00511-8 [DOI] [PubMed] [Google Scholar]
- Hernández-Negrete I., Carretero-Ortega J., Rosenfeldt H., Hernández-García R., Calderón-Salinas J.V., Reyes-Cruz G., Gutkind J.S., Vázquez-Prado J. 2007. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J. Biol. Chem. 282:23708–23715 10.1074/jbc.M703771200 [DOI] [PubMed] [Google Scholar]
- Hoekstra M.F. 1997. Responses to DNA damage and regulation of cell cycle checkpoints by the ATM protein kinase family. Curr. Opin. Genet. Dev. 7:170–175 10.1016/S0959-437X(97)80125-6 [DOI] [PubMed] [Google Scholar]
- Huang J., Manning B.D. 2008. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem. J. 412:179–190 10.1042/BJ20080281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Li Y., Zhu T., Wu J., Guan K.L. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648–657 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- Inoki K., Li Y., Xu T., Guan K.L. 2003a. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17:1829–1834 10.1101/gad.1110003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Zhu T., Guan K.L. 2003b. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 115:577–590 10.1016/S0092-8674(03)00929-2 [DOI] [PubMed] [Google Scholar]
- Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., et al. 2006. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 126:955–968 10.1016/j.cell.2006.06.055 [DOI] [PubMed] [Google Scholar]
- Jacinto E., Lorberg A. 2008. TOR regulation of AGC kinases in yeast and mammals. Biochem. J. 410:19–37 10.1042/BJ20071518 [DOI] [PubMed] [Google Scholar]
- Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M.A., Hall A., Hall M.N. 2004. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6:1122–1128 10.1038/ncb1183 [DOI] [PubMed] [Google Scholar]
- Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S.Y., Huang Q., Qin J., Su B. 2006. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 127:125–137 10.1016/j.cell.2006.08.033 [DOI] [PubMed] [Google Scholar]
- Jaffe A.B., Hall A. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247–269 10.1146/annurev.cellbio.21.020604.150721 [DOI] [PubMed] [Google Scholar]
- Jiang X., Yeung R.S. 2006. Regulation of microtubule-dependent protein transport by the TSC2/mammalian target of rapamycin pathway. Cancer Res. 66:5258–5269 10.1158/0008-5472.CAN-05-4510 [DOI] [PubMed] [Google Scholar]
- Julien L.A., Carriere A., Moreau J., Roux P.P. 2010. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell. Biol. 30:908–921 10.1128/MCB.00601-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Jung U.S., Piotrowski J., Levin D.E. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559–1571 10.1101/gad.9.13.1559 [DOI] [PubMed] [Google Scholar]
- Kamada Y., Qadota H., Python C.P., Anraku Y., Ohya Y., Levin D.E. 1996. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271:9193–9196 10.1074/jbc.271.16.9193 [DOI] [PubMed] [Google Scholar]
- Kamada Y., Fujioka Y., Suzuki N.N., Inagaki F., Wullschleger S., Loewith R., Hall M.N., Ohsumi Y. 2005. Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol. Cell. Biol. 25:7239–7248 10.1128/MCB.25.16.7239-7248.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura Y., Xiong Y., Iglesias P.A., Hoeller O., Bolourani P., Devreotes P.N. 2008. PIP3-independent activation of TorC2 and PKB at the cell’s leading edge mediates chemotaxis. Curr. Biol. 18:1034–1043 10.1016/j.cub.2008.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantidakis T., Ramsbottom B.A., Birch J.L., Dowding S.N., White R.J. 2010. mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc. Natl. Acad. Sci. USA. 107:11823–11828 10.1073/pnas.1005188107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandoga A., Kessler J.S., Hanschen M., Khandoga A.G., Burggraf D., Reichel C., Hamann G.F., Enders G., Krombach F. 2006. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J. Leukoc. Biol. 79:1295–1305 10.1189/jlb.0805468 [DOI] [PubMed] [Google Scholar]
- Kim D.H., Sarbassov D.D., Ali S.M., King J.E., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 110:163–175 10.1016/S0092-8674(02)00808-5 [DOI] [PubMed] [Google Scholar]
