Abstract
Sandwich-cultured rat hepatocytes are used in drug discovery for pharmacological and toxicological assessment of drug candidates, yet their utility as a functional model for drug transporters has not been fully characterized. To evaluate the system as an in vitro model for drug transport, expression changes of hepatic transporters relative to whole liver and freshly isolated hepatocytes (day 0) were examined by real-time quantitative reverse transcription-polymerase chain reaction for 4 consecutive days of culture. No significant differences in transporter expression levels were observed between freshly isolated hepatocytes and whole liver. Two distinct mRNA profiles were detected over time showing 1) a more than 5-fold decline in levels of uptake transporters such as Na+-taurocholate cotransporting polypeptide (Ntcp), organic anion transporter (Oat) 2, organic anion-transporting polypeptide (Oatp) 1a1, Oatp1a4, and Oatp1b2 and 2) a greater than 5-fold increase of efflux transporters P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp), and multidrug resistance-related proteins (Mrp) 1, 2, 3, and 4. In addition, protein levels and functional activities for selected transporters were also determined. Protein levels for Mrp2, Bcrp, P-gp, Ntcp, and Oatp1a4 corresponded to changes in mRNA. Functional activities of Oatps and Oct1 exhibited a 3- and 4-fold decrease on day 2 and day 4, respectively, relative to that on day 0, whereas a more than 10-fold reduction in Oat2 activity was observed. These results indicate that the cell culture conditions used herein did not provide an optimal environment for expression of all hepatic transporters. Significant time-dependent alterations in basal gene expression patterns of transporters were detected compared with those in liver or freshly isolated hepatocytes. Further work and new strategies are required to improve the validity of this model as an in vitro tool for in vivo drug transport or biliary clearance prediction.
Introduction
Drug transporters are membrane proteins that play important roles in the absorption, distribution, and elimination of a wide range of drugs, nutrients, and metabolites. They are distributed and expressed in many tissues including intestine, liver, kidney, and brain (Kusuhara and Sugiyama, 2002). In particular, hepatic drug transporters contribute significantly to the hepatic exposure and biliary excretion of various endogenous and exogenous compounds. Uptake (OATPs, OCT1, and OAT2) and efflux transporters (MRP3, 4, and 5) expressed at sinusoidal (basolateral) membranes mediate the translocation of drugs or endogenous metabolites from blood into liver and from liver into blood, respectively. Transporters located at canalicular (apical) membranes (P-gp, BCRP, and MRP2) serve to pump small molecules from liver into the bile (Müller and Jansen, 1997; Suzuki and Sugiyama, 1999; Sai, 2005).
The exact roles of many drug transporters are still under active investigation; however, several in vitro, preclinical, and clinical studies have demonstrated that interactions with transporters contribute to the toxicity and therapeutic efficacy of small molecules. Similar to the liabilities observed in drug-metabolizing enzymes, transporter-mediated drug-drug interactions have been reported in the clinic (Giacomini et al., 2010). Working alone or in concert with drug-metabolizing enzymes, these interactions are known to have a significant impact on the exposure, efficacy, and safety profiles of drugs (Greiner et al., 1999; Wu and Benet, 2005; Lau et al., 2007). Therefore, it has become critically important to characterize a drug candidate as a substrate, an inhibitor, and/or an inducer of transporters during the selection of new chemical entities in drug discovery.
A variety of well established in vitro approaches have been used in lead identification and optimization to study drug transporter activities, such as cells overexpressing individual or multiple transporters and primary cultured hepatocytes (Sahi, 2005). Primary hepatocytes cultured in a sandwich configuration (between two layers of extracellular matrix) represent a relevant in vitro experimental model for assessment of hepatic metabolism and transport, drug-drug interactions, and hepatotoxicity (Annaert and Brouwer, 2005; Shitara et al., 2005). In contrast with transfected cells, which express only one or two specific transporters and often lack drug-metabolizing enzymes, hepatocytes contain all the complement of transporters and metabolizing enzymes and retain regulation mechanisms as well. They are extensively used as an experimental model to investigate metabolism, transport, and hepatotoxicity of investigational drugs (Liu et al., 1999; Ansede et al., 2010). Furthermore, they are also used to explore the dynamic interplay between drug-metabolizing enzymes and transporters (Lau et al., 2002; Shitara et al., 2005; Wu and Benet, 2005; Hewitt et al., 2007) and regulation of hepatic transporters by nuclear receptor ligands (Jigorel et al., 2006). In addition, they are used to predict in vivo hepatobiliary disposition of drug candidates (Liu et al., 1999; Annaert and Brouwer, 2005; Hoffmaster et al., 2005; Hewitt et al., 2007).
Whereas the expression of hepatic membrane transporters has been investigated in primary cultured hepatocytes to some extent (Hoffmaster et al., 2005; Zhang et al., 2005; Jørgensen et al., 2007), a comprehensive understanding of transporter expression relative to culture time is limited. Distribution and elimination of drugs are often mediated by multiple transporters; hence, the coordinated expression and activities of transporters at the sinusoidal and canaliculi side of hepatocytes could significantly influence drug disposition, drug-drug interaction risk, and toxicity. Thus, to further characterize this system and gain more insights into its utility and limitations, in the present communication, the gene expression profiles and functionality of major hepatic drug transporters (uptake or efflux) were studied in sandwich-cultured rat hepatocytes, a widely used in vitro model. Furthermore, because gene expression is a dynamic and highly regulated process and may change in response to the functional requirements of the hepatocytes, the expression of less important xenobiotic transporters (Mrp1 and Mrp6) was also evaluated. The temporal changes in overall transporter gene expression profiles were examined over a period of 4 days of culture time. To investigate whether changes in mRNA levels corresponded to the changes in protein expressions, Western blot analysis was performed for selected transporters. In addition, functional activities of uptake transporters (Oatps, Octs, and Oats) at different culture time points were also assessed for comparison. With respect to canalicular transporters, their functional activity has been well characterized elsewhere (Liu et al., 1999; Annaert and Brouwer, 2005).
Materials and Methods
Chemicals and Reagents.
