Abstract
Transporters located on the sinusoidal and canalicular membranes of hepatocytes regulate the efflux of drugs and metabolites into blood and bile, respectively. Changes in the expression or function of these transporters during liver disease may lead to a greater risk of adverse drug reactions. Nonalcoholic fatty liver disease (NAFLD) is a progressive condition encompassing the relatively benign steatosis and the more severe, inflammatory state of nonalcoholic steatohepatitis (NASH). Here, we present an analysis of the effect of NAFLD progression on the major ATP-binding cassette (ABC) efflux transport proteins ABCC1–6, ABCB1, and ABCG2. Human liver samples diagnosed as normal, steatotic, NASH (fatty), and NASH (not fatty) were analyzed. Increasing trends in mRNA expression of ABCC1, ABCC4–5, ABCB1, and ABCG2 were found with NAFLD progression, whereas protein levels of all transporters exhibited increasing trends with disease progression. Immunohistochemical staining of ABCC3, ABCB1, and ABCG2 revealed no alterations in cellular localization during NAFLD progression. ABCC2 staining revealed an alternative mechanism of regulation in NASH in which the transporter appears to be internalized away from the canalicular membrane. This correlated with a preferential shift in the molecular mass of ABCC2 from 200 to 180 kDa in NASH, which has been shown to be associated with a loss of glycosylation and internalization of the protein. These data demonstrate increased expression of multiple efflux transporters as well as altered cellular localization of ABCC2 in NASH, which may have profound effects on the ability of patients with NASH to eliminate drugs in an appropriate manner.
Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses various stages of hepatocellular damage ranging from steatosis (simple fatty liver) to nonalcoholic steatohepatitis (NASH), which may then progress to cirrhosis and end-stage liver failure (Ali and Cusi, 2009). NAFLD is reported to affect approximately 17 to 40% of the U.S. population (McCullough, 2006; Ali and Cusi, 2009), whereas NASH itself is present in 5.7 to 17% (McCullough, 2006), making each a serious concern among healthcare professionals. Patients with NAFLD typically exhibit one or more symptoms of the metabolic syndrome such as hypertriglyceridemia, dyslipidemia, insulin resistance, obesity, or hypertension with each often necessitating separate and sometimes multiple medications (Angulo, 2002). Furthermore, the severity of NAFLD has been shown to correlate with the number of components of the metabolic syndrome diagnosed in the patient (McCullough, 2006). More alarming is the estimate that 30 to 50% of obese individuals may actually have the more severe form of the disease, NASH (Cheung and Sanyal, 2009). The strong association of NAFLD with type II diabetes mellitus and obesity, all of which are steadily increasing within the population, has begun to make each of these diseases a considerable clinical burden.
NAFLD initially develops as simple steatosis, which is an accumulation of triglycerides primarily within zone 3 hepatocytes termed microvesicular steatosis (Clark et al., 2002). This is considered the first insult in NAFLD pathogenesis and is believed to be relatively benign (Clark et al., 2002). The progression from steatosis to NASH has been the subject of extensive investigation and is postulated to result from a second insult mediated by one or a combination of the following: oxidative stress, proinflammatory cytokine induction, lipid peroxidation, or Fas ligand activation (Angulo, 2002). NASH is characterized by macrovesicular steatosis, which, as opposed to that seen in the steatotic stage, consists of enlarged lipid vesicles that may encompass most the hepatocyte cytoplasm (Marra et al., 2008). In addition to macrovesicular steatosis, the progression from steatosis to NASH is characterized by inflammation, oxidative damage, fibrosis, and more widespread zonal damage than that seen in steatosis (Marra et al., 2008). The significance of the progression to NASH is exacerbated by the propensity for NASH to further progress to cirrhosis and liver failure in 15 to 25% of patients (McCullough, 2006).
Because of the significant transformations in liver pathology and function occurring with the progression of NAFLD, as well as the likelihood of NAFLD patients to be medicated for one or more symptoms of the metabolic syndrome, it is reasonable to question the ability of these patients to manage the elimination of xenobiotics. Extrusion of compounds from the hepatocyte is mediated largely by the ATP-binding cassette (ABC) family of membrane transporters (Klaassen and Aleksunes, 2010). The ABC family of transporters consists of a wide variety of proteins that hydrolyze ATP to actively transport xenobiotics, endobiotics, and conjugates across cellular membranes (Klaassen and Aleksunes, 2010) and include such members as the ABCC subfamily also known as multidrug resistance-associated proteins, ABCB1 or P-glycoprotein, and ABCG2 or breast cancer resistance protein. Collectively, ABC transporters are large proteins possessing anywhere from 12 to 17 membrane-spanning regions, with the exception of ABCG2 (breast cancer resistance protein), which is considered a half-transporter (Gu and Manautou, 2010; Klaassen and Aleksunes, 2010). These efflux transporters reside on the sinusoidal and canalicular membranes of hepatocytes and are responsible for substrate transport into the blood and bile, respectively (Klaassen and Aleksunes, 2010). Because of their pivotal arrangement within hepatocytes, efflux transporters have considerable influence on overall drug exposure and elimination. Disruption of the expression or function of membrane transporters has the potential to significantly alter patient drug management. The purpose of the current study was to investigate the effect of the progression of human NAFLD on efflux drug transporter expression.