- Kim D.H., Sarbassov D.D., Ali S.M., Latek R.R., Guntur K.V., Erdjument-Bromage H., Tempst P., Sabatini D.M. 2003. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell. 11:895–904 10.1016/S1097-2765(03)00114-X [DOI] [PubMed] [Google Scholar]
- King J.S., Insall R.H. 2009. Chemotaxis: finding the way forward with Dictyostelium. Trends Cell Biol. 19:523–530 10.1016/j.tcb.2009.07.004 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Kishimoto T., Kamata S., Otsuka M., Miyazaki M., Ishikura H. 2007. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci. 98:726–733 10.1111/j.1349-7006.2007.00439.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltin Y., Faucette L., Bergsma D.J., Levy M.A., Cafferkey R., Koser P.L., Johnson R.K., Livi G.P. 1991. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol. Cell. Biol. 11:1718–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel P.W., Barr V.A., Rericha E.C., Zhang G., Parent C.A. 2008. Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J. Cell Biol. 183:949–961 10.1083/jcb.200808105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn H.S., Jung M.Y., Beaven M.A., Metcalfe D.D., Gilfillan A.M. 2011. Prostaglandin E2 activates and utilizes mTORC2 as a central signaling locus for the regulation of mast cell chemotaxis and mediator release. J. Biol. Chem. 286:391–402 10.1074/jbc.M110.164772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J., Schneider U., Howald I., Schmidt A., Hall M.N. 2000. HEAT repeats mediate plasma membrane localization of Tor2p in yeast. J. Biol. Chem. 275:37011–37020 10.1074/jbc.M007296200 [DOI] [PubMed] [Google Scholar]
- Kupfer A., Louvard D., Singer S.J. 1982. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc. Natl. Acad. Sci. USA. 79:2603–2607 10.1073/pnas.79.8.2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K., Matsuda M. 2005. Localized RhoA activation as a requirement for the induction of membrane ruffling. Mol. Biol. Cell. 16:4294–4303 10.1091/mbc.E04-12-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Sabatini D.M. 2009. mTOR signaling at a glance. J. Cell Sci. 122:3589–3594 10.1242/jcs.051011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clainche C., Carlier M.F. 2008. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 88:489–513 10.1152/physrev.00021.2007 [DOI] [PubMed] [Google Scholar]
- Lee S., Parent C.A., Insall R., Firtel R.A. 1999. A novel Ras-interacting protein required for chemotaxis and cyclic adenosine monophosphate signal relay in Dictyostelium. Mol. Biol. Cell. 10:2829–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Comer F.I., Sasaki A., McLeod I.X., Duong Y., Okumura K., Yates J.R., III, Parent C.A., Firtel R.A. 2005. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol. Biol. Cell. 16:4572–4583 10.1091/mbc.E05-04-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li F., Cardelli J.A., Martin K.A., Blenis J., Huang S. 2006. Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene. 25:7029–7040 10.1038/sj.onc.1209691 [DOI] [PubMed] [Google Scholar]
- Liu L., Chen L., Chung J., Huang S. 2008. Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene. 27:4998–5010 10.1038/onc.2008.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Das S., Losert W., Parent C.A. 2010a. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev. Cell. 19:845–857 10.1016/j.devcel.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Luo Y., Chen L., Shen T., Xu B., Chen W., Zhou H., Han X., Huang S. 2010b. Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J. Biol. Chem. 285:38362–38373 10.1074/jbc.M110.141168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J.L., Bonenfant D., Oppliger W., Jenoe P., Hall M.N. 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell. 10:457–468 10.1016/S1097-2765(02)00636-6 [DOI] [PubMed] [Google Scholar]
- Luan F.L., Ding R., Sharma V.K., Chon W.J., Lagman M., Suthanthiran M. 2003. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 63:917–926 10.1046/j.1523-1755.2003.00805.x [DOI] [PubMed] [Google Scholar]
- Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P.P. 2005. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 121:179–193 10.1016/j.cell.2005.02.031 [DOI] [PubMed] [Google Scholar]
- Marston D.J., Goldstein B. 2006. Actin-based forces driving embryonic morphogenesis in Caenorhabditis elegans. Curr. Opin. Genet. Dev. 16:392–398 10.1016/j.gde.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Masri J., Bernath A., Martin J., Jo O.D., Vartanian R., Funk A., Gera J. 2007. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Res. 67:11712–11720 10.1158/0008-5472.CAN-07-2223 [DOI] [PubMed] [Google Scholar]
- McDonald P.C., Oloumi A., Mills J., Dobreva I., Maidan M., Gray V., Wederell E.D., Bally M.B., Foster L.J., Dedhar S. 2008. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 68:1618–1624 10.1158/0008-5472.CAN-07-5869 [DOI] [PubMed] [Google Scholar]
- McMahon L.P., Choi K.M., Lin T.A., Abraham R.T., Lawrence J.C., Jr 2002. The rapamycin-binding domain governs substrate selectivity by the mammalian target of rapamycin. Mol. Cell. Biol. 22:7428–7438 10.1128/MCB.22.21.7428-7438.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meili R., Ellsworth C., Firtel R.A. 2000. A novel Akt/PKB-related kinase is essential for morphogenesis in Dictyostelium. Curr. Biol. 10:708–717 10.1016/S0960-9822(00)00536-4 [DOI] [PubMed] [Google Scholar]
- Michels A.A., Robitaille A.M., Buczynski-Ruchonnet D., Hodroj W., Reina J.H., Hall M.N., Hernandez N. 2010. mTORC1 directly phosphorylates and regulates human MAF1. Mol. Cell. Biol. 30:3749–3757 10.1128/MCB.00319-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell D.J. 2006. The social lives of migrating cells in Drosophila. Curr. Opin. Genet. Dev. 16:374–383 10.1016/j.gde.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Mora A., Komander D., van Aalten D.M., Alessi D.R. 2004. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 15:161–170 10.1016/j.semcdb.2003.12.022 [DOI] [PubMed] [Google Scholar]
- Moss S.C., Lightell D.J., Jr, Marx S.O., Marks A.R., Woods T.C. 2010. Rapamycin regulates endothelial cell migration through regulation of the cyclin-dependent kinase inhibitor p27Kip1. J. Biol. Chem. 285:11991–11997 10.1074/jbc.M109.066621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka T.T., Rahbar R., Platanias L.C., Fish E.N. 2008. CCL5-mediated T-cell chemotaxis involves the initiation of mRNA translation through mTOR/4E-BP1. Blood. 111:4892–4901 10.1182/blood-2007-11-125039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navé B.T., Ouwens M., Withers D.J., Alessi D.R., Shepherd P.R. 1999. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 344:427–431 10.1042/0264-6021:3440427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo A.F., Eng C.H., Schlaepfer D.D., Marcantonio E.E., Gundersen G.G. 2004. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 303:836–839 10.1126/science.1091325 [DOI] [PubMed] [Google Scholar]
- Parsons J.T., Horwitz A.R., Schwartz M.A. 2010. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11:633–643 10.1038/nrm2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L.R., Huang X., Boudeau J., Pawłowski R., Wullschleger S., Deak M., Ibrahim A.F., Gourlay R., Magnuson M.A., Alessi D.R. 2007. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 405:513–522 10.1042/BJ20070540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L.R., Komander D., Alessi D.R. 2010. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11:9–22 10.1038/nrm2822 [DOI] [PubMed] [Google Scholar]
- Pearson R.B., Dennis P.B., Han J.W., Williamson N.A., Kozma S.C., Wettenhall R.E., Thomas G. 1995. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 14:5279–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J., Kleckner N. 2003. The ATRs, ATMs, and TORs are giant HEAT repeat proteins. Cell. 112:151–155 10.1016/S0092-8674(03)00033-3 [DOI] [PubMed] [Google Scholar]
