Abstract
Intestinal secretory movement of the fluoroquinolone antibiotic, ciprofloxacin, may limit its oral bioavailability. Active ATP-binding cassette (ABC) transporters such as breast cancer resistance protein (BCRP) have been implicated in ciprofloxacin transport. The aim of this study was to test the hypothesis that BCRP alone mediates intestinal ciprofloxacin secretion. The involvement of ABC transport proteins in ciprofloxacin secretory flux was investigated with the combined use of transfected cell lines [bcrp1/BCRP-Madin-Darby canine kidney II (MDCKII) and multidrug resistance-related protein 4 (MRP4)-human embryonic kidney (HEK) 293] and human intestinal Caco-2 cells, combined with pharmacological inhibition using 3-(6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6, 7,12,12a-octahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester (Ko143), cyclosporine, 3-[[3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl]-(2-dimethylcarbamoylethylsulfanyl)methylsulfanyl] propionic acid (MK571), and verapamil as ABC-selective inhibitors. In addition, the regional variation in secretory capacity was investigated using male Han Wistar rat intestine mounted in Ussing chambers, and the first indicative measurements of ciprofloxacin transport by ex vivo human jejunum were made. Active, Ko143-sensitive ciprofloxacin secretion was observed in bcrp1-MDCKII cell layers, but in low-passage (BCRP-expressing) Caco-2 cell layers only a 54% fraction was Ko143-sensitive. Ciprofloxacin accumulation was lower in MRP4-HEK293 cells than in the parent line, indicating that ciprofloxacin is also a substrate for this transporter. Ciprofloxacin secretion by Caco-2 cell layers was not inhibited by MK571. Secretory flux showed marked regional variability in the rat intestine, increasing from the duodenum to peak in the ileum. Ciprofloxacin secretion was present in human jejunum and was reduced by Ko143 but showed marked interindividual variability. Ciprofloxacin is a substrate for human and rodent BCRP. An additional pathway for ciprofloxacin secretion exists in Caco-2 cells, which is unlikely to be MRP(4)-mediated. BCRP is likely to be the dominant transport mechanism for ciprofloxacin efflux in both rat and human jejunum.
Introduction
Fluoroquinolone antibiotics such as ciprofloxacin are widely used antimicrobial agents displaying broad-spectrum activity against both Gram-positive and Gram-negative bacteria. At physiological pH (7.4), ciprofloxacin exists predominantly as a zwitterion, with a shift toward the cationic form at pH 6.5 (Sörgel and Kinzig, 1993). The octanol-water partition coefficient (logP) value for this compound is −1.08, suggesting moderately hydrophilic properties, which would serve to reduce lipid partitioning and passive transcellular absorptive permeability (Zhao et al., 2002). In agreement with this assessment, ciprofloxacin has been classified as a low-permeability compound (Volpe, 2004; Zakelj et al., 2006). In vivo it displays variable oral bioavailability of between 50 and 80% (Sörgel et al., 1989a). Ciprofloxacin plasma clearance in vivo is predominantly renal (Jaehde et al., 1989; Rohwedder et al., 1990), but it is also subject to intestinal elimination (Sörgel et al., 1989b, 1991) without undergoing significant metabolism (Sörgel et al., 1989b). The intestinal elimination pathway becomes increasingly important for patients with reduced renal function (Rohwedder et al., 1990).
The mechanisms responsible for ciprofloxacin secretion in the gut have been widely investigated. As has been recently highlighted, many fluoroquinolones are subject to ATP-binding cassette (ABC) transporter-mediated efflux (Alvarez et al., 2008). In a previous study, Griffiths et al. (1993) demonstrated saturable ATP-dependent secretory transport in human intestinal Caco-2 cells with a number of fluoroquinolones sharing a common secretory pathway (Griffiths et al., 1993, 1994). Measurements of basolateral and apical transport suggested the existence of distinct transporters at either membrane domain (Griffiths et al., 1994) although transport characteristics were distinct from those of vinblastine, a known P-glycoprotein (P-gp) substrate (Cavet et al., 1997). Lowes and Simmons (2002) confirmed the lack of involvement of MDR1 in ciprofloxacin secretion using wild-type and MDR1-transfected MDCKII cells. In addition, Merino et al. (2006) have shown ciprofloxacin secretion using murine bcrp1-transfected MDCKII epithelial layers, whereas in human BCRP-transfected MDCKII cells ciprofloxacin secretion was evident only at extended incubation times. In both cases, net secretion was inhibited by the selective BCRP inhibitor 3-(6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6, 7,12,12a-octahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester (Ko143) (Merino et al., 2006). In bcrp knockout mice, ciprofloxacin plasma concentrations were 2-fold greater after oral administration, suggesting significant involvement of bcrp1 in modulating systemic drug levels (Merino et al., 2006).
Ciprofloxacin transport may occur through additional ABC transporters. A recent investigation of bacterial resistance to fluoroquinolones has studied the impact of MRP transporters on ciprofloxacin accumulation in murine J774 macrophages (Marquez et al., 2009). It was noted that the resistant populations displayed increased expression of both mrp2 and mrp4 and reduced levels of intracellular ciprofloxacin. With the use of small interfering RNA to selectively deplete either MRP2 or MRP4, it was shown that mrp4 alone acts to reduce ciprofloxacin accumulation (Marquez et al., 2009).
There are few direct measurements of ciprofloxacin secretion across intestinal segments in vitro. Active secretion of ciprofloxacin was observed in rat small intestinal tissue mounted in Ussing chambers, with higher secretory flux being observed in distal than in proximal small intestine (Zakelj et al., 2006). However, Rodríguez-Ibáñez et al. (2006) demonstrated no regional variation in absorptive ciprofloxacin permeability in the rat using the in situ gut loop technique. There are no ex vivo measurements of ciprofloxacin transport in human intestine, and the impact of differential ciprofloxacin secretory capacity along the proximal-distal axis of the gut remains unclear.
The objectives of the current study were 2-fold. First, the question whether BCRP alone may mediate intestinal secretion of ciprofloxacin was addressed by the use of murine bcrp1-, hBCRP-, and hMRP4-transfected cell lines, together with Caco-2 epithelial layers in which the selective BCRP inhibitor, Ko143, was used. Second, the extent of BCRP-mediated ciprofloxacin secretion by human and rat intestine, including investigation of regional variation in ciprofloxacin efflux in the rat has been determined in excised intestinal tissue mounted in Ussing chambers.
Materials and Methods
Materials.
[14C]Mannitol (specific activity 56 mCi · mmol−1) was from PerkinElmer Life and Analytical Sciences (Waltham, MA). [14C]Ciprofloxacin was a generous gift from Bayer (Wuppertal, Germany). Cell culture media and supplements were from Sigma-Aldrich (Poole, Dorset, UK), and tissue culture plastic flasks and culture plates were supplied by Costar (High Wycombe, UK). All other chemicals were obtained from Sigma-Aldrich.
Ussing Chamber Experiments.
