Abstract
Although sex differences in asthma severity are recognized, the mechanisms by which sex steroids such as estrogen influence the airway are still under investigation. Airway tone, a key aspect of asthma, represents a balance between bronchoconstriction and dilation. Nitric oxide (NO) from the bronchial epithelium is an endogenous bronchodilator. We hypothesized that estrogens facilitate bronchodilation by generating NO in bronchial epithelium. In acutely dissociated human bronchial epithelial cells from female patients exposure to 17β-estradiol (E2; 10 pM–100 nM) resulted in rapid increase of diaminofluorescein fluorescence (NO indicator) within minutes, comparable with that induced by ATP (20 μM). Estrogen receptor (ER) isoform-specific agonists (R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol (THC) (ERα) and diaryl-propionitrile (DPN) (ERβ) stimulated NO production to comparable levels and at comparable rates, whereas the ER antagonist 7α,17β-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol (ICI 182,780) (1 μM) was inhibitory. Estrogen effects on NO were mediated via caveolin-1 (blocked using the caveolin-1 scaffolding domain peptide) and by increased intracellular calcium concentration [prevented by 20 μM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester but not by blocking Ca2+ influx using LaCl3]. Estrogen increased endothelial NO synthase activation (inhibited by 100 μM NG-nitro-l-arginine methyl ester) and phosphorylated Akt. In epithelium-intact human bronchial rings contracted with acetylcholine (1 μM), E2, THC, and DPN all produced acute bronchodilation in a dose-dependent fashion. Such bronchodilatory effects were substantially reduced by epithelial denudation. Overall, these data indicate that estrogens, acting via ERα or ERβ, can acutely produce NO in airway epithelium (akin to vascular endothelium). Estrogen-induced NO and its impairment may contribute to altered bronchodilation in women with asthma.
Introduction
Asthma is more common in women than men (Schatz and Camargo, 2003; Melgert et al., 2007), with symptoms exacerbated during the menstrual cycle and pregnancy (Vrieze et al., 2003; Farha et al., 2009). These data suggest that sex steroids (e.g., estrogens) play a role in asthma. However, the mechanisms by which sex steroids influence the airway in a beneficial or detrimental fashion are still under investigation. An important aspect of asthma pathophysiology is enhanced airway tone, which represents a balance between bronchodilation and bronchoconstriction, both of which could potentially be modulated by estrogens on a rapid timescale, thus dynamically altering airway tone. In this regard, we have shown previously that clinically relevant concentrations of estrogens can acutely (i.e., within minutes) reduce intracellular calcium concentration ([Ca2+]i) in airway smooth muscle (ASM) (Townsend et al., 2010), thus rapidly reducing bronchoconstriction. However, the mechanisms by which estrogens may influence bronchodilation have not been well studied.
Nitric oxide (NO) is a major endogenous as well as exogenous bronchodilator (Nijkamp and Folkerts, 1995; Feletou et al., 2001; Folkerts and Nijkamp, 2006). In airways, NO may be derived from airway epithelium (Di Maria et al., 2000; Shaul, 2002; Bove and van der Vliet, 2006), nonadrenergic/noncholinergic innervation (Lammers et al., 1992; Belvisi et al., 1995), and ASM (Nijkamp and Folkerts, 1995). All three NOS isoforms [neuronal NOS, endothelial NOS (eNOS), and inducible NOS (iNOS)] are known to be expressed within the respiratory tract (Shaul, 2002; Ricciardolo et al., 2004; Bove and van der Vliet, 2006), with airway epithelium being a major site for eNOS and iNOS, the latter especially in the asthmatic airway (Ortiz and Garvin, 2003; Jiang et al., 2009). Although NO has several mechanisms of action, the bronchodilatory properties of NO on ASM are of particular interest in terms of regulation of airway tone and its dysfunction in airway hyper-reactivity and inflammation.
The effects of sex steroids such as estrogen or progesterone on the airway epithelium have not been well studied (Ricciardolo et al., 2004; Bove and van der Vliet, 2006). In vascular endothelium, estrogens, acting via estrogen receptors (ERs), induce rapid NO production (and vasodilation) by facilitating caveolar dissociation from eNOS (Hisamoto and Bender, 2005). Whether a similar set of mechanisms is involved or estrogens actually produce NO in airway epithelium is not known. ERs are known to be present in airway epithelium (Ivanova et al., 2009) with both cytoplasmic and nuclear localization. A previous study found levels of exhaled NO production, a marker of inflammation in asthma, to be higher during the late follicular phase (Kharitonov et al., 1994), but this finding probably reflects iNOS activity (the major source of exhaled NO). A single study using NCI-H441 lung adenocarcinoma epithelial cells showed that acute exposure to estradiol increases the conversion of [3H]l-arginine to [3H]l-citrulline (Kirsch et al., 1999), but did not demonstrate NO production. In the present study, using acutely dissociated, normal human bronchial epithelial cells (BECs) and epithelium-intact versus -denuded bronchial rings from females, we tested the hypothesis that estrogen enhances rapid (probably nongenomic) NO production via eNOS activation, thereby potentiating bronchodilation.
Materials and Methods
Isolation of Human BECs.
Human bronchial epithelium was isolated from bronchial tissue obtained from lung samples incidental to thoracic surgery (lobectomy, pneumenectomy) in female patients at the Mayo Clinic, Rochester, MN. After completion of analysis by the pathologist for surgical purposes, the discarded lung sample was dissected to identify normal-appearing airways (confirmed via review of clear margins by the pathologist). Patient ages ranged between 45 and 75 years. Patient histories were noted, but samples were de-identified, thus considered exempt by the Mayo Institutional Review Board and approved for research use. Airway samples were restricted to patients with focal disease not involving the airway (e.g., focal nonairway cancers or granuloma) and without pre-existing airway disease (asthma, emphysema, chronic obstructive pulmonary disease) at the time of surgery and thus considered normal. No attempts were made to determine hormonal status (premenopausal or postmenopausal) of the patients at the time of surgery; however, ER expression was confirmed in all samples before further experimentation.
Airway samples were rapidly transferred to the laboratory in ice-cold Hanks' balanced salt solution. The bronchial epithelium was dissected from the smooth muscle and cartilage on ice, minced, and transferred to T25 flasks or eight-well LabTek chambers (Nalge Nunc International, Rochester, NY). Explants were grown in a 95% air/5% CO2 humidified incubator using basal epithelial growth medium supplemented with 2 ml of bovine pituitary extract, 0.5 ml of hydrocortisone, 0.5 ml of human epidermal growth factor, 0.5 ml of epinephrine, 0.5 ml of transferrin, 0.5 ml of insulin, 0.5 ml of retinoic acid, 0.5 ml of triiodothyronine, and 0.5 ml of GA-1000 (gentamicin/amphotericin B; Lonza, Portsmouth, NH). Explants were removed from culture at confluence. The BECs were then trypsinized and replated for experiments. All experiments were performed in cells from primary explants and passages 1 and 2 of subculture. Cell purity was verified by periodic Western analyses for expression of E-cadherin, but the absence of fibroblast surface protein or smooth muscle myosin.
Isolation of Cellular Fractions.