- Peterson T.R., Laplante M., Thoreen C.C., Sancak Y., Kang S.A., Kuehl W.M., Gray N.S., Sabatini D.M. 2009. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 137:873–886 10.1016/j.cell.2009.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon M., Marx S.O., Gallo R., Badimon J.J., Taubman M.B., Marks A.R. 1996. Rapamycin inhibits vascular smooth muscle cell migration. J. Clin. Invest. 98:2277–2283 10.1172/JCI119038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner S., Sherrod W.S., Lichlyter D. 1997. Microtubule retraction into the uropod and its role in T cell polarization and motility. J. Immunol. 159:1063–1067 [PubMed] [Google Scholar]
- Ridley A.J., Schwartz M.A., Burridge K., Firtel R.A., Ginsberg M.H., Borisy G., Parsons J.T., Horwitz A.R. 2003. Cell migration: integrating signals from front to back. Science. 302:1704–1709 10.1126/science.1092053 [DOI] [PubMed] [Google Scholar]
- Roux P.P., Ballif B.A., Anjum R., Gygi S.P., Blenis J. 2004. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. USA. 101:13489–13494 10.1073/pnas.0405659101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saci A., Cantley L.C., Carpenter C.L. 2011. Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol. Cell. 42:50–61 10.1016/j.molcel.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara K., Liu B., Hollenbeck S., Kent K.C. 2005. Rapamycin inhibits fibronectin-induced migration of the human arterial smooth muscle line (E47) through the mammalian target of rapamycin. Am. J. Physiol. Heart Circ. Physiol. 288:H2861–H2868 10.1152/ajpheart.00561.2004 [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14:1296–1302 10.1016/j.cub.2004.06.054 [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 307:1098–1101 10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Ali S.M., Sengupta S., Sheen J.H., Hsu P.P., Bagley A.F., Markhard A.L., Sabatini D.M. 2006. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 22:159–168 10.1016/j.molcel.2006.03.029 [DOI] [PubMed] [Google Scholar]
- Schmidt A., Kunz J., Hall M.N. 1996. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc. Natl. Acad. Sci. USA. 93:13780–13785 10.1073/pnas.93.24.13780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Bickle M., Beck T., Hall M.N. 1997. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell. 88:531–542 10.1016/S0092-8674(00)81893-0 [DOI] [PubMed] [Google Scholar]
- Scott P.H., Brunn G.J., Kohn A.D., Roth R.A., Lawrence J.C., Jr 1998. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA. 95:7772–7777 10.1073/pnas.95.13.7772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulić A., Hudson C.C., Homme J.L., Yin P., Otterness D.M., Karnitz L.M., Abraham R.T. 2000. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 60:3504–3513 [PubMed] [Google Scholar]
- Shiota C., Woo J.T., Lindner J., Shelton K.D., Magnuson M.A. 2006. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev. Cell. 11:583–589 10.1016/j.devcel.2006.08.013 [DOI] [PubMed] [Google Scholar]
- Shor B., Wu J., Shakey Q., Toral-Barza L., Shi C., Follettie M., Yu K. 2010. Requirement of the mTOR kinase for the regulation of Maf1 phosphorylation and control of RNA polymerase III-dependent transcription in cancer cells. J. Biol. Chem. 285:15380–15392 10.1074/jbc.M109.071639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer A., Lei K., Johannessen C.M., Ellisen L.W. 2005. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol. Cell. Biol. 25:5834–5845 10.1128/MCB.25.14.5834-5845.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Milne L., Hawkins P. 2008. Moving towards a better understanding of chemotaxis. Curr. Biol. 18:R485–R494 10.1016/j.cub.2008.04.048 [DOI] [PubMed] [Google Scholar]
- Sun J., Marx S.O., Chen H.J., Poon M., Marks A.R., Rabbani L.E. 2001. Role for p27(Kip1) in vascular smooth muscle cell migration. Circulation. 103:2967–2972 [DOI] [PubMed] [Google Scholar]
- Swaney K.F., Huang C.H., Devreotes P.N. 2010. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu Rev Biophys. 39:265–289 10.1146/annurev.biophys.093008.131228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Hara K., Inoue H., Kawa Y., Tokunaga C., Hidayat S., Yoshino K., Kuroda Y., Yonezawa K. 2000. Carboxyl-terminal region conserved among phosphoinositide-kinase-related kinases is indispensable for mTOR function in vivo and in vitro. Genes Cells. 5:765–775 10.1046/j.1365-2443.2000.00365.x [DOI] [PubMed] [Google Scholar]
