Abstract
Mild therapeutic hypothermia is emerging clinically as a neuroprotection therapy for individuals experiencing cardiac arrest (CA); however, its effects combined with disease pathogenesis on drug disposition and response have not been fully elucidated. We determined the activities of four major hepatic-metabolizing enzymes (CYP3A, CYP2C, CYP2D, and CYP2E) during hypothermia after experimental CA in rats by evaluating the pharmacokinetics of their probe drugs as a function of altered body temperature. Animals were randomized into sham normothermia (37.5–38°C), CA normothermia, sham hypothermia (32.5–33°C), and CA hypothermia groups. Probe drugs (midazolam, diclofenac, dextromethorphan, and chlorzoxazone) were given simultaneously by intravenous bolus after temperature stabilization. Multiple blood samples were collected between 0 and 8 h after drug administration. Pharmacokinetic (PK) analysis was conducted using a noncompartmental approach and population PK modeling. Noncompartmental analysis showed that the clearance of midazolam (CYP3A) in CA hypothermia was reduced from sham normothermia rats (681.6 ± 190.0 versus 1268.8 ± 348.9 ml · h−1 · kg−1, p < 0.05). The clearance of chlorzoxazone (CYP2E) in CA hypothermia was also reduced from sham normothermia rats (229.6 ± 75.6 versus 561.89 ± 215.9 ml · h−1 · kg−1, p < 0.05). Population PK analysis further demonstrated the decreased clearance of midazolam (CYP3A) was associated with CA injury (p < 0.05). The decreased clearance of chlorzoxazone (CYP2E1) was also associated with CA injury (p < 0.01). Hypothermia was found to be associated with the decreased volume of distribution of midazolam (V1), dextromethorphan (V1), and peripheral compartment for chlorzoxazone (V2) (p < 0.05, p < 0.05, and p < 0.01, respectively). Our data indicate that hypothermia, CA, and their interaction alter cytochrome P450-isoform specific activities in an isoform-specific manner.
Introduction
Brain injury is a common cause of morbidity and mortality after resuscitation from cardiac arrest (CA) (Geocadin et al., 2008; Manole et al., 2009). Therapeutically reducing body temperature to 32–35°C over 12 to 24 h after out-of-hospital CA is the proven neuroprotective strategy in these highly susceptible patients (Bernard et al., 2002; Hypothermia after Cardiac Arrest Study Group, 2002; Holzer, 2010). Despite the benefit of therapeutic hypothermia, whole-body cooling after resuscitation complicates drug therapy because of its potential effects on both drug disposition and response. Previous studies have found significant elevated drug levels and prolonged drug response during hypothermia for drugs such as phenytoin, vecuronium, morphine, propofol, and fentanyl (Leslie et al., 1995; Caldwell et al., 2000; Iida et al., 2001; Fritz et al., 2005; Róka et al., 2008). Given the large number of medications used in critically ill patients after CA and possible adverse drug effects, close pharmacokinetic (PK) and pharmacodynamic monitoring is required by critical care practitioners (Cullen et al., 1997; Lazarou et al., 1998; Vargas et al., 1998). At present, the specific changes in drug metabolism and disposition during hypothermia after CA are largely unknown.
Many of the drugs used in CA patients do not have readily measurable pharmacodynamic endpoints and have narrow therapeutic indices. This fact necessitates the evaluation of a systemic drug metabolism and pharmacokinetics as a tool to ensure drug safety and efficacy. Cytochrome P450 (P450) enzymes play an essential role in metabolizing medications commonly used in critical care medicine including benzodiazepines, calcium channel blockers, anesthetics, and opioids (Tortorici et al., 2007). The magnitude of altered activity of specific P450 isoforms in CA with changes in body temperature is poorly understood. In addition, during hypothermia after CA, the alterations in normal drug metabolism can be quite complex because of suppressed effects from heart failure, multiple organ dysfunction, and temperature. Various pathophysiological disturbances including hepatic ischemia and changes in hepatic blood flow may significantly alter the PK behavior of drugs. Therefore, a thorough understanding of both the temperature and pathophysiological effects of CA on individual P450 isoform activity is necessary to delineate the alterations in drug metabolism and disposition.
In this study, we estimated the activities of four major P450 isoforms, CYP3A, CYP2C, CYP2D, and CYP2E. A probe cocktail of midazolam, diclofenac, dextromethorphan, and chlorzoxazone was used for the phenotypic assessment of CYP3A, CYP2C, CYP2D, and CYP2E1 activity, respectively. In vivo validation of all these probe drugs or in combination in cocktail has been previously performed (Scott et al., 1999; Blakey et al., 2004; Krösser et al., 2006; Ryu et al., 2007; Ghassabian et al., 2009; Xia et al., 2010). Midazolam, diclofenac, dextromethorphan, and chlorzoxazone were also chosen on the basis of their relative specificity, their relatively short half-life, and their prior clinical use as probe drugs (Streetman et al., 2000; Frye 2004). In addition, these drugs were selected because mass spectrometric detection limits allowed for lower doses of these drugs in rats to decrease the likelihood of probe drug interactions upon coadministration. We hypothesized that the metabolism of P450 probe substrates decreases during cooling because of the acute effect of hypothermia on the enzyme activity and that these alterations would be isoform-specific. The first endpoint of the study was to evaluate systemic clearance of probe drugs within different groups by noncompartmental PK analysis. The secondary endpoint was to determine the specific correlation between hypothermia and CA with probe drug pharmacokinetics by nonlinear mixed-effect PK modeling.
Materials and Methods
Animals.
Animal care and experimental protocols were approved by the University of Pittsburgh Animal Care and Use Committee. Male Sprague-Dawley rats (300–350 g) were purchased from Hilltop Laboratories (Scottdale, PA). The animals were maintained on a 12-h light/dark cycle and were allowed free access to food and water. All animals were acclimated for 1 week before the experiment.
Surgery and Cardiac Arrest Procedure.
The animals were randomly divided into four groups: 1) sham normothermia, 2) sham hypothermia, 3) CA normothermia, and 4) CA hypothermia (n = 6). Rats were anesthetized with 1 to 1.5% isoflurane via a nose cone, tracheally intubated, and mechanically ventilated to maintain eucapnia (PaCO2 between 35 and 45 mmHg) to start surgery and cannulation. Anesthesia was maintained throughout the experiment with isoflurane and oxygen. The left femoral artery was cannulated for measurement of mean arterial pressure, and the femoral vein was cannulated bilaterally for drug administration and blood sampling. Neuromuscular blockade was induced and maintained with 2 mg/kg i.v. vecuronium before the cannulation. After preparative surgery, isoflurane was washed out with 100% oxygen for 3 min and room air for 2 min. CA was initiated by asphyxiation via disconnection of the mechanical ventilation. Asphyxial CA was continued for 8 min, with approximately 6 min of asystole, confirmed by electrocardiogram. Resuscitation was initiated by reconnecting the rat to the ventilator, administering intravenous epinephrine (0.005 mg/kg) and sodium bicarbonate (1 mEq/kg), and performing manual chest compressions for approximately 40 to 60 s until restoration of spontaneous circulation. Sham animals received all of the above surgical procedures for blood sample collection but were not made hypothermic or subjected to cardiac arrest.
Hypothermia and Temperature Measurement.
Hypothermia was induced starting 60 min after resuscitation. Systemic hypothermia with a target temperature of 32.5–33°C was initiated by surface cooling for 30 min, followed by 15 min of stabilization. The animals were maintained in a hypothermic state for 8 h after temperature stabilization. Rectal temperature of the normothermic group was maintained at 37.5–38°C. Body temperature was continuously measured using a rectal temperature probe. A heating pad, heating light, cooling fan, and ice bags were used to adjust and maintain the target temperature.