Human BECs were harvested, and cellular fractions were prepared by separation into heavy (nuclear/Golgi), cytosolic, and membrane fractions by using the FractionPREP Cell Fractionation System (BioVision, Mountain View CA; manufacturer-provided protocol) as described previously for ASM (Townsend et al., 2010).
Western Blot Analysis.
Standard SDS-polyacrylamide gel electrophoresis (Criterion Gel System; Bio-Rad Laboratories, Hercules, CA; 10% gradient gels) and polyvinylidene difluoride membrane (Bio-Rad Laboratories) transfer techniques were used. Membranes were blotted for ERα [1 μg/ml (1:200 dilution) rabbit anti-ERα; Santa Cruz Biotechnology Inc., Santa Cruz, CA], ERβ [1 μg/ml (1:200 dilution) mouse anti-ERβ; Santa Cruz Biotechnology Inc.], eNOS [1 μg/ml (1:250 dilution); mouse anti-eNOS; BD Biosciences Transduction Laboratories, Lexington, KY], p-eNOS [1 μg/ml (1:250 dilution); mouse anti-p-eNOS (pSer1177); BD Biosciences Transduction Laboratories], iNOS [1.5 mg/ml (1:650 dilution); rabbit anti-iNOS; Cell Signaling Technology, Danvers, MA], AKT [1 μg/ml (1:1000 dilution); rabbit anti-AKT; Cell Signaling Technology], and phosphorylated Akt (p-AKT) [1 μg/ml (1:1000 dilution); rabbit anti-p-AKT; Cell Signaling Technology]. GAPDH [1 μg/ml (1:1000 dilution); rabbit anti-GAPDH; Cell Signaling Technology] was used for normalization. Primary antibodies were detected by using horseradish peroxidase-conjugated secondary antibodies and signals developed by Supersignal West Pico or West Femto (p-eNOS, p-AKT) Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA). Blots were imaged on a Kodak ImageStation 4000 mm (Carestream Health, Rochester, NY) and quantified using densitometry.
Immunofluorescence Microscopy.
ER expression in BECs was verified by using immunofluorescence as described for other proteins (Townsend et al., 2010). In brief, BECs grown on eight-well LabTek chambers were fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked in 4% normal donkey serum, and incubated overnight with an antibody against eNOS along with an antibody against either ERα or ERβ. Immunopositivity was detected with appropriate Alexa Fluor 488- and Cy3-conjugated secondary antibodies (donkey anti-rabbit or anti-mouse IgG; Jackson Immunoresearch Laboratories Inc., West Grove, PA; 1:200 dilution). Samples were visualized with an Olympus (Tokyo, Japan) FluoView laser scanning confocal microscope equipped with Ar and Kr lasers and appropriate filters (1024 × 1024 pixel images, 0.4-μm optical section thickness; 40×/1.3 numerical aperture oil-immersion lens).
Real-Time NO imaging.
BECs grown in eight-well LabTek chambers were loaded with the NO-sensitive fluorescent dye 4,5-diaminofluorescein diacetate (DAF-2 DA; Calbiochem, San Diego, CA; 5 μM; 60 min at room temperature). The NO-insensitive dye 4-aminofluorescein diacetate (DAF-4) (5 μM) was used as a negative control for NO detection. Cells were imaged as described previously (Meuchel et al., 2011) by using a real-time fluorescence imaging system (MetaFluor; Molecular Devices, Sunnyvale, CA) mounted on a Nikon (Tokyo, Japan) Diaphot inverted microscope, with a 40×/1.3 numerical aperture oil immersion lens (Fryer Instruments, Edina, MN). Cells were initially perfused with 2.5 mM Ca2+ Hanks' balanced salt solution, and baseline fluorescence was established. The responses of ∼10 cells per chamber (average number of cells in field of view) were obtained from software-defined regions of interest [0.2-Hz acquisition of 510-nm emission after 488-nm excitation using a Photometric Cascade digital camera system (Roper Scientific, Trenton, NJ)].
Real-Time Simultaneous NO and Ca2+ Imaging.
BECs grown in eight-well LabTek chambers were loaded with DAF-2 DA as well as the fluorescent calcium indicator X-Rhod-1 AM (Invitrogen, Carlsbad, CA; 1 μM; 45 min at room temperature). Each dye was sequentially visualized using the appropriate excitation/emission filter sets in the imaging system as above (quality-control experiments done to verify absence of bleed through between the fluorescence channels). Calcium calibration was performed by using X-Rhod-1 tripotassium salt (Anaspec, Fremont, CA) according to the calcium calibration kit protocol (Invitrogen).
Force Measurements.
Bronchial samples for force studies were collected from third- to sixth-generation bronchi of female patients (as above). The connective tissue was removed by microdissection, and the bronchial epithelium was either retained (epithelium-intact) or mechanically removed (epithelium-denuded) by abrasion within the bronchial lumen via a fine-wire brush. The bronchial rings (epithelium-intact or -denuded) were then suspended in a 5-ml organ bath (Radnoti Systems, Monrovia, CA), and force measurements were performed by using calibrated force transducers (FT03; Grass Instruments, Quincy, MA). Rings were maintained at 37°C in physiological saline solution. Each ring was stretched to optimal length by using repeated contractions with 1 μM acetylcholine (ACh). The rings were then contracted with 1 μM ACh until a stable contraction was reached. Samples were then exposed to vehicle (time control) or increasing concentrations of 17β-estradiol (E2), (R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol (THC), and diaryl-propionitrile (DPN), and the extent of bronchodilation was measured. Force responses were measured by using custom-built software based on LabView (National Instruments, Austin, TX).
Materials.
Compounds were obtained from sources as indicated. Chemical structure and formal name were indicated by the companies. THC, DPN, and (±)-1-[(3aR*, 4S*, 9bS*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone (G-1) were obtained from Tocris Bioscience (Ellisville MO). MAHMA-NONOate, diethylamine-NONOate (DEANO), and sulfo-NONOate were obtained from Cayman Chemical (Ann Arbor, MI). NG-nitro-l-arginine methyl ester (l-NAME), 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester (BAPTA), DAF-2 DA, DAF-4, and caveolin-1 scaffolding domain (CSD) peptide were purchased from Calbiochem (San Diego, CA). E2, 7α,17β-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol (ICI 182,780), N-([3-(Aminomethyl)phenyl]methyl)ethanimidamide dihydrochloride (1400W), and other chemicals were obtained from Sigma (St. Louis, MO) unless otherwise noted.
Statistical Analysis.
Bronchial samples were obtained from six female patients and used for BEC isolation. NO experiments were performed in at least 15 cells each from four different patient samples, although not all protocols were performed in each sample obtained. Force responses were measured from four patient samples. Results were compared by using unpaired t test or one-way analysis of variance with repeated measures as appropriate. Bonferroni correction was applied for multiple comparisons. Statistical significance was established at p < 0.05. Values are expressed as means ± S.E.
Results
ER Expression in Human BECs.