[3H]Estradiol 17β-d-glucuronide (E17BG) (45.2 Ci/mmol), p-[glycyl-14C]aminohippuric acid (PAH) (55 mCi/mmol), and [3H]1-methyl-4-phenylpyridinium acetate (MPP+) (83 Ci/mmol) were obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). Cell culture reagents and products were purchased from Mediatech (Manassas, VA). Williams' E medium (WEM) was purchased from Sigma-Aldrich (St. Louis, MO). Standard Hanks' balanced salt solution and insulin were obtained from Invitrogen (Carlsbad, CA). Matrigel and fibronectin were purchased from BD Biosciences (San Jose, CA). The supplement of insulin, transferrin, and selenium (500×) was from Lonza Inc. (Allendale, NJ). The reagents for total protein measurement were from Thermo Fisher Scientific (Waltham, MA). Primary antibodies, Mrp2 (M2III-6), and Bcrp (BXP-53) were purchased from Enzo Life Sciences (Farmingdale, WA), human MDR1 (D-11) that cross-reacts with rodent Mdr1a/b was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), Oatp1a1 (Slco1a1) and Oatp1a4 (Slco1a4) were from Millipore Corporation (Billerica, MA), POU domain, class 2, transcription factor 1 (POU class 2 homeobox 1), Bsep, Ntcp, and Mrp1 were from Abcam Inc. (Cambridge, MA), DPP IV-CD26-adenosine deaminase binding protein was from Thermo Fisher Scientific, GAPDH was obtained from Sigma-Aldrich, and ZO-1 was from Invitrogen. Secondary antibodies and Western blot reagents were from LI-COR Biosciences (Lincoln, NE). RT-PCR enzymes and reagents were from Invitrogen. RNA extraction and purification kits were from QIAGEN (Valencia, CA). Fluorescein isothiocyanate, Alexa 488, and 4′,6′-diamidino-2-phenylindole (DAPI) were from Invitrogen.
Animals.
Hepatocytes were isolated from male Sprague-Dawley rats weighing approximately 250 to 300 g obtained from Charles River Laboratories, Inc. (Wilmington, MA). Animals were provided free access to food and water before surgery. All animal procedures were compliant with the guidelines of the Institutional Animal Care and Use Committee of Amgen Inc.
Isolation and In Vitro Culture of Primary Rat Hepatocytes.
Freshly isolated rat hepatocytes were prepared according to a published method (Moldéus et al., 1978) based on collagenase digestion and separation of liver parenchymal cells. The isolated cells were resuspended in WEM containing l-glutamine (4 mM) and then were centrifuged at 50g (2 min at 4°C) to remove collagenase and cell debris. Three additional washes provided platable cells with viability (as assessed by trypan blue exclusion testing) ranging from 87 to 95%. The hepatocyte culture condition was adapted from a published method with minor modifications (Turncliff et al., 2006). In brief, hepatocytes were resuspended in plating medium, Dulbecco's modified Eagle medium, containing insulin (1 μg/ml), fibronectin (5 μg/ml), dexamethasone (0.1 μM), 50 U/ml penicillin, 50 mg/ml streptomycin, and 10% fetal bovine serum, and seeded at a density of 400,000 cells/well in 24-well BD BioCoat collagen-coated plates (BD Biosciences). Cells were incubated at 37°C in 5% CO2-95% air and allowed to attach for 2 to 4 h. After attachment, plating medium was discarded along with unattached hepatocytes, and the cells were washed once with 1× PBS. Ice-cold hepatocyte plating medium (Dulbecco's modified Eagle's medium, 0.5 ml) supplemented with 50 U/ml penicillin, 50 mg/ml streptomycin, 1× glutamine, insulin-transferrin-selenium (1%), dexamethasone (0.1 μM), and 5% fetal bovine serum containing 0.25 mg/ml Matrigel was then added to each well. After an overnight incubation with a Matrigel overlay, medium was replaced by culture medium (WEM without phenol red) supplemented with 50 U/ml penicillin, 50 mg/ml streptomycin, 1× glutamine, insulin-transferrin-selenium (1%), and dexamethasone (0.1 μM). Medium was replaced daily, and cultured hepatocytes were maintained at 37°C in 5% CO2-95% air.
RNA Isolation and Real-Time qRT-PCR.
Total RNA was isolated from rat livers and cultured hepatocytes using QIAGEN RNA minikits. On-column digestion was performed to remove all traces of residual DNA. The purified RNA was quantified by spectrophotometry on a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific), and the quality was evaluated by visualization of the isolated RNA on a capillary electrophoresis column using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). For each of the test and reference samples, 1 μg of total RNA was reverse-transcribed according to the manufacturer's directions, using random hexamer primers with the SuperScript III First-Strand Synthesis System for RT-PCR kit (Invitrogen). The resulting cDNA was treated with RNase H to remove residual RNA.
Customized TaqMan Arrays using 384-well Micro Fluidic Cards (Applied Biosystems, Foster City, CA) were used to determine the relative expression and profiling of the selected transporters. The probe and primer sets for 20 transporter genes, 3 metabolism enzyme genes, and 18S rRNA were selected from predesigned gene expression assays. The selected PCR primers were gene-specific and were designed to span an exon-exon junction. Two microliters of single-stranded cDNA of each sample (equivalent to 100 ng of total RNA) were mixed with 48 μl of nuclease-free water and 50 μl of TaqMan Universal PCR Master Mix. The mixture was then transferred into a loading port on the card. The card was centrifuged and sealed, and PCR amplification was then performed using an Applied Biosystems Prism 7900HT sequence detection system under the following thermal cycler conditions: 10 min at 95°C (activation), 40 cycles of denaturation at 95°C for 15 s, and annealing and extension at 60°C for 1 min.
TaqMan Data Analysis and Statistical Procedures.
RQ Manager 1.2 software (Applied Biosystems) was used to determine the amplification curves on the basis of the comparative threshold cycle (Ct) method (Bookout and Mangelsdorf, 2003). Relative quantitation of the transcription levels was analyzed by RealTime StatMiner software (Integromics; Granada, Spain) for automated data analysis. Gene expression values were normalized to 18S rRNA.