Materials and Methods
Materials.
Tris-HCl, EDTA, NaCl, glycerol, and Nonidet P-40 were obtained from Sigma-Aldrich (St. Louis, MO).
Human Liver Samples and Tissue Preparations.
Frozen and formalin-fixed, paraffin-embedded (FFPE) adult human liver tissue was obtained from the Liver Tissue Cell Distribution System that is coordinated through the University of Minnesota, Virginia Commonwealth University, and the University of Pittsburgh. All samples were scored and categorized by a medical pathologist within the Liver Tissue Cell Distribution System. Scoring was performed according to the NAFLD activity score system developed by Kleiner et al. (2005) followed by confirmation via histological examination at the University of Arizona. Donor information, including age and gender, was published previously (Fisher et al., 2009). The samples were diagnosed as either normal (n = 20), steatotic (n = 12), NASH with fatty liver (NASH fatty, n = 11), and NASH without fatty liver (NASH not fatty/cirrhosis, n = 11). Those samples exhibiting >10% fatty infiltration of hepatocytes were staged as steatotic. Samples were diagnosed as NASH (fatty) when >5% fatty infiltration of hepatocytes occurred with significant inflammation and fibrosis. NASH (not fatty) samples were diagnosed based on a reduction in fatty deposits within the hepatocytes to <5% with more marked inflammation and fibrotic branching. Total RNA was isolated from human liver tissue using RNAzol B reagent (Tel-Test Inc., Friendswood, TX) per the manufacturer's protocol. RNA concentrations were determined by UV spectrophotometry, and the integrity of the RNA was confirmed by ethidium bromide staining after agarose gel electrophoresis. Whole-cell lysate preparations of human liver tissue were prepared from tissue homogenized in NP-40 buffer [20 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 1% nonidet P-40, and 2 mM EDTA with one protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN) per 25 ml] at 4°C. Homogenized tissue was then agitated at 4°C for 2 h, centrifuged at 10,000g for 30 min, and the supernatant was transferred to a clean collection tube. Protein concentrations were determined using the Pierce BCA protein quantitation assay (Thermo Fisher Scientific, Waltham, MA) per the manufacturer's recommendations.
Branched DNA Assay.
Specific oligonucleotide probes for ABCC1–6, ABCB1, and ABCG2 (see Supplemental Data Table 1) were diluted in lysis buffer supplied by the Quantigene HV Signal Amplification Kit (Genospectra, Fremont, CA). Substrate solution, lysis buffer, capture hybridization buffer, amplifier, and label probe buffer used in the analysis were all obtained from the Quantigene Discovery Kit (Genospectra). The assay was performed in 96-well format with 10 μg of total RNA added to the capture hybridization buffer and 50 μl of the diluted probe set. The total RNA was then allowed to hybridize to the probe set overnight at 53°C. Hybridization steps were performed per the manufacturer's protocol the following day. Luminescence of the samples was measured with a Quantiplex 320 bDNA luminometer interfaced with Quantiplex Data Management Software, version 5.02 (Bayer, Walpole, MA).
Immunoblot Protein Analysis.
Whole-cell lysate proteins (50 μg/well) were prepared in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) without β-mercaptoethanol or boiling and separated by SDS-polyacrylamide gel electrophoresis using either Mini-PROTEAN TGX 4 to 15% gradient gels (ABCC2; Bio-Rad Laboratories) or 7.5% Tris-glycine gels (ABCC1, ABCC3–6, ABCB1, and ABCG2), followed by overnight transfer to polyvinylidene difluoride membranes. The following mouse monoclonal antibodies were obtained from Abcam Inc. (Cambridge, MA) and used to determine relative protein levels: ABCC1 (MRPm5, 1:5000), ABCC3 (M3II-9, 1:7000), and ABCB1 (C219, 1:15,000). ABCC2 (M2III-5, 1:2000) and ABCG2 (BXP-21, 1:1000) protein levels were determined using mouse monoclonal antibodies obtained from Kamiya Biomedical (Thousand Oaks, CA). Protein levels of ABCC4 (M4I-10, 1:2000) and ABCC6 (M6II-68, 1:10,000) were analyzed with rat monoclonal antibodies generated by George L. Scheffer (Amsterdam, The Netherlands). ABCC5 (M5I-1, 1:1000) protein levels were determined using rat monoclonal antibodies obtained from Abcam Inc. Quantification of relative protein expression was determined using image processing and analysis with Image J software (National Institutes of Health, Bethesda, MD) and normalized to total pan-cadherin (1:7000; Abcam Inc.).
Immunohistochemistry.