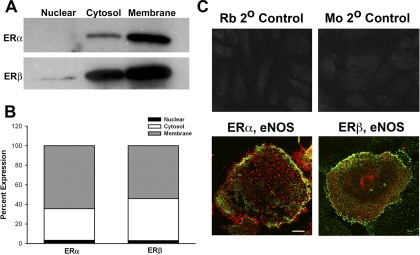
Western analyses of cell fractions from unstimulated BECs revealed that both ERα and ERβ were expressed in the cytosol as well as plasma membrane (Fig. 1), with a relatively higher membrane expression of ERα compared with ERβ. Nuclear expression of ERα or ERβ was small. Two-color fluorescent immunostaining confirmed the colocalization of both ERs with eNOS within the plasma membrane (Fig. 1C). However, it should be noted that ERβ was also observed separately from eNOS, suggesting a noncaveolar expression of this isoform.
Fig. 1.
Human BECs express ERs. A, Western analyses of fractions of unstimulated BECs show that ERα and ERβ both localize to the plasma membrane and cytosol, with a small degree of expression in the nucleus. B, bar graph summarizes results from four patients. C, fluorescent immunostaining demonstrated ERα and ERβ expression in BECs, with substantial colocalization of eNOS with either isoform. An Alexa Fluor 488 dye was used to visualize ERs, whereas Cy3 was used for eNOS. Rb, rabbit; Mo, mouse. Scale bar, 10 μm (top panels), 2 μm (bottom panels).
Sensitivity and Specificity of DAF-2 in BECs.
The NO-sensitive dye DAF-2 DA is intracellularly metabolized and converted to DAF-2, which fluoresces in proportion to the total NO that the dye binds over time (i.e., a cumulative response). To establish sensitivity and specificity of DAF-2 in isolated BECs for NO, a variety of NO and nitrous oxide (N2O) donors were used.
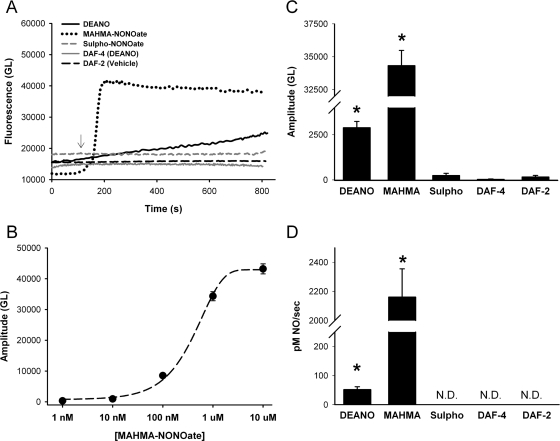
The fast-acting NO donor MAHMA-NONOate was perfused and exhibited a rapid increase in intracellular fluorescence that plateaued in approximately 3 min (Fig. 2A). Sensitivity of DAF-2 for NO within BECs per se was determined as described recently for pulmonary artery endothelial cells (Meuchel et al., 2011) by constructing an empirical curve of amplitudes of fluorescence changes (difference from baseline at 15 min) using a range of MAHMA-NONOate concentrations (1 nM to 10 μM). Concentrations of 1 nM MAHMA-NONOate showed minimal changes over baseline fluorescent levels and were comparable with vehicle controls. Maximal fluorescence as well as rate increased in a dose-dependent manner with saturation occurring at 10 μM MAHMA-NONOate. The data were fitted with a four-parameter sigmoid curve with R2 value equal to 0.996 (Fig. 2B). The empirical calibration was used to determine the rate of NO production in subsequent protocols.
Fig. 2.
Validation and calibration of the NO-sensitive fluorescent dye DAF-2 DA in BECs. A, exposure of DAF-2 DA-loaded cells to NO donors such as MAHMA-NONOate (1 μM) or DEANO (50 μM) resulted in rapid increase in fluorescence, consistent with the NO release properties of each agent. The nitrous oxide donor sulfo-NONOate had no effect on DAF-2 fluorescence compared with vehicle controls. The NO-insensitive indicator DAF-4 showed no change in fluorescence with 50 μM DEANO. Overall, these results were used to validate the use of DAF-2 in human BECs. B, a concentration-response curve of the NO donor MAHMA-NONOate was constructed (1 nM-10 μM). Data points were maximal amplitudes taken after 15 min and corrected for baseline. The empirical response curve was fitted with a four-parameter sigmoid (R2 = 0.996). C, amplitude of changes in gray level (GL) of DAF-2 fluorescence with exposure to different agents. D, rate of change in DAF-2 fluorescence reflecting rate of NO production with exposure to different agents. In C and D, values are means ± S.E. (n = four patients). * indicates significant difference from vehicle control (p < 0.05). N.D., not determinable (rate of change was negligible).
We verified that the rate of change in DAF-2 fluorescence reflected NO release or production. At similar concentrations, the NO donor DEANO showed a slower rate of increase (Fig. 2D) and reached a smaller amplitude (Fig. 2C) compared with MAHMA-NONOate and did not saturate at 15 min. The N2O donor sulfo-NONOate had no effect on DAF-2 fluorescence in BECs (thereby establishing selectivity of DAF-2 for NO). In addition, lack of change in fluorescence of the NO-insensitive dye DAF-4 in BECs with exposure to DEANO was verified.
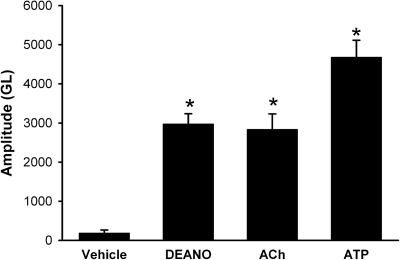
DAF-2 fluorescence was low at baseline in untreated BECs, indicating a low level of NO. Both Ach (1 μM) and ATP (20 μM), well known inducers of NO in airway epithelium (Ricciardolo et al., 2004; Bove and van der Vliet, 2006), increased NO levels (Fig. 3; p < 0.05 compared with vehicle), further demonstrating the applicability of DAF-2. The amount of NO produced by ACh and ATP was comparable with the NO donor DEANO (50 μM).
Fig. 3.
NO induction in BECs. Exposure of DAF-2 DA-loaded cells to the sustained NO donor DEANO (50 μM) or the known endogenous inducers of epithelial NO such as ACh (1 μM) and ATP (20 μM) resulted in substantial increases in fluorescence. Amplitude of changes in GL of DAF-2 fluorescence was calculated as the maximal fluorescence achieved after 15 min of exposure corrected for baseline fluorescence. ACh-induced NO was comparable with that by DEANO in rate (not shown) and amplitude, whereas ATP produced even greater NO. Values are means ± S.E. (n = four patients). * indicates significant difference from vehicle control (p < 0.05),.
ER Activation Induces NO Production in BECs.