Drug Administration and Pharmacokinetics Sampling.
Once rectal temperature was stabilized, the four probe drugs were given intravenously at doses of 0.2 mg/kg midazolam, 0.5 mg/kg dextromethorphan, 0.5 mg/kg diclofenac, and 0.5 mg/kg chlorzoxazone simultaneously. The probe substrate cocktail formulation was prepared in saline with ethanol (<20% v/v). Normal saline solution (1.0 ml) was injected to flush the catheter after the drug administration. Baseline blood samples and samples (0.3 ml) at 5, 15, 30, 60, 120, 180, 240, 300, 360, and 480 min were obtained via a heparinized syringe. The blood sample volume was replaced with an equal volume of normal saline. The blood samples were centrifuged, and the supernatant plasma was transferred to a labeled microcentrifuge tube and immediately placed into a −20°C freezer.
Arterial Blood Gas Sampling.
Multiple arterial blood gas samples (100 μl) were collected (at baseline before CA, 10, 30, and 60 min after return of spontaneous circulation, at the end of hypothermia induction, and every hour during the blood sampling) for the purpose of monitoring the status of the animals over the time. Physiological parameters including pH, MAP, PaCO2, pH, lactate, hematocrit, K+, Mg2+, glucose, and blood urea nitrogen (BUN) were measured at each time point. A maintenance infusion of saline (3 ml · h−1 · kg−1) was started beginning at 20 min after drug administration and continued for 8 h. Doses of bicarbonate were given as needed to correct acidosis.
Bioanalytical Analysis by UPLC-Triple Quadrupole Mass Spectrometry.
Probe drug plasma concentrations were measured simultaneously by UPLC-triple quadrupole mass spectrometry. A UPLC system with an ACQUITY BEH C18 (1.7 μm × 2.1 mm × 50 mm) column (Waters, Milford, MA) was used. The mobile phase consisted of 0.1% formic acid in water (A) and pure acetonitrile (B), a gradient from 90:10 (A:B) to 70:30 (A:B) within 3 min, and a return to 90:10 (A:B) at 5.0 min at a flow rate of 0.25 ml/min. A triple-quadrupole mass spectrometer, equipped with electrospray ionization source was used for mass analysis and detection (Quantum Ultra; Thermo Fisher Scientific, Waltham, MA). All plasma samples were extracted by solid-phase extraction using 1cc Oasis HLB cartridges (Waters) with the manufacturer's standard protocol. The specific ion transition for midazolam, diclofenac, dextromethorphan, and chlorzoxazone were 325.6 > 291, 294 > 250, 272 > 171, and 168.6 > 132, respectively. The assay has been validated with the linearity range from 0.2 to 250 ng/ml for midazolam and 2.5 to 500 ng/ml for diclofenac, dextromethorphan, and chlorzoxazone. Assay accuracy (3 days) is greater than 86.4% for all four probe drugs. Interday and intraday variability were <20.0 and <18.1%, respectively. Xcalibur 2.0 software was used for data acquisition and analysis.
Noncompartmental Model Analysis.
Noncompartmental pharmacokinetic analysis was used for calculating the area under curve (AUC0–inf) and systemic clearance (CL) from each individual time concentration curve of midazolam, diclofenac, dextromethorphan, and chlorzoxazone by WinNonlin 5.2 (Pharsight, Mountain View, CA). AUC was calculated from drug plasma concentration data based on the trapezoidal rule. The clearance was calculated from the dose and AUC.
Population Pharmacokinetic Modeling.
The population pharmacokinetic model was constructed using a nonlinear mixed-effects approach as implemented in NONMEM VI (Icon, Hanover, MD). The first-order conditional estimation method with interaction was used to estimate all parameters. The population pharmacokinetic model consisted of a pharmacokinetic structural model and a statistical model in which between-subject and within-subject variability were described. One- and two-compartment structural pharmacokinetic models were investigated as base model structures. The one-compartment model structure was evaluated using the ADVAN1 TRANS2 subroutine (estimate CL and V) and the two-compartment model structure was implemented as the ADVAN3 TRANS4 subroutine [estimate CL, central volume of distribution (V1), peripheral volume of distribution (V2), and intercompartment clearance (Q)]. The interindividual variability in the pharmacokinetic parameters was assumed to be log normally distributed for all population parameters. Interindividual variability in model parameters were modeled using an exponential term: θi = θ × eη with ηi∼N(0, ω2), where θ is the population value, and ηi is the variable accounting for interindividual variability with mean zero and variance ω2. Residual variability (σ2), the discrepancy between the individual observed (Cobs, ij) and the individual model-predicted (Cpred, ij) plasma concentrations, was evaluated using additive, proportional, or a combined additive and proportional model: Cobs, ij = Cpred, ij × (1 + εij1+ εij2), where Cobs, ij is the jth measured observation in individual i, Cpred, ij is the jth model predicted value in individual i, and εij is the residual error. Further refinement of the error model was performed as needed. The model-building process was guided by analyzing the goodness-of-fit plots, plausibility of parameter estimates, precision of parameter estimates, and the lowest objective function value (OFV) provided by NONMEM. Final model parameters were reported with estimated means with S.E. (S.E. %).
Covariate Model.
After a base model was chosen for each drug, covariate effects on the parameters of that model were evaluated in the following way. Factors treated as categorical covariates were temperature (TEMG) (0 = normothermia, 1 = hypothermia) and CA injury (CAG) (0 = sham, 1 = CA). They have been tested in forward addition in either of the following ways: PTV = θ1 + θ2 × (Cov) or PTV = θ1 × θ2(Cov), where θ1 and θ2 are estimated fixed-effect parameters and Cov is the subject-specific value of the categorical covariate. Three physiological parameters (glucose, BUN, and K+) were tested in forward fashion as continuous covariates, which has been tested in the following: PTV = θ1 × θ2(Cov/Medcov), where θ1 and θ2 are estimated fixed-effect parameters, Cov is the subject-specific value of the continuous covariate, and MedCov is the median value of the Cov. To assess whether the model with covariates statistically improved the fit to the data from the base model, the difference between their objective function values, referred to as the log likelihood ratio, was calculated. This ratio was assumed to be χ2 distributed. A decrease in the OFV of 3.84 and 6.63 is considered to be significant at p = 0.05 and p = 0.01, respectively, with 1 df. For the final model, stepwise forward addition and then a backward elimination process was used.
The final covariate models were also evaluated using goodness-of-fit diagnostic plots, successful minimization, precision for all parameters (S.E. %), and a visual predictive check. For a predictive check, 1000 Monte Carlo simulation replicates of the original data set were generated using the final population model estimates. These simulated curves were compared with the observed data. The empirical Bayes estimates from NONMEM represented the parameters for individual subjects for each time point. The individual clearances generated for each time point from each of the four groups were visualized as a graph for further evaluation.
Other Statistical Analysis.
Physiological variables were compared by repeated-measures ANOVA (among groups and across time) with Bonferroni post hoc testing. Noncompartmental analyses for pharmacokinetic variables of each probe drug from different treatments were compared by one-way ANOVA with Bonferroni post hoc testing. Significance was denoted by p < 0.05. All figures and data are expressed as means ± S.D. as determined from Prism 5 (GraphPad Software Inc., San Diego, CA) or Microsoft Excel.
Results
Temperature Curves and Physiological Parameters.