Immunohistochemical (IHC) staining for all proteins was performed on FFPE human liver samples. In brief, tissue sections were deparaffinized in xylene and rehydrated in ethanol, followed by antigen retrieval in citrate buffer (pH 6, ABCC2, ABCB1, and ABCG2) or Tris-EDTA buffer (pH 9, ABCC3). Endogenous peroxidase activity was blocked with 0.3% (v/v) H2O2 in methanol for 20 min. Immunohistochemical staining for ABCC2, ABCB1, and ABCG2 was performed with the MACH3 staining kit (Biocare Medical, Concord, CA) per the manufacturer's protocol. Samples were incubated in a primary antibody solution overnight at 4°C. ABCC2 (M2III-5, 1:20) mouse monoclonal antibody was obtained from Kamiya Biomedical. ABCB1 (C-219, 1:40) and ABCG2 (BXP-21, 1:50) mouse monoclonal antibodies were acquired through Abcam Inc. Immunohistochemical staining for ABCC3 was performed with the MACH4 staining kit (Biocare Medical) per the manufacturer's recommendations. Samples were incubated in an ABCC3 antibody solution (M3II-9, 1:500; Abcam Inc.) for 2 h at room temperature. All slides were counterstained with hematoxylin (Sigma-Aldrich) after color development with Betazoid DAB (Biocare Medical). All slides were imaged with a Nikon Eclipse E4000 microscope and a Sony Exwave DXC-390 camera (Nikon Instruments, Melville, NY).
Statistical Analysis.
All human liver samples used in the study were categorized and ordered by disease progression: normal < steatosis < NASH (fatty) < NASH (not fatty). The data obtained from this study exhibited continuous outcomes with skewed distributions. The data were presented as box and whisker plots, and median values were compared. Data were analyzed by a nonparametric trend analysis with a significance level of p ≤ 0.05, thus allowing for the determination of significant increases or decreases with disease progression. All analyses were performed with Stata9 software (StataCorp LP, College Station, TX).
Results
Human NAFLD Histology.
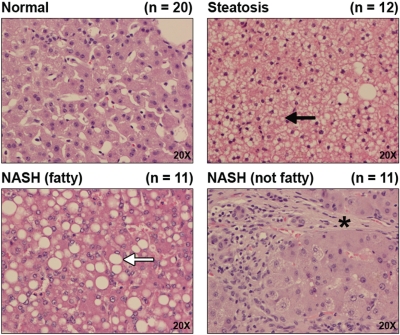
Liver samples stained with hematoxylin and eosin are shown in Fig. 1. Representative images are shown at magnification, 20×. Steatosis was diagnosed when samples possessed >10% fatty infiltration of hepatocytes, whereas a diagnosis of NASH (fatty) was made when samples possessed >5% fatty infiltration of hepatocytes accompanied by significant inflammation and fibrosis. NASH (not fatty) was differentiated from NASH (fatty) when samples presented with a reduction in fatty deposits to <5% accompanied by increased inflammation and branching fibrosis. Microvesicular lipid deposits within hepatocytes, indicated by a black arrow, are clearly visible in steatotic samples, whereas macrovesicular deposits, indicated by a white arrow, are apparent throughout NASH (fatty) samples. In addition to macrovesicular deposits, the NASH (fatty) stage is further characterized by the infiltration of inflammatory cells within the liver and fibrosis. The progression from NASH (fatty) to NASH (not fatty) involves a reduction in fatty deposition within hepatocytes accompanied by an increase in inflammation. Furthermore, fibrosis (indicated by an asterisk) in the NASH (not fatty) stage is much more prevalent and often presents as branching fibrosis, which significantly disrupts the hepatic architecture and segregates hepatocytes into small populations.
Fig. 1.
Histology of human NAFLD. Hematoxylin and eosin staining of formalin-fixed, paraffin-embedded human liver samples diagnosed as normal, steatotic, NASH (fatty), and NASH (not fatty) are shown at magnification 20×. Steatosis was diagnosed as >10% fatty infiltration of hepatocytes with microvesicular steatosis (black arrow). NASH (fatty) was staged as >5% fatty infiltration with macrovesicular steatosis (white arrow), inflammation, and fibrosis. NASH (not fatty) was diagnosed as <5% fatty infiltration with more significant inflammation and fibrosis (*).
ATP-Binding Cassette Transporter Expression in Human NAFLD.
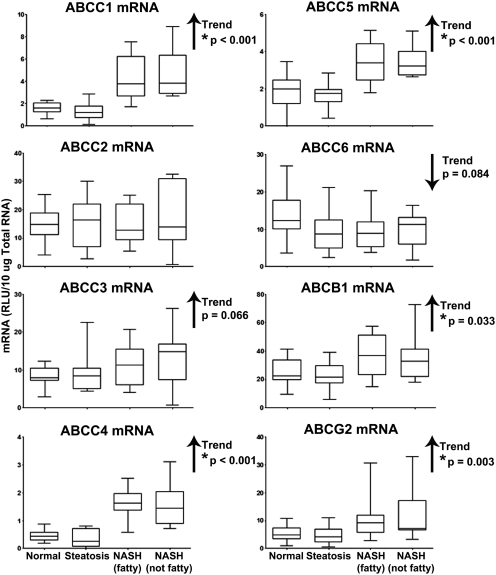
mRNA levels of ABCC1–6, ABCB1, and ABCG2 transporters were assessed by the branched DNA method of mRNA quantification, and the results are shown in Fig. 2. There was a significant increasing trend in mRNA expression of the sinusoidal transporters ABCC1 (p < 0.001), ABCC4 (p < 0.001), and ABCC5 (p < 0.001) with progression of NAFLD. We observed an increase in ABCC3 (p = 0.066) and a decrease in ABCC6 (p = 0.084) mRNA levels; however, these trends were not statistically significant. The biliary transporters ABCB1 and ABCG2 were also significantly upregulated with disease progression (p = 0.033 and 0.003, respectively). mRNA levels of ABCC2 remained unchanged throughout progression of NAFLD.