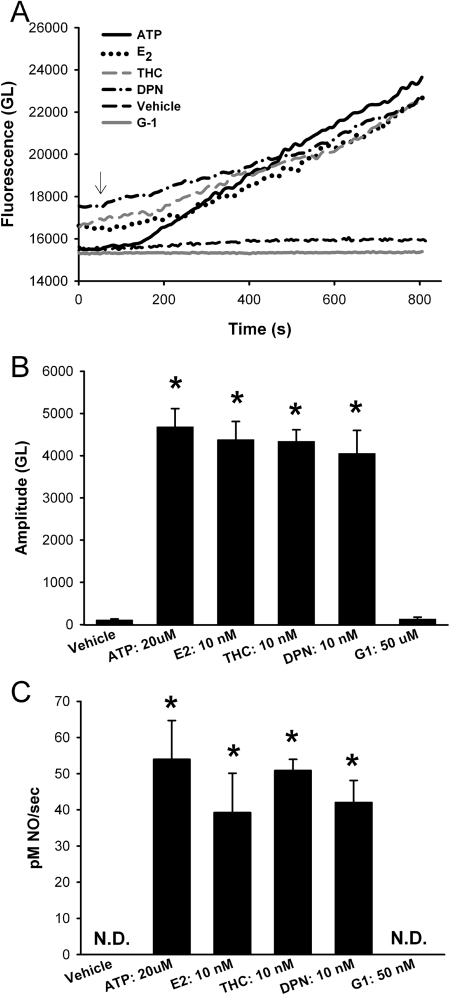
Exposure of BECs to E2 (10 nM; ERα and ERβ activation), THC (10 nM; ERα activation), or DPN (10 nM; ERβ activation) all increased DAF-2 fluorescence (Fig. 4A) such that by 15 min these ER agonists had produced significant increases in NO, comparable with that achieved by 20 μM ATP (Fig. 4B; p < 0.05). No differences in maximal amplitude measured after 15 min or the rate of NO production were observed between ERα versus ERβ stimulation (Fig. 4, B and C).
Fig. 4.
ERα and ERβ activation results in NO production in human BECs. A, in DAF-2-loaded human BECs 20 μM ATP (shown) and 1 μM ACh (not shown) both induced an increase in NO production (15 min). Treatment with the nonselective physiologically relevant E2 (10 nM) or the ERα- versus ERβ-selective agonists (THC and DPN, respectively; 10 nM each) caused an increase in fluorescence comparable with that of ATP (20 μM). In comparison, the GPCR30 agonist G1 had no effect on fluorescence. B, amplitude of agonist or estrogen induced DAF-2 response. Based on the DAF-2 calibration in Fig. 2, NO levels were in the range of 30 to 85 nM. Vehicle control as well as G1 showed minimal change in fluorescence. C, rate of NO production. Estrogen-induced NO production was only slightly slower than that by ATP. In B and C, values are means ± S.E. (n = five patients). * indicates significant difference from vehicle control (p < 0.05). N.D., not determinable (rate of change was negligible).
There is recent evidence in nonairway tissues for involvement of G protein-coupled receptor 30 (GPCR30 or GPER) in nongenomic estrogen signaling (Langer et al., 2010). Whether GPCR30 is present and functional in BECs is not known. However, the GPCR30-specific agonist G-1 (50 μM) had no effect on NO levels (Fig. 4). In addition, Western analyses of BECs for GPCR30 showed no detectable expression of this receptor (data not shown). Accordingly, this avenue of estrogen signaling was not explored further.
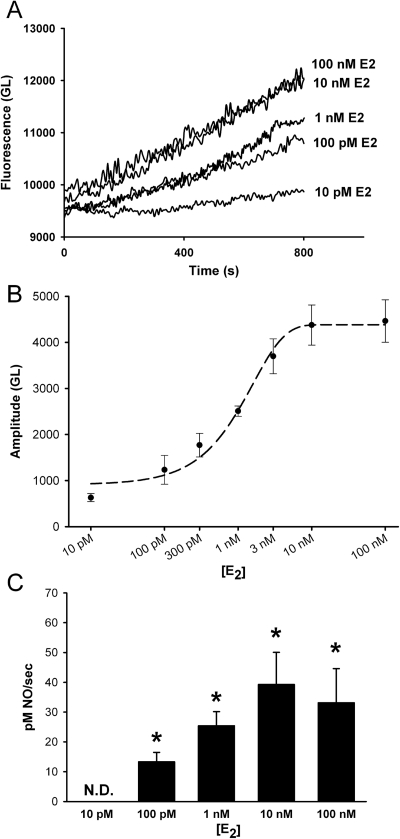
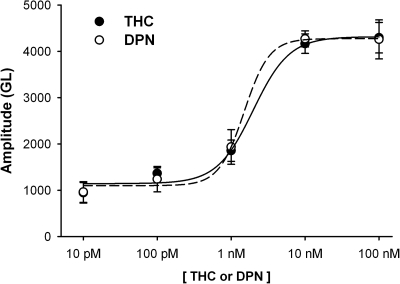
A concentration response for E2-induced NO production (10 pM to 100 nM E2) revealed minimal NO production at 10 pM E2 and maximal response at 10 nM (Fig. 5). Qualitatively similar results were obtained for ER-specific agonists THC (ERα) and DPN (ERβ) (Fig. 6). Both of these ER isoform-specific agonists showed minimal NO production at 10 pM. DPN-induced NO response saturated at 10 nM, whereas THC-induced response was maximal at 100 nM. Maximal amplitudes were taken at 15 min, and the curve was fitted with a four-parameter sigmoid (R2 = 0.993, E2; R2 = 0.992, THC; R2 = 0.996, DPN).
Fig. 5.
E2 effects on NO production are concentration-dependent. A, in DAF-2 loaded BECs increasing physiologic concentrations of E2 (10 pM–100 nM) increased NO production in a concentration-dependent manner. No difference was observed between 10 and 100 nM concentrations. B, summary of E2-induced NO amplitudes taken after 15 min. The curve was fitted with a four-parameter sigmoid (R2 = 0.993). C, rate of NO production. Increasing E2 concentrations until 10 nM resulted in greater rates of NO production. Values are means ± S.E. (n = five patients). * indicates significant difference from vehicle control (p < 0.05). N.D., not determinable (rate of change was negligible).
Fig. 6.
ER isoform-specific agonist effects on NO production are concentration-dependent. In DAF-2 loaded BECs increasing concentrations of ERα-specific agonist THC and ERβ-specific agonist DPN (10 pM–100 nM) resulted in progressively increased NO production (measured at 15 min). DPN-induced NO response saturated at 10 nM, whereas THC-induced response was maximal at 100 nM. The effects of DPN and THC were comparable overall. The curves were fitted with a four-parameter sigmoid (R2 = 0.992, THC; R2 = 0.996, DPN).
Mechanisms of E2-Induced NO Production in BECs.
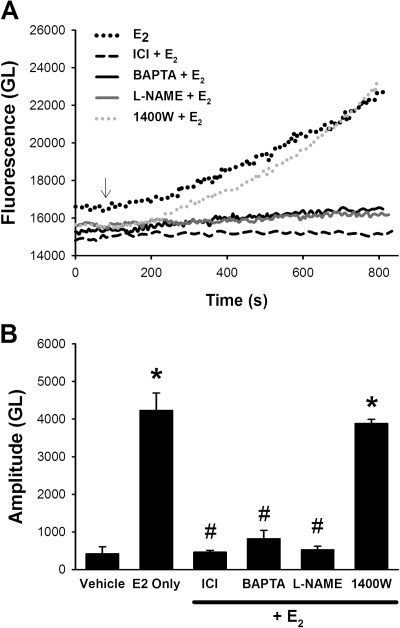
Pretreatment of BECs with the nonspecific ER antagonist ICI 182,780 (1 μM; 30 min) completely abrogated subsequent E2-induced increase in NO (Fig. 7; p < 0.05). In addition, chelation of [Ca2+]i using the cell-permeant BAPTA-AM (20 μM, 30 min) resulted in substantially blunted NO response to 10 nM E2 (Fig. 7; p < 0.05). Finally, inhibition of NOS with l-NAME (100 μM; 45 min) severely blunted E2-induced increase in NO (Fig. 7; p < 0.05).