Ethics approval for the use of human intestinal samples from patients undergoing pancreatoduodenectomy to remove tumors of the head of the pancreas surplus to histopathological requirements was granted (Integrated Research Application System reference: 09/H1006/2). Human tissue samples were collected from three such male patients with their informed consent. None of the patients had undergone radiotherapy or chemotherapy before surgery. Blood perfusion of the intestine was maintained until the moment of excision. Approximately 10 cm of proximal jejunum was removed and immediately placed in ice-cold Krebs bicarbonate-Ringer solution, presaturated with carbogen. The tissue was then transported from the hospital operating theater to the laboratories at AstraZeneca (Macclesfield, Cheshire, UK). The mean time period between excision of intestinal tissue and arrival at the laboratory was 88 ± 19 min. Tissue was prepared for use in the Ussing chambers as described previously (Haslam et al., 2011).
Male Han Wistar rats (Harlan, UK), aged approximately 100 days and weighing 250 to 400 g, were used in the experiments. Animal dosing and surgical/anesthetic procedures were performed in accordance with UK law and ethical guidelines described in the National Institutes of Health Guide for the Care and Use of Laboratory Animal Care (NIH publication 85-23, revised 1985). For surgical procedures, rats were anesthetized using inhaled isoflurane (induction 5%, 2 l/min and thereafter 3%, 0.7 l/min). After abdominal incisions, intestinal lumens were occluded by thin cotton thread proximal to the sections of tissue taken from duodenum (within 15 cm proximal to the pyloric sphincter), jejunum (5 cm distal to the ligament of Treitz), ileum (2 cm proximal to the ileocecal junction), and colon (2 cm distal to the cecum). The segments were placed in a chamber containing ice-cold KBR perfused with carbogen for 30 min. Removal by dissection of the serosal layer of the duodenal, jejunal, and ileal sections and the serosa and muscularis externa of the colonic sections was performed with the aid of a stereo microscope (Wild M8) and a light source (Schott KL 1500).
Intestinal segments (rat and human) were mounted as flat sheets in a modified Ussing chamber (Navicyte; Harvard Apparatus Inc., Holliston, MA). The exposed tissue surface area was 0.64 or 1.78 cm2 for rat and human sections, respectively. A four-electrode system was used for recording electrical parameters. This consisted of two Ag/AgCl electrodes for potential difference (PD) measurement and two Ag/AgCl electrodes for current passage. Asymmetry in the PD sensing electrodes was zeroed before tissue mounting. Correction for the series fluid resistance was also made in assembled chambers before tissue mounting. After 30 to 40 min of equilibration at 37°C, mean PD values were comparable with those in previous studies (Polentarutti et al., 1999), indicative of viable tissue segments.
To initiate experiments, the KBR in “donor” or “receiver” wells was replaced with 5 ml of KBR containing ciprofloxacin (1–100 μM) or KBR alone. Inhibitors, where used, were added to both apical and basal wells. Samples were removed at 30-min intervals up to 150 min, for analysis by HPLC-mass spectrometry. Net secretion (Jnet) was calculated from paired adjacent tissues by subtracting apical-to-basal (Ja-b) from basal-to-apical (Jb-a) flux. Fluxes were expressed as nanomoles per centimeter squared per hour or as apparent permeability coefficients (Papp, Pa-b, and Pb-a) and were calculated as reported previously (Söderholm et al., 1998). Pa, Pb, and Papp are expressed as centimeters per second.
Cell Culture.
All cell culture was performed in a class II laminar flow hood (SafeFlow 1.2; BioAir Instruments, Pavia, Italy) under aseptic conditions. Caco-2 cells were maintained in high-glucose (4500 mg/l d-glucose) Dulbecco's modified Eagle's medium supplemented with fetal calf serum (10% v/v), l-glutamine (1 mM), nonessential amino acids (1% v/v), and the antibiotic gentamicin (30 μg/ml). Two Caco-2 cell strains were used: a high-passage (PA) strain (115–120 passages) originating from Dr. I. Hassan as described previously (Cavet et al., 1997) and a low-PA strain (passages 34–40) originating from AstraZeneca, displaying rapid growth and higher values of transepithelial resistance (American Type Culture Collection, Manassas, VA). Caco-2 cells were seeded onto 12-well Transwell supports (12-mm diameter, 0.4-μm pore size, 1.14-cm2 growth area) at high density (5 × 105 cells/cm2). Cells were maintained at 37°C in a humidified incubator with 5% CO2 in air. Cells were grown to confluence for 14 days, and transepithelial electrical resistance was measured using a EVOM voltohmmeter (World Precision Instruments, Stevenage, Hertfordshire, UK). Typical resistance values were 250 Ω · cm2 for low-PA Caco-2 cells and 400 Ω · cm2 for high-PA Caco-2 cells.
MDCKII native, human BCRP-MDCKII, and mouse bcrp1-MDCKII cells were a gift from Alfred Schinkel (Netherlands Cancer Institute, Amsterdam, The Netherlands). MDCKII cell lines were cultured in minimum essential Eagle's medium with fetal calf serum (10% v/v), nonessential amino acids (1% v/v), and l-glutamine (1% v/v) and a penicillin/streptomycin mix (1% v/v). Medium was replaced every 3 to 4 days. MDCKII cells were seeded onto 12-well Transwell supports and left for 5 to 7 days.
Native HEK (293/4.59) and MRP4-transfected HEK cells (293/4.63) were a gift from Prof. P. Borst of the Netherlands Cancer Institute (Reid et al., 2003). HEK293 cell lines were maintained in high-glucose (4500 mg/l d-glucose) Dulbecco's modified Eagle's medium supplemented with fetal calf serum (10% v/v) and penicillin/streptomycin (1%, v/v).
Transepithelial Transport Experiments in Cultured Monolayers.
Bidirectional transepithelial transport experiments were performed using Caco-2 and MDCKII cell monolayers. Upon reaching confluence, cell monolayers grown on Transwell plates were washed (three times) in a modified Krebs' buffer consisting of 137 mM NaCl, 5.4 mM KCl, 1 mM MgSO4, 0.3 mM NaH2PO4, 0.3 mM KH2PO4, 10 mM glucose, and 10 mM HEPES (buffered to pH 7.4 at 37°C with Trizma base). Monolayers were placed in fresh base plates and were allowed a 15-min equilibration period at 37°C before monolayer integrity measurements. Acceptable Caco-2 monolayers generated transepithelial electrical resistance values that were typically >200 Ω · cm2. Because MDCK II monolayers display low RT values, monolayer integrity was also assessed by eliciting a dilution transepithelial PD by replacing the Krebs' solution NaCl with 274 mM mannitol in the basolateral compartment, resulting in a basal electropositive PD. PD values were typically in the range of 60 to 80 mV for confluent MDCKII monolayers.
Flux experiments were performed using identical apical and basal Krebs' buffer composition with the addition of radiolabeled [14C]ciprofloxacin (total ciprofloxacin concentration 10 μM) or other substrate to the donor (apical or basal) compartment. Samples were taken to determine absorptive (apical-to-basal, Ja-b) and secretory (basal-to-apical, Jb-a) fluxes at hourly intervals up to 3 h. Jnet was calculated from paired epithelial monolayers by subtracting Ja-b from Jb-a flux. Flux calculations were performed as described previously (Cavet et al., 1997). The passive paracellular flux was determined by concurrent measurement of [3H]mannitol movement. Movement of mannitol into the contralateral compartment was generally <2%. Monolayers in which contralateral mannitol movement was >3% were discounted from the results, based on compromised monolayer integrity. [14C]Ciprofloxacin activities were determined by liquid scintillation counting.
Cellular Ciprofloxacin Accumulation in HEK293 Cells.