Fig. 2.
mRNA expression of ABC transporters in Human NAFLD. mRNA levels of ABCC1–6, ABCB1, and ABCG2 in human liver samples staged as normal, steatotic, NASH (fatty), and NASH (not fatty) are shown. mRNA levels were measured by the branched DNA assay and expressed as relative light units (RLU) per 10 μg of total RNA. Arrows, increasing or decreasing trend with NAFLD progression. *, significant trends (p ≤ 0.05).
Protein Expression of ATP-Binding Cassette Transporters in Human NAFLD.
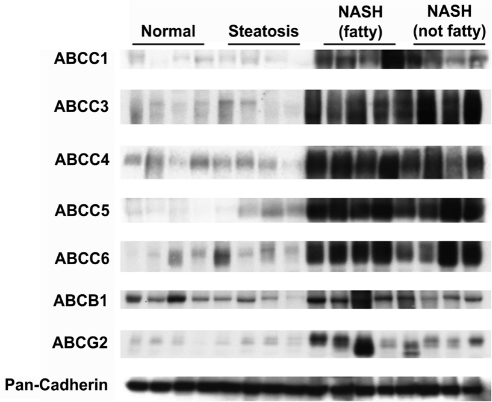
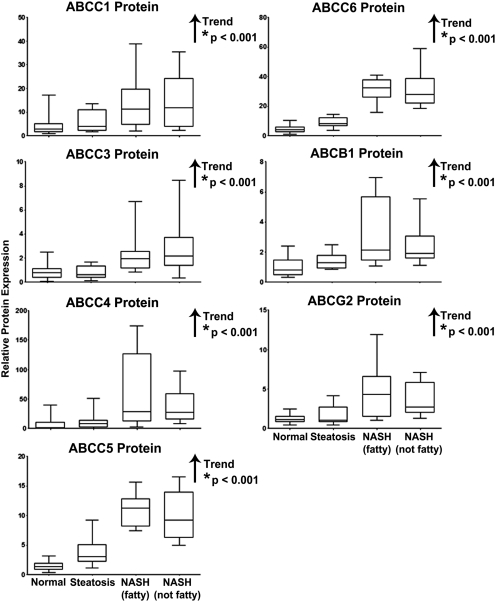
Protein levels of ABCC1, ABCC3–6, ABCB1, and ABCG2 were assessed by immunoblot analysis in human liver whole-cell lysate preparations and normalized to the control protein, pan-cadherin (protein levels of ABCC2 will be discussed later). Representative immunoblots of the transporters depicting four samples in each diagnostic category are shown in Fig. 3 with pan-cadherin. The relative protein expression of each transporter was determined by densitometric analysis in all 54 human liver samples within the study. Densitometric results are shown in Fig. 4. There were significant increasing trends in relative protein expression for all efflux transporters currently examined: ABCC1 (p < 0.001), ABCC3 (p < 0.001), ABCC4 (p < 0.001), ABCC5 (p < 0.001), ABCC6 (p < 0.001), ABCB1 (p < 0.001), and ABCG2 (p < 0.001).
Fig. 3.
Representative protein expression of ABC Transporters in Human NAFLD. Immunoblot analysis of ABC transporters was performed with 50 μg whole-cell lysate protein obtained from human liver samples staged as normal, steatotic, NASH (fatty), and NASH (not fatty). Representative immunoblots are shown for each transporter (ABCC1, ABCC3–6, ABCB1, and ABCG2) and the control protein pan-cadherin with four samples from each diagnostic category.
Fig. 4.
Relative protein expression of ABC transporters in human NAFLD. Relative protein levels of ABCC1, ABCC3–6, ABCB1, and ABCG2 in human liver whole-cell lysate preparations staged as normal, steatotic, NASH (fatty), and NASH (not fatty) were determined by densitometric analysis of all samples and expressed as relative to control protein (pan-cadherin). Arrows, increasing or decreasing trend with NAFLD progression. *, significant trends (p ≤ 0.05).
Immunohistochemical Staining of ATP-Binding Cassette Transporters in FFPE Human Liver Samples.