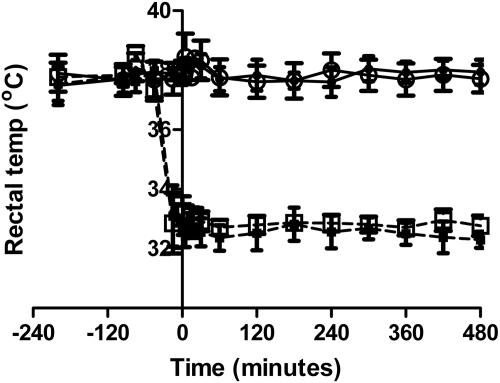
The body temperatures for sham normothermia, sham hypothermia, CA normothermia, and CA hypothermic groups were 37.9 ± 0.4, 32.5 ± 0.4, 37.8 ± 0.5, and 32.8 ± 0.3°C, respectively. Time-temperature curves of the four treatment groups are depicted in Fig. 1.
Fig. 1.
Temperature-time histories of the four treatment groups (mean ± S.D.). – – –, hypothermia groups; ——, normothermia groups. ●, sham normothermia; ■, sham hypothermia; ○, CA normothermia; □, CA hypothermia.
Physiological parameters MAP, PaCO2, pH, glucose, lactate, BUN, hematocrit, Mg2+, and K+ from each treatment group at each time point (baseline, 1-h after resuscitation, end of cooling induction, 4-h cooling, and 8-h cooling) are shown in Table 1. At 1 h after resuscitation, MAP in both CA groups was lower than in the sham control groups and then recovered with time. The PaCO2 level was lower in the CA groups than in sham groups at 1 h after resuscitation. At 1 h after resuscitation, the glucose level in the CA hypothermia group was the lowest among groups. BUN increased over time in all four groups, especially in the CA groups. pH in the CA hypothermia group decreased from the end of cooling induction compared with baseline. Mg2+ and K+ levels in CA groups both increased over time.
TABLE 1.
Physiological parameters at different time points in four groups
| Baseline before CA | 1 H after Resuscitation | End of Cooling Induction, PK Baseline | 4 H after Cooling | 8 H after Cooling | |
|---|---|---|---|---|---|
| MAP | |||||
| Sham normothermia | 101.7 ± 7.5 | 92.7 ± 8.8 | 102.8 ± 13.8 | 125.8 ± 16.4 | 115 ± 15.2 |
| Sham hypothermia | 110.6 ± 29.5 | 100.3 ± 11.5 | 119.5 ± 17.4 | 131.7 ± 21.9 | 129.2 ± 14.5 |
| CA normothermia | 109 ± 27.9 | 59.3 ± 14.4† | 86.3 ± 21.3 | 110 ± 5.8 | 136.5 ± 11.9# |
| CA hypothermia | 88 ± 11.5 | 69.2 ± 16.8#,† | 138.6 ± 12.9#,† | 130.5 ± 20.5# | 126.3 ± 9.9# |
| PaCO2 | |||||
| Sham normothermia | 37.9 ± 5.9 | 36.4 ± 4.4 | 35.8 ± 4.2 | 35.2 ± 3.4 | 34.8 ± 4.4 |
| Sham hypothermia | 37.1 ± 4.5 | 37.2 ± 2.4 | 40.1 ± 5.1 | 36.8 ± 7.1 | 30.8 ± 3.7# |
| CA normothermia | 39.3 ± 3.2 | 32.4 ± 5.4# | 36.7 ± 4.1 | 39.6 ± 8.0 | 35.4 ± 5.3 |
| CA hypothermia | 36.5 ± 2.6 | 29.1 ± 2.3#,† | 49.7 ± 5.9#,† | 42.9 ± 5.5#,† | 36.9 ± 8.3 |
| Glucose | |||||
| Sham normothermia | 86.8 ± 20.2 | 65.7 ± 8.07# | 69.7 ± 10.9# | 78.2 ± 18.6 | 75.3 ± 23.5 |
| Sham hypothermia | 113.5 ± 30.7 | 76.8 ± 16.6# | 87.8 ± 19.1# | 83.8 ± 18.2# | 89.2 ± 17.4# |
| CA normothermia | 112 ± 39.9 | 63.6 ± 11.8# | 56 ± 5.5#,† | 85.2 ± 28.2# | 71 ± 30.3# |
| CA hypothermia | 92.6 ± 17.0 | 53 ± 13.4#,† | 72.8 ± 29.6# | 80.7 ± 16.7# | 73.3 ± 38.1# |
| Lactate | |||||
| Sham normothermia | 0.78 ± 0.23 | 0.78 ± 0.29 | 0.82 ± 0.36 | 0.32 ± 0.30# | 0.5 ± 0.25# |
| Sham hypothermia | 0.96 ± 0.24 | 0.9 ± 0.35 | 0.56 ± 0.23# | 0.62 ± 0.23#,† | 0.4 ± 0.34# |
| CA normothermia | 0.82 ± 0.18 | 1.40 ± 0.7† | 0.7 ± 0.4 | 0.8 ± 0.23† | 1.32 ± 1.5#,† |
| CA hypothermia | 0.96 ± 0.31 | 0.88 ± 0.48 | 0.25 ± 0.13#,† | 0.47 ± 0.25#,† | 0.38 ± 0.11#,† |
| BUN | |||||
| Sham normothermia | 24.7 ± 7.3 | 22.5 ± 4.5 | 23.5 ± 6.0 | 38 ± 12.7# | 57.8 ± 19.7# |
| Sham hypothermia | 23.2 ± 5.6 | 24 ± 3.8 | 23.2 ± 4.6 | 37.2 ± 10.3# | 49.2 ± 16.7# |
| CA normothermia | 26.5 ± 8.3 | 30.1 ± 6.2† | 32.3 ± 7.5#,† | 45 ± 9.8#,† | 66.8 ± 18.0#,† |
| CA hypothermia | 26.8 ± 7.9 | 32.2 ± 9.0#,† | 31.7 ± 5.7#,† | 46.2 ± 10.2#,† | 60.6 ± 16.8# |
| Hematocrit % | |||||
| Sham normothermia | 36.7 ± 2.9 | 35.3 ± 1.2 | 34.2 ± 1.6 | 32.3 ± 2.7# | 35 ± 1.0 |
| Sham hypothermia | 35.8 ± 1.6 | 35.4 ± 2.4 | 35 ± 1.0 | 31.6 ± 1.0# | 30.8 ± 1.7#,† |
| CA normothermia | 37 ± 2.8 | 35.5 ± 1.5 | 33.3 ± 3.2# | 30 ± 1.9# | 30.8 ± 1.6#,† |
| CA hypothermia | 37 ± 4.3 | 36.8 ± 2.1 | 37.5 ± 1.6† | 31 ± 1.3# | 32.8 ± 3.7#,† |
| pH | |||||
| Sham normothermia | 7.38 ± 0.03 | 7.38 ± 0.03 | 7.39 ± 0.03 | 7.36 ± 0.06 | 7.36 ± 0.04 |
| Sham hypothermia | 7.38 ± 0.02 | 7.45 ± 0.16 | 7.36 ± 0.01 | 7.37 ± 0.03 | 7.39 ± 0.03 |
| CA normothermia | 7.37 ± 0.03 | 7.44 ± 0.03 | 7.39 ± 0.01 | 7.38 ± 0.02 | 7.35 ± 0.06 |
| CA hypothermia | 7.39 ± 0.03 | 7.43 ± 0.03 | 7.29 ± 0.04#,† | 7.32 ± 0.05#,† | 7.33 ± 0.03# |
| Mg2+ | |||||
| Sham normothermia | 0.36 ± 0.03 | 0.37 ± 0.03 | 0.37 ± 0.04 | 0.41 ± 0.05 | 0.41 ± 0.06 |
| Sham hypothermia | 0.39 ± 0.03 | 0.39 ± 0.04 | 0.40 ± 0.03 | 0.41 ± 0.05 | 0.43 ± 0.05 |
| CA normothermia | 0.36 ± 0.04 | 0.39 ± 0.04 | 0.41 ± 0.02 | 0.43 ± 0.04# | 0.46 ± 0.06#,† |
| CA hypothermia | 0.34 ± 0.05 | 0.43 ± 0.05#,† | 0.42 ± 0.02#,† | 0.41 ± 0.05# | 0.46 ± 0.09#,† |
| K+ | |||||
| Sham normothermia | 4.75 ± 0.53 | 4.74 ± 0.40 | 4.61 ± 0.37 | 5.25 ± 0.66# | 5.62 ± 0.98# |
| Sham hypothermia | 4.91 ± 0.30 | 4.79 ± 0.24 | 4.12 ± 0.35 | 3.75 ± 0.34#,† | 4.83 ± 0.28 |
| CA normothermia | 4.78 ± 0.35 | 4.84 ± 0.31 | 4.84 ± 0.37 | 5.43 ± 0.47 | 5.97 ± 0.42# |
| CA hypothermia | 4.6 ± 0.48 | 4.73 ± 0.45 | 3.89 ± 0.36#,† | 4.83 ± 0.18 | 5.67 ± 0.9# |
P < 0.05 compared with the baseline time point within the same treatment group (compare within row), by one-way repeated-measures ANOVA.