Statistical comparison of groups was performed using the parametric t test functions of the Limma package (RealTime StatMiner software; Integromics Inc., Philadelphia, PA) (Smyth, 2004). The Benjamini-Hochberg method was used to calculate the false discovery rate and to pick out genes whose expression levels are significantly different between two groups (Benjamini and Hochberg, 1995). For each gene the false discovery rate was estimated and used to determine the corresponding adjusted (adj) p value. Only genes exhibiting expression changes at a significant level (adj p < 0.05, fold change of 2) were considered to be differentially expressed. All samples were run in duplicate from three separate experiments.
Western Blot Analysis.
Plated hepatocytes were washed twice with ice-cold PBS and incubated with ice-cold Complete Lysis-M buffer containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) for 10 min. Cells were scraped off the plates and transferred into a centrifuge tube. The suspension was gently mixed on an orbital shaker at 4°C for 15 min to lyse the cells and then centrifuged at 14,000g at 4°C for 15 min. The supernatant was transferred to a fresh centrifuge tube and diluted at least 1:10 before the protein concentration was determined by using the BCA method (Pierce Chemical) (Smith et al., 1985). Samples were divided into aliquots and stored frozen at −80°C until subsequent analysis.
Protein samples were heat-denatured, and 20 μg was loaded for separation on SDS NuPAGE Bis-Tris gels (Invitrogen). After transfer, the membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences) for 45 min and treated with primary antibodies (Supplemental Table 1), followed by anti-mouse or anti-rabbit conjugated secondary antibodies according to the manufacturer's instructions (IRDye 800CW or IRDye 680LT; LI-COR Biosciences). The protein bands were visualized using an Odyssey Imager near-infrared fluorescence detection imaging system (LI-COR Biosciences). A monoclonal antibody against GAPDH (1:5000) (Sigma-Aldrich) served as a loading control. The protein level of the bile canalicular protein, DPP IV, was used to assess the functional polarization, whereas POU domain, class 2, transcription factor 1 (OTF1) was used as a control for transcriptional activities. All immunoblots were repeated at least three times.
In Vitro Uptake Experiments.
Activity of sinusoidal drug transporters was determined by cellular accumulation of radiolabeled probe substrates (Zamek-Gliszczynski et al., 2003). In brief, after attachment (2–4 h), plated cells were washed with the assay buffer (Hanks' balanced salt solution containing 10 mM HEPES and allowed to equilibrate for 20 min at 37°C with or without inhibitor. Dosing solutions containing the probe substrates [14C]PAH (1 μM) for Oat2, [3H]E17BG (1 μM) for Oatps, and [3H]MPP+ (1 μM) for Oct1 in the presence or absence of the respective inhibitors, probenecid (50 μM), estrone 3-sulfate (100 μM), and tetraethylammonium (250 μM), were added at 37°C or 4°C for 3 min. To terminate the active uptake process (3 min), cells were washed with ice-cold assay buffer. Cells were lysed, and substrate accumulation was determined by liquid scintillation counting analysis. Passive diffusion was determined by performing parallel experiments at 4°C. The protein content of the hepatocytes in each well was measured with the BCA method. For analysis of differences between the groups, an unpaired t test with the Bonferroni correction for multiple comparisons was used after two-way analysis of variance. Differences were considered significant at the p < 0.05 level.
Light Microscopy.
Light microscopy was used to confirm the integrity of the canalicular networks. Photographic images were taken with a Fisher Scientific Micromaster Digital Inverted Microscope at 200× magnification (Thermo Fisher Scientific).
Immunofluorescence.
Immunolocalization of selected transporter proteins on day 4 postculture was investigated by confocal microscopy. Hepatocytes plated onto glass chamber slides in the sandwich configuration were fixed with ice-cold acetone for 10 min. After three 0.5-ml washes with PBS, cells were incubated with 1% ultrapure bovine serum albumin for 60 min. Blocking buffer was removed, and cells were incubated with the following diluted specific primary antibodies for rat Mrp2, Bcrp, Oatp1, P-gp, or ZO-1. Samples were washed three times with 1 ml of PBS containing 0.05% Tween 20 (PBST) and then were incubated with the fluorophore-conjugated secondary antibodies (Alexa Fluor 488 or fluorescein isothiocyanate). After three washes with 1 ml of PBST-SDS (containing 0.01% SDS), the cells were counterstained with DAPI to label the nucleus. Cells were then mounted onto glass coverslips and examined using a Leica SP2 confocal microscope (Leica Microsystems, Bannockburn, IL), and differential interference contrast images were collected. After sequential excitation, blue and green fluorescent images of the same cell were merged for colocalization. Background fluorescence was determined by applying the secondary conjugated antibody alone and by replacement of the primary antibody with nonimmune serum.
Results
Morphological Assessment of Cultured Rat Hepatocytes.
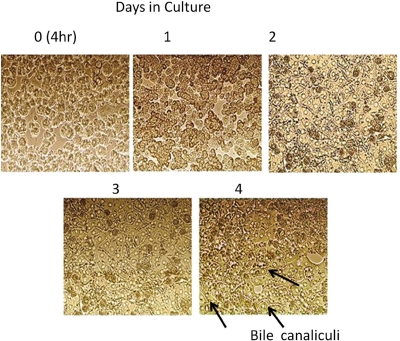
Figure 1 illustrates the morphological changes in cultured hepatocytes occurring over 4 days of cell culture. Microscopic observations showed that hepatocytes displayed mostly spherical and singular shapes at early stages after seeding. After incubation for 24 h, hepatocytes assumed the characteristic cuboidal shape with visible intercellular boundaries. Reaggregation of cell clusters and reestablishment of cell-to-cell contact was also apparent 24 h postseeding. Repolarization and formation of an extensive bile canalicular network was apparent at 72 h postseeding (Fig. 1).
Fig. 1.
Time course morphology images of primary rat hepatocytes. Light microscopic images show pattern of time-dependent differentiation and polarization of cultured hepatocytes as well as bile canalicular formation. The bile canalicular network is visible as bright dots or belts between adjacent hepatocytes.
Fluorescence Immunocytochemistry Using Confocal Microscopy in Cultured Rat Hepatocytes.