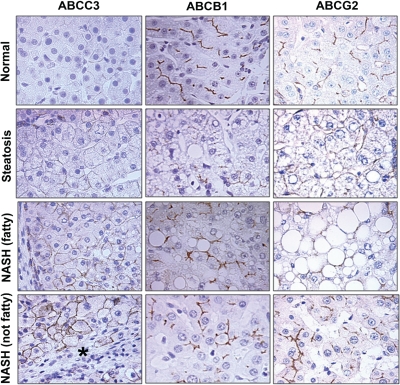
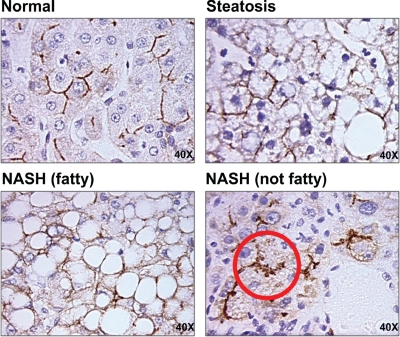
IHC staining for ABCC2, ABCC3, ABCB1, and ABCG2 was performed on FFPE human liver samples and is shown in Fig. 5 at magnification 40×, whereas staining for ABCC2 is shown in Fig. 6 (magnification, 40×). Minimal staining of ABCC3 was observed in normal and steatotic samples. However, staining became more prominent in both stages of NASH and was localized primarily to fibrotic areas (indicated by an asterisk). ABCB1 staining appeared to be more prominent in NASH (fatty) and NASH (not fatty) samples; however, no significant changes to cellular localization were observed. Likewise, staining for ABCG2 appeared most prominent in NASH (not fatty) samples; however, the cellular localization of ABCG2 did not appear to be significantly altered. In end-stage NASH (not fatty) samples it appears that ABCC2 may be internalized into intracellular vesicles away from the biliary membrane (circled in red). This phenomenon was observed only in the end-stage NASH samples and was not present in normal or steatotic human livers.
Fig. 5.
Immunohistochemical staining of ABC transporters in FFPE human NAFLD samples. IHC staining of ABCC3, ABCB1, and ABCG2 in formalin-fixed, paraffin-embedded human liver samples staged as normal, steatotic, NASH (fatty), and NASH (not fatty) is shown at 40× magnification. Antibody binding was detected by either the MACH3 (ABCB1 and ABCG2) or MACH4 (ABCC3) method. Color development was performed using Betazoid DAB. *, fibrosis in ABCC3 staining.
Fig. 6.
Immunohistochemical staining of ABCC2 in FFPE human NAFLD samples. IHC staining of ABCC2 in formalin-fixed, paraffin-embedded human liver samples staged as normal, steatotic, NASH (fatty), and NASH (not fatty) is shown at magnification, 40×. Antibody binding was detected by the MACH3 method. Color development was performed using Betazoid DAB.
Protein Expression and Glycosylation Status of ABCC2 in Human NAFLD.
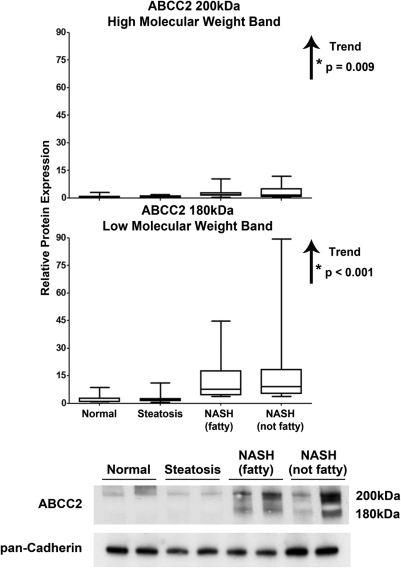
Protein levels of ABCC2 were assessed by immunoblot analysis using 4 to 15% gradient gels for separation of proteins followed by normalization to the control protein, pan-cadherin. The use of gradient gels to aid in the separation of the high- (200 kDa) and low- (180 kDa) molecular-mass forms of ABCC2 was previously developed by Zhang et al. (2005). The shift in molecular mass from 200 to 180 kDa was identified in sandwich-cultured hepatocytes as a loss of glycosylation by a treatment regimen using a glycosylation inhibitor (Zhang et al., 2005). In addition, the loss of glycosylation was positively associated with internalization of ABCC2 in sandwich-cultured hepatocytes (Zhang et al., 2005). Densitometric analysis of ABCC2 for all samples within the current study along with representative immunoblots depicting two samples within each diagnostic category are shown in Fig. 7. The fully glycosylated high- and lesser-glycosylated low-molecular-mass forms of ABCC2 were significantly increased with progression of NAFLD (p = 0.009 and <0.001, respectively). However, the low-molecular-mass (180 kDa) form of ABCC2 was rarely detected in normal and steatotic samples, but it was readily apparent in all NASH (fatty) and NASH (not fatty) samples.
Fig. 7.
Representative and relative protein expression of the high- and low-molecular-mass forms of ABCC2 in human NAFLD. Immunoblot analysis and relative protein expression of ABCC2 in human liver samples staged as normal, steatotic, NASH (fatty), and NASH (not fatty). Separation of whole-cell lysate proteins was performed on 4 to 15% gradient gels, which allows for separation of the high- (∼200 kDa) and low- (∼180 kDa) molecular-mass forms of ABCC2. Representative blots are shown for ABCC2 and control protein, pan-cadherin, with two samples from each diagnostic category. Relative protein levels were determined by densitometric analysis of all samples and expressed as relative to control protein. Arrows, increasing or decreasing trend with NAFLD progression. *, significant trends (p ≤ 0.05).