Fig. 7.
Mechanisms of E2-induced NO production in BECs. A, in DAF-2-loaded BECs pretreatment with the ER antagonist ICI 182,780 (1 μM; 30 min), the calcium chelator BAPTA (20 μM; 30 min), or the eNOS inhibitor l-NAME (100 μM; 45 min) substantially attenuated the subsequent effect of 10 nM E2 on NO production. In contrast, the iNOS inhibitor 1400W (10 μM; 30 min) did not alter the amount of NO produced by E2 (or that by THC or DPN; not shown). B, values are means ± S.E. (n = five patients). * indicates significant difference from vehicle control; # indicates significant inhibitor effect (p < 0.05).
In a separate set of experiments, BECs were pretreated with the iNOS-specific antagonist 1400W (10 μM) for 30 min before NO imaging. There was no difference in DAF-2 fluorescence achieved with ATP, E2, THC, or DPN in 1400W-treated cells compared with values obtained in the absence of this iNOS inhibitor (Fig. 7). Furthermore, Western analyses showed minimal iNOS expression in BECs grown in growth media (Supplemental Fig. 1). Fifteen-minute pretreatment with E2 (to match functional NO imaging experiments) did not change iNOS expression compared with vehicle control. Overnight treatment with inflammatory cytokine TNFα (20 ng/ml; used as a positive control) significantly up-regulated iNOS expression in these cells (Supplemental Fig. 1; p < 0.05).
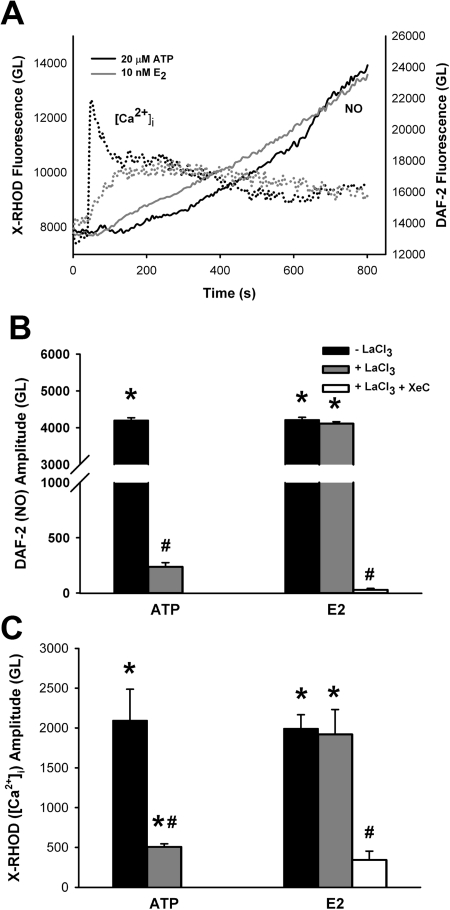
In cells dual-labeled with the Ca2+ indicator X-Rhod-1 AM as well as DAF-2 DA, exposure to either 20 μM ATP or 10 nM E2 resulted in an increase in [Ca2+]i that preceded NO production (Fig. 8). After the peak of the [Ca2+]i response, a plateau level was reached that was higher than basal levels. NO production continued to increase, whereas [Ca2+]i levels remained elevated (Fig. 8).
Fig. 8.
Estrogen increases [Ca2+]i via intracellular store depletion. A, in cells dual-labeled with the Ca2+ indicator X-Rhod-1 AM, as well as with DAF-2 DA, exposure to either 20 μM ATP or 10 nM E2 resulted in an increase in Ca2+ that preceded NO production. After peak [Ca2+]i response, a plateau level was reached that was higher than basal calcium levels. NO production continued to increase, whereas [Ca2+]i levels remained elevated. To determine the role of intracellular stores in E2-induced elevation in [Ca2+]i, 1 mM LaCl3 was added for 20 min before introduction of either ATP or E2, thus nonspecifically inhibiting plasma membrane Ca2+ influx. B and C, subsequent treatment with ATP resulted in no change in DAF-2 fluorescence (B) and only a small increase in [Ca2+]i (C). In contrast, pretreatment with LaCl3 did not inhibit increases in [Ca2+]i caused by 10 nM E2 nor did it abolish the increase in NO. However, the additional presence of the IP3 receptor antagonist XeC (1 μM) completely abolished E2-induced NO response as well as elevation of [Ca2+]i, indicating an intracellular source for Ca2+. Values are means ± S.E. (n = four patients). * indicates significant difference from vehicle control; # indicates significant inhibitor effect (p < 0.05).
In separate sets of experiments to determine the mechanism of E2-induced increase in [Ca2+]i, 1 mM LaCl3 was added for 20 min before exposure to either ATP or E2 to nonspecifically inhibit Ca2+ influx across the plasma membrane. In such cells, treatment with ATP resulted in minimal changes in [Ca2+]i (suggesting a predominant role for influx), and no increase in DAF-2 fluorescence was detected (Fig. 8). In contrast, pretreatment with LaCl3 did not inhibit increases in [Ca2+]i caused by 10 nM E2 nor did it abolish the observed increase in NO production (Fig. 8). To further determine the source of E2-induced increases in Ca2+, BECs were pretreated with LaCl3 as well as the IP3 receptor antagonist xestospongin C (XeC; 1 μM). The presence of both inhibitors resulted in substantial blunting of the Ca2+ response and complete abolishment of E2-induced NO production (Fig. 8; p < 0.05).
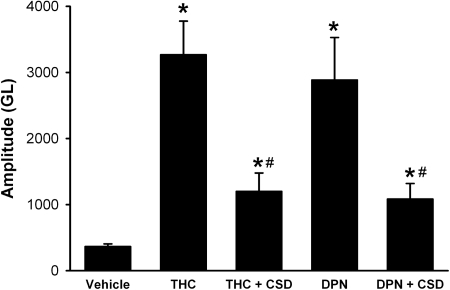
Previous studies have already established the association between caveolae and eNOS (García-Cardeña et al., 1997; Ju et al., 1997) and the importance of Ca2+-induced dissociation of eNOS in subsequent NO production (Kone, 2000). Human bronchial epithelium is known to express the constitutive caveolar protein caveolin-1 (Krasteva et al., 2006). Pretreatment of BECs with the CSD peptide (5 μM; 4-h pretreatment), which inhibits caveolin-1 function, significantly attenuated both THC- and DPN-induced NO production by ∼50% (p < 0.05; Fig. 9).
Fig. 9.
Inhibition of caveolin-1 attenuates ER activation-induced NO production. In DAF-2-loaded BECs, pretreatment with the inhibitory CSD peptide (5 μM; 4 h) significantly attenuated the subsequent effect of 10 nM THC or DPN on NO production, but did not abolish it. * indicates significant difference from vehicle control; # indicates significant inhibitor effect (p < 0.05).
Effect of E2 on Akt and eNOS Phosphorylation.