P < 0.05 compared with the sham normothermia within the same time point (compare within column) by one-way repeated-measures ANOVA.
Time-Concentration Curves and Systemic Clearance from Noncompartmental Model Analysis.
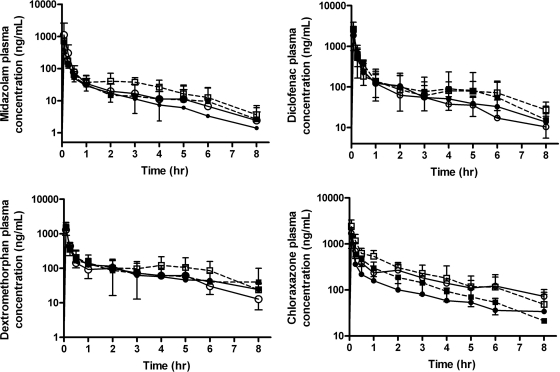
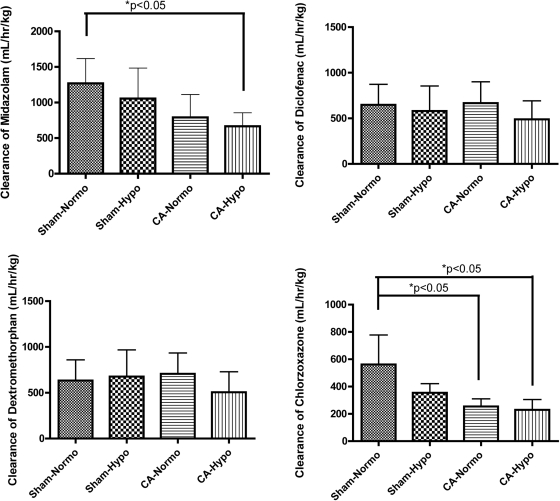
Time-concentration curves from the four groups for midazolam, diclofenac, dextromethorphan, and chlorzoxazone are shown in Fig. 2. Based on the time-concentration profile, systemic clearances calculated by noncompartmental analysis are shown in Fig. 3 and Table 2. The clearances of midazolam in sham hypothermia, CA normothermia, and CA hypothermia groups were 83.2, 62.4, and 52.5% of those in the sham normothermia group, respectively. A significant reduction in the clearance of midazolam in CA hypothermia was found compared with the sham normothermic control (681.6 ± 190.0 versus 1268.8 ± 348.9 ml · h−1 · kg−1, p < 0.05). The clearances of diclofenac in sham hypothermia, CA normothermia, and CA hypothermia groups were 89.6, 102.8, and 75.5% compared with those in the sham normothermia group. There was no significant difference found in clearances of diclofenac among the four groups. The clearances of dextromethorphan in sham hypothermia, CA normothermia, and CA hypothermia groups were 106.9, 111.5, and 79.9% of those in the sham normothermia group, respectively. There was no significant difference found in clearances of dextromethorphan among four groups. The clearances of chlorzoxazone in sham hypothermia, CA normothermia, and CA hypothermia groups were 63.1, 45.3, and 40.9% of the sham normothermia group, respectively. Significant reductions in clearances of chlorzoxazone for CA normothermia and CA hypothermia were observed compared with those in sham normothermia (254.7 ± 55.5 and 229.6 ± 75.6 versus 561.8 ± 215.9 ml · h−1 · kg−1, p < 0.05).
Fig. 2.
Time course of plasma concentrations obtained for midazolam, diclofenac, dextromethorphan, and chlorzoxazone from 0 to 8 h after intravenous bolus of 0.2 mg/kg midazolam, 0.5 mg/kg diclofenac, 0.5 mg/kg dextromethorphan, and 0.5 mg/kg chlorzoxazone. – – –, hypothermia groups; ——, normothermia groups. ●, sham normothermia; ■, sham hypothermia; ○, CA normothermia; □, CA hypothermia.
Fig. 3.
Systemic clearances of midazolam, diclofenac, dextromethorphan, and chlorzoxazone in four groups by noncompartmental PK analysis. Significant difference between groups was tested using one-way ANOVA with Bonferroni post hoc testing. *, p < 0.05. Normo, normothermia; Hypo, hypothermia.
TABLE 2.
Pharmacokinetic variables of four treatments from noncompartmental analysis for midazolam, diclofenac, dextromethorphan, and chlorzoxazone
One-way repeated-measures ANOVA with a Bonferroni post hoc test was used to detect the differences among the four treatment groups. Data are presented as means ± SD.
| Groups | AUC0–T | AUC0–inf | AUCextrap | Vz | CLs |
|---|---|---|---|---|---|
| ng × h/ml | % | l/kg | ml · h−1 · kg−1 | ||
| Midazolam | |||||
| Sham normothermia | 164 ± 43 | 167 ± 44 | 0.39–1.3 | 2.3 ± 1.1 | 1268 ± 349 |
| Sham hypothermia | 230 ± 139 | 234 ± 144 | 0.39–3.0 | 1.5 ± 0.75 | 1055 ± 428 |
| CA normothermia | 327 ± 271 | 336 ± 268 | 0.28–14.8 | 2.1 ± 1.5 | 791 ± 321 |
| CA hypothermia | 321 ± 128 | 329 ± 131 | 0.05–5.8 | 1.4 ± 1.3 | 682 ± 190* |
| Diclofenac | |||||
| Sham normothermia | 838 ± 364 | 873 ± 361 | 1.8–12.7 | 1.5 ± 0.91 | 647 ± 226 |
| Sham hypothermia | 1169 ± 915 | 1195 ± 941 | 0.87–3.3 | 0.99 ± 0.5 | 580 ± 273 |
| CA normothermia | 818 ± 368 | 852 ± 359 | 0.71–18.8 | 1.9 ± 1.1 | 660 ± 235 |
| CA hypothermia | 1252 ± 551 | 1213 ± 579 | 2.9–7.3 | 1.5 ± 0.98 | 489 ± 203 |
| Dextromethorphan | |||||
| Sham normothermia | 782 ± 431 | 869 ± 269 | 1.4–19.8 | 1.8 ± 0.87 | 633 ± 226 |
| Sham hypothermia | 675 ± 109 | 702 ± 106 | 2.1–5.0 | 2.7 ± 2.4 | 677 ± 292 |
| CA normothermia | 748 ± 299 | 786 ± 304 | 2.3–10.9 | 2.1 ± 1.1 | 706 ± 228 |
| CA hypothermia | 1192 ± 811 | 1264 ± 815 | 1.5–12.9 | 1.5 ± 1.2 | 506 ± 223 |
| Chlorzoxazone | |||||
| Sham normothermia | 926 ± 442 | 1112 ± 742 | 1.0–16.7 | 2.4 ± 2.3 | 562 ± 216 |
| Sham hypothermia | 1167 ± 617 | 1449 ± 262 | 2.5–4.8 | 0.83 ± 0.2 | 355 ± 66 |
| CA normothermia | 1748 ± 444 | 2055 ± 521* | 6.3–19.7 | 1.3 ± 0.6 | 255 ± 55* |
| CA hypothermia | 2254 ± 835* | 2409 ± 863* | 0.2–12.7 | 0.61 ± 0.3 | 230 ± 76* |
P < 0.05.