Discussion
Adverse drug reactions (ADRs) have gained increasingly greater attention over the last several years. Defined by the World Health Organization as an unintentional, harmful reaction to an administered medication, ADRs remain a significant contributor to causes of death in several countries (Finney, 2008; Wooten, 2010). Type B ADRs are those that are unpredictable and are thus considered idiosyncratic (Wooten, 2010). Because of their unpredictability and often unknown etiology, type B ADRs pose a formidable threat to patient medication management. In contrast, type A ADRs are typically predictable on the basis of the known pharmacokinetic and pharmacodynamic profiles of the drug (Wooten, 2010). Predictions of drug disposition can be made based on the identification of a drug as a substrate for specific transporters. However, this predictability may prove useless for a patient population or disease demographic in which basic drug metabolism and disposition data are unavailable. Thus, for diseases that alter drug metabolizing enzyme and transporter expression and function, the predictability of type A ADRs fails. This leads to an inability to identify a particular demographic as an at-risk population for ADRs. Given that NAFLD is becoming an increasingly prevalent disease, understanding the capacity for hepatic drug metabolism and disposition in each stage of the disease is necessary to reduce the risk of ADRs in this patient population.
Our laboratory has previously investigated the effect of human NAFLD on cytochrome P450 and GSH transferase enzyme expression and functionality (Fisher et al., 2009; Hardwick et al., 2010). However, little information is available on the expression or functionality of the ABC transporters in human NAFLD. These patients represent a population that is very likely to be prescribed various pharmaceuticals to treat symptoms of the metabolic syndrome, yet little is understood about the ability of these patients to manage the metabolism and elimination of xenobiotics. To date, animal models have been used to gain an understanding of the effects of NAFLD on transporter expression and subsequent drug disposition. The current data provide new insight to the effect of NAFLD on the regulation of efflux transporter expression in human patients that could, in turn, enable better prediction of pharmacokinetics in these patients, thereby leading to a more positive therapeutic outcome.
Previous studies within our laboratory using diet-induced rodent models of NAFLD have shown that disease progression leads to significant induction of Abcc3, Abcc4, and Abcg2 mRNA in NASH, but not steatosis (Lickteig et al., 2007). Likewise, protein levels of Abcc2–4 and Abcg2 were induced in NASH rodents (Lickteig et al., 2007). These expression changes in hepatic ABC transporters were found to result in functional alterations of drug metabolite disposition. Upon administration of acetaminophen, NASH rodents exhibited a significant reduction in the biliary excretion of glucuronide-, sulfate-, and GSH-acetaminophen conjugates with a concomitant shift to sinusoidal excretion (Lickteig et al., 2007). The link between altered efflux transporter expression and changes in drug disposition is clearly demonstrated in this rodent model of NASH; however, until now, similar studies in human patients have been severely lacking.
The data presented in the current study provide a generous foundation for further investigation into drug disposition outcomes during NAFLD progression. Similar to rodent NAFLD models, ABCC4 and ABCG2 gene induction was observed in human NASH patients (Fig. 2). Additional transporters, including ABCC1, 5, and ABCB1, were also found to be induced with disease progression (Fig. 2). In general, most transporters of the ABC family followed an increasing trend in mRNA expression as the disease progressed from steatosis to end-stage NASH (not fatty). Constitutive androstane receptor (CAR) and pregnane X receptor are known to induce ABCC2, 3, ABCB1, and ABCG2 in humans (Klaassen and Aleksunes, 2010). We have previously identified induction of the CAR target gene CYP2B6 and the pregnane X receptor target genes CYP2A6 and GSTA1 in this same set of human samples (Fisher et al., 2009; Hardwick et al., 2010). Nuclear translocation of CAR and induction of CAR target genes has been shown to worsen histological features of NASH in a rodent model of the disease and possibly play a role in the transition from steatosis to NASH (Yamazaki et al., 2007). The current study has demonstrated induction of the CAR target genes ABCB1 and ABCG2, as well as a modest increase in ABCC3 mRNA. In addition, NF-E2-related factor 2 (Nrf2), in response to oxidative stress conditions, is believed to induce human ABCC2, 3, ABCB1, and ABCG2 (Klaassen and Aleksunes, 2010). We have previously shown activation of Nrf2 and several of its downstream target genes in this same set of NAFLD samples (Hardwick et al., 2010). Here, we have identified induction of several transporters that have been identified as Nrf2 target genes, including ABCB1 and ABCG2. In addition, although not significant (p = 0.066), ABCC3 exhibited a noticeable increasing trend in mRNA expression similar to ABCB1 and ABCG2. The observed induction of ABC transporter genes may be due to initiators of the second hit mediating the transition from steatosis to NASH through activation of various nuclear receptors and transcription factors.