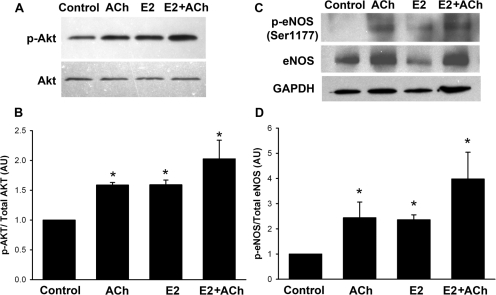
Phosphorylation of Akt and eNOS are known to be key steps in the production of NO (Hurt et al., 2002). In airway epithelium this pathway is initiated by many agonists including ACh. Exposure of bronchial epithelial tissue to ACh (1 μM; 2 min) or E2 (10 nM; 2 min) resulted in increased phosphorylation of Akt compared with vehicle controls (Fig. 10A; p < 0.05). Phosphorylation induced by E2 was comparable with that induced by 1 μM ACh. The combination of E2 and ACh resulted in greater phosphorylation of Akt compared with either treatment individually (Fig. 10, A and B; p < 0.05). A similar trend in ACh versus E2 effects was observed for phosphorylation of eNOS at Ser1177 when exposed to ACh (1 μM; 10 min) or E2 (10 nM; 20 min) (Fig. 10C; p < 0.05). Phosphorylation induced by E2 was comparable with that induced by 1 μM ACh. The combination of E2 and ACh resulted in greater phosphorylation of eNOS compared with either treatment individually (Fig. 10, C and D; p < 0.05).
Fig. 10.
Estrogen increases Akt and eNOS phosphorylation. A, to determine E2 effects on Akt phosphorylation BECs were treated with ACh (1 μM; 2 min), E2 (10 nM; 2 min), or a combination of E2 and ACh (2 min). Western analyses indicated that ACh or E2 alone or in combination increased p-Akt levels. B, in summary bar graph values are means ± S.E. (n = four patients) relative to total Akt expression. To determine eNOS phosphorylation, BECs were treated with ACh (1 μM; 10 min), E2 (10 nM; 20 min), or a combination of E2 and ACh (E2 alone, 10 min; E2+Ach, additional 10 min). C, Western analyses indicated increased phosphorylation of eNOS at Ser1177. D, in summary bar graph values are means ± S.E. (n = four patients). * indicates significant difference from unstimulated controls (p < 0.05).
E2 Effects on Bronchodilation.
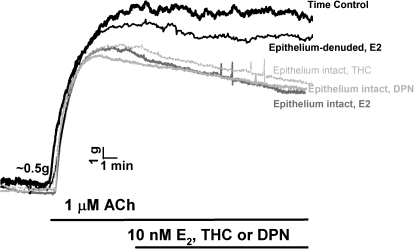
In epithelium-intact human bronchial rings from female patients, exposure to 1 μM ACh produced a typical, sustained force response (Fig. 11). Exposure to 10 nM E2 THC or DPN produced rapid relaxation within a 15-min time frame that was comparable between treatments (Fig. 11). It should be noted that a 15-min time period was selected to match the NO imaging data. However, longer exposure to ER agonists resulted in >50% relaxation (not shown). Denudation of epithelium blunted E2 effects on bronchodilation (Fig. 11 shows E2, but a similar lack of effect was observed for THC and DPN).
Fig. 11.
Estrogen acutely induces bronchodilation. In epithelium-intact human bronchial rings (from female patients), 1 μM ACh produced a typical, sustained force response. Exposure to 10 nM E2, THC, or DPN produced rapid relaxation within a 15-min time frame (selected to match the NO imaging data). Longer exposure to ER agonists resulted in >50% relaxation (not shown). Denudation of epithelium blunted E2 effects on bronchodilation.
Discussion
In this study, biologically relevant concentrations of estrogen as well as ER-specific agonists induced physiologically significant levels of NO in human BECs and produced bronchodilation via an epithelium-dependent mechanism. Estrogen acts on the bronchial epithelium via both major ER isoforms, which are expressed in the membrane and cytosol, and induce NO within a matter of minutes. These data point to nongenomic mechanisms (involving Akt and eNOS phosphorylation), providing novel evidence for a functional role of non-nuclear ERs in the airway epithelium. Combined with our previous study in human ASM cells (Townsend et al., 2010), the novel findings of E2-induced NO production suggest an important bronchodilatory role for sex steroids. The relevance of such effects lies in the normal effects of estrogens on the airway and the dysregulation of estrogen signaling in the setting of diseases such as asthma, which involves impaired NO-mediated bronchodilation and enhanced ASM contractility.
Estrogen signaling involves the potent ER agonist E2. Although airway ER expression, especially in humans, has not been well studied, based on perimenstrual fluctuations in asthma symptoms, ER expression is likely. One previous study reported ERα and ERβ expression in human BECs (Ivanova et al., 2009), but examined only genomic actions of ER activation in these cells. Genomic responses to estrogens are known to be complex, cell-specific, and estrogen concentration- and duration-dependent and involve a host of signaling proteins and pathways (Heldring et al., 2007). Given these complexities, and the relative lack of knowledge on estrogen signaling within the airway epithelium, we did not address the issue of prolonged estrogen exposure in BECs in this study, while recognizing its importance in vivo. In this regard, the results of our present study are novel, highlighting the localization of both plasma membrane receptors as well as cytosolic in human BECs and focusing on rapid, nongenomic mechanisms of ER action.
Although rapid and likely nongenomic effects of E2 occurring within seconds to minutes have been observed in other tissues (Levin, 2002; Simoncini and Genazzani, 2003), the underlying mechanisms are still under investigation. Previous work, but not in the lung, has focused on nongenomic actions of ERα and ERβ (Levin, 2002; Heldring et al., 2007). The presence of both ER isoforms in the BEC plasma membrane, and the current results on NO production and bronchodilation using ER isoform-specific agonists (THC versus DPN), suggest that both ERα and ERβ are functional in terms of nongenomic regulation of NO in airway epithelium. Blocking ER activation with the ER antagonist ICI 182,780 attenuated NO production in BECs, further confirming that rapid, nongenomic ERα or ERβ activation was responsible for the observed NO effects. In other tissues, there is recent evidence for a G protein-coupled receptor (GPCR30 or GPER) in nongenomic estrogen signaling (Langer et al., 2010). This receptor is not blocked by ICI 182,780. To date, we have not detected GPCR30 in the human airway, and the GPCR30 agonist G-1 had no effect on BEC-induced NO production, suggesting that this receptor is not a key player in estrogen-induced NO effects in the airway.
Although the present study has demonstrated that BECs have ERs, there is currently limited data on estrogen signaling per se within the airway epithelium. A previous study using H441 epithelial cells found that acute exposure to E2 increases conversion of [3H]l-arginine to [3H]l-citrulline (Kirsch et al., 1999), although NO production was not measured. We now show that estradiol rapidly increases NO production in acutely dissociated BECs from female patients and increases phosphorylation of Akt and eNOS (Ser1177). In the previous study, the authors noted the necessity of determining which ERs (ERα and/or ERβ) are responsible for eNOS activation and subsequent NO production. Using ER-specific agonists THC (ERα) and DPN (ERβ), we addressed this question, finding that both receptor types can increase NO levels in a concentration-dependent manner.