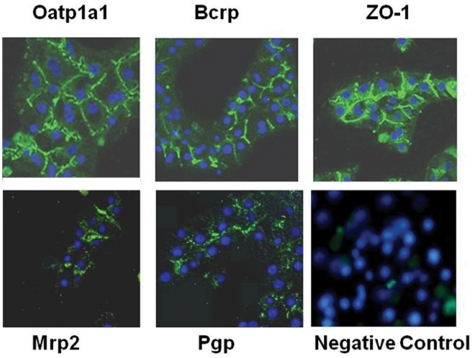
Cellular distribution of selected transporter proteins (P-gp, Bcrp, Oatp1a1, and Mrp2) in day 4 cultures of hepatocytes was assessed by indirect immunofluorescence (Fig. 2). A characteristic fluorescent pattern (green) restricted to the canalicular domain was observed in the hepatocytes immunostained for Bcrp. Immunofluorescence signals of Mrp2 and P-gp indicated expression and localization of these proteins primarily on the canalicular domain of adjacent cells and areas near cell junctions. Oatp1a1 immunoreactivity was demonstrated with well demarcated surface staining (green).
Fig. 2.
Immunofluorescence localization of ZO-1 (tight junction marker), Bcrp, P-gp, Mrp2, and Oatp1a1. Low-magnification confocal microscopic views show the localization of apical Oatp1a1, ZO-1, and basolateral Bcrp of cultured rat hepatocytes on day 4. To compare the expression level of Oatp1a1, Bcrp, and ZO-1 in the rat hepatocytes, the labeling conditions and settings of the confocal microscope were identical for all observations. A microscopic image with nonimmune serum (negative control) is also shown as an example.
Samples stained with the labeled secondary antibody alone as negative control did not exhibit nonspecific staining, suggesting minimum contribution of the background to the observed signal in hepatocytes (Fig. 2). Nuclei were stained with the blue fluorescent stain, DAPI. ZO-1, a marker protein for tight junctions (Stevenson et al., 1989), showed a visible fine network on the membrane areas of cell-cell contact, demonstrating defined membrane boundaries reflecting normal development of tight junctions and epithelial polarization.
Time-Dependent Changes of Basal Transporter Expression Levels in Primary Rat Hepatocytes.
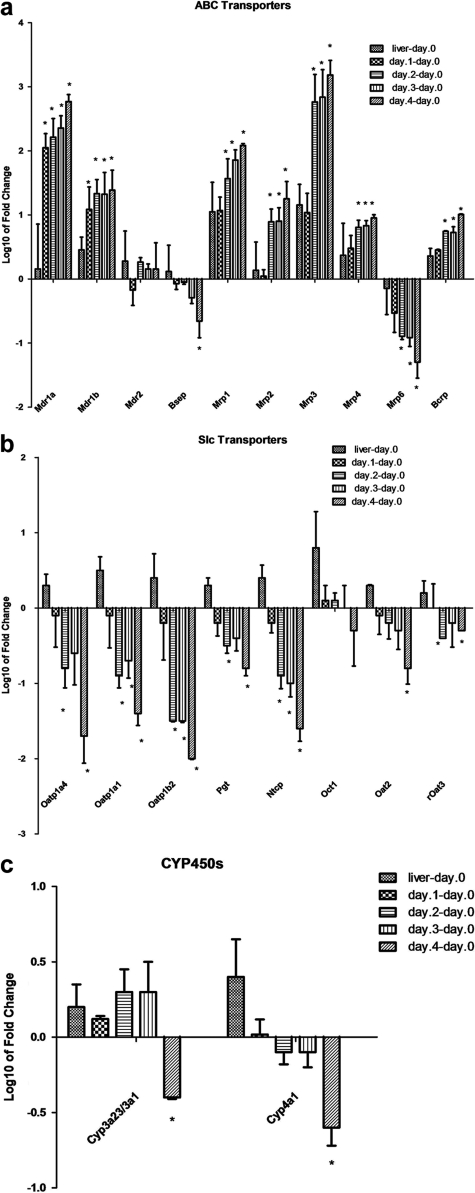
Examination of the hepatic transporter gene expression levels in primary rat hepatocytes cultured in sandwich configuration showed no significant changes between whole liver and freshly isolated hepatocytes on day 0, except for Mrp1 (∼18-fold difference), indicating that the isolation procedure had minimal impact on expression levels (Figs. 3 and 4). Most of the transcripts remained relatively constant as well on day 1 compared with day 0. No significant change in relative mRNA levels of transporters was detected on day 1, except for Mdr1, for which a significant increase was evident (Fig. 3).
Fig. 3.
Time-dependent changes in membrane transporters expression in cultured rat hepatocytes. a–c, gene expression changes of sinusoidal and canalicular membrane transporters or Cyp450s in rat primary hepatocytes cultures on day 1 to day 4 and total liver compared with day 0 (freshly isolated hepatocytes). Total RNA was isolated from freshly isolated rat hepatocytes (day 0), whole liver, and 1-, 2-, 3-, and 4-day-old cultured primary hepatocytes as described under Materials and Methods. The values of columns are expressed as the log 10 of mean of fold change. Statistical comparison of groups was performed using the parametric t test functions. The Benjamini-Hochberg method was used to determine the adjusted p values and statistical significance of each point *, adj p < 0.05, as described under Materials and Methods. Slc, solute carrier organic transporter; ABC, ATP-binding cassette.
Fig. 4.
Hierarchical cluster analysis of differentially expressed transporters genes in primary rat hepatocytes cultures. Data were represented as expression values relative to the freshly isolated hepatocytes (day 0) at p < 0.05. Each column represents a selected culture time point (day 1, 2, 3, and 4) and whole liver (column 1), shades of red and green indicate up- or down-regulation of genes according to the color scheme shown. Black indicates no change. Genes represented by rows were clustered according to their similarities in pattern of expression. Slc, solute carrier organic transporter; ABC, ATP-binding cassette; DDCt, ΔΔCt.