In this study, we have identified an overarching increase in ABC transporter protein levels with progression of NAFLD. ABCC1–6, ABCB1, and ABCG2 protein all exhibited significant increasing trends (Figs. 3 and 4) with disease progression. A noticeable elevation in protein levels of ABCC1, ABCC3–6, and ABCG2 was observed between steatosis and NASH samples (Fig. 3). The increase in protein levels of numerous efflux transporters suggests a heightened ability for NASH patients to excrete drugs and drug conjugates out of hepatocytes. However, due to the overlapping substrate specificity, yet differing affinity for substrates of several of these transporters, these effects could have a variety of implications upon the hepatic elimination of xenobiotics.
Of particular note in this study is the potential altered regulation of ABCC2 in NASH (not fatty) patients. Internalization of Abcc2 to intracellular vesicles has been extensively studied in rodents, and linked to oxidative stress and cholestatic conditions. In particular, vesicular retrieval of Abcc2 has been demonstrated during estradiol-17β-glucuronide (E217-G)-induced cholestasis (Mottino et al., 2002), lipopolysaccharide-induced oxidative stress (Saeki et al., 2011), bile duct ligation (Kojima et al., 2008b), and following ethacrynic acid treatment (Ji et al., 2004). ABCC2 internalization has also been identified in several forms of human liver injury including drug-induced liver injury, obstructive jaundice, autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cirrhosis (Kojima et al., 2003, 2008a). Here, we provide the first evidence that ABCC2 may be internalized in the later stages of NAFLD. Previous studies have shown that internalization of hepatic Abcc2 is correlated to GSH depletion in vivo (Sekine et al., 2008). We have previously shown in these same NAFLD samples that disease progression leads to significant depletion of hepatic GSH stores (Hardwick et al., 2010). It is likely that GSH depletion in these samples is the mechanism responsible for the proposed internalization of ABCC2 observed in the current study; however, further analyses are needed to identify the specific mechanism underlying this alternative method of ABCC2 regulation.
Zhang et al. (2005) identified a role for glycosylation in the regulation of Abcc2 cellular localization. Rat hepatocytes were isolated and placed in a sandwich-culture system. At day 0 of culture, hepatocytes expressed a 180- to 190-kDa form of Abcc2, but by day 4 the molecular mass of Abcc2 had increased to 200 kDa (Zhang et al., 2005). It was discovered that the glycosylation status determined the molecular mass shift of Abcc2, and that the fully glycosylated, 200-kDa form was properly localized to the canalicular membrane (Zhang et al., 2005). In contrast, the low-molecular-mass, 180- to 190-kDa, lesser-glycosylated form was not localized in the canalicular membrane (Zhang et al., 2005). To further test our hypothesis of ABCC2 internalization in NASH, we investigated the protein expression of these two glycosylation states of ABCC2. Normal and steatotic human samples solely exhibited the high-molecular-mass, 200-kDa form of ABCC2, indicative of membrane localization. We identified the appearance of a 180-kDa band of ABCC2 protein that is preferentially expressed in NASH samples (Fig. 7), which correlates with the observation of ABCC2 internalization identified by immunohistochemical staining. This suggests that there is a portion of ABCC2 protein in NASH samples that is not fully glycosylated and not properly localized in the canalicular membrane.
The functional consequence of this method of ABCC2 regulation is extremely important in drug disposition. In conjunction with their studies identifying the association between glycosylation status and cellular localization, Zhang et al. (2005) determined that the loss of glycosylation of the ABCC2 protein results in diminished transport function due to improper localization. The biliary excretion of 5-(6)-carboxy-2′,7′-dichlorofluorescein was reduced when Abcc2 was internalized (Zhang et al., 2005). However, when the fully glycosylated, high-molecular-mass form was expressed in sandwich-cultured rat hepatocytes, Abcc2 was properly localized to the canalicular membrane and able to excrete substrates into the canalicular space (Zhang et al., 2005). The disruption of Abcc2 cellular localization and its effect on transport function has been demonstrated in vivo in estradiol-17-β-d-glucuronide-induced cholestasis. Upon administration of estradiol-17-β-d-glucuronide, Abcc2 was retrieved to pericanalicular vesicles and the biliary excretion of dinitrophenyl-S-GSH was diminished (Mottino et al., 2002). These studies indicate that the internalization of ABCC2 that was observed in our NASH samples may limit these patients' ability to excrete ABCC2 substrates into bile.