HEK293 cells were seeded at 5 × 104 onto 12-well plates (Corning Life Sciences, Lowell, MA). Medium was aspirated and replaced with transport buffer (Krebs' buffer with additional radiolabel and inhibiting agents). Cell were incubated at 37°C for 1 h and were then washed with ice-cold Krebs' buffer. Cells were then treated with 500 μl of lysis buffer (0.05% Triton X-100), and samples were collected for measurement and protein correction via a Bradford assay. Cellular accumulation of [14C]ciprofloxacin (10 μM) was determined by liquid scintillation counting.
RNA Isolation and Quantitative PCR.
RNA was isolated from confluent flasks of epithelial cells or mucosal scrapings from intestinal tissue samples, using a RNeasy Mini Kit (QIAGEN, Valencia, CA), according to the manufacturer's standard protocol. Any contaminating DNA was digested with Turbo DNA-free (Ambion, Huntingdon, UK). RNA was quantified using a ND-1000 spectrophotometer (Nanodrop Technologies Inc., Wilmington, DE), and integrity was checked by measurement of the A260/280 nm ratio, which was routinely in the range of 1.8 to 2.0. cDNA synthesis from 1 μg of total RNA was performed using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. Product amplification by qPCR was performed with 2 μl of cDNA reaction, using qPCR MasterMix plus Low ROX (Eurogentec, Seraing, Belgium) according to the manufacturer's guidelines. Negative controls involved omission of RNA from the reverse transcription reactions and amplification with specific primer/probe sets to confirm the lack of genomic DNA contamination. Gene-specific TaqMan primer/probe sets for ABCB1 (MDR1), ABCC2, ABCC4, and ABCG2 were purchased from Invitrogen.
Statistics.
Results are expressed as mean ± S.E.M. (of n experiments). For statistics relating to Jnet, individual values of net flux from paired monolayers were used. Individual experiments were conducted with at least three replicates per condition, mean values being computed for n separate experiments. Statistical analysis was performed using one- or two-tailed Student's t tests (paired or unpaired as appropriate) with post hoc power calculations as appropriate or one-way analysis of variance with a Bonferroni post-test for multiple comparisons (SigmaPlot 11; Systat Software, Inc., Chicago, IL). A post hoc power calculation was made. Kinetic constants for Michaelis-Menten kinetics and IC50 values were calculated by nonlinear regression with the method of least squares (GraphPad Instat; GraphPad Software Inc., San Diego, CA).
Results
Ciprofloxacin Secretory Transport in Cultured Cell Lines.
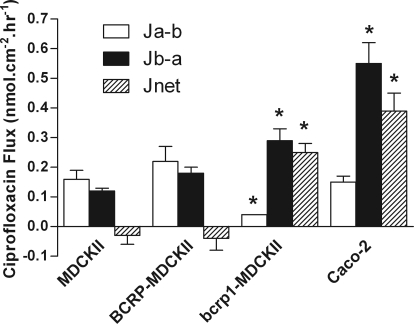
Ciprofloxacin flux was determined in the absorptive (Ja-b) and secretory (Jb-a) directions in MDCKII and Caco-2 cell layers (Fig. 1). In wild-type MDCKII monolayers, the bidirectional fluxes were similar with no significant net secretory flux evident (Fig. 1). The same pattern was also observed in BCRP-MDCKII cells. In contrast, murine bcrp1-transfected MDCKII cell monolayers showed a marked net secretion of ciprofloxacin compared with wild-type cell layers (n = 3, P < 0.05). Efflux ratios (Jb-a/Ja-b) for ciprofloxacin transport (Fig. 1) were 0.8, 0.8, 7.6, and 3.6 for WT-MDCKII, BCRP-MDCKII, bcrp1-MDCKII, and Caco-2 cells, respectively.
Fig. 1.
Transepithelial [14C]ciprofloxacin fluxes across confluent monolayers of MDCKII, bcrp1-MDCKII, and Caco-2 cells grown on permeable Transwell supports. Total ciprofloxacin concentration was 10 μM. Fluxes were determined in the apical-to-basal (Ja-b) and basal-to-apical (Jb-a) directions, where secretory net flux Jnet = Jb-a − Ja-b. *, significant differences in flux values in relation to MDCKII monolayers, P < 0.05. n = 3 separate experiments.
Table 1 confirms high levels of murine bcrp1 and human BCRP mRNA expression in the respective transfected MDCKII cell lines. Ciprofloxacin secretion was also evident across both high-passage (Figs. 1 and 2C) and low-passage (Fig. 2B) Caco-2 cell monolayers. Table 1 shows detection of hBCRP expression in both Caco-2 cell strains although hBCRP mRNA expression relative to GAPDH in low-passage Caco-2 cells is greater than that seen in high-passage cells.
TABLE 1.
Transporter mRNA expression levels (relative to GAPDH) in cultured epithelial cells
Results are n = 3 repeats.
| Cell Line | BCRP (ABCG2) | bcrp1 (abcg2) | MRP4 (ABCC4) |
|---|---|---|---|
| WT-MDCKII | 0.71 ± 0.16 | ||
| bcrp1-MDCKII | 0.02 ± 0.01 | 3803.80 ± 504.12 | |
| BCRP-MDCKII | 20389.59 ± 377.91 | ||
| WT-HEK293 | 0.42 ± 0.05 | ||
| MRP4-HEK293 | 94.67 ± 2.39 | ||
| Low PA Caco-2 | 26.87 ± 0.69 | 2.12 ± 0.18 | |
| High PA Caco-2 | 0.43 ± 0.08 | 0.08 ± 0.01 |
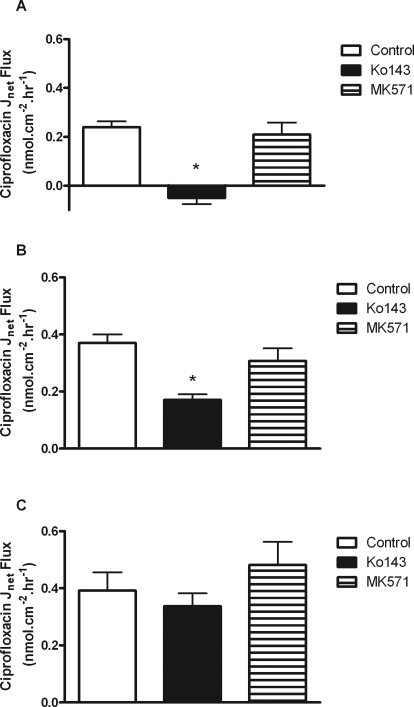
Fig. 2.
Net transepithelial [14C]ciprofloxacin flux (Jnet) across confluent monolayers of bcrp1-MDCKII cells (A), low-PA Caco-2 cells (B), and high-PA Caco-2 cells (C) grown on permeable Transwell supports. Fluxes were determined in the presence and absence of Ko143 (1 μM) and MK571 (10 μM). Other experimental details are as in the legend for Fig. 1. *, significant reductions in Jnet compared with control values, P < 0.05. n = 3 separate experiments.