Large variations in expression levels between day 0 and day 2 to day 4 were present. A clear difference in gene expression pattern between the uptake and efflux transporters was observed. Gene expression levels of most uptake transporters decreased during culture (indicated in green in Fig. 4), whereas most efflux transporters increased over time (as shown in red in Fig. 4). For example, transcripts for uptake transporters including Oatp1a4, Oatp1b2, and Ntcp were significantly reduced (more than 10-fold) over time relative to day 0. The opposite pattern was observed for the efflux transporters such as Mrp1, Mrp2, Mrp3, Mdr1a, Mdr1b, and Bcrp, with an increase of more than 10-fold in expression levels (Fig. 3). The mRNA levels for Oct1 and Mdr2 remained relatively unchanged or less significant (Oct1, day 4). For Mrp5 and Oat1, however, the mRNA expression levels were very low, and the real changes in gene expression could not be determined.
To assess the impact of culture time on phase I enzyme mRNA expression levels, selected metabolic enzymes were also included in this analysis. Cyp4a1 relative expression levels continuously declined, whereas Cyp3a11 remained relatively constant on day 0 to day 3 with approximately a 2-fold decrease by day 4. Cyp1a1 transcript levels were not detectable in freshly isolated hepatocytes and were detected at very low levels in rat liver. However, an increase in the Cyp1a1 mRNA levels was observed at later culture time points (days 2, 3, and 4). Taken together, these results indicate that the expression profiles of cultured rat hepatocytes are different from those of rat liver or freshly isolated rat hepatocytes.
Time-Dependent Changes of Transporter Protein Levels.
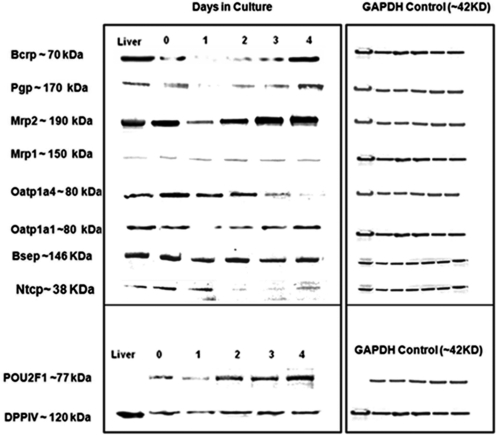
To determine whether the changes in mRNA levels correspond to protein expression levels, Western blot analysis of uptake transporters Oatp1a1, Oatp1a4, Ntcp, and efflux transporters Bcrp, Mdr1, Mrp1, Mrp2, Bsep, and Mdr2 was performed. Protein levels for the canalicular marker DPP IV and the transcription factor OTF1 were assessed as measurement of apical functional polarization and normal transcriptional activities. Consistent with changes in mRNA levels, efflux transporters Mdr1, Mrp2, and Bcrp showed a time-dependent increase in protein amount after an initial decrease on day 1 (Fig. 5). Protein levels of Mrp1 remained relatively constant (Fig. 5). In concert with the mRNA transcription levels, a time-dependent decline in the protein levels of Oatp1a4 and Ntcp levels was also observed. After an initial decline on day 1, the protein levels of the uptake transporter Oatp1a1 improved on day 4 of culture despite down-regulation of the mRNA level. A minor decrease in the protein levels of Bsep was only observed on day 3, which appeared to recover on day 4 of culture (Fig. 5).
Fig. 5.
Western blotting analysis of the expression of transporter proteins in cultured rat hepatocytes. Western immunoblots were performed using either cultured hepatocyte lysate samples or total liver homogenate (20 mg protein/lane) as indicated under Materials and Methods. GAPDH was used as the loading control, DPP IV as the canalicular marker, and OTF1 as transcriptional control for housekeeping genes.
Sinusoidal Membrane Transporter Activities.
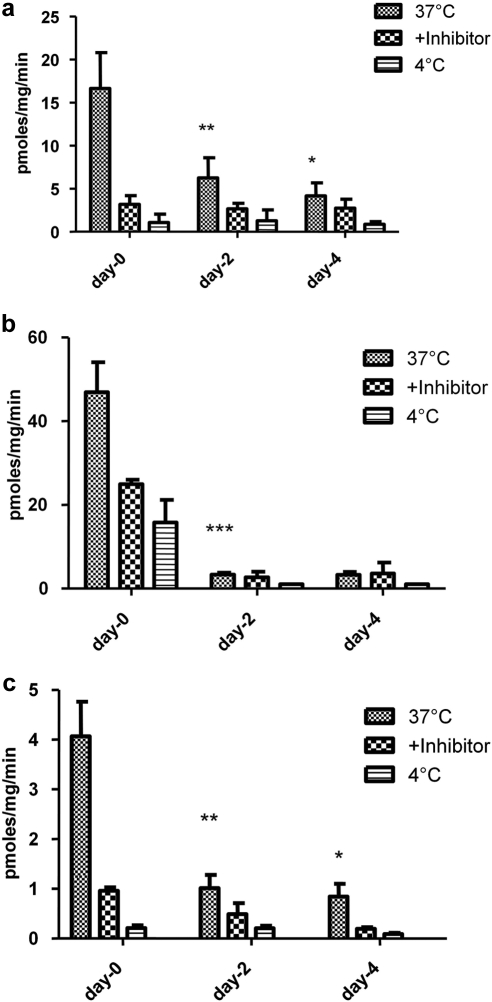
The influence of culture time on selected uptake transporter activities was dramatic (Fig. 6). Uptake of E17BG (Oatp substrate) and MPP+ (Oct substrate) decreased approximately 4-fold. Uptake of PAH, a marker for Oat2 activity was decreased by approximately 8-fold. The decline in activities generally paralleled the observed decrease in the mRNA levels except for Oct1, for which mRNA levels remained relatively unchanged.
Fig. 6.