Population Pharmacokinetic Modeling Analysis.
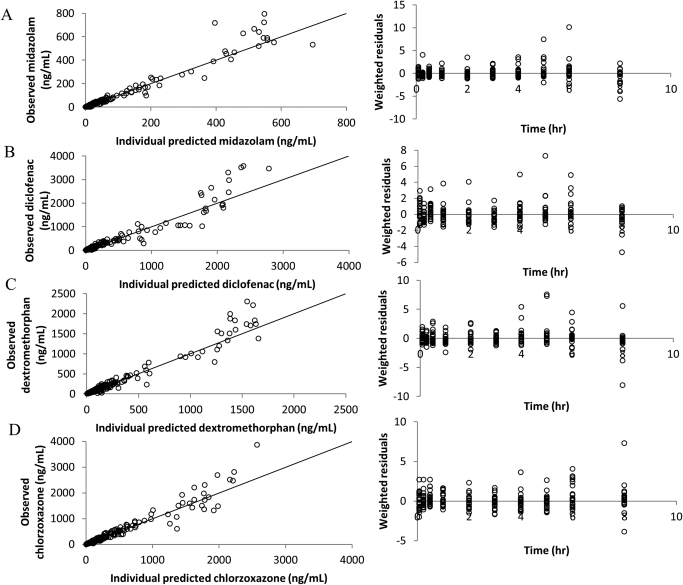
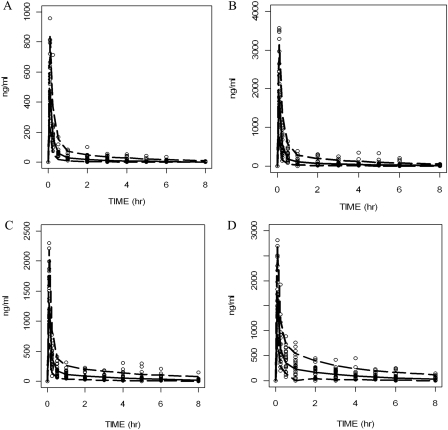
The two-compartment model structure was significantly better than the one-compartment model for the disposition of midazolam, diclofenac, dextromethorphan, and chlorzoxazone (OFV difference at least 16.0 points, 2 df, p < 0.001). Interindividual variability (ω2) terms were needed for CL, V1, Q, and V2 in models for midazolam, diclofenac, and dextromethorphan and for CL, Q, and V2 in the model for chlorzoxazone. The residual error model used for all four models was Cobs, ij = Cpred, ij × (1 +εij1). Each significant covariate in the final model has a lowered OFV value greater than 3.84 (p < 0.05) than the OFV value of the base model. Parameter estimates and S.E.s (S.E. %) calculated from the covariance step in NONMEM are shown in Table 3. Goodness-of-fit diagnostic plots, including individual predicted concentration-observed concentration and weighted residuals-time of the final models for midazolam, diclofenac, dextromethorphan and chlorzoxazone are depicted in Fig. 4.
TABLE 3.
Population pharmacokinetic parameter estimates from a nonlinear mixed-effect model for midazolam, diclofenac, dextromethorphan, and chlorzoxazone
Data are estimates (S.E. %).
| Parameters | Midazolam CL = θ1 + CAG × θ5 V1 = θ2 + TEMG × θ6 | Diclofenac CL = θ1 + TEMG × θ5 | Dextromethorphan V1 = θ2 + TEMG × θ5 | Chlorzoxazone CL = θ1 + CAG × θ5 V2 = θ4 + TEMG × θ6 |
|---|---|---|---|---|
| CL (θ1) (l · h−1 · kg−1) | 0.990 (12.7) | 0.587 (6.55) | 0.504 (5.33) | 0.433 (5.64) |
| V1 (θ2) (l/kg) | 0.258 (2.42) | 0.138 (2.31) | 0.204 (2.63) | 0.142 (1.68) |
| Q (θ3) (l · h−1 · kg−1) | 0.644 (10.6) | 0.507 (8.22) | 1.01 (12.5) | 0.889 (10.6) |
| V2 (θ4) (l/kg) | 1.0 (11.9) | 0.854 (15.1) | 1.42 (15.5) | 0.970 (15.1) |
| Covariate θ5 | −0.318 (11.5) | −0.184 (9.06) | −0.0685 (3.2) | −0.204 (6) |
| Covariate θ6 | −0.0786 (16.4) | −0.478 (15.8) | ||
| w2 (CL) | 0.119 (6.21) | 0.149 (5.48) | 0.269 (11.3) | 0.126 (3.54) |
| ω2 (V1) | 0.018 (14.6) | 0.082 (12.4) | 0.0385 (2.86) | |
| ω2 (Q) | 0.460 (12.4) | 0.312 (12.6) | 0.234 (8.99) | 0.218 (9.44) |
| ω2 (V2) | 0.214 (14.7) | 0.406 (11.8) | 0.170 (5.73) | 0.170 (5.91) |
| σ2 | 0.107 (12.5) | 0.119 (1.87) | 0.0838 (1.47) | 0.0662 (0.877) |
| Final OFV | 1268.8 | 1939.5 | 1880.9 | 2094.5 |
| OFV change | 10.4 | 6.0 | 4.7 | 23.6 |
| p value | <0.05 | <0.05 | <0.05 | <0.01 |
CL, clearance; V1, central compartment of distribution; Q, intercompartment clearance; V2, peripheral compartment of distribution; CAG, CA categorical covariate (0 = sham, 1 = CA); TEMG, temperature categorical covariate (0 = normothermia, 1 = hypothermia); ω2, interindividual variability; σ2, residual error; OFV, objective function value; covariate θ5, effect of the first significant covariate on the specific PK parameters (CA for midazolam and chlorzoxazone and temperature for dextromethorphan and diclofenac); covariate θ6, effect of the second significant covariate found on specific PK parameters (temperature for midazolam and chlorzoxazone).
Fig. 4.
Goodness-of-fit diagnostic plots: individual predicted concentration-observed concentration and weighted residuals-time of midazolam (A), diclofenac (B), dextromethorphan (C), and chlorzoxazone (D) from their final pharmacokinetic models. The solid line in the plot is the line of unity.
The final pharmacokinetic model for midazolam included a CA categorical (CAG) covariate in systemic clearance (CL) and a temperature categorical (TEMG) covariate in central volume of distribution (V1). CA groups showed decreased clearance of midazolam, which is described in the equation CL (liters per hour per kilogram) = 0.990 + CAG × (−0.318). The systemic clearances of midazolam in the CA groups were estimated to be 67.9% of those in the sham groups. Hypothermia was associated with a decrease in the V1 of midazolam. The specific relationship is shown in the equation V1 (liters per hour) = 0.258 + TEMG × (−0.0786). The V1 of midazolam in the hypothermic groups was estimated to be 69.5% of that seen in normothermia. The final pharmacokinetic model for diclofenac included a TEMG covariate in CL, which can be described in the equation CL (liters per hour per kilogram) = 0.587 + TEMG × (−0.184). The CL of diclofenac in the hypothermic groups was estimated to be 68.7% of that in normothermia. The final pharmacokinetic model for dextromethorphan included a TEMG covariate in V1, which can be described as V1 (liters per kilogram) = 0.204 + TEMG × (−0.0685). The central volume of distribution of dextromethorphan in hypothermia group was estimated to be 66.4% of that seen in normothermia. The final pharmacokinetic model for chlorzoxazone included a CAG covariate in CL and TEMG covariate in peripheral volume of distribution (V2). The CA groups showed decreased clearance of chlorzoxazone, which can be described as CL (liters per hour per kilogram) = 0.433 + CAG × (−0.204). Systemic clearance of chlorzoxazone in the CA groups was estimated to be 52.9% of those in the sham groups. In addition, hypothermia was associated with a decreased V2 for chlorzoxazone, described as V2 (liters per kilogram) = 0.970 + TEMG × (−0.478). The peripheral compartment volume of chlorzoxazone in hypothermia was estimated to be 50.7% of that seen in normothermia. In addition, three physiological parameters (glucose, BUN, and K+) were tested individually as continuous variables, and no significant association was found with any of the PK parameters.