- Tee A.R., Manning B.D., Roux P.P., Cantley L.C., Blenis J. 2003. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13:1259–1268 10.1016/S0960-9822(03)00506-2 [DOI] [PubMed] [Google Scholar]
- Toschi A., Lee E., Xu L., Garcia A., Gadir N., Foster D.A. 2009. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol. Cell. Biol. 29:1411–1420 10.1128/MCB.00782-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treins C., Warne P.H., Magnuson M.A., Pende M., Downward J. 2010. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 29:1003–1016 10.1038/onc.2009.401 [DOI] [PubMed] [Google Scholar]
- Turner H.E., Harris A.L., Melmed S., Wass J.A. 2003. Angiogenesis in endocrine tumors. Endocr. Rev. 24:600–632 10.1210/er.2002-0008 [DOI] [PubMed] [Google Scholar]
- Vaillant C., Meissirel C., Mutin M., Belin M.F., Lund L.R., Thomasset N. 2003. MMP-9 deficiency affects axonal outgrowth, migration, and apoptosis in the developing cerebellum. Mol. Cell. Neurosci. 24:395–408 10.1016/S1044-7431(03)00196-9 [DOI] [PubMed] [Google Scholar]
- Wan X., Mendoza A., Khanna C., Helman L.J. 2005. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 65:2406–2411 10.1158/0008-5472.CAN-04-3135 [DOI] [PubMed] [Google Scholar]
- Wang F. 2009. The signaling mechanisms underlying cell polarity and chemotaxis. Cold Spring Harb. Perspect. Biol. 1:a002980 10.1101/cshperspect.a002980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Harris T.E., Roth R.A., Lawrence J.C., Jr 2007. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J. Biol. Chem. 282:20036–20044 10.1074/jbc.M702376200 [DOI] [PubMed] [Google Scholar]
- Weijer C.J. 2009. Collective cell migration in development. J. Cell Sci. 122:3215–3223 10.1242/jcs.036517 [DOI] [PubMed] [Google Scholar]
- Wong A.S., Roskelley C.D., Pelech S., Miller D., Leung P.C., Auersperg N. 2004. Progressive changes in Met-dependent signaling in a human ovarian surface epithelial model of malignant transformation. Exp. Cell Res. 299:248–256 10.1016/j.yexcr.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Wong K., Van Keymeulen A., Bourne H.R. 2007. PDZRhoGEF and myosin II localize RhoA activity to the back of polarizing neutrophil-like cells. J. Cell Biol. 179:1141–1148 10.1083/jcb.200706167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S.Y., Kim D.H., Jun C.B., Kim Y.M., Haar E.V., Lee S.I., Hegg J.W., Bandhakavi S., Griffin T.J., Kim D.H. 2007. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J. Biol. Chem. 282:25604–25612 10.1074/jbc.M704343200 [DOI] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Oppliger W., Hall M.N. 2005. Molecular organization of target of rapamycin complex 2. J. Biol. Chem. 280:30697–30704 10.1074/jbc.M505553200 [DOI] [PubMed] [Google Scholar]
- Yang Q., Inoki K., Ikenoue T., Guan K.L. 2006a. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 20:2820–2832 10.1101/gad.1461206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Inoki K., Kim E., Guan K.L. 2006b. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc. Natl. Acad. Sci. USA. 103:6811–6816 10.1073/pnas.0602282103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Zhang X., Li M., Chen P., Zhang B., Guo H., Cao W., Wei X., Cao X., Hao X., Zhang N. 2010. mTOR complex component Rictor interacts with PKCzeta and regulates cancer cell metastasis. Cancer Res. 70:9360–9370 10.1158/0008-5472.CAN-10-0207 [DOI] [PubMed] [Google Scholar]
- Zhou H.Y., Wong A.S. 2006. Activation of p70S6K induces expression of matrix metalloproteinase 9 associated with hepatocyte growth factor-mediated invasion in human ovarian cancer cells. Endocrinology. 147:2557–2566 10.1210/en.2005-1404 [DOI] [PubMed] [Google Scholar]
- Zoncu R., Efeyan A., Sabatini D.M. 2011. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12:21–35 10.1038/nrm3025 [DOI] [PMC free article] [PubMed] [Google Scholar]