Time-dependent changes in sinusoidal membrane transporter activities. Primary rat hepatocytes were incubated with radiolabeled transporter probe substrates: [3H]E17BG (1 μM), [14C]PAH (1 μM), and [3H]MPP+ (1 μM), which are transported by Oatps (a), Oat2 (b), and Oct1 (c), respectively. The incubations were performed as described under Materials and Methods in the presence or absence of estrone 3-sulfate (100 μM), probenecid (50 μM), and tetraethylammonium (250 μM), which are known inhibitors for Oatps, Oat2, and Oct1, respectively. In addition, 4°C incubations were evaluated to determine passive diffusion of the substrate. Cells were lysed in lysis buffer and cellular accumulation of the radiolabeled compounds was determined by scintillation counting. Data were normalized relative to the protein values and are expressed as picomoles per milligram per minute. Data represented are the mean values of three separate experiments performed in triplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
Primary sandwich-cultured hepatocytes are the closest model to the in vivo situation and are recognized to maintain some liver-specific functions (Lau et al., 2002; Shitara et al., 2005; Wu et al., 2005; Hewitt et al., 2007). Nevertheless, isolation of hepatocytes results in loss of cell polarity and architecture. Although culturing hepatocytes in the sandwich configuration restores cell polarization and excretory function (LeCluyse et al., 1994; Hoffmaster et al., 2005), temporal transcriptional changes in gene and protein expression have been reported (Boess et al., 2003; Richert et al., 2006; Swift et al., 2010). The current study was performed to determine an optimal culture time point at which the expression of sinusoidal or canalicular membrane transporters is comparable to that of freshly isolated hepatocytes. Hence, basal expression levels of hepatic drug transporters was examined over a period of 4 days postculture by real-time qRT-PCR. Protein expression of selected transporters with commercially available antibodies was also determined for comparison. Finally, activities of major hepatic uptake transporters were assessed to investigate the impact of expression change on their functions.
The culture conditions in the present study were adapted from published methods optimized in other laboratories (Chandra et al., 2001; Turncliff et al., 2006) except that fibronectin was included in the initial culture stages to aid hepatocyte attachment and differentiation (Pankov and Yamada, 2002). Phase-contrast microscopic analysis confirmed that culture conditions used herein were optimal for hepatocyte viability, morphology, and canalicular network formation. Cultured hepatocytes demonstrated intact bile canaliculi reconstruction after day 2 (Fig. 1), indicative of propagation and polarized distribution of apical transporters. Immunocytochemistry demonstrated correct regional localization and distribution of selected transporters at either basolateral (Oatp1a1) or apical (P-gp, Mrp2, and Bcrp) domains of the hepatocytes (Fig. 2). Visualization of the tight junction protein ZO-1 showed an organized network on the membrane areas of cell-cell contact, further validating restoration of bile canaliculi and bipolar configuration (Fig. 2) (Stevenson et al., 1989).
Gene expression data confirmed that transporter expression levels were not influenced by the hepatocyte isolation procedures (Luttringer et al., 2002). In contrast, pronounced differences in transporter gene expression profiles were observed at different culture stages. Opposite time-dependent expression profiles were found for uptake and efflux transporters. In general, mRNA levels of most uptake transporters were suppressed (Fig. 3), whereas a sharp increase in most of the efflux transporters was observed, relative to that of freshly isolated hepatocytes or liver. In particular, expression levels of Mdr1a, Mdr1b, Mrp1, Mrp2, Mrp3, Mrp4, and Bcrp improved, those of Mdr2 did not change, and those of Bsep and Mrp6 declined.
Transporter expression data at the mRNA levels are limited in sandwich-cultured hepatocytes. Borlak and Klutcka (2004) reported a substantial reduction in basolateral transporter levels (Oatp1a1, Oatp1a4, and Ntcp) and an increase in Mdr1b levels. These observations are in accordance with the current results. For Mrp2, Bsep, Mdr2, and Mdr1a, the report indicated no significant changes although high variability in expression levels was noted. Luttringer et al. (2002) examined changes in transporter (uptake or efflux) expression levels using microarray analysis. Although these changes were to some extent in accordance with the present observations, the magnitude of the changes was less significant in some cases (Mdr1a, Bsep, Mrp1, Mrp2, Oatp1a4, and Oatp1a1). It is well documented that qRT-PCR is more sensitive than microarray. Microarray results may underestimate actual changes in gene expression and account for the observed discrepancy (Morey et al., 2006). Furthermore, down-regulation of uptake transporters (Oatp1a1 and Oatp1a4) and up-regulation of efflux transporters (Mdr1a/b) were observed in both conventional and sandwich cultures (Jørgensen et al., 2007). Therefore, the results of this study confirm previous reports and provide a direct observation of global hepatic transporter transcriptional changes as a function of time in sandwich-cultured rat hepatocytes.
With respect to protein expression, a decrease on day 1 of culture was detected for Mrp2, Bcrp, and P-gp (Fig. 5), which was followed by a time-dependent increase that paralleled mRNA expression. The protein level decline on day 1 could be caused by the rapid protein degradation or loss of cell polarity and architecture shortly after hepatocyte isolation. Bsep and Mrp1 protein levels remained relatively constant over different culture stages. Because Mrp1 protein expression is considerably low and barely detectable in hepatocytes, the observed levels of Mrp1 protein suggest an apparent increase in hepatic Mrp1 protein. As an alternative, it may be also caused by cross-reactivity of the Mrp1 antibody to other proteins, leading to an increase in the background signal. Similar to mRNA expression, protein levels of uptake transporters Oatp1a4 and Ntcp declined over time, whereas Oatp1a1 levels were relatively well maintained after an initial decline on day 1 (Fig. 5). The fast recovery of Oatp1a1 protein levels may be related to regulated control mechanisms that stabilize the protein in response to the functional requirements of the hepatocytes. The protein expression results (uptake and efflux) were consistent with previously published data (Hoffmaster et al., 2005; Swift et al., 2010). A comparable protein level or higher levels were attained for Mrp2, Mrp1, Bcrp, P-gp, and Bsep in cultured hepatocytes on day 4, which indicated that hepatocytes were able to synthesize and maintain certain proteins under the current culture conditions.
The uptake activity measured herein declined in parallel to the mRNA (Oatp, Oct, and Oat) and protein levels (Oatp). Uptake across the sinusoidal membrane is an important first step in hepatic exposure and hepatobiliary excretion of small molecules. In some cases, as in highly polar drugs (e.g., pravastatin), the hepatocellular uptake is carrier-mediated and the rate-limiting step for hepatic elimination (Treiber et al., 2004; Kotani et al., 2011). The impaired hepatic uptake could therefore limit the influx and hepatocellular exposure of such molecules and result in decreased metabolism or in vitro biliary clearance. Indeed, Kotani et al. (2011) recently showed that the hepatic clearances of taurocholate, digoxin, pravastatin, and rosuvastatin predicted from uptake clearances in 4 day sandwich-cultured rat hepatocytes markedly underestimated in vivo hepatic clearance due to down-regulation of Oatps. These observations should be considered when sandwich cultured rat hepatocytes are used to evaluate therapeutic agents for hepatic uptake or in vitro biliary clearance. Likewise, changes in the expression levels of efflux transporters (Mrp2, Bcrp, P-gp, Mrp3, and Bsep) may lead to inaccurate estimation of in vitro biliary clearance of actively transported compounds, thus limiting the predictability of this model for in vivo biliary clearance.