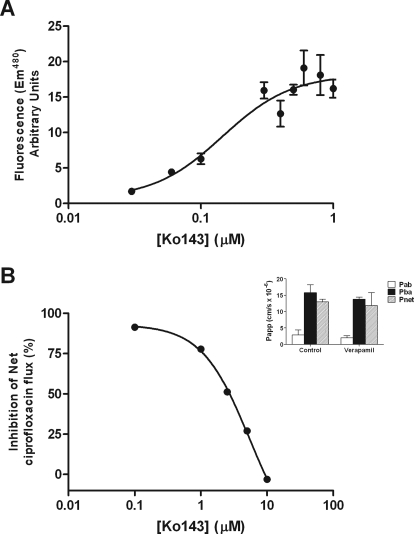
The pharmacological sensitivity of ciprofloxacin secretion by bcrp1-MDCKII and Caco-2 cell layers was assessed. Ko143, a potent and specific BCRP inhibitor (Allen et al., 2002), has an IC50 of 0.15 μM for increasing Hoechst 33342 accumulation in bcrp1-MDCKII cells due to inhibition of efflux (Fig. 5A). At 1 μM, Ko143 completely abolished net secretory ciprofloxacin flux in bcrp1-MDCKII cells (0.24 ± 0.02 to −0.05 ± 0.03 nmol · cm−2 · h−1, n = 3; P < 0.05) (Fig. 2A). Ko143 (1 μM) reduced net ciprofloxacin secretion in low passage Caco-2 cells from 0.37 ± 0.03 to 0.17 ± 0.02 nmol · cm−2 · h−1 (P < 0.05) (Fig. 2B) but had no significant impact on net flux in the high passage Caco-2 layers (Fig. 2C) (from 0.39 ± 0.06 to 0.33 ± 0.04 nmol · cm−2 · h−1 plus inhibitor, n = 9; NS). This result correlates with the different expression levels of BCRP mRNA between Caco-2 cell strains (Table 1).
Fig. 5.
Comparison of the dose-dependent inhibition of rodent bcrp by Ko143. A, increase in Hoechst 33342 cellular accumulation in bcrp1-MDCKII cells. B, inhibition of net ciprofloxacin flux across excised sections of rat ileum expressed as a percentage of control ciprofloxacin secretion with a donor concentration of 100 μM. Other details as in the legend to Fig. 4. Data are mean ± S.E.M. of n = 3 separate animals. Inset, ciprofloxacin permeability across excised rat ileum in the absence and presence of 100 μM verapamil.
Inhibition of ciprofloxacin secretion in Caco-2 cells was also investigated using cyclosporine (CsA). It is now known that CsA inhibits both P-gp (Loor et al., 2002) and also BCRP, albeit at lower affinity (Gupta et al., 2006; Matsson et al., 2009). Application of high-dose (50 μM) CsA abolished net secretory ciprofloxacin flux in bcrp1-MDCKII cells (from 0.24 ± 0.02 to 0.04 ± 0.02 nmol · cm−2 · h−1, n = 3; P < 0.05 versus controls) but inhibited only a fraction of net secretory flux in low passage Caco-2 cell monolayers (from 0.39 ± 0.06 to 0.22 ± 0.04 nmol · cm−2 · h−1, n = 3; P < 0.05). Taken together with Ko143 inhibition, these data suggest that BCRP cannot account for all of ciprofloxacin secretion noted in either low- or high-passage Caco-2 cells.
Previously, we have shown that the anion transport inhibitor 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS) at 0.4 mM may inhibit ciprofloxacin secretion across Caco-2 monolayers (Cavet et al., 1997). Secretory ciprofloxacin flux was reduced but not abolished, in both bcrp1-MDCKII cells (from 0.24 ± 0.02 to 0.18 ± 0.02 nmol · cm−2 · h−1, n = 3; P < 0.05) and low-passage Caco-2 monolayers (from 0.39 ± 0.06 to 0.14 ± 0.03 nmol · cm−2 · h−1, n = 3; P < 0.05), suggesting that DIDS is therefore not a definitive pharmacological agent with respect to the inhibition of ciprofloxacin secretion.
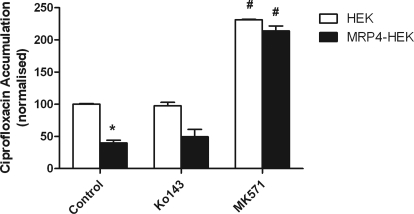
To test the possible involvement of MRP4 (Marquez et al., 2009), ciprofloxacin accumulation was investigated in WT-HEK293 and MRP4-transfected HEK293 cells. Uptake was significantly reduced in the MRP4-overexpressing line in relation to the wild type (Fig. 3). Addition of the MRP inhibitor, 3-[[3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl]-(2-dimethylcarbamoylethylsulfanyl)methylsulfanyl] propionic acid (MK571), increased ciprofloxacin accumulation in MRP4-HEK293 cells to the levels seen in untransfected cells (Fig. 3). Ko143 at 1 μM was without effect on ciprofloxacin accumulation in both control and MRP4-HEK293 cells, confirming the relative specificity of this agent. MRP4 mRNA expression was detected in low passage Caco-2 cells (Table 1). In both low- and high-passage Caco-2 cell monolayers, MK571 (10 μM) was without effect (Fig. 2, B and C), confirming no MRP(4) involvement in ciprofloxacin secretion in this model system. MK571 had no significant effect on ciprofloxacin secretion in bcrp1-MDCKII cells (Fig. 2A).
Fig. 3.
Cellular accumulation of [14C]ciprofloxacin (external concentration 10 μM) in HEK293 and MRP4-HEK293 cells. Uptake was measured in the presence and absence of the MRP4 inhibitor MK571 (10 μM) and the BCRP inhibitor Ko143 (1 μM). *, significant reduction in accumulation in the MRP4-HEK293 cells versus wild-type HEK293 cells, P < 0.05. n = 3 separate experiments. #, significant increases in accumulation through inhibition, P < 0.05. n = 3 separate experiments.
Ciprofloxacin Secretion in Rat Intestinal Tissue.
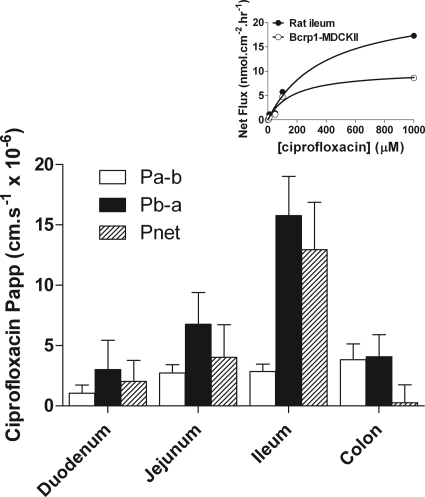
Figure 4 shows that excised rat small intestine is able to maintain a substantial net ciprofloxacin secretion from basal (blood) to apical (lumen) solutions. Asymmetric permeability was noted in all regions of the small intestine (duodenum, jejunum, and ileum), but no net secretion was observed in the colon. Apical-to-basal permeability (Pa-b) displayed a marginal increase along the proximal-distal axis of the gut (1.04 ± 0.68, 2.73 ± 0.67, 2.84 ± 0.62, and 3.82 ± 1.32 cm/s × 10−6 for duodenum, jejunum, ileum, and colon, respectively); however, secretory movement (Pb-a) differed substantially (3.01 ± 2.44, 6.75 ± 2.65, 15.78 ± 2.84, and 4.07 ± 1.84 cm/s × 10−6 for duodenum, jejunum, ileum, and colon, respectively). Secretory permeability was greatest in the ileum which resulted in the largest transepithelial asymmetry. Ratios of secretory (Pb-a) to absorptive (Pa-b) permeabilities were therefore 2.9, 2.5, 5.6, and 1.1 in the duodenum, jejunum, ileum, and colon, respectively. The higher absorptive permeability in the colon combined with the absence of net secretion indicates that colonic delivery of ciprofloxacin may increase fractional absorption.