Visual Predictive Check.
A predictive check from 1000 simulations using the final model estimates was performed for each model. The predictive check curves demonstrated that the simulated distribution of median and 95% confidence interval values was in agreement with the observed values (Fig. 5). The simulation, as well as the diagnostic plots, revealed that the final models provided a reliable description of the data with good precision of the structure model estimates and covariate effect.
Fig. 5.
Predictive check from 1000 simulations using the final estimated parameter of each model. A, midazolam; B, diclofenac; C, dextromethorphan; D, chlorzoxazone. ——, median of all simulated concentrations; – – –, 5th and 95th percentiles of all simulated values. All circles are observed values from the study. The simulated data and the observed data are in good agreement with each other.
CA-Hypothermia Interaction.
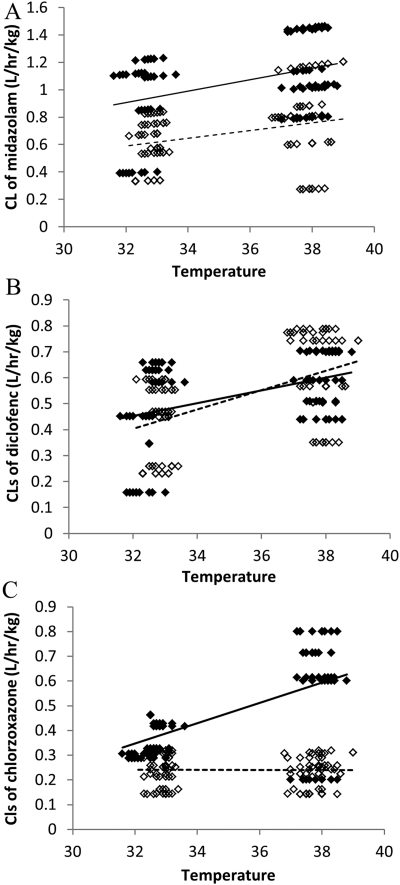
The individual clearances of midazolam, diclofenac, and chlorzoxazone from their final models were generated by NONMEM. Temperature and individual clearance correlations are plotted in Fig. 6. Within the same temperature groups, sham and CA have been separated. Two trend lines across sham normothermia-sham hypothermia and across CA normothermia-CA hypothermia were added separately (Fig. 6). A temperature-CA interaction was found for clearances of chlorzoxazone when the two trend lines were not parallel with each other (not in the same trend). The interaction indicated that when both CA and hypothermia exist, the metabolism would be different than if either occurred alone.
Fig. 6.
Individual generated clearances versus temperature from final pharmacokinetic models for midazolam (A), diclofenac (B), and chlorzoxazone (C). ♦ with solid trend line, sham groups; ♢ with dashed trend line, CA groups.
Discussion
Our study evaluated the effects of systemic hypothermia-mediated alterations on the activity of multiple P450 isoforms in vivo after cardiac arrest. Our results have revealed that the magnitude of the CA and temperature effects are P450 isoform-specific with the greatest alterations in the metabolism observed for CYP3A and CYP2E1 isoforms. Our results suggest that the combination of hypothermia with CA was more likely to be associated with decreased enzyme activities. Although other reports, by our laboratory and others, have focused largely on the effects of hypothermia on drug metabolism, we believe that CA and temperature-CA interaction findings may have clinical implications for patients with CA in whom therapeutic hypothermia is used.
CA is more likely to be correlated with the decreased activity of CYP3A observed in the hypothermic CA group. CA and its associated sequelae can cause severe metabolic disturbances. These effects on hepatic drug metabolism are currently also poorly understood. Previous studies have shown that the metabolism and elimination of drugs are often altered in critically ill patients. Shelly et al. (1987) demonstrated the failure of critically ill patients to metabolize midazolam. The clearance of midazolam was reduced, and the plasma concentration of its metabolite 1′-hydroxymidazolam was decreased in septic shock patients. Spina and Ensom (2007) showed that therapeutic monitoring of midazolam in the intensive care unit is warranted. Kirwan et al. (2009) showed that midazolam can be used to monitor changes in hepatic drug metabolism in critically ill patients. The concentration of midazolam at 4 h after administration was significantly greater in critically ill patients compared with that in normal subjects. A previous study by Vree et al. (1989) reported the decreased elimination rate of midazolam in the intensive care patients, which may due to decreased albumin and altered tissue binding. The CA effect as well as possible CA-temperature interactions observed in our study thus may be related to hepatic ischemia, liver dysfunction, compromised renal function, or decreased albumin. The use of hypothermia in patients with CA could thus result in an unrecognized interaction if it is translated to the clinical conditions for predicting metabolism of CYP3A in critical illness.
Previous studies demonstrated reduced metabolism of CYP3A substrates during hypothermia (Caldwell et al., 2000; Fukuoka et al., 2004; Fritz et al., 2005; Hostler et al., 2010). Our previous study predicted approximately 11.1% midazolam clearance decrease per degree Celsius change below 36.5°C in healthy volunteers. Altered pharmacokinetic parameters including both clearance and volume of distribution have been reported during hypothermia. Hypothermia decreased the central compartment volume of midazolam in this study and therefore also explained the observed higher plasma concentration during hypothermia. Our laboratory also previously tested midazolam metabolism during hypothermia after intravenous infusion, and there were approximately 20% decreases in clearance in patients in hypothermic CA compared with those in normothermic CA, which was consistent with our current study. Our current understanding is that with the combination of hypothermia and CA both may affect the activity of CYP3A and the metabolism and distribution of midazolam.
Likewise, the combination of hypothermia and CA affects the activity of CYP2E1. There was a hypothermia-CA interaction effect on the clearance of chlorzoxazone. Hypothermia reduced the peripheral volume of distribution. This result is consistent with a previous study in our laboratory, in which no significant difference was observed between CA normothermia and CA hypothermia at 33°C for chlorzoxazone metabolism with a 5-hour hypothermia treatment (Tortorici et al., 2009). Twenty-four hours after CA and rewarming, the activity of CYP2E1 in the CA normothermia group was found to be significantly lower than that in the control and CA hypothermia groups. A previous study in our laboratory showed that hypothermia significantly reduced the CYP2E1 activity at 30°C after cardiac arrest (Tortorici et al., 2006). In addition, hypothermia reduced binding affinity (Km) rather than capacity (Vmax) for chlorzoxazone at 30°C in vitro. Others have shown that the metabolism of chlorzoxazone and mephenytoin was correlated with the multiple organ dysfunction score and the multiple organ failure score for CYP2C19 and CYP2E1 activities in critically ill trauma patients (Harbrecht et al., 2005). The P450 isoform activity was differentially altered as has been suggested.