Many elements could contribute to the dynamic changes observed in mRNA, protein levels, and activity. After isolation, hepatocytes lose many of their liver-specific functions and are placed in a setting that is dramatically different from the native liver architecture; thus, the transcriptional changes could be related to loss of native physiology and architecture of hepatocytes in culture and subsequent adaptive changes to reduce stress and enhance cell survival rate. On the other hand, it could be the result of protective mechanisms to reduce toxic waste materials including nutrient remnants generated during ordinary cellular metabolism and maintain homeostasis. Therefore, a down-regulation in the expression of absorptive mechanisms would limit the uptake of potentially toxic substances, whereas an increase in or maintenance of the expression levels of P-gp, Bcrp, Bsep, Mrp2, and Mdr2 at the canalicular membrane could facilitate the secretion of potentially hepatotoxic endogenous compounds (Zollner et al., 2003; Nies et al., 2009).
Related human hepatocytes studies are scarce. In general, a similar trend in the expression (protein and gene) of uptake and efflux transporters has been described in human hepatocytes (Hoffmaster et al., 2005; Jigorel et al., 2005). Although overall better maintenance of some uptake transporters (NTCP) compared with that in rat hepatocytes has been reported (Jigorel et al., 2005), a comprehensive evaluation of all major hepatocellular transporters as described in the present studies may be necessary to determine the time profile expression of human hepatic transporters in the cultured system.
In summary, this report presents a comprehensive survey of culture time-related changes in relative gene or protein expression levels of transporters in sandwich hepatocytes. Our temporal analysis defined 18 hepatic transporter genes displaying dynamic expression changes across the time course studied. These data show concordance compared with previous reports (Borlak and Klutcka 2004; Jigorel et al., 2005; Richert et al., 2006; Swift et al., 2010). The findings from the present study indicate that under the current culture conditions an optimum environment for the simultaneous expression of all of the transporters was not attained. Further optimization of culture conditions is needed to attain basolateral transporters levels comparable to those of liver.
Supplementary Material
Acknowledgments
We thank Dr. Wesley Chan (Amgen Inc.) for assistance with the three-dimensional and confocal microscopy imaging and Dr. Bradley K. Wong (Amgen Inc.) for critically reviewing this manuscript.
This work was supported in part by National Institutes of Health National Heart, Lung, and Blood Institute [Training Grant T32-HL07013] (to J.S.H.).
Parts of this work were previously presented at the following conference: Tchaparian EH, Houghton JS, Uyeda C, Grillo MP, and Jin L (2010) Effect of culture time on basal expression level of drug transporters in sandwich-cultured primary rat hepatocytes. 2010 International Pharmaceutical Federation Pharmaceutical Sciences World Conference/American Association of Pharmaceutical Sciences Annual Meeting and Exposition; 2010 Nov 14–18; New Orleans, LA. Abstract M1222. Amgen Inc., South San Francisco, CA.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/dmd.111.039545.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- OATP/Oatp
- organic anion transporting polypeptide
- OCT/Oct
- organic cation transporter
- OAT/Oat
- organic anion transporter 2
- MRP/Mrp
- multidrug resistance-related protein
- P-gp
- P-glycoprotein
- BCRP/Bcrp
- breast cancer resistant protein
- E17BG
- estradiol 17β-d-glucuronide
- PAH
- p-aminohippuric acid
- MPP+
- 1-methyl-4-phenylpyridinium
- WEM
- Williams' E medium
- MDR/Mdr
- multidrug resistance protein
- Slc
- solute carrier organic transporter
- Bsep
- bile salt export pump
- NTCP/Ntcp
- Na+-taurocholate cotransporting polypeptide
- DPP IV
- dipeptidyl peptidase IV
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- ZO-1
- zonula occludens protein 1
- RT
- reverse transcription
- PCR
- polymerase chain reaction
- DAPI
- 4′,6′-diamidino-2-phenylindole
- PBS
- phosphate-buffered saline
- q
- quantitative
- OTF1
- POU domain, class 2, transcription factor 1.
Authorship Contributions
Participated in research design: Tchaparian and Jin.
Conducted experiments: Tchaparian and Houghton.
Performed data analysis: Tchaparian.
Wrote or contributed to the writing of the manuscript: Tchaparian.
Other: Uyeda and Grillo.