Fig. 4.
Ciprofloxacin permeability across excised sections of rat duodenum, jejunum, ileum, and colon. Ciprofloxacin permeability was determined in the apical-to-basal (Pa-b) and basal-to-apical (Pb-a) directions in adjacent tissue segments giving net permeability (Pnet = Pb-a − Pa-b). Total ciprofloxacin concentration in the donor compartment was 30 μM. Ciprofloxacin concentrations were determined by HPLC-tandem mass spectrometry. Data are mean ± S.E.M. of n = 3 separate animals. Inset, dose-response curves showing the net flux (Jnet) of increasing concentrations of ciprofloxacin in excised rat ileum (●) and bcrp1-MDCKII monolayers (○).
Table 2 and Fig. 4 (inset) compare the kinetics of net ciprofloxacin secretion (Jnet) in bcrp1-MDCKII epithelia with those for rat ileum. The maximal secretory capacity of rat ileum is 2-fold greater than that of bcrp1-transfected MDCKII cells (Table 2). For comparison, kinetic data for ciprofloxacin secretion by Caco-2 intestinal epithelia are shown (Table 2) emphasizing that a lower affinity secretion is seen (Griffiths et al., 1993).
TABLE 2.
Ciprofloxacin net flux kinetics in cultured cell lines and rat ileum
| Tissue | Km | Vmax |
|---|---|---|
| μM | nmol · cm−2 · h−1 | |
| Rat ileum | 130.6 ± 1.2 | 17.3 ± 4.6 |
| Caco-2a | 890 ± 230 | 44.3 ± 4.9 |
| bcrp1-MDCKII | 94.7 ± 1.1 | 8.6 ± 2.0 |
Caco-2 data are from Griffiths et al. (1993).
No significant effect of 100 μM verapamil on the absorptive (Pa-b), secretory (Pb-a), and net (Pnet) ciprofloxacin permeabilities was observed in rat ileum (Fig. 5B, inset), indicating that ciprofloxacin secretion in rat intestine is not mediated by MDR1. In contrast, marked attenuation of ciprofloxacin secretion in the ileum was observed using the BCRP inhibitor Ko143 (Fig. 5B). Inhibition was only evident at Ko143 concentrations in excess of 1 μM, with complete inhibition of secretion at 10 μM with an IC50 of 5.45 ± 0.16 μM (Fig. 5B). This contrasts with the ability of Ko143 to inhibit bcrp activity, as assayed by increased cellular accumulation of Hoechst 33342 in bcrp1-MDCKII cells (Fig. 5A), in which the IC50 was 0.15 ± 0.06 μM.
ABC Transporter mRNA Expression along the Rat Intestinal Tract.
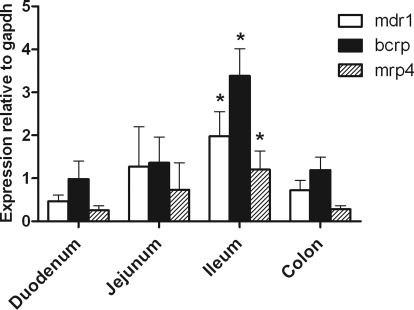
Intestinal segmental variations in the expression of mdr1a, mrp4, and bcrp mRNA was determined by quantitative PCR (Fig. 6). For bcrp, the greatest level of expression (relative to GAPDH) was observed in the ileum. This correlates to the magnitude of ciprofloxacin secretion (Fig. 6). mdr1a, bcrp1, and mrp4 all showed a similar aboral increase in expression, with a peak evident in the ileum (P < 0.05; n = 3–5 animals, relative to duodenum). Expression of all transcripts was lower in the colon than in the ileum. These results confirm data reported in previous studies for bcrp (MacLean et al., 2008). In contrast to these data, the same authors have reported higher mdr1a expression in the colon than in the ileum, although these data were not significant. Stephens et al. (2002) reported higher P-gp protein levels in the ileum and distal colon than in proximal colon.
Fig. 6.
Relative changes in mdr1a, bcrp1, and mrp4 expression in the four separate regions of rat small intestine determined by TaqMan analysis after amplification using gene-specific primers and probes. Expression is presented relative to the housekeeping gene GAPDH. *, significant differences in expression level relative to the duodenum was noted for all genes of interest, P < 0.05. n = 3 tissue extracts from separate animals.
Ciprofloxacin Secretion in Human Intestinal Tissue.
Table 3 displays the Pa-b, Pb-a, and Pnet of ciprofloxacin, prazosin, and atenolol across excised sections of human jejunum sourced from pancreatoduodenectomy from three individual donors. Whereas all three patient samples showed net ciprofloxacin secretion, there is considerable interpatient variability. Patient 2 shows values of Pb-a and net secretion that are ∼10-fold higher than those of patients 1 and 3, despite control values for Pa-b being of similar magnitude. In the presence of 10 μM Ko143, a concentration sufficient to completely attenuate active efflux in the rat intestine, net ciprofloxacin secretion was reversed in patients 1 and 3, whereas it was markedly reduced in patient 2. Expressing the effect of Ko143 on net ciprofloxacin secretion as a percentage of change, net ciprofloxacin was changed by 134, 73, and 132% for patients 1, 2, and 3, respectively, with a mean of 113 ± 20% (S.E.M.). This change was significant at P = 0.03 (one-sided t test versus no reduction) with a power of 0.80 (post hoc test α = 0.05). It may be concluded that there is a Ko143-dependent reduction in ciprofloxacin secretion in human jejunum.
TABLE 3.
Individual donor Papp values for ciprofloxacin and prazosin and atenolol movement across excised human jejunum mounted in Ussing chambers
Ciprofloxacin, prazosin, and atenolol permeability across excised sections of human jejunum from pancreatoduodenectomy. Permeability was determined in the apical-to-basal (Pa-b) and basal-to-apical (Pb-a) directions in adjacent tissue segments, giving net permeability (Pnet = Pb-a − Pa-b). Total drug concentrations in donor compartments were 30 μM for ciprofloxacin, 10 μM for prazosin, and 100 μM for atenolol. Ko143 was present at 10 μM. All receiver drug concentrations were determined by HPLC-tandem mass spectrometry. Individual data from three separate human donors.