In conclusion, we have observed a general increase in the mRNA and protein expression of numerous ABC transporters after the transition from steatosis to NASH in human NAFLD samples. However, internalization of ABCC2 in end-stage NASH could lead to a reduction in functionality and ultimately elicit alterations in the hepatic elimination of xenobiotics. Considering NAFLD patients are frequently medicated for symptoms of the metabolic syndrome, such as hypertension, dyslipidemia, and insulin resistance, these patients could be at increased risk for ADRs. Despite greater recognition of this disease, the field of NAFLD research is in great need of further studies on the ability of these patients to metabolize and eliminate various pharmaceutical agents to ensure safety and improve therapeutic outcome.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health-funded Liver Tissue Cell Distribution System for continued assistance and procurement of liver tissue samples from patients with various stages of NAFLD. We especially thank Marion Namenwirth (University of Minnesota), Melissa Thompson (Virginia Commonwealth University), and Dr. Stephen C. Strom and Kenneth Dorko (University of Pittsburgh). In addition, we thank Alejandra Curiel for contributions to this work.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK068039]; the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES006694]; the National Institutes of Health National Center for Complementary and Alternative Medicine [Grant AT002842]; and the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant HD062489]. The Liver Tissue Cell Distribution System was sponsored by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Contract N01-DK70004/HHSN267200700004C].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/dmd.111.041012.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- ABC
- ATP-binding cassette
- FFPE
- formalin-fixed, paraffin-embedded
- IHC
- immunohistochemical
- ADR
- adverse drug reaction
- CAR
- constitutive androstane receptor
- Nrf2
- NF-E2-related factor 2.
Authorship Contributions
Participated in research design: Hardwick and Cherrington.
Conducted experiments: Hardwick, Fisher, and Canet.
Contributed new reagents or analytic tools: Scheffer.
Performed data analysis: Hardwick and Cherrington.
Wrote or contributed to the writing of the manuscript: Hardwick and Cherrington.
References
- Ali R, Cusi K. (2009) New diagnostic and treatment approaches in non-alcoholic fatty liver disease (NAFLD). Ann Med 41:265–278 [DOI] [PubMed] [Google Scholar]
- Angulo P. (2002) Nonalcoholic fatty liver disease. N Engl J Med 346:1221–1231 [DOI] [PubMed] [Google Scholar]
- Cheung O, Sanyal AJ. (2009) Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol 25:230–237 [DOI] [PubMed] [Google Scholar]
- Clark JM, Brancati FL, Diehl AM. (2002) Nonalcoholic fatty liver disease. Gastroenterology 122:1649–1657 [DOI] [PubMed] [Google Scholar]
- Finney L. (2008) Safety of Medicines—Adverse Drug Reactions. Fact Sheet No. 293 World Health Organization, Geneva, Switzerland [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, Cherrington NJ. (2009) Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos 37:2087–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Manautou JE. (2010) Regulation of hepatic ABCC transporters by xenobiotics and in disease states. Drug Metab Rev 42:482–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. (2010) Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 38:2293–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Ito K, Sekine S, Tajima A, Horie T. (2004) Ethacrynic-acid induced glutathione depletion and oxidative stress in normal and MRP2-deficient rat liver. Free Radic Biol Med 37:1718–1729 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62:1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. , and Nonalcoholic Steatohepatitis Clinical Research Network (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321 [DOI] [PubMed] [Google Scholar]
- Kojima H, Nies AT, König J, Hagmann W, Spring H, Uemura M, Fukui H, Keppler D. (2003) Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J Hepatol 39:693–702 [DOI] [PubMed] [Google Scholar]
- Kojima H, Sakurai S, Uemura M, Kitamura K, Kanno H, Nakai Y, Fukui H. (2008a) Disturbed colocalization of multidrug resistance protein 2 and radixin in human cholestatic liver diseases. J Gastroenterol Hepatol 23:e120–e128 [DOI] [PubMed] [Google Scholar]
- Kojima H, Sakurai S, Yoshiji H, Uemura M, Yoshikawa M, Fukui H. (2008b) The role of radixin in altered localization of canalicular conjugate export pump Mrp2 in cholestatic rat liver. Hepatol Res 38:202–210 [DOI] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, Manautou JE, Cherrington NJ. (2007) Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos 35:1970–1978 [DOI] [PubMed] [Google Scholar]
- Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. (2008) Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med 14:72–81 [DOI] [PubMed] [Google Scholar]
- McCullough AJ. (2006) Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol 40:S17–S29 [DOI] [PubMed] [Google Scholar]
- Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. (2002) Altered localization and activity of canalicular Mrp2 in estradiol-17beta-d-glucuronide-induced cholestasis. Hepatology 35:1409–1419 [DOI] [PubMed] [Google Scholar]
- Saeki J, Sekine S, Horie T. (2011) LPS-induced dissociation of multidrug resistance-associated protein 2 (Mrp2) and radixin is associated with Mrp2 selective internalization in rats. Biochem Pharmacol 81:178–184 [DOI] [PubMed] [Google Scholar]
- Sekine S, Ito K, Horie T. (2008) Canalicular Mrp2 localization is reversibly regulated by the intracellular redox status. Am J Physiol Gastrointest Liver Physiol 295:G1035–G1041 [DOI] [PubMed] [Google Scholar]
- Wooten JM. (2010) Adverse drug reactions: Part I. South Med J 103:1025–1028; quiz 1029 [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Kakizaki S, Horiguchi N, Sohara N, Sato K, Takagi H, Mori M, Negishi M. (2007) The role of the nuclear receptor constitutive androstane receptor in the pathogenesis of non-alcoholic steatohepatitis. Gut 56:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Tian X, Chandra P, Brouwer KL. (2005) Role of glycosylation in trafficking of Mrp2 in sandwich-cultured rat hepatocytes. Mol Pharmacol 67:1334–1341 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.