Only a single report in airway epithelium (Kirsch et al., 1999) has reported that estradiol effects on NOS are Ca2+-dependent. Our results showing the blunting effects of the Ca2+ chelator BAPTA on E2-induced NO are consistent with this idea. The novel aspect of our study here is exploration of the mechanisms by which estrogens alter [Ca2+]i. Dual imaging of [Ca2+]i and NO allowed for time resolution of the Ca2+ events leading to NOS activation. Inhibition of influx with LaCl3 blunted ATP-induced Ca2+ elevation and prevented changes in NO levels. In contrast, LaCl3 did not substantially affect E2-induced elevation in Ca2+ or NO, suggesting an intracellular source for Ca2+. This is in contrast to previous work (Kirsch et al., 1999) showing a role for influx. However, inhibition of IP3 receptor channels with XeC abolished E2-induced Ca2+ and NO changes. Previous studies have established a role for IP3 receptor channels in BEC [Ca2+]i regulation (Boitano et al., 1992). However, this is the first evidence for estrogen effects on BEC NO via mobilization of intracellular Ca2+ stores.
Caveolin-1 is an important regulator of membrane localization of eNOS with dissociation of eNOS from caveolae being an important step in NO production (Kone, 2000). Accordingly, interactions of caveolin-1 scaffolding domain with eNOS inhibits NO production (Ju et al., 1997). In this regard, our results with CSD are consistent. What is novel is the caveolar association of both ERα and ERβ in human BEC. Studies in vascular endothelium have established the importance of ERα-caveolin-1 association that influences eNOS activity (Klinge et al., 2008). However, little is known about the interaction of ERβ with caveolin-1 and eNOS, especially in the airway. Our studies showed that inhibition of caveolin-1 with CSD reduced eNOS activation by 50% in BECs, presumably by dissociating caveolin-1 from eNOS. However, significant amounts of NO were still produced by ERα or ERβ activation in the presence of CSD, suggesting a novel role for ER-induced eNOS signaling independent of caveolin-1.
The functional relevance of E2-induced epithelial NO lies in modulation of airway tone: a balance between bronchoconstriction and bronchodilation. Our novel findings in epithelium-intact bronchial rings show that ER activation produces rapid and sufficient epithelial NO to induce bronchodilation even in the presence of ACh, a well known bronchoconstrictor. It should, however, be noted that ACh can also induce epithelial NO generation (as shown in this study), and thus estrogens could enhance this epithelial effect. On the other hand, by reducing ASM [Ca2+]i (Townsend et al., 2010), estrogens may counteract the bronchoconstricting action of ACh on ASM itself, albeit less effectively than if the epithelium was present. This latter aspect may be particularly important in the role of estrogens in determining the balance between bronchoconstriction and bronchodilation in the setting of dysfunctional epithelium in airway diseases.
The clinical relevance of our study lies in diseases such as asthma, which are more prevalent in women (Melgert et al., 2007; Postma, 2007), with increased severity and frequency of exacerbations (Becklake and Kauffmann, 1999), and catamenial variations in airway reactivity in ∼40% of women with pre-existing disease (Chhabra, 2005; Murphy and Gibson, 2008). Clinical data suggest that estrogens are detrimental in asthma; however, asthma exacerbations are greater during late luteal phase when estrogen levels are lowest (Hanley, 1981; Gibbs et al., 1984) and can be alleviated in postmenopausal women receiving hormone replacement therapy (Bellia and Augugliaro, 2007). We and others have shown that estrogen can exhibit relaxant effects directly on the ASM, in part by decreasing [Ca2+]i via L-type (Townsend et al., 2010) and BKCa channels (Dimitropoulou et al., 2005) (the latter being responsive to cGMP, a downstream effector of NO). Indeed, it is likely that estrogen-induced epithelial NO stimulates guanylyl cyclase in the underlying ASM, producing bronchodilation in humans similar to murine models (Dimitropoulou et al., 2005). Our study suggests that the epithelial-derived NO may stimulate guanylyl cyclase in ASM. Thus, estrogens may have a two-pronged effect in inducing bronchodilation.
A potential confounding factor in examining the role of estrogens is the role of airway inflammation and how estrogens interact with inflammatory mediators in modulating airway tone. Here, inflammation-induced alterations in NO [e.g., due to enhanced epithelial iNOS (Ortiz and Garvin, 2003; Jiang et al., 2009)] may also be relevant. Asthma severity correlates with increasing levels of exhaled NO, a marker of airway inflammation. Menstrual variations in exhaled NO have been reported (Mandhane et al., 2009) that suggest regulation of NO by sex steroids; however, the underlying mechanisms remain unknown. It must be noted that in our study we examined only normal BECs not exposed to inflammatory mediators. Accordingly, we found very low levels of iNOS, and as expected, iNOS inhibition did not alter NO production by E2, THC, or DPN. Whether estrogens influence epithelial NO production via iNOS in the presence of inflammation remains to be determined. Furthermore, other sex steroids (and their metabolites) may play roles in modulating airway reactivity. Here, the interaction between progesterone and estrogen may be important in women, whereas testosterone-induced changes in airway tone may be relevant in men. These avenues are largely unexplored.
Supplementary Material
Acknowledgments
We thank Sarah VanOosten for assistance with the human airway force measurements.
This work was supported by the National Institutes of Health National Heart, Lung and Blood Institute [Grants HL090595, HL088029]; and the Mayo Graduate School (Rochester, MN).
This article represents partial fulfillment for E.A.T.'s PhD thesis in Physiology and Biomedical Engineering.
Portions of this work were presented previously: Townsend EA, Thompson MA, Cassivi SD, Pabelick CM, and Prakash YS (2010) Estrogen increases nitric-oxide production in human bronchial epithelial cells, at the 2010 Experimental Biology Meeting; 2010 April 24–28; Anaheim, CA. FASEB J 24:612.3.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.184416.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- [Ca2+]i
- intracellular calcium concentration
- ACh
- acetylcholine
- AM
- acetoxymethyl
- ASM
- airway smooth muscle
- BAPTA
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester
- BEC
- bronchial epithelial cell
- CSD
- caveolin-1 scaffolding domain
- DAF-2
- 4,5-diaminofluorescein
- DAF-4
- 4-aminofluorescein diacetate
- DA
- diacetate
- DEANO
- diethylamine-NONOate
- DPN
- diaryl-propionitrile
- E2
- 17β-estradiol
- ER
- estrogen receptor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GL
- gray level
- GPCR30
- G protein-coupled receptor 30
- ICI 182,780
- 7α,17β-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol
- IP3
- inositol trisphosphate
- l-NAME
- NG-nitro-l-arginine methyl ester
- MAHMA
- 6-(2-hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-hexanamine
- NO
- nitric oxide
- NOS
- NO synthase
- eNOS
- endothelial NOS
- p-ENOS
- phosphorylated ENOS
- iNOS
- inducible NOS
- p-Akt
- phosphorylated Akt
- THC
- (R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol
- XeC
- xestospongin C
- 1400W
- N-([3-(Aminomethyl)phenyl]methyl)ethanimidamide dihydrochloride
- GA-1000
- gentamicin/amphotericin B
- G-1
- (±)-1-[(3aR*, 4S*, 9bS*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone.
Authorship Contributions
Participated in research design: Townsend, Meuchel, Thompson, Pabelick, and Prakash.