| Pa-b | Pb-a | Pnet | Efflux Ratio (Pb-a/Pa-b) | |
|---|---|---|---|---|
| cm/s × 10−6 | ||||
| Donor 1 | ||||
| Ciprofloxacin | 10.14 | 20.67 | 10.53 | 2.04 |
| Ciprofloxacin + Ko143 | 11.02 | 7.45 | −3.57 | 0.68 |
| Prazosin | 0.50 | 3.01 | 2.51 | 6.02 |
| Atenolol | 0.24 | 0.40 | 0.16 | 1.68 |
| Donor 2 | ||||
| Ciprofloxacin | 13.07 | 118.25 | 105.18 | 9.05 |
| Ciprofloxacin + Ko143 | 66.07 | 94.42 | 28.35 | 1.43 |
| Prazosin | 1.61 | 2.59 | 0.98 | 1.61 |
| Atenolol | 0.65 | 0.61 | −0.04 | 0.94 |
| Donor 3 | ||||
| Ciprofloxacin | 11.60 | 19.57 | 7.97 | 1.69 |
| Ciprofloxacin + Ko143 | 15.61 | 13.07 | −2.54 | 0.84 |
| Prazosin | 0.55 | 1.85 | 1.3 | 3.36 |
| Atenolol | 0.59 | 0.89 | 0.30 | 1.51 |
In contrast to the data for ciprofloxacin, data for prazosin are consistent among individuals. Prazosin displayed net active secretion in all three donors (mean values of 0.89 ± 0.04 cm/s × 10−6 for Pa-b and 2.48 ± 0.03 cm/s × 10−6 for Pb-a), giving a mean efflux ratio of 2.8. The variability in prazosin permeability between donors was not as great as that seen for ciprofloxacin. Atenolol was included as an internal measure of tissue integrity. Values of transintestinal atenolol permeability were low compared with those for ciprofloxacin and similar in both absorptive and secretory directions (mean values being 0.49 ± 0.13 and 0.63 ± 0.14 cm/s × 10−6, respectively).
Discussion
Previous studies have highlighted the importance of active efflux transporters in the secretion of ciprofloxacin and other fluoroquinolones into the intestinal lumen (Cavet et al., 1997; Lowes and Simmons, 2002), a clinically important route for elimination (Sörgel et al., 1989, 1991). We have confirmed that murine bcrp1 mediates active efflux of ciprofloxacin across the apical membrane of bcrp1-overexpressing MDCKII cell monolayers, which showed a significant increase in secretion relative to that of wild-type cells (Merino et al., 2006). A statistically significant ciprofloxacin secretion in the human BCRP-transfected MDCKII cell line was not seen at 2 h, in agreement with Merino et al. (2006), who reported only modest levels of net ciprofloxacin secretion by BCRP-MDCKII monolayers, with a small statistically significant difference between Ja-b and Jb-a only being observed with prolonged incubation at 4 h with no difference reported at 2 h. Comparative studies have shown lower levels of substrate efflux in BCRP-MDCKII cells compared with those in bcrp1-MDCKII cells for a number of compounds, including benzimidazoles, fluoroquinolones, troglitazone sulfate, and mitoxantrone (Merino et al., 2005, 2006; Enokizono et al., 2007; An and Morris, 2010). The original hBCRP-MDCKII cell line (Pavek et al., 2005) was generated from a full-length human BCRP cDNA (a gift from Susan Bates, National Cancer Institute, Bethesda, MD) (Miyake et al., 1999; Ozvegy et al., 2001). The hBCRP transfected to form the hBCRP-MDCKII cell line is the wild type with arginine at position 482 variant (Pavek et al., 2005). An R482G mutation is acquired during mitoxantrone selection and results in structural (transmembrane domain) and functional (substrate recognition) changes in the BCRP protein; other studies highlight the impact of the amino acid mutations at position 482 (Honjo et al., 2001; Volk et al., 2002), but this does not explain the differences noted in this and other studies. Because high levels of BCRP mRNA were detected in BCRP-MDCKII cells in this study, differences in transepithelial secretory transport between the human and murine cell lines may result from differences in post-translational modifications of mouse/human proteins in the dog kidney cell line or from inefficient translation of BCRP mRNA into similarly high levels of functional protein.
Because of the possibility of false-negative results for substrate specificity arising from the use of BCRP overexpressing cell lines to determine substrate-transporter interactions, a pharmacological approach to identify BCRP-mediated transport can be used. With use of mouse bcrp1-transfected cells, Ko143 gave a dose-dependent increase in Hoechst 33342 accumulation with an IC50 of 0.15 μM, similar to the previously reported values for bcrp inhibition (Allen et al., 2002; Muenster et al., 2008). Furthermore, the net secretion of ciprofloxacin was completely abolished by the specific BCRP inhibitor Ko143, in contrast with the partial inhibition evident with CsA and DIDS, P-gp/BCRP, and anion exchange inhibitors, respectively. Ko143 inhibition is therefore a useful tool for defining BCRP-mediated transport in intact epithelial layers.
Because ciprofloxacin shows net secretory transport in Caco-2 cell monolayers, it is apparent that an interaction with a human efflux transporter(s) exist. Secretion of ciprofloxacin in low-passage Caco-2 cells is only partially reduced by Ko143 (53%). The inability to fully inhibit net ciprofloxacin flux in Caco-2 cells indicates that additional and distinct mechanisms other than BCRP exist to maintain active ciprofloxacin secretion. Evidence for a distinct mechanism can be found in the data from the high-passage Caco-2 cells, in which Ko143-insensitive ciprofloxacin secretion is maintained in the presence of only minor expression of BCRP mRNA (relative to GAPDH).
Marquez et al. (2009) have recently demonstrated a role of MRP4 in limiting ciprofloxacin uptake in macrophages, with a lesser role for BCRP-mediated efflux. We therefore investigated the role of MRP4 by looking at ciprofloxacin uptake into MRP4-overexpressing HEK293 cells (Wielinga et al., 2002). There was a significant reduction in ciprofloxacin accumulation in the MRP4-HEK293 cells relative to the wild-type controls. In addition, an increase in ciprofloxacin accumulation (inhibition of MRP-mediated efflux) was evident after the inclusion of the known MRP(4) antagonist MK571 (van Aubel et al., 2002; Reid et al., 2003; Wu et al., 2005) in both the wild-type and transfected cells. The impact of MK571 in both wild-type and transfected cell models suggested that HEK293 cells express endogenous levels of MRP4, which has been confirmed by qPCR analysis. Because Ko143 does not affect accumulation, it is evident that MRP4 is responsible for mediating the export of ciprofloxacin from these cells (limiting cellular accumulation).
In low-passage Caco-2 cells, MRP4 mRNA expression was also confirmed. Net ciprofloxacin secretion across Caco-2 cells, although partially responsive to inhibition by Ko143, was insensitive to the MRP inhibitor MK571. This indicates a likely role for BCRP in active ciprofloxacin transport in Caco-2 cells and also makes the existence of an MRP-mediated route for active efflux unlikely. It has recently been convincingly demonstrated that MRP2 plays no role in mediating ciprofloxacin uptake in macrophages (Marquez et al., 2009), whereas MRP4 can mediate ciprofloxacin efflux. Taking together the difference in apparent affinity for ciprofloxacin secretion seen in Caco-2 epithelia compared with that seen for bcrp-transfected MDCKII epithelial layers (Table 2), we may conclude that in human intestinal Caco-2 epithelia, a major component of secretion is not via BCRP and an alternative transport pathway remains to be identified. The alternative role of organic anion-transporting polypeptide (Vanwert et al., 2008; Kalliokoski and Niemi, 2009) transport systems now needs to be investigated.