References
- Annaert PP, Brouwer KL. (2005) Assessment of drug interactions in hepatobiliary transport using rhodamine 123 in sandwich-cultured rat hepatocytes. Drug Metab Dispos 33:388–394 [DOI] [PubMed] [Google Scholar]
- Ansede JH, Smith WR, Perry CH, St Claire RL, 3rd, Brouwer KR. (2010) An in vitro assay to assess transporter-based cholestatic hepatotoxicity using sandwich-cultured rat hepatocytes. Drug Metab Dispos 38:276–280 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser C Appl Stat 57:289–300 [Google Scholar]
- Boess F, Kamber M, Romer S, Gasser R, Muller D, Albertini S, Suter L. (2003) Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci 73:386–402 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Mangelsdorf DJ. (2003) Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal 1:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlak J, Klutcka T. (2004) Expression of basolateral and canalicular transporters in rat liver and cultures of primary hepatocytes. Xenobiotica 34:935–947 [DOI] [PubMed] [Google Scholar]
- Chandra P, Lecluyse EL, Brouwer KL. (2001) Optimization of culture conditions for determining hepatobiliary disposition of taurocholate in sandwich-cultured rat hepatocytes. In Vitro Cell Dev Biol Anim 37:380–385 [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, Kroemer HK. (1999) The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest 104:147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt NJ, Lechón MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Lohmann C, Skonberg C, et al. (2007) Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev 39:159–234 [DOI] [PubMed] [Google Scholar]
- Hoffmaster KA, Zamek-Gliszczynski MJ, Pollack GM, Brouwer KL. (2005) Multiple transport systems mediate the hepatic uptake and biliary excretion of the metabolically stable opioid peptide [d-penicillamine2,5]enkephalin. Drug Metab Dispos 33:287–293 [DOI] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Bertrand M, Fardel O. (2005) Functional expression of sinusoidal drug transporters in primary human and rat hepatocytes. Drug Metab Dispos 33:1418–1422 [DOI] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. (2006) Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos 34:1756–1763 [DOI] [PubMed] [Google Scholar]
- Jørgensen L, Van Beek J, Lund S, Schousboe A, Badolo L. (2007) Evidence of Oatp and Mdr1 in cryopreserved rat hepatocytes. Eur J Pharm Sci 30:181–189 [DOI] [PubMed] [Google Scholar]
- Kotani N, Maeda K, Watanabe T, Hiramatsu M, Gong LK, Bi YA, Takezawa T, Kusuhara H, Sugiyama Y. (2011) Culture period-dependent changes in the uptake of transporter substrates in sandwich-cultured rat and human hepatocytes. Drug Metab Dispos 39:1503–1510 [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. (2002) Role of transporters in the tissue-selective distribution and elimination of drugs: transporters in the liver, small intestine, brain and kidney. J Control Release 78:43–54 [DOI] [PubMed] [Google Scholar]
- Lau YY, Huang Y, Frassetto L, Benet LZ. (2007) Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther 81:194–204 [DOI] [PubMed] [Google Scholar]
- Lau YY, Sapidou E, Cui X, White RE, Cheng KC. (2002) Development of a novel in vitro model to predict hepatic clearance using fresh, cryopreserved, and sandwich-cultured hepatocytes. Drug Metab Dispos 30:1446–1454 [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Audus KL, Hochman JH. (1994) Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol 266:1764–1774 [DOI] [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KL. (1999) Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol 277:G12–G21 [DOI] [PubMed] [Google Scholar]
- Luttringer O, Theil FP, Lavé T, Wernli-Kuratli K, Guentert TW, de Saizieu A. (2002) Influence of isolation procedure, extracellular matrix and dexamethasone on the regulation of membrane transporters gene expression in rat hepatocytes. Biochem Pharmacol 64:1637–1650 [DOI] [PubMed] [Google Scholar]
- Moldéus P, Högberg J, Orrenius S. (1978) Isolation and use of liver cells. Methods Enzymol 52:60–71 [DOI] [PubMed] [Google Scholar]
- Morey JS, Ryan JC, Van Dolah FM. (2006) Microarray validation: factors influencing correlation between oligonucleotide microarrays and real-time PCR. Biol Proced Online 8:175–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Jansen PL. (1997) Molecular aspects of hepatobiliary transport. Am J Physiol 272:G1285–G1303 [DOI] [PubMed] [Google Scholar]
- Nies AT, Koepsell H, Winter S, Burk O, Klein K, Kerb R, Zanger UM, Keppler D, Schwab M, Schaeffeler E. (2009) Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 50:1227–1240 [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. (2002) Fibronectin at a glance. J Cell Sci 115:3861–3863 [DOI] [PubMed] [Google Scholar]
- Richert L, Liguori MJ, Abadie C, Heyd B, Mantion G, Halkic N, Waring JF. (2006) Gene expression in human hepatocytes in suspension after isolation is similar to the liver of origin, is not affected by hepatocyte cold storage and cryopreservation, but is strongly changed after hepatocyte plating. Drug Metab Dispos 34:870–879 [DOI] [PubMed] [Google Scholar]
- Sahi J. (2005) Use of in vitro transporter assays to understand hepatic and renal disposition of new drug candidates. Expert Opin Drug Metab Toxicol 1:409–427 [DOI] [PubMed] [Google Scholar]
- Sai Y. (2005) Biochemical and molecular pharmacological aspects of transporters as determinants of drug disposition. Drug Metab Pharmacokinet 20:91–99 [DOI] [PubMed] [Google Scholar]
- Shitara Y, Sato H, Sugiyama Y. (2005) Evaluation of drug-drug interaction in the hepatobiliary and renal transport of drugs. Annu Rev Pharmacol Toxicol 45:689–723 [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85 [DOI] [PubMed] [Google Scholar]
- Smyth GK. (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:Article 3 [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Heintzelman MB, Anderson JM, Citi S, Mooseker MS. (1989) ZO-1 and cingulin: tight junction proteins with distinct identities and localizations. Am J Physiol 257:C621–C628 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sugiyama Y. (1999) Transporters for bile acids and organic anions. Pharm Biotechnol 12:387–439 [DOI] [PubMed] [Google Scholar]
- Swift B, Pfeifer ND, Brouwer KL. (2010) Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab Rev 42:446–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber A, Schneiter R, Delahaye S, Clozel M. (2004) Inhibition of organic anion transporting polypeptide-mediated hepatic uptake is the major determinant in the pharmacokinetic interaction between bosentan and cyclosporin A in the rat. J Pharmacol Exp Ther 308:1121–1129 [DOI] [PubMed] [Google Scholar]
- Turncliff RZ, Tian X, Brouwer KL. (2006) Effect of culture conditions on the expression and function of Bsep, Mrp2, and Mdr1a/b in sandwich-cultured rat hepatocytes. Biochem Pharmacol 71:1520–1529 [DOI] [PubMed] [Google Scholar]
- Wu CY, Benet LZ. (2005) Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res 22:11–23 [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Xiong H, Patel NJ, Turncliff RZ, Pollack GM, Brouwer KL. (2003) Pharmacokinetics of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein and its diacetate promoiety in the liver. J Pharmacol Exp Ther 304:801–809 [DOI] [PubMed] [Google Scholar]
- Zhang P, Tian X, Chandra P, Brouwer KL. (2005) Role of glycosylation in trafficking of Mrp2 in sandwich-cultured rat hepatocytes. Mol Pharmacol 67:1334–1341 [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, Marschall HU, Zatloukal K, Denk H, et al. (2003) Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol 39:480–488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.