Furthermore, there was no significant difference found for diclofenac (CYP2C) clearance among the four groups by noncompartmental model analysis. However, in the population PK model, after we grouped hypothermia groups versus normothermia groups, there was a weak association between hypothermia and reduced clearance of diclofenac. In this model, ω block structure (on CL, Q, and V2) was used, which indicated an interaction between clearance and volume of distribution. This result may indicate a differential effect from that for the mathematic calculation, and the temperature effect on CYP2C activity needs further investigation. Likewise, there was no significant difference in clearance found for dextromethorphan (CYP2D) among the four groups. Hypothermia was correlated with the reduced central volume of distribution of dextromethorphan. During hypothermia, the volume of distribution of many drugs is affected, among which pentobarbital and rocuronium are CYP2D substrates (Schaible et al., 1982; Beaufort et al., 1995). Compared with other P450 isoforms, CYP2D as well as CYP2C can interact with specific functional groups in the substrate binding pocket (e.g., a basic nitrogen for CYP2D6 and an acid for CYP2C9) and help orient them and possibility maintain the metabolic rate and the clearance of the substrates better than other P450 isoforms (Redlich et al., 2008). Potential mechanisms for altered volume of distribution during hypothermia include global blood perfusion changes, blood pH alterations, altered lipid solubility, and potential alterations in drug tissue binding (van den Broek et al., 2010). At present, no studies have been conducted to explore the exact mechanisms of these alterations; however, the reduction in volume of distribution has been consistent across studies and warrants future mechanistic evaluation.
In general, the clearance of hepatically metabolized drugs is either dependent on hepatic blood flow or dependent on liver enzyme activity/plasma protein binding on the basis of the classic article of Pang and Rowland (1977), which describes the well stirred model. Because the goal of this study was to evaluate P450 isoform-specific metabolism via the use of probe drugs, all of the selected drugs have low hepatic extraction ratios; therefore, their clearance is highly dependent on enzyme intrinsic activity rather than on hepatic blood flow. In addition, the most severe changes in hepatic blood flow would be expected to occur during the acute period of cardiac arrest. Because our drug compounds were not administered until 90 min after resuscitation, it is highly unlikely that hepatic blood flow changes at this later time point would have a significant effect on the hepatic clearance of low hepatic extraction probe drugs. However, the effects of cardiac arrest and hypothermia on hepatic elimination of high and low extraction drugs are important, given the significant number of high extraction ratio drugs, such as propofol and fentanyl, that are administered to this patient population. Further research to elucidate clearance changes and alterations in hepatic blood flow in these models represents an important area of study.
One limitation of this study is the use of probe drugs as indices of given P450 phenotypic activities. Although the best possible probes were selected for this analysis, it is important to note the significant limitations of the use of these probes as phenotypic measures of P450 isoform-specific activity. In particular, diclofenac has been shown in both rats and humans to undergo significant glucuronidation as a major route of elimination. Indeed, several researchers have demonstrated that acyl glucuronidation of diclofenac is the predominant route of elimination in nonhuman primates and human liver microsomes (Kumar et al., 2002; Prueksaritanont et al., 2006). In the rat, diclofenac elimination is more dependent on CYP2C; however, glucuronidation is a significant contributor to the overall elimination, and, therefore, our results with diclofenac are probably due to alterations in CYP2C metabolism and/or acyl glucuronidation. Likewise, other probes have demonstrated involvement of other P450 isoforms, and results may not be solely due to changes in a single isoform (Wang and Unadkat, 1999; Kuo et al., 2003). Although these limitations of probe drug specificity exist, the selected drugs do represent different pathways of elimination, and, therefore, changes in clearance are reflective of differing changes in their individual elimination routes. The translational relevance of these alterations to humans will require further investigation with the important consideration that several of these probe drugs are even less selective in humans. Even with these limitations, it is clear from this study that differential effects are seen with drugs that predominantly rely on differing routes of hepatic metabolism during cardiac arrest and hypothermia.
In conclusion, our current results have shown the effect of CA and hypothermia on isoform-specific activity. Given the prominent role of the use of mild hypothermia in the management of patients with CA, further translational studies using clinically relevant drugs and pharmacokinetic-pharmacodynamic modeling are vital to further explain and confirm the isoform-specific effect in different disease and temperature states. Ultimately, the effect of therapeutic hypothermia on drug metabolism in patients with CA must be further studied to optimize therapy in these highly vulnerable patients.
Acknowledgments
We acknowledge John Melick for his expertise in the technical aspects of these animal models.
This work was supported by the National Institutes of Health National Center for Research Resources [Grant S10-RR023461, Award KL2-RR024154] (to S.M.P. and P.E.E., respectively); the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM073031] (to S.M.P.); and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS30318] (to P.M.K.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/dmd.111.040642.
- CA
- cardiac arrest
- PK
- pharmacokinetic
- P450
- cytochrome P450
- PaCO2
- partial pressure of carbon dioxide
- MAP
- mean arterial pressure
- BUN
- blood urea nitrogen
- UPLC
- ultraperformance liquid chromatography
- AUC
- area under the curve
- OFV
- objective function value
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Zhou, Empey, Kochanek, and Poloyac.
Conducted experiments: Zhou.
Contributed new reagents or analytic tools: Zhou and Poloyac.
Performed data analysis: Zhou and Bies.
Wrote or contributed to the writing of the manuscript: Zhou, Empey, Bies, Kochanek, and Poloyac.
References
- Beaufort AM, Wierda JM, Belopavlovic M, Nederveen PJ, Kleef UW, Agoston S. (1995) The influence of hypothermia (surface cooling) on the time-course of action and on the pharmacokinetics of rocuronium in humans. Eur J Anaesthesiol Suppl 11:95–106 [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346:557–563 [DOI] [PubMed] [Google Scholar]
- Blakey GE, Lockton JA, Perrett J, Norwood P, Russell M, Aherne Z, Plume J. (2004) Pharmacokinetic and pharmacodynamic assessment of a five-probe metabolic cocktail for CYPs 1A2, 3A4, 2C9, 2D6 and 2E1. Br J Clin Pharmacol 57:162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JE, Heier T, Wright PM, Lin S, McCarthy G, Szenohradszky J, Sharma ML, Hing JP, Schroeder M, Sessler DI. (2000) Temperature-dependent pharmacokinetics and pharmacodynamics of vecuronium. Anesthesiology 92:84–93 [DOI] [PubMed] [Google Scholar]
- Cullen DJ, Sweitzer BJ, Bates DW, Burdick E, Edmondson A, Leape LL. (1997) Preventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care units. Crit Care Med 25:1289–1297 [DOI] [PubMed] [Google Scholar]