To investigate ciprofloxacin secretion by native intestine, we have used excised sections of rat intestinal tissue mounted in Ussing chambers. Given the ambiguous evidence reported in previous publications (Rodríguez-Ibáñez et al., 2006; Zakelj et al., 2006), it was interesting to note that regional differences in secretory flux and permeability do exist. Net secretion of ciprofloxacin peaked in the ileum, suggesting higher active transport in this region. There was no net secretion evident in the colon. Expression analysis by qPCR has also indicated a regional difference in transporter expression, with MDR1, BCRP, and MRP4 all showing peak expression in the ileum. Previous studies have confirmed the trend for higher expression of P-gp and BCRP in the distal small intestine (Englund et al., 2006; MacLean et al., 2008) or colon (Stephens et al., 2002), although some studies have reported similar levels of protein across all regions (Berggren et al., 2007; Lowes et al., 2010). The expression data presented here correlate with the functional data, indicating that the maximal secretory transport capacity noted in the ileum is matched to the regions of highest efflux transporter expression. Ciprofloxacin secretion in the rat ileum is completely inhibited by Ko143; however, the potency of this inhibition is much lower compared with Ko143 inhibition of Hoechst 33342 accumulation in the bcrp1-MDCKII cells. Verapamil had no impact on ciprofloxacin secretion, contradicting previous in vitro Caco-2 studies in which complete inhibition of efflux was noted (Rodríguez-Ibáñez et al., 2003). The pharmacological specificity for Ko143 inhibition of ciprofloxacin secretion indicated by the present data, combined with the segmental distribution of ciprofloxacin secretion and BCRP mRNA expression suggests that, at least in rodent intestine, ciprofloxacin secretion is mediated primarily by BCRP. Previous reports have discounted the involvement of MDR1 and MRP2 in ciprofloxacin secretion (Lowes and Simmons, 2002; Marquez et al., 2009) and the present study has confirmed no involvement of MDR1 in the rat ileum.
This study has for the first time also confirmed active secretion of ciprofloxacin in the human intestine using the Ussing chamber technique. Absorptive permeability is of a magnitude similar to that of the rat duodenum; however, the secretory component is larger, giving rise to a higher efflux ratio. Although absorptive permeability was very similar for each individual donor, there was considerable variability in secretory ciprofloxacin movement (Table 3). This result is in contrast with the data generated for the dual P-gp/BCRP substrate, prazosin, which displays substantially lower variability. Addition of the specific BCRP inhibitor Ko143 reduces net ciprofloxacin secretion by 113%, an effect that is statistically significant despite the interpatient variability in ciprofloxacin transport data. It may be concluded that, as with the rat small intestine, ciprofloxacin secretion is likely to be mediated predominantly via BCRP in the human jejunum. Further experiments are required to substantiate the Ko143-independent ciprofloxacin secretory component suggested by the data from donor 2. Because a substantial increase in absorptive permeability may also be observed after Ko143 inhibition, we suggest that BCRP may play a role in partially limiting human absorption of ciprofloxacin in vivo.
In conclusion, this study has demonstrated that ciprofloxacin is likely to be a substrate for BCRP in human intestinal cells (Caco-2), as indicated by sensitivity to Ko143 inhibition. It is also likely that alternative transport pathways exist. Both rodent and human intestine display active secretion of ciprofloxacin, which is completely attenuated after the addition of a specific BCRP inhibitor (Ko143). It is therefore likely that BCRP is the predominant transporter responsible for ciprofloxacin secretion in both rat and human intestine.
J.A.W. was supported by a Biotechnology and Biological Sciences Research Council - Collaborative Awards in Science and Engineering award in conjunction with AstraZeneca.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/dmd.111.038323.
- ABC
- ATP-binding cassette
- P-gp
- P-glycoprotein
- MDR/mdr
- multidrug resistance protein
- MDCKII
- Madin-Darby canine kidney II
- BCRP/bcrp
- breast cancer resistance protein
- Ko143
- 3-(6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester
- MRP/mrp
- multidrug resistance-related protein
- h
- human
- KBR
- Krebs-Ringer bicarbonate
- PD
- potential difference
- HPLC
- high-performance liquid chromatography
- a
- apical
- b
- basal
- PA
- passage
- HEK
- human embryonic kidney
- PCR
- polymerase chain reaction
- q
- quantitative
- WT
- wild-type
- CsA
- cyclosporine
- DIDS
- 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid
- MK-571
- 3-[[3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl]-(2-dimethylcarbamoylethylsulfanyl)methylsulfanyl] propionic acid
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase.
Authorship Contributions
Participated in research design: Haslam, Wright, O'Reilly, Sherlock, Coleman, and Simmons.
Conducted experiments: Haslam and Wright.
Contributed new reagents or analytic tools: O'Reilly and Sherlock.
Performed data analysis: Haslam, Wright, Coleman, and Simmons.
Wrote or contributed to the writing of the manuscript: Haslam, Wright, O'Reilly, Sherlock, Coleman, and Simmons.
References
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. (2002) Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther 1:417–425 [PubMed] [Google Scholar]
- Alvarez AI, Pérez M, Prieto JG, Molina AJ, Real R, Merino G. (2008) Fluoroquinolone efflux mediated by ABC transporters. J Pharm Sci 97:3483–3493 [DOI] [PubMed] [Google Scholar]
- An G, Morris ME. (2010) Effects of single and multiple flavonoids on BCRP-mediated accumulation, cytotoxicity and transport of mitoxantrone in vitro. Pharm Res 27:1296–1308 [DOI] [PubMed] [Google Scholar]
- Berggren S, Gall C, Wollnitz N, Ekelund M, Karlbom U, Hoogstraate J, Schrenk D, Lennernäs H. (2007) Gene and protein expression of P-glycoprotein, MRP1, MRP2, and CYP3A4 in the small and large human intestine. Mol Pharm 4:252–257 [DOI] [PubMed] [Google Scholar]
- Cavet ME, West M, Simmons NL. (1997) Fluoroquinolone (ciprofloxacin) secretion by human intestinal epithelial (Caco-2) cells. Br J Pharmacol 121:1567–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund G, Rorsman F, Rönnblom A, Karlbom U, Lazorova L, Gråsjö J, Kindmark A, Artursson P. (2006) Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci 29:269–277 [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Sugiyama Y. (2007) Involvement of breast cancer resistance protein (BCRP/ABCG2) in the biliary excretion and intestinal efflux of troglitazone sulfate, the major metabolite of troglitazone with a cholestatic effect. Drug Metab Dispos 35:209–214 [DOI] [PubMed] [Google Scholar]
- Griffiths NM, Hirst BH, Simmons NL. (1993) Active secretion of the fluoroquinolone ciprofloxacin by human intestinal epithelial Caco-2 cell layers. Br J Pharmacol 108:575–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths NM, Hirst BH, Simmons NL. (1994) Active intestinal secretion of the fluoroquinolone antibacterials ciprofloxacin, norfloxacin and pefloxacin; a common secretory pathway? J Pharmacol Exp Ther 269:496–502 [PubMed] [Google Scholar]
- Gupta A, Dai Y, Vethanayagam RR, Hebert MF, Thummel KE, Unadkat JD, Ross DD, Mao Q. (2006) Cyclosporin A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother Pharmacol 58:374–383 [DOI] [PubMed] [Google Scholar]