Conducted experiments: Townsend, Meuchel, Pabelick, and Prakash.
Contributed new reagents or analytic tools: Pabelick and Prakash.
Performed data analysis: Townsend and Meuchel.
Wrote or contributed to the writing of the manuscript: Townsend, Meuchel, Thompson, Pabelick, and Prakash.
References
- Becklake MR, Kauffmann F. (1999) Gender differences in airway behaviour over the human life span. Thorax 54:1119–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellia V, Augugliaro G. (2007) Asthma and menopause. Monaldi Arch Chest Dis 67:125–127 [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Ward JK, Mitchell JA, Barnes PJ. (1995) Nitric oxide as a neurotransmitter in human airways. Arch Int Pharmacodyn Ther 329:97–110 [PubMed] [Google Scholar]
- Boitano S, Dirksen ER, Sanderson MJ. (1992) Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science 258:292–295 [DOI] [PubMed] [Google Scholar]
- Bove PF, van der Vliet A. (2006) Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic Biol Med 41:515–527 [DOI] [PubMed] [Google Scholar]
- Chhabra SK. (2005) Premenstrual asthma. Indian J Chest Dis Allied Sci 47:109–116 [PubMed] [Google Scholar]
- Di Maria GU, Spicuzza L, Mistretta A, Mazzarella G. (2000) Role of endogenous nitric oxide in asthma. Allergy 55(Suppl 61):31–35 [DOI] [PubMed] [Google Scholar]
- Dimitropoulou C, White RE, Ownby DR, Catravas JD. (2005) Estrogen reduces carbachol-induced constriction of asthmatic airways by stimulating large-conductance voltage and calcium-dependent potassium channels. Am J Respir Cell Mol Biol 32:239–247 [DOI] [PubMed] [Google Scholar]
- Farha S, Asosingh K, Laskowski D, Hammel J, Dweik RA, Wiedemann HP, Erzurum SC. (2009) Effects of the menstrual cycle on lung function variables in women with asthma. Am J Respir Crit Care Med 180:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Lonchampt M, Coge F, Galizzi JP, Bassoullet C, Merial C, Robineau P, Boutin JA, Huang PL, Vanhoutte PM, et al. (2001) Regulation of murine airway responsiveness by endothelial nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol 281:L258–L267 [DOI] [PubMed] [Google Scholar]
- Folkerts G, Nijkamp FP. (2006) Nitric oxide in asthma therapy. Curr Pharm Des 12:3221–3232 [DOI] [PubMed] [Google Scholar]
- García-Cardeña G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. (1997) Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem 272:25437–25440 [DOI] [PubMed] [Google Scholar]
- Gibbs CJ, Coutts II, Lock R, Finnegan OC, White RJ. (1984) Premenstrual exacerbation of asthma. Thorax 39:833–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley SP. (1981) Asthma variation with menstruation. Br J Dis Chest 75:306–308 [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, et al. (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931 [DOI] [PubMed] [Google Scholar]
- Hisamoto K, Bender JR. (2005) Vascular cell signaling by membrane estrogen receptors. Steroids 70:382–387 [DOI] [PubMed] [Google Scholar]
- Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, Snyder SH, Burnett AL. (2002) Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A 99:4061–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova MM, Mazhawidza W, Dougherty SM, Minna JD, Klinge CM. (2009) Activity and intracellular location of estrogen receptors α and β in human bronchial epithelial cells. Mol Cell Endocrinol 305:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Malavia N, Suresh V, George SC. (2009) Nitric oxide gas phase release in human small airway epithelial cells. Respir Res 10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. (1997) Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272:18522–18525 [DOI] [PubMed] [Google Scholar]
- Kharitonov SA, Logan-Sinclair RB, Busset CM, Shinebourne EA. (1994) Peak expiratory nitric oxide differences in men and women: relation to the menstrual cycle. Br Heart J 72:243–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch EA, Yuhanna IS, Chen Z, German Z, Sherman TS, Shaul PW. (1999) Estrogen acutely stimulates endothelial nitric oxide synthase in H441 human airway epithelial cells. Am J Respir Cell Mol Biol 20:658–666 [DOI] [PubMed] [Google Scholar]
- Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. (2008) Resveratrol stimulates nitric oxide production by increasing estrogen receptor α-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J 22:2185–2197 [DOI] [PubMed] [Google Scholar]
- Kone BC. (2000) Protein-protein interactions controlling nitric oxide synthases. Acta Physiol Scand 168:27–31 [DOI] [PubMed] [Google Scholar]
- Krasteva G, Pfeil U, Drab M, Kummer W, König P. (2006) Caveolin-1 and -2 in airway epithelium: expression and in situ association as detected by FRET-CLSM. Respir Res 7:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers JW, Barnes PJ, Chung KF. (1992) Nonadrenergic, noncholinergic airway inhibitory nerves. Eur Respir J 5:239–246 [PubMed] [Google Scholar]
- Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. (2010) A critical review of fundamental controversies in the field of GPR30 research. Steroids 75:603–610 [DOI] [PubMed] [Google Scholar]
- Levin ER. (2002) Cellular functions of plasma membrane estrogen receptors. Steroids 67:471–475 [DOI] [PubMed] [Google Scholar]
- Mandhane PJ, Hanna SE, Inman MD, Duncan JM, Greene JM, Wang HY, Sears MR. (2009) Changes in exhaled nitric oxide related to estrogen and progesterone during the menstrual cycle. Chest 136:1301–1307 [DOI] [PubMed] [Google Scholar]
- Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. (2007) Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep 7:143–150 [DOI] [PubMed] [Google Scholar]
- Meuchel LW, Thompson MA, Cassivi SD, Pabelick CM, Prakash YS. (2011) Neurotrophins induce nitric oxide generation in human pulmonary artery endothelial cells. Cardiovasc Res 91:668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Gibson PG. (2008) Premenstrual asthma: prevalence, cycle-to-cycle variability and relationship to oral contraceptive use and menstrual symptoms. J Asthma 45:696–704 [DOI] [PubMed] [Google Scholar]
- Nijkamp FP, Folkerts G. (1995) Nitric oxide and bronchial hyperresponsiveness. Arch Int Pharmacodyn Ther 329:81–96 [PubMed] [Google Scholar]
- Ortiz PA, Garvin JL. (2003) Trafficking and activation of eNOS in epithelial cells. Acta Physiol Scand 179:107–114 [DOI] [PubMed] [Google Scholar]
- Postma DS. (2007) Gender differences in asthma development and progression. Gend Med 4(Suppl B):S133–S146 [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. (2004) Nitric oxide in health and disease of the respiratory system. Physiol Rev 84:731–765 [DOI] [PubMed] [Google Scholar]
- Schatz M, Camargo CA., Jr (2003) The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol 91:553–558 [DOI] [PubMed] [Google Scholar]
- Shaul PW. (2002) Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 64:749–774 [DOI] [PubMed] [Google Scholar]
- Simoncini T, Genazzani AR. (2003) Non-genomic actions of sex steroid hormones. Eur J Endocrinol 148:281–292 [DOI] [PubMed] [Google Scholar]
- Townsend EA, Thompson MA, Pabelick CM, Prakash YS. (2010) Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 298:L521–L530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze A, Postma DS, Kerstjens HA. (2003) Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol 112:271–282 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.