- Fritz HG, Holzmayr M, Walter B, Moeritz KU, Lupp A, Bauer R. (2005) The effect of mild hypothermia on plasma fentanyl concentration and biotransformation in juvenile pigs. Anesth Analg 100:996–1002 [DOI] [PubMed] [Google Scholar]
- Frye RF. (2004) Probing the world of cytochrome P450 enzymes. Mol Interv 4:157–162 [DOI] [PubMed] [Google Scholar]
- Fukuoka N, Aibiki M, Tsukamoto T, Seki K, Morita S. (2004) Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation 60:225–230 [DOI] [PubMed] [Google Scholar]
- Geocadin RG, Koenig MA, Jia X, Stevens RD, Peberdy MA. (2008) Management of brain injury after resuscitation from cardiac arrest. Neurol Clin 26:487–506, ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian S, Chetty M, Tattam BN, Chem MC, Glen J, Rahme J, Stankovic Z, Ramzan I, Murray M, McLachlan AJ. (2009) A high-throughput assay using liquid chromatography-tandem mass spectrometry for simultaneous in vivo phenotyping of 5 major cytochrome p450 enzymes in patients. Ther Drug Monit 31:239–246 [DOI] [PubMed] [Google Scholar]
- Harbrecht BG, Frye RF, Zenati MS, Branch RA, Peitzman AB. (2005) Cytochrome P-450 activity is differentially altered in severely injured patients. Crit Care Med 33:541–546 [DOI] [PubMed] [Google Scholar]
- Holzer M. (2010) Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 363:1256–1264 [DOI] [PubMed] [Google Scholar]
- Hostler D, Zhou J, Tortorici MA, Bies RR, Rittenberger JC, Empey PE, Kochanek PM, Callaway CW, Poloyac SM. (2010) Mild hypothermia alters midazolam pharmacokinetics in normal healthy volunteers. Drug Metab Dispos 38:781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study Group (2002) Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 346:549–556 [DOI] [PubMed] [Google Scholar]
- Iida Y, Nishi S, Asada A. (2001) Effect of mild therapeutic hypothermia on phenytoin pharmacokinetics. Ther Drug Monit 23:192–197 [DOI] [PubMed] [Google Scholar]
- Kirwan CJ, Lee T, Holt DW, Grounds RM, MacPhee IA, Philips BJ. (2009) Using midazolam to monitor changes in hepatic drug metabolism in critically ill patients. Intensive Care Med 35:1271–1275 [DOI] [PubMed] [Google Scholar]
- Krösser S, Neugebauer R, Dolgos H, Fluck M, Rost KL, Kovar A. (2006) Investigation of sarizotan's impact on the pharmacokinetics of probe drugs for major cytochrome P450 isoenzymes: a combined cocktail trial. Eur J Clin Pharmacol 62:277–284 [DOI] [PubMed] [Google Scholar]
- Kumar S, Samuel K, Subramanian R, Braun MP, Stearns RA, Chiu SH, Evans DC, Baillie TA. (2002) Extrapolation of diclofenac clearance from in vitro microsomal metabolism data: role of acyl glucuronidation and sequential oxidative metabolism of the acyl glucuronide. J Pharmacol Exp Ther 303:969–978 [DOI] [PubMed] [Google Scholar]
- Kuo BP, Hu OY, Hsiong CH, Pao LH, Chen TS, Hung CF. (2003) Single-point plasma or urine dextromethorphan method for determining CYP3A activity. Biopharm Drug Dispos 24:367–373 [DOI] [PubMed] [Google Scholar]
- Lazarou J, Pomeranz BH, Corey PN. (1998) Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279:1200–1205 [DOI] [PubMed] [Google Scholar]
- Leslie K, Sessler DI, Bjorksten AR, Moayeri A. (1995) Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg 80:1007–1014 [DOI] [PubMed] [Google Scholar]
- Manole MD, Kochanek PM, Fink EL, Clark RS. (2009) Postcardiac arrest syndrome: focus on the brain. Curr Opin Pediatr 21:745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang KS, Rowland M. (1977) Hepatic clearance of drugs. I. Theoretical considerations of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J Pharmacokinet Biopharm 5:625–653 [DOI] [PubMed] [Google Scholar]
- Prueksaritanont T, Li C, Tang C, Kuo Y, Strong-Basalyga K, Carr B. (2006) Rifampin induces the in vitro oxidative metabolism, but not the in vivo clearance of diclofenac in rhesus monkeys. Drug Metab Dispos 34:1806–1810 [DOI] [PubMed] [Google Scholar]
- Redlich G, Zanger UM, Riedmaier S, Bache N, Giessing AB, Eisenacher M, Stephan C, Meyer HE, Jensen ON, Marcus K. (2008) Distinction between human cytochrome P450 (CYP) isoforms and identification of new phosphorylation sites by mass spectrometry. J Proteome Res 7:4678–4688 [DOI] [PubMed] [Google Scholar]
- Róka A, Melinda KT, Vásárhelyi B, Machay T, Azzopardi D, Szabó M. (2008) Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics 121:e844–849 [DOI] [PubMed] [Google Scholar]
- Ryu JY, Song IS, Sunwoo YE, Shon JH, Liu KH, Cha IJ, Shin JG. (2007) Development of the “Inje cocktail” for high-throughput evaluation of five human cytochrome P450 isoforms in vivo. Clin Pharmacol Ther 82:531–540 [DOI] [PubMed] [Google Scholar]
- Schaible DH, Cupit GC, Swedlow DB, Rocci ML., Jr (1982) High-dose pentobarbital pharmacokinetics in hypothermic brain-injured children. J Pediatr 100:655–660 [DOI] [PubMed] [Google Scholar]
- Scott RJ, Palmer J, Lewis IA, Pleasance S. (1999) Determination of a ‘GW cocktail’ of cytochrome P450 probe substrates and their metabolites in plasma and urine using automated solid phase extraction and fast gradient liquid chromatography tandem mass spectrometry. Rapid Commun Mass Spectrom 13:2305–2319 [DOI] [PubMed] [Google Scholar]
- Shelly MP, Mendel L, Park GR. (1987) Failure of critically ill patients to metabolise midazolam. Anaesthesia 42:619–626 [DOI] [PubMed] [Google Scholar]
- Spina SP, Ensom MH. (2007) Clinical pharmacokinetic monitoring of midazolam in critically ill patients. Pharmacotherapy 27:389–398 [DOI] [PubMed] [Google Scholar]
- Streetman DS, Bertino JS, Jr, Nafziger AN. (2000) Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics 10:187–216 [DOI] [PubMed] [Google Scholar]
- Tortorici MA, Kochanek PM, Bies RR, Poloyac SM. (2006) Therapeutic hypothermia-induced pharmacokinetic alterations on CYP2E1 chlorzoxazone-mediated metabolism in a cardiac arrest rat model. Crit Care Med 34:785–791 [DOI] [PubMed] [Google Scholar]
- Tortorici MA, Kochanek PM, Poloyac SM. (2007) Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 35:2196–2204 [DOI] [PubMed] [Google Scholar]
- Tortorici MA, Mu Y, Kochanek PM, Xie W, Poloyac SM. (2009) Moderate hypothermia prevents cardiac arrest-mediated suppression of drug metabolism and induction of interleukin-6 in rats. Crit Care Med 37:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek MP, Groenendaal F, Egberts AC, Rademaker CM. (2010) Effects of hypothermia on pharmacokinetics and pharmacodynamics: a systematic review of preclinical and clinical studies. Clin Pharmacokinet 49:277–294 [DOI] [PubMed] [Google Scholar]
- Vargas E, Simón J, Martin JC, Puerro M, Gonzalez-Callejo MA, Jaime M, Gomez-Mayoral B, Duque F, Gomez-Delgado A, Moreno A. (1998) Effect of adverse drug reactions on length of stay in intensive care units. Clin Drug Investig 15:353–360 [DOI] [PubMed] [Google Scholar]
- Vree TB, Shimoda M, Driessen JJ, Guelen PJ, Janssen TJ, Termond EF, van Dalen R, Hafkenscheid JC, Dirksen MS. (1989) Decreased plasma albumin concentration results in increased volume of distribution and decreased elimination of midazolam in intensive care patients. Clin Pharmacol Ther 46:537–544 [DOI] [PubMed] [Google Scholar]
- Wang Y, Unadkat JD. (1999) Enzymes in addition to CYP3A4 and 3A5 mediate N-demethylation of dextromethorphan in human liver microsomes. Biopharm Drug Dispos 20:341–346 [DOI] [PubMed] [Google Scholar]
- Xia CH, Sun JG, Wang GJ, Shang LL, Zhang XX, Zhang R, Peng Y, Wang XJ, Hao HP, Xie L, et al. (2010) Herb-drug interactions: in vivo and in vitro effect of Shenmai injection, a herbal preparation, on the metabolic activities of hepatic cytochrome P450 3A1/2, 2C6, 1A2, and 2E1 in rats. Planta Med 76:245–250 [DOI] [PubMed] [Google Scholar]