- Haslam IS, O'Reilly DA, Sherlock DJ, Kauser A, Womack C, Coleman T. (2011) Pancreatoduodenectomy as a source of human small intestine for Ussing chamber investigations and comparative studies with rat tissue. Biopharm Drug Dispos 32:210–221 [DOI] [PubMed] [Google Scholar]
- Honjo Y, Hrycyna CA, Yan QW, Medina-Pérez WY, Robey RW, van de Laar A, Litman T, Dean M, Bates SE. (2001) Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res 61:6635–6639 [PubMed] [Google Scholar]
- Jaehde U, Sörgel K, Naber KG, Reiter A, Seelmann R, Sigl G, Muth P, Schunack W. (1989) Gastrointestinal secretion of ciprofloxacin (CIP) in healthy volunteers, in Program and Abstracts of the 29th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1989 Sept 17–20; Houston, TX Abstract 202, American Society for Microbiology, Washington, DC [Google Scholar]
- Kalliokoski A, Niemi M. (2009) Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 158:693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F, Tiberghien F, Wenandy T, Didier A, Traber R. (2002) Cyclosporins: structure-activity relationships for the inhibition of the human MDR1 P-glycoprotein ABC transporter. J Med Chem 45:4598–4612 [DOI] [PubMed] [Google Scholar]
- Lowes S, Simmons NL. (2002) Multiple pathways for fluoroquinolone secretion by human intestinal epithelial (Caco-2) cells. Br J Pharmacol 135:1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes S, Haslam IS, Fihn BM, Hilgendorf C, Karlsson JE, Simmons NL, Ungell AL. (2010) The effects of pregnenolone 16α-carbonitrile dosing on digoxin pharmacokinetics and intestinal absorption in the rat. Pharmaceutics 2:61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean C, Moenning U, Reichel A, Fricker G. (2008) Closing the gaps: a full scan of the intestinal expression of P-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein 2 in male and female rats. Drug Metab Dispos 36:1249–1254 [DOI] [PubMed] [Google Scholar]
- Marquez B, Caceres NE, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. (2009) Identification of the efflux transporter of the fluoroquinolone antibiotic ciprofloxacin in murine macrophages: studies with ciprofloxacin-resistant cells. Antimicrob Agents Chemother 53:2410–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsson P, Pedersen JM, Norinder U, Bergström CA, Artursson P. (2009) Identification of novel specific and general inhibitors of the three major human ATP-binding cassette transporters P-gp, BCRP and MRP2 among registered drugs. Pharm Res 26:1816–1831 [DOI] [PubMed] [Google Scholar]
- Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG. (2006) Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab Dispos 34:690–695 [DOI] [PubMed] [Google Scholar]
- Merino G, Jonker JW, Wagenaar E, Pulido MM, Molina AJ, Alvarez AI, Schinkel AH. (2005) Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2). Drug Metab Dispos 33:614–618 [DOI] [PubMed] [Google Scholar]
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, et al. (1999) Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res 59:8–13 [PubMed] [Google Scholar]
- Muenster U, Grieshop B, Ickenroth K, Gnoth MJ. (2008) Characterization of substrates and inhibitors for the in vitro assessment of Bcrp mediated drug-drug interactions. Pharm Res 25:2320–2326 [DOI] [PubMed] [Google Scholar]
- Ozvegy C, Litman T, Szakacs G, Nagy Z, Bates S, Varadi A, Sarkadi B. (2001) Functional characterization of human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun 285:111–117 [DOI] [PubMed] [Google Scholar]
- Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH. (2005) Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther 312:144–152 [DOI] [PubMed] [Google Scholar]
- Polentarutti BI, Peterson AL, Sjöberg AK, Anderberg EK, Utter LM, Ungell AL. (1999) Evaluation of viability of excised rat intestinal segments in the Ussing chamber: investigation of morphology, electrical parameters, and permeability characteristics. Pharm Res 16:446–454 [DOI] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P. (2003) Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol 63:1094–1103 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ibáñez M, Nalda-Molina R, Montalar-Montero M, Bermejo MV, Merino V, Garrigues TM. (2003) Transintestinal secretion of ciprofloxacin, grepafloxacin and sparfloxacin: in vitro and in situ inhibition studies. Eur J Pharm Biopharm 55:241–246 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ibáñez M, Sánchez-Castaño G, Montalar-Montero M, Garrigues TM, Bermejo M, Merino V. (2006) Mathematical modelling of in situ and in vitro efflux of ciprofloxacin and grepafloxacin. Int J Pharm 307:33–41 [DOI] [PubMed] [Google Scholar]
- Rohwedder RW, Bergan T, Thorsteinsson SB, Scholl H. (1990) Transintestinal elimination of ciprofloxacin. Diagn Microbiol Infect Dis 13:127–133 [DOI] [PubMed] [Google Scholar]
- Söderholm JD, Hedman L, Artursson P, Franzén L, Larsson J, Pantzar N, Permert J, Olaison G. (1998) Integrity and metabolism of human ileal mucosa in vitro in the Ussing chamber. Acta Physiol Scand 162:47–56 [DOI] [PubMed] [Google Scholar]
- Sörgel F, Jaehde U, Naber K, Stephan U. (1989a) Pharmacokinetic disposition of quinolones in human body fluids and tissues. Clin Pharmacokinet 16 (Suppl 1):5–24 [DOI] [PubMed] [Google Scholar]
- Sörgel F, Kinzig M. (1993) Pharmacokinetics of gyrase inhibitors, Part 2: renal and hepatic elimination pathways and drug interactions. Am J Med 94:56S–69S [PubMed] [Google Scholar]
- Sörgel F, Naber KG, Jaehde U, Reiter A, Seelmann R, Sigl G. (1989b) Gastrointestinal secretion of ciprofloxacin. Evaluation of the charcoal model for investigations in healthy volunteers. Am J Med 87: 62S–65S [DOI] [PubMed] [Google Scholar]
- Sörgel F, Naber KG, Kinzig M, Mahr G, Muth P. (1991) Comparative pharmacokinetics of ciprofloxacin and temafloxacin in humans: a review. Am J Med 91:51S–66S [DOI] [PubMed] [Google Scholar]
- Stephens RH, Tanianis-Hughes J, Higgs NB, Humphrey M, Warhurst G. (2002) Region-dependent modulation of intestinal permeability by drug efflux transporters: in vitro studies in mdr1a(−/−) mouse intestine. J Pharmacol Exp Ther 303:1095–1101 [DOI] [PubMed] [Google Scholar]
- van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. (2002) The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol 13:595–603 [DOI] [PubMed] [Google Scholar]
- Vanwert AL, Srimaroeng C, Sweet DH. (2008) Organic anion transporter 3 (oat3/slc22a8) interacts with carboxyfluoroquinolones, and deletion increases systemic exposure to ciprofloxacin. Mol Pharmacol 74:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe DA. (2004) Permeability classification of representative fluoroquinolones by a cell culture method. AAPS PharmSci 6:e13. [DOI] [PubMed] [Google Scholar]
- Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. (2002) Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res 62:5035–5040 [PubMed] [Google Scholar]
- Wielinga PR, Reid G, Challa EE, van der Heijden I, van Deemter L, de Haas M, Mol C, Kuil AJ, Groeneveld E, Schuetz JD, et al. (2002) Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol 62:1321–1331 [DOI] [PubMed] [Google Scholar]
- Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. (2005) Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J 272:4725–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakelj S, Sturm K, Kristl A. (2006) Ciprofloxacin permeability and its active secretion through rat small intestine in vitro. Int J Pharm 313:175–180 [DOI] [PubMed] [Google Scholar]
- Zhao YH, Abraham MH, Le J, Hersey A, Luscombe CN, Beck G, Sherborne B, Cooper I. (2002) Rate-limited steps of human oral absorption and QSAR studies. Pharm Res 19:1446–1457 [DOI] [PubMed] [Google